Abstract
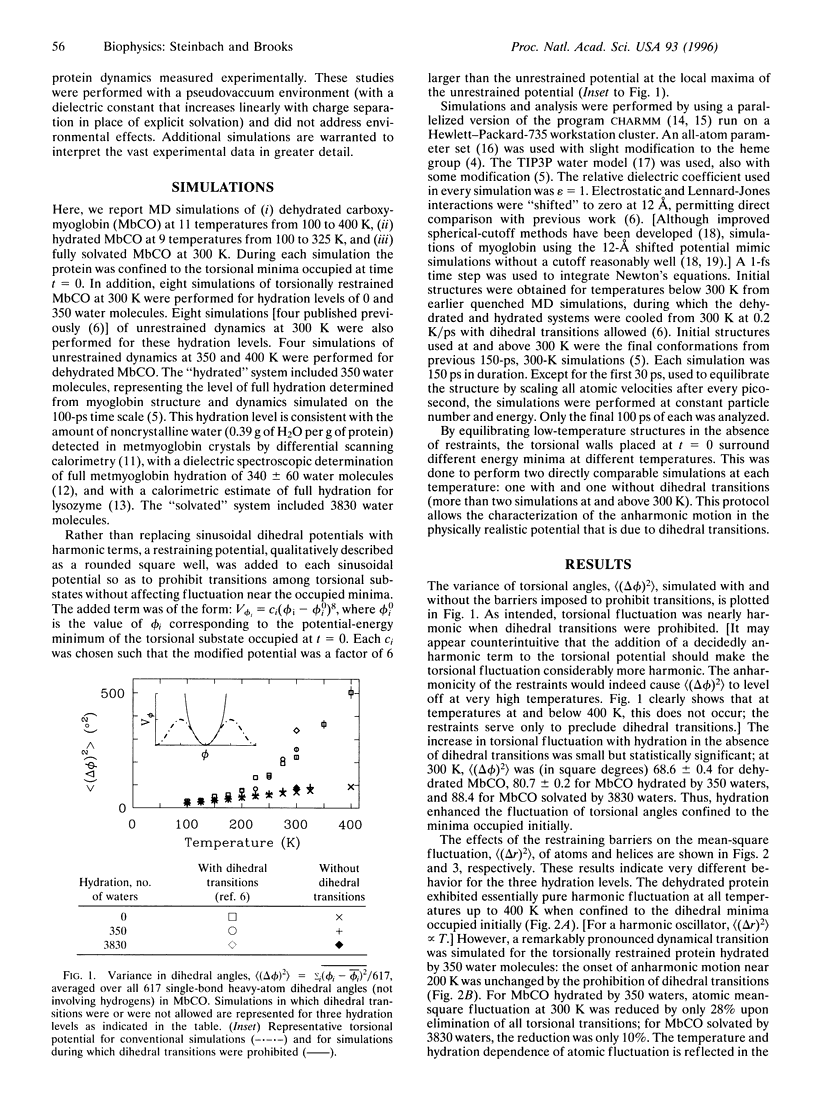
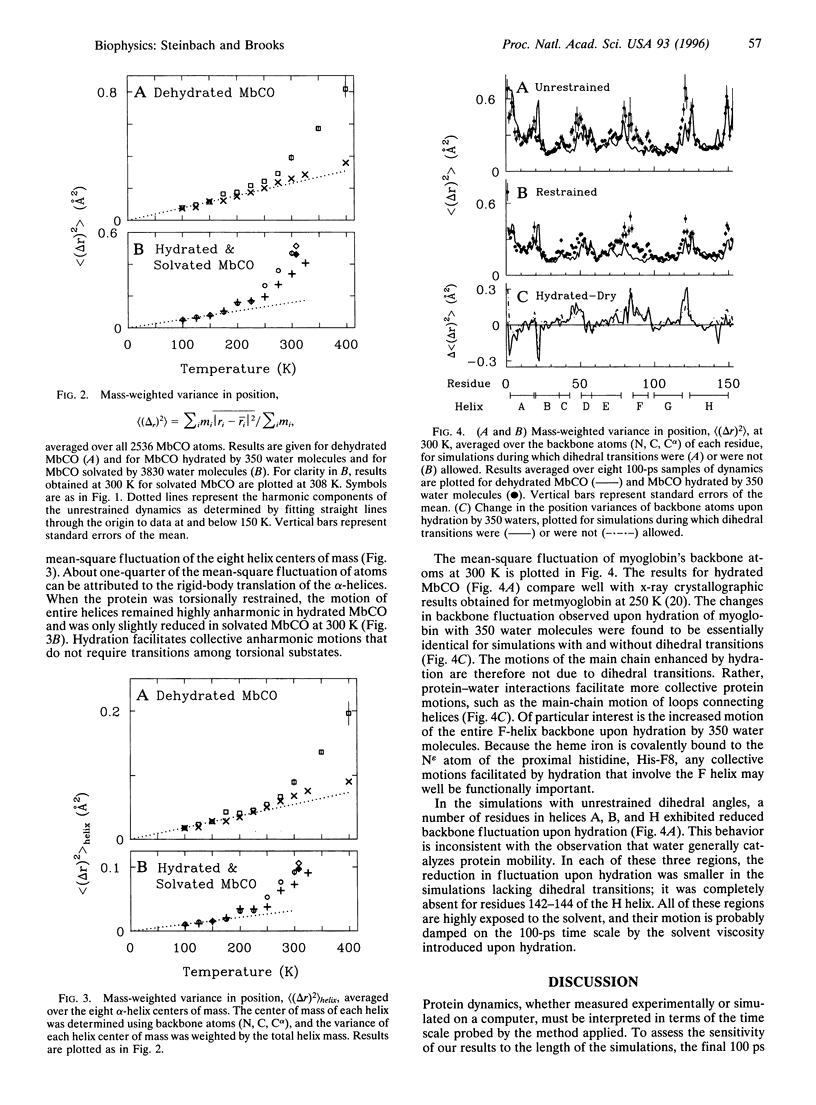
To characterize the functionally important anharmonic motions of proteins, simulations of carboxymyoglobin (MbCO) dynamics have been performed during which dihedral transitions were prohibited. Comparison of torsionally restrained and unrestrained protein dynamics simulated at three levels of hydration and at temperatures ranging from 100 to 400 K suggests that hydration "catalyzes" protein mobility by facilitating collective anharmonic motions that do not require dihedral transitions. When dihedral transitions were prohibited, dehydrated MbCO, to a good approximation, exhibited only harmonic fluctuations, whereas hydrated MbCO exhibited both harmonic and anharmonic motions. The fluctuation of helix centers of mass also remained highly anharmonic in the torsionally restrained hydrated system. Atomic mean-square fluctuation at 300 K was reduced upon prohibition of dihedral transitions by only 28% and 10% for MbCO hydrated by 350 and 3830 water molecules, respectively.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreani C., Filabozzi A., Menzinger F., Desideri A., Deriu A., Di Cola D. Dynamics of hydrogen atoms in superoxide dismutase by quasielastic neutron scattering. Biophys J. 1995 Jun;68(6):2519–2523. doi: 10.1016/S0006-3495(95)80434-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin R. H., Beeson K. W., Eisenstein L., Frauenfelder H., Gunsalus I. C. Dynamics of ligand binding to myoglobin. Biochemistry. 1975 Dec 2;14(24):5355–5373. doi: 10.1021/bi00695a021. [DOI] [PubMed] [Google Scholar]
- Chirgadze Y. N., Ovsepyan A. M. Hydration mobility in peptide structures. Biopolymers. 1972;11(10):2179–2186. doi: 10.1002/bip.1972.360111017. [DOI] [PubMed] [Google Scholar]
- Doster W., Bachleitner A., Dunau R., Hiebl M., Lüscher E. Thermal properties of water in myoglobin crystals and solutions at subzero temperatures. Biophys J. 1986 Aug;50(2):213–219. doi: 10.1016/S0006-3495(86)83455-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doster W., Cusack S., Petry W. Dynamical transition of myoglobin revealed by inelastic neutron scattering. Nature. 1989 Feb 23;337(6209):754–756. doi: 10.1038/337754a0. [DOI] [PubMed] [Google Scholar]
- Doster W, Cusack S, Petry W. Dynamic instability of liquidlike motions in a globular protein observed by inelastic neutron scattering. Phys Rev Lett. 1990 Aug 20;65(8):1080–1083. doi: 10.1103/PhysRevLett.65.1080. [DOI] [PubMed] [Google Scholar]
- Ferrand M., Dianoux A. J., Petry W., Zaccaï G. Thermal motions and function of bacteriorhodopsin in purple membranes: effects of temperature and hydration studied by neutron scattering. Proc Natl Acad Sci U S A. 1993 Oct 15;90(20):9668–9672. doi: 10.1073/pnas.90.20.9668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frauenfelder H., Parak F., Young R. D. Conformational substates in proteins. Annu Rev Biophys Biophys Chem. 1988;17:451–479. doi: 10.1146/annurev.bb.17.060188.002315. [DOI] [PubMed] [Google Scholar]
- Frauenfelder H., Petsko G. A., Tsernoglou D. Temperature-dependent X-ray diffraction as a probe of protein structural dynamics. Nature. 1979 Aug 16;280(5723):558–563. doi: 10.1038/280558a0. [DOI] [PubMed] [Google Scholar]
- Kneller G. R., Smith J. C. Liquid-like side-chain dynamics in myoglobin. J Mol Biol. 1994 Sep 23;242(3):181–185. doi: 10.1006/jmbi.1994.1570. [DOI] [PubMed] [Google Scholar]
- Krupyanskii YuF, Parak F., Goldanskii V. I., Mössbauer R. L., Gaubman E. E., Engelmann H., Suzdalev I. P. Investigation of large intramolecular movement within metmyoglobin by Rayleigh scattering of Mössbauer radiation (RSMR). Z Naturforsch C. 1982 Jan-Feb;37(1-2):57–62. doi: 10.1515/znc-1982-1-211. [DOI] [PubMed] [Google Scholar]
- Loncharich R. J., Brooks B. R. Temperature dependence of dynamics of hydrated myoglobin. Comparison of force field calculations with neutron scattering data. J Mol Biol. 1990 Oct 5;215(3):439–455. doi: 10.1016/s0022-2836(05)80363-8. [DOI] [PubMed] [Google Scholar]
- Loncharich R. J., Brooks B. R. The effects of truncating long-range forces on protein dynamics. Proteins. 1989;6(1):32–45. doi: 10.1002/prot.340060104. [DOI] [PubMed] [Google Scholar]
- Rasmussen B. F., Stock A. M., Ringe D., Petsko G. A. Crystalline ribonuclease A loses function below the dynamical transition at 220 K. Nature. 1992 Jun 4;357(6377):423–424. doi: 10.1038/357423a0. [DOI] [PubMed] [Google Scholar]
- Steinbach P. J., Brooks B. R. Protein hydration elucidated by molecular dynamics simulation. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):9135–9139. doi: 10.1073/pnas.90.19.9135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P. H., Rupley J. A. Protein--water interactions. Heat capacity of the lysozyme--water system. Biochemistry. 1979 Jun 12;18(12):2654–2661. doi: 10.1021/bi00579a035. [DOI] [PubMed] [Google Scholar]



