Abstract
Background: A relationship between hypothyroidism and depression has been assumed for many years; however, the true nature of this association has been difficult to define with many conflicting studies. In recent years, our knowledge in this area has increased significantly with large cohort studies and genetically driven studies being published. Objectives: We reviewed the literature on thyroid function and depression to determine if this relationship has been clarified. Methods: We performed a search on the Pubmed database using the terms ‘thyroid ’ and ‘mental health ’, ‘depression ’ and ‘well-being ’. Results: Large epidemiological studies generally suggest no association between thyroid function and depression in subjects without thyroid disease. Subjects on thyroxine have poorer psychological well-being than subjects with no thyroid disease even if biochemically euthyroid, they also show an association between thyroid function and well-being. Whilst there is some early evidence that genetic factors can influence well-being on thyroxine and response to combination therapy, there is also evidence to suggest that much morbidity on thyroxine may be due to initial misdiagnosis and mis-attribution of symptoms. Conclusion: Despite the large number of studies, the relationship between thyroid function and depression remains poorly defined. Clarification of the proportion of subjects on thyroxine incorrectly may assist the large (perhaps genetically driven) studies needed to move forward in this area, as it is expected that they cloud the results.
Key Words: Thyroid, Hypothyroidism, Depression, Thyroxine
Introduction
An association between hypothyroidism and depression has been accepted and taught in medicine for many years, although the nature of this relationship and what determines it have not been convincingly proven. Observations on which this association was first derived are the similarity of symptoms in severely depressed and hypothyroid patients, the therapeutic use of thyroid hormones in the management of depression and the apparent abnormalities in the hypothalamic-pituitary-thyroid axis of subjects with depression. However, there have been many conflicting studies in this area and this review seeks to define what our current understanding of this relationship is and where further research is required.
Physiology
The importance of thyroid hormones on cerebral development and functioning are highlighted by the devastating neurological consequences of severe iodine deficiency [1,2], MCT8 mutations [3,4] and untreated congenital hypothyroidism [5,6]. Furthermore, we have come to realise the importance of thyroid hormone transporters and iodothyronine deiodinases in delivering the hormones to where they have their actions [7].
In animal studies, thyroid hormones influence noradrenergic and serotonergic neurotransmission which play a key role in the pathogenesis of depression and are targets for current antidepressant therapies [8,9,10,11,12,13]. These studies have shown increased serotonin levels in the cerebral cortex of rats after T3 administration and also decreased serotonin synthesis in the brain with hypothyroidism. Serotonin also has an inhibitory effect on thyrotropin-releasing hormone (TRH) secretion suggesting a feedback loop which allows activation of the hypothalamus-pituitary-thyroid axis when serotonin levels in the brain are low.
Does Hypothyroidism (and Subclinical Hypothyroidism) Affect Mood and Psychological Well-Being?
Classic Epidemiology
There are many studies in this area with varied and often conflicting results. The reason for this is likely partly methodological in that the larger studies are likely to have less extensively defined both thyroid (TSH but not T4) and mood (self-reported questionnaire rather than clinical diagnosis) phenotypes. Sample selection is also important as population studies are more likely to have higher prevalence of (asymptomatic) subclinical hypothyroidism versus case control studies where patients are selected due to thyroid abnormalities.
It should be noted that studies differ in their method of measuring psychological impairment and this may also influence their comparability. This is due to the difficulty of making psychological diagnoses in a study of thousands of subjects. Some studies have looked at actual diagnoses of ‘depressive disorders ’, these are usually through database records (admissions or appointments for depression) or self-reported previous or current diagnoses of depression. Other studies have used validated questionnaires which pick up symptoms of depression; these questionnaires have generally shown good correlation with clinical diagnoses of depression. Still others use questionnaires designed to assess psychological well-being, these assess general well-being, however, can often be divided into depressive symptoms, anxiety symptoms, etc. and have also been shown to correlate with clinical diagnoses. Throughout this review we will attempt to identify which study uses which method of assessment; however, a longer interpretation of each study's relevance to clinical diagnosis is beyond the scope of this review.
The larger studies have shown either no effect or conversely an inverse relationship between thyroid function and mood. The HUNT 2 study from Norway is clearly the largest of these, looking at the relationship between TSH and Hospital Anxiety and Depression Scale (HADS) scores (self-reported symptom questionnaire) in over 30,000 individuals. The investigators found no association with HADS depression or anxiety in subjects with no history of thyroid disease [14]. Our reanalysis of this data considering only subjects with a TSH within the reference range (to exclude selection bias of subjects being put on thyroxine) again showed no association between TSH and HADS depression, in fact in males there was an indication that higher TSH was associated with slightly less HADS depression [15]. Two other large studies have shown this unexpected association with either lower T4 and/or higher TSH and current depressive syndrome (from depression section of Diagnostic Interview Schedule) in 6,869 young adults from the US [16] and worse psychological well-being (by self-reported questionnaire) in 2,269 men from the UK [17]. Two large studies in elderly subjects from the UK [18] and Australia (in men only) [19] showed no association with depression in 5,857 and 3,932 subjects, respectively.
All the studies in this area are summarised in table 1 (this does not include subjects on thyroid hormone replacement or pregnant subjects). The analysis column in this table explains the method by which statistical analysis was performed. ‘Continuous ’ suggests analysing for a linear association across thyroid hormone range, generally these have included not only subjects within the reference range but also those outside with no history of thyroid disease or taking thyroid-related medications. There is a potential bias here whereby the more symptomatic subjects may have been put on thyroxine and therefore removed from the analysis. Grouped analysis whereby groups of abnormal thyroid function are compared to the euthyroid reference population is the other common method of analysis; however, the small number of subjects in the abnormal groups often affects the power of these studies. In addition, those on thyroxine are left out of the analysis again leading to loss of the more symptomatic subjects. Overall, the evidence suggests no association of depression in subjects with thyroid function within or slightly outside the reference range. In fact, in males and in younger subjects there may be a small inverse association (less depression with lower T4 or higher TSH). There is some evidence for an association in more significantly hypothyroid subjects (TSH >10 or plus low T4) [20] and in subjects specifically chosen for thyroid dysfunction [21,22]. It is likely that in the larger, population-based studies these subjects are not prevalent enough to affect the analysis.
Table 1.
Studies investigating the relationship between thyroid function and depression/psychological well-being
| Study | n | Thyroid measure | Psychiatric measure | Analysis | Association | Direction | p |
|---|---|---|---|---|---|---|---|
| Engum et al. [14] | 30,589 | TSH | HADS | abnormal vs. euthyroid | no | – | |
| Forman-Hoffman and Philibert [16] | 6,869 | free T4/TSH | DIS | continuous | yes | ↓ ↓ | 0.001 TSH 0.03 free T4 |
| Roberts et al. [18] | 5,857 | free T4/TSH | HADS | continuous | no (depression) | – | |
| Almeida et al. [19] | 3,932 | free T4/TSH | GDS, ICD depression | continuous | no | ||
| Grabe et al. [74] | 3,790 | free T4/T3/TSH | Zersson Complaint Scale | abnormal vs. euthyroid | no | – | |
| Williams et al. [17] | 2,269 | total T4 | GHQ-30 | continuous | yes | ↓ | 0.03 |
| Philibert et al. [24] | 1,555 | free T4/TSH | lifetime MD | continuous | no | ||
| Guimaraes et al. [20] | 1,298 | free T4/TSH | PRIME-MD | abnormal vs. euthyroid | TSH >10 yes Clin Hypo yes SC Hypo no | ↑ ↑ – | |
| de Jongh et al. [75] | 1,219 | TSH +/− T4/T3 | CES-D | abnormal vs. euthyroid | ns | ||
| van de Ven et al. [23] | 1,125 | free T4/TSH | BDI, current/ever depression | groups of thyroid | ns | ||
| Kritz-Silverstein et al. [76] | 1,110 | TSH | BDI | continuous | men yes women no | ↓ – | 0.03 |
| Gussekloo et al. [77] | 599 | free T4/TSH | GDS | continuous | no | – | |
| Pop et al. [25] | 538 | free T4/TSH | EDS | abnormal vs. euthyroid | no | – | |
| Kim et al. [78] | 495 | TSH | GMS | continuous | ns | ||
| Jorde et al. [38] | 243 | free T4/TSH | GHQ-30 | SC hypothyroid vs. euthyroid | yes | ↓ | <0.01 |
| Gulseren et al. [21] | 160 | free T4/T3/TSH | HDRS, SF-36 | abnormal vs. euthyroid | yes | ↑ | 0.001 |
| Frey et al. [79] | 121 | free T4/T3/TSH | NEO depressive trait | continuous | TSH & neuro | ↓ | 0.0002 TSH |
| van Boxtel et al. [80] | 120 | TSH | SCL | continuous | no | – | |
| Bono et al. [22] | 36 | free T4/T3/TSH | HDRS | SC hypothyroid vs. treated | yes | ↑ | <0.05 |
HADS = Hospital anxiety and depression scale; DIS = diagnostic interview schedule; GDS = geriatric depression scale; ICD = international classification of diseases; GHQ = general health questionnaire; PRIME-MD = primary care evaluation of mental disorders; CES-D = centre for epidemiological studies-depression scale; BDI = becks depression index; EDS = edinburgh depression scale; GMS = geriatric mental scale; HDRS = hamilton depression rating scale; SF-36 = short form 36; NEO = neurotism, extraversion, openness personality inventory; SCL = symptomatic check list; SC = subclinical; ↑ = positive association; ↓ = inverse association.
An important bias introduced into these larger studies is that subjects with depressive symptoms and subclinical hypothyroidism are likely to have been commenced on thyroxine in countries where medical care and thyroid function testing are readily available. As these subjects are usually removed from these studies, the remaining subjects with subclinical hypothyroidism are likely to be less symptomatic; this hypothesis was supported by the study of Engum et al. [14] who showed the rate of psychological morbidity to be lower in those with a previously undiagnosed raised TSH than in the euthyroid population. This process would also increase the number of symptomatic subjects in the population on thyroxine, potentially contributing to the excess psychological morbidity in patients on thyroxine (discussed in more detail below). We tried to eliminate this bias by investigating this population across the normal range only and again found no association [15]; this appears to be true of other studies looking at groups across the reference range also [23].
A recent study by Philibert et al. [24] looked at several genetic polymorphisms known to be related to thyroid function and their influence on lifetime major depressive disorder and found an association with a polymorphism in the iodothyronine deiodinase 1 gene (DIO1) in Caucasian females. This study used 3 ethnically diverse, independent cohorts (combined n = 1,555) and looked at the relationship between 12 polymorphisms previously suspected to influence thyroid function. The polymorphism in DIO1 but not the others influenced lifetime depression, DIO2 polymorphisms were not tested. This is yet to be replicated.
Autoimmune Thyroid Disease
Several studies have investigated whether the presence of thyroid autoimmunity with thyroid hormone levels within the reference range is associated with depression. It is recognised that stressful events can exacerbate or bring on episodes of autoimmune diseases, and also there appears to be an association between thyroid autoimmunity and postpartum depression. Two studies have shown an association between thyroid autoimmunity and depression: Pop et al. [25] which showed an association in 583 women between TPO antibody levels >100 mU/l and Edinburgh Depression Scale scores and Kirim et al. [26] which showed an association in 201 subjects with TPO or Tg antibody positivity and Hamilton Depression Rating Scale scores. Against these, however, a study using the HUNT 2 data in 745 subjects with normal thyroid function did not show any association between presence of TPO antibodies and HADS score [27]. All three studies are small in terms of number of subjects with antibody positivity and have the potential for selection bias. Larger, unselected cohorts with thyroid antibody assessment are required to resolve this issue.
Subjects on Thyroid Hormone Replacement
The situation is different in subjects on thyroid hormone replacement. It has been clearly documented that these subjects have poorer well-being compared to the general population (see below) [14,15,28]. It also appears that the association between thyroid function and depression is more clearly seen in this population.
An association between thyroid function and depression in subjects on thyroxine was first shown by Saravanan et al. [29] who found a clear association between higher TSH and lower free T4 and poorer psychological well-being in 697 subjects on thyroxine from the UK. This persisted when only subjects with a TSH within the reference range (indicating adequate replacement) were included. Findings were similar in the HUNT 2 study from Norway [15] and a study from the Netherlands [30].
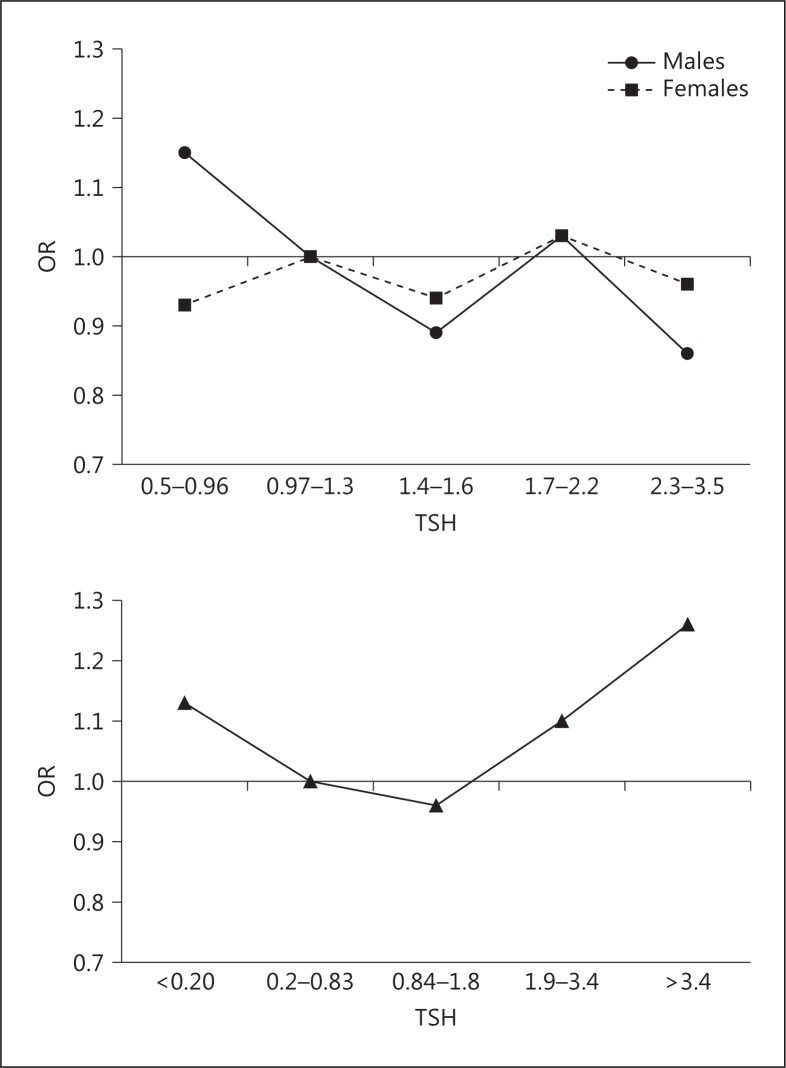
As there appeared to be a clear difference in the associations between thyroid function and depression in subjects who were and were not on thyroxine, we used the large sample size of the HUNT 2 study to determine if we could show a statistical difference in these relationships. We looked for an interaction of being on thyroxine on the relationship between TSH and HADS depression and found that this was the case in females (p = 0.02) indicating significantly different associations in the two populations [15]. This is best shown in figure 1.
Fig. 1.
Association of HADS depression by quintiles of TSH in males and females with no thyroid disease and females on thyroxine from the HUNT 2 cohort. The association in subjects with no thyroid disease is confined to the reference range to exclude selection bias (see text).
Against a causative association between thyroid function and psychological well-being is a study from Walsh et al. [31] in subjects on thyroid hormone replacement whereby alterations in thyroid hormone levels by alterations of dose did not appear to influence psychological well-being in these blinded subjects. The size and trial length suggest that a larger study with longer treatment times may be required for confirmation of this finding.
In addition to an association between thyroid function and depression in this population it has also been convincingly shown that subjects on thyroxine have poorer psychological well-being than their counterparts with no thyroid disease, even when only including subjects who's replacement is considered biochemically adequate. This finding further suggests an association between thyroid function and depression. Several studies have consistently shown poorer psychological well-being in subjects on thyroxine, even when considering only those subjects whose thyroxine replacement is considered to be adequate biochemically [28,30,32]. A study from the Rancho Bernado study in elderly subjects did not find increased depression but significantly higher antidepressant use in those on thyroxine [33]. In the HUNT study which is the largest of these, the OR of HADS depression in females on thyroxine (n = 1,546) to those with no thyroid disease (n = 18,137) was 1.46 (p < 0.001) [15]. The analysis was not performed in males due to the low numbers of males on thyroxine.
Why Might Subjects on Thyroxine Have Reduced Psychological Well-Being?
The association between thyroxine use and impaired psychological well-being has been replicated on at least 3 occasions and appears robust. This stands in contrast with the lack of association between thyroid function and psychological well-being in large community-based studies. Possible explanations for this apparent paradox might include the following.
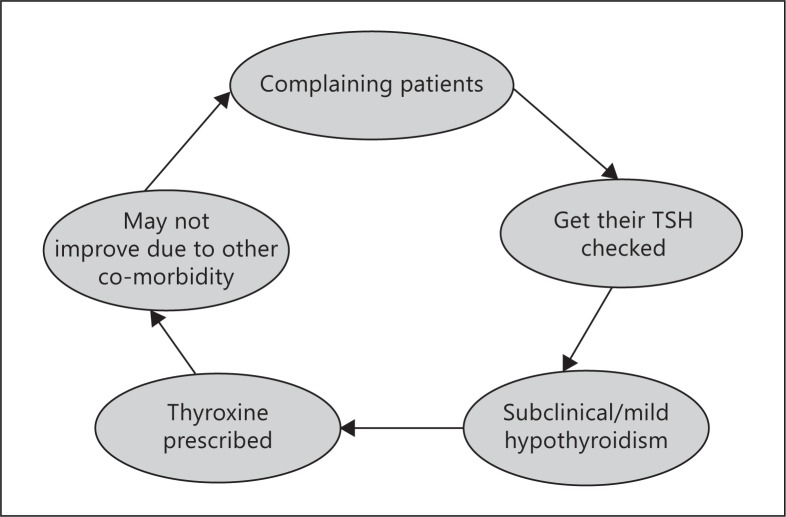
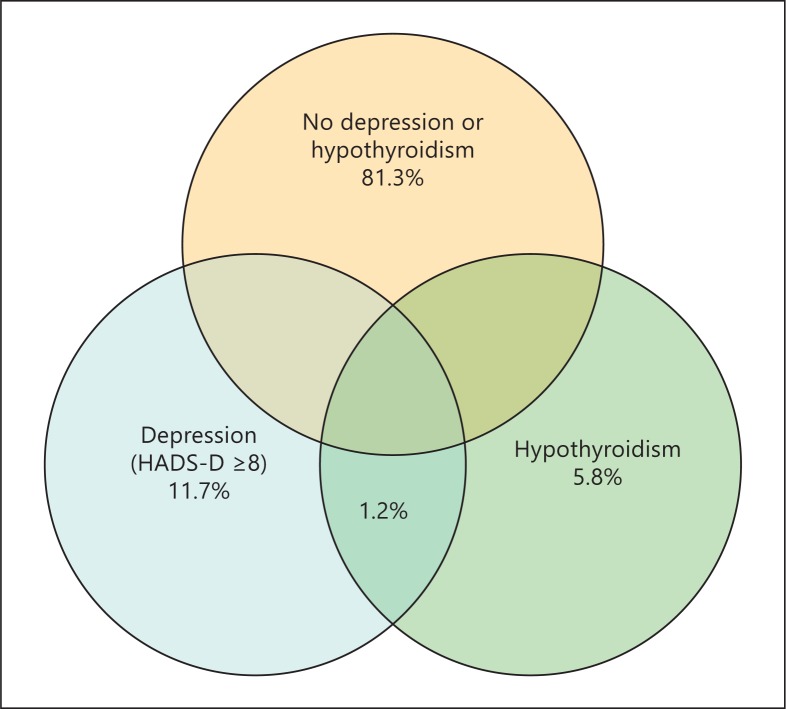
One possibility is the mis-attribution of symptoms due to a separate cause of low mood to incidentally discovered thyroid disease followed by treatment with thyroid hormone resulting in apparent, poorer psychological well-being of subjects on thyroxine. The lack of improvement of symptoms on thyroxine in these subjects results in a ‘cycle of misattribution ’ (fig. 2). Both depression and biochemical subclinical hypothyroidism are common, particularly in an older female population. Therefore, there is a reasonable prevalence of overlap between the two conditions, as show in a diagram drawn from the HUNT 2 data (fig. 3). Medical teaching and guidelines recommend looking for thyroid disease in subjects with depressive symptoms, and the finding of a mild elevation in TSH (shown above in large studies to not be associated with depression) may often lead to a diagnosis of hypothyroidism which is more palatable to both patient and clinician. There is indirect evidence for this occurrence, although the extent to which it contributes to poorer well-being on thyroxine cannot be estimated currently. In a study looking at mental health in general practice patients referred for thyroid function testing in the UK, we discovered that these patients had a higher rate of psychological morbidity than seen in all presentations (54.2 vs. 19%), suggesting that depressive symptoms were a common reason for assessing thyroid function. However, this practice did not diagnose any more thyroid disease than population screening, suggesting that the symptoms were mostly unrelated to thyroid disease [34]. This is reflected in the Colorado thyroid disease prevalence study which shows that low mood is a poor predictor of hypothyroidism [35]. Furthermore, despite guidelines on when to commence thyroxine (TSH >10 mIU/l or plus low T4) there is evidence from Scotland that the number of subjects who are commenced on thyroxine with a TSH less than 6.0 mIU/l is almost 50% [36], and a recent primary care database study in England of over 53,000 subjects on thyroxine indicated that around 64% had started therapy with a TSH <10 mIU/l [37]. Therefore, the majority of patients commenced on thyroxine have a TSH in the subclinical range (<10 mIU/l) and a considerable proportion of these could have been treated due to depressive symptoms. The problem with this practice is twofold: firstly, the patients' symptoms are unlikely to improve on thyroxine treatment resulting in dissatisfaction on thyroxine treatment and requests for increased doses or alternative treatments. Secondly, it is likely to significantly delay the correct diagnosis of depression with appropriate treatment.
Fig. 2.
Cycle of mis-attribution of symptoms to thyroid disease and continued symptoms. Proposed mechanism to explain why many subjects do not respond to thyroid hormone replacement despite adequate levels of TSH.
Fig. 3.
Overlap of subjects with both depression and raised TSH in the HUNT 2 cohort.
However, there are other explanations that could underlie the association between poor psychological well-being and thyroid hormone treatment. Subjects with a chronic disease and on lifelong medication are more likely to report poorer mental health than those with no illnesses, and certainly those on thyroxine generally have more comorbidities (e.g. heart disease) [28] and indeed the thyroid disease may have been detected in routine testing in association with these other conditions (e.g. routine testing of TSH in cardiac clinics). But it also remains possible that a subset of individuals respond poorly to exogenous thyroxine as replacement and/or that the presence of thyroid autoimmunity, irrespective of thyroid function, contributes to low mood.
Does Thyroid Hormone Replacement Correct Mood and Well-Being Deficit?
Treatment of Subclinical Hypothyroidism
Several relatively small studies (n = 36-70) have looked at the effect of treatment of subclinical hypothyroidism with thyroxine on psychological well-being/depression [21,38,39,40,41,42]. The studies are listed in table 2. The first four studies are placebo controlled and show no benefit from thyroxine replacement, except for Meier et al. [41] which shows improvement but only in those who started with a TSH >12 mIU/l. It could be questioned whether this represents subclinical or overt hypothyroidism. The other two are not placebo controlled, but have results compared to controls (with no thyroid disease) [21,42]. They both show improvement in well-being scores back down to levels seen in controls without thyroid disease; however, the lack of a placebo arm raises concerns about the placebo effect. Overall, the evidence is not convincing for a significant benefit of treatment, however there is a lack of larger placebo-controlled studies.
Table 2.
Studies investigating the effect of thyroxine treatment on psychological well-being in subclinical hypothyroidism
| Study | n (treated/placebo) | Duration | Measure | Result |
|---|---|---|---|---|
| Jorde et al. [38] | 36/33 | 1 year | BDI, GHQ-30 | ns |
| Jaeschke et al. [39] | 18/19 | >6 months | CTQ, SIP | ns |
| Kong et al. [40] | 23/17 | 6 months | HADS, GHQ-30 | ns |
| Meier et al. [41] | 31/32 | 50 weeks | Billewicz/Zulewski scores | significant improvement only in those with TSH >12 |
| Gulseren et al. [21] | 36/0 | 9–10 months | BDQ, HDRS, SF-36 | improved to control group |
| Baldini et al. [42] | 38/0 | 6 months | SF36 | improved to control group |
BDI = Becks depression index; GHQ = general health questionnaire; CTQ = chronic thyroid questionnaire; SIP = sickness impact profile; HADS = hospital anxiety and depression score, BDQ = brief disability questionnaire, HDRS = hamilton depression rating scale; SF-36 = short form 36.
Combination T4/T3 Therapy
The use of a combination of both thyroxine (T4) and tri-iodothyronine (T3) for thyroid hormone replacement in hypothyroidism (either synthetic human or derived from animal tissue) has long been shrouded in controversy. The vast majority of patients on thyroid hormone replacement are on T4 only due to distinct advantages including: (1) long half-life and stability, (2) easy monitoring of levels with TSH assessment, and (3) numerous studies showing the effectiveness of this practice in reversing hypothyroid symptoms and consequences. It is also clear that T4 is readily changed into T3 by the iodothyronine deiodinases 1 and 2. However, there remain patients who do not feel back to their pre-illness level of well-being on T4 only and advocates for combination therapy suggest this is due to a lack of T3, with evidence from animal studies that only a combination regimen of T3 and T4 can achieve normal T4 and T3 serum and tissue levels [43,44]. There have been eleven placebo-controlled studies of combination T4/T3 versus T4 alone on a variety of outcomes including quality of life scores, depression and fatigue [45,46,47,48,49,50,51,52,53,54,55]. Whilst the initial study appeared to show a benefit of combination therapy, all the following studies and three subsequent meta-analyses have shown no benefits [56,57,58]. A more recent placebo-controlled crossover study which was completed after these meta-analyses did show a positive effect of combination therapy; however, it was small (n = 59) and used a higher dose of T3 than most of the other studies [59]. Although the meta-analyses and majority of the studies have shown negative benefit from combination therapy there continues to be doubts due to uncertainty on the appropriate replacement ratio of T3:T4 and how to assess this. The largest study from Bristol, UK showed a significant placebo effect on well-being in these subjects, lasting out to at least 1 year [55], suggesting that anecdotal reports of improvement on combination therapy must be viewed with some scepticism.
Genetic Predisposition to Response to Combination Therapy
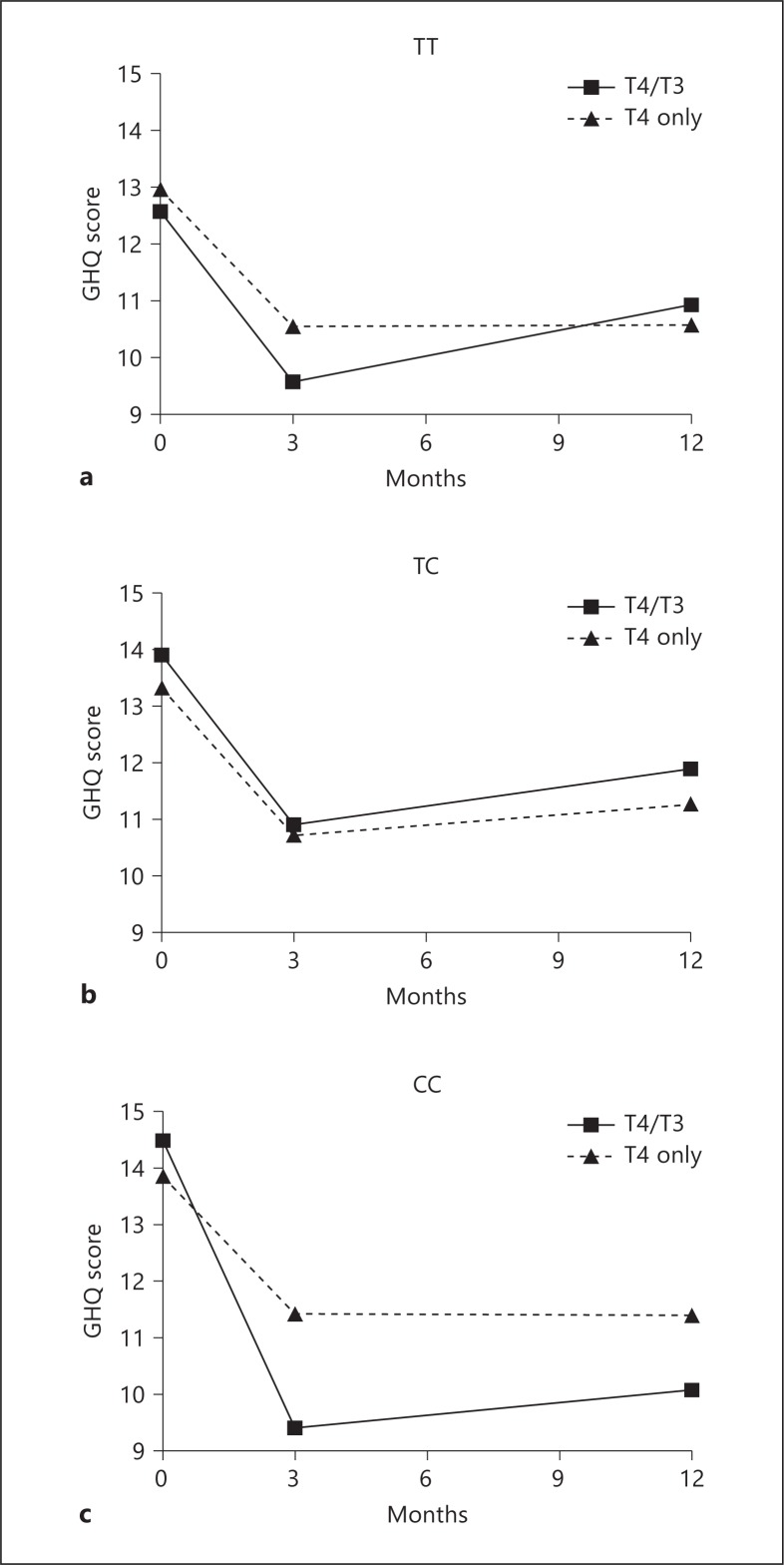
Whilst overall studies of combination therapy have not shown any benefits there remain subjects who feel better on a combination. A possible explanation for this, other than a placebo effect, could be a genetic predisposition to feeling worse on T4 monotherapy and better with T3. Mechanistically, two pathways which could produce such an effect would be the iodothyronine deiodinases (due to a lesser ability to change T4 to T3) and the specific thyroid hormone transporters (due to a detriment in transporting T4 but not T3 into cells). Two studies have investigated this possibility. We reanalysed the large combination trial from Bristol, UK, looking to see if genetic polymorphisms within the deiodinase genes would be associated with response to different therapies. We observed that a likely functional polymorphism in DIO2 (rs225014) was associated with poorer psychological well-being in subjects on thyroxine, but also to an improved response with combination T4/T3 therapy (not seen in subjects with the same polymorphism given T4 and placebo) [60] (fig. 4). This polymorphism therefore may be selecting out subjects who do not respond to T4 only. The biological function of this gene is consistent with that in that there is only D2 in the brain to convert T4 to T3 (not D1); therefore, potentially decreased function of this enzyme may limit supply of T3 to brain cells, but only in subjects who need to receive all of their T3 from T4 deiodination. The frequency of this polymorphism in the population is approximately 12% suggesting that these subjects could have been lost in the later, non-genetically based studies. Furthermore, there is no way to identify these subjects without genetic testing (no change in biochemical tests). This initial study is yet to be replicated. We attempted to find further evidence for this by analysing the DIO2 polymorphism in subjects from the UK who had chosen to be on combined T4/T3 therapy versus those who had tried this therapy but gone back to T4 monotherapy. Although there were small numbers (15 subjects 9 controls), the proportion of subjects with the rare genotype in the group that had remained on T4/T3 therapy was not significantly increased [unpubl. data]. Potential reasons for this are: (1) that other SNPs/genes are also involved, and (2) the possibility that the small sample included subjects with misattribution of symptoms and placebo effect of the treatment. This emphasizes the need for replication of the initial study.
Fig. 4.
Response to combination T4/T3 therapy versus T4 monotherapy by DIO2 genotype. From Panicker et al. [60]. Copyright 2009, The Endocrine Society, used with permission.
A smaller study found a polymorphism in the gene for thyroid hormone transporter OATP1C1, thought to be important for transport of T4 into the brain, to be associated with symptoms of fatigue and depression in subjects on T4 monotherapy [61]. There was no treatment arm in this study and its findings have not been replicated.
Are There Risks of Thyroid Hormone Replacement or Overreplacement?
Theoretically there should be no adverse effects from replacing a hormone that the body is missing. Many of the arguments for the use of thyroxine in subjects where no clear benefit has been shown is the apparent lack of adverse events caused by treating someone with thyroxine at an appropriate level. There are, however, potential risks which must be considered. Firstly, it has been shown that as many as 32% of subjects on a stable dose of thyroxine have a TSH outside the reference range, even though in this study all participants had had a TSH performed in the previous 15 months. Furthermore, most of these (24.5%) had a low TSH suggestive of over replacement [29]. This is in keeping with other studies [62,63] which indicate that approximately 20% of individuals on levothyroxine have a suppressed TSH. Given that this is a lifelong treatment and most patients are on it for many years there is a potential for harm here. Reassuring, however, is the paper by Flynn et al. [63,] which shows that in subjects on long-term thyroxine therapy, a suppressed or high TSH was associated with an increased risk of cardiovascular disease, dysrhythmias and fractures; however, a low but not suppressed TSH was not associated with any increase risk compared to those with a TSH within the reference range. In the Saravanan study above, those with a suppressed or raised TSH made up 9% of the population on thyroxine.
The risks of combination T4/T3 therapy have not been studied in the long term and can therefore not be estimated. Indeed, many of the trials on combination therapy have used different replacement doses/ratio's reflecting that we do not know the ideal replacement ratio or dosing schedule, given the shorter half-life of T3. Indeed, in the largest of these studies from Bristol in which 10 μg of T3 was substituted for 50 μg of T4 in the treatment group, this group had a significantly higher TSH level on treatment suggesting that they may have been undertreated compared to controls [55]. Furthermore, the short half-life of T3 makes the monitoring of adequacy of replacement difficult to monitor (a morning serum TSH is unlikely to accurately reflect the varying levels of T3 throughout the day). Therefore, if further trials are suggestive of benefit of combination therapy, long-term outcomes will need to be assessed, particularly in the areas of cardiovascular disease, arrhythmia and osteoporosis.
There are also risks associated with treating subjects misdiagnosed with thyroid disease with thyroxine; these are covered above.
Do Thyroid Hormones Improve Response to Antidepressant Therapy?
As mentioned above animal studies have shown that thyroid hormone levels in the brain can influence neurotransmitter concentrations (serotonin and noradrenaline); furthermore, antidepressants can influence deiodinase action potentially leading to altered local thyroid hormone concentrations. Therefore, it seems reasonable that thyroid hormones could be used to augment treatment for depression, and this practice has been used for some years. There have been many studies in this area, unfortunately mostly small and with methodological problems. The two main areas of study are: (1) does augmentation of antidepressants with thyroid hormones speed the time to response, and (2) does the addition of thyroid hormones to patients with resistant depression already on antidepressants increase response?
Two early meta-analyses looked into these questions. Altshuler et al. [64] found an accelerated response to antidepressants with the addition of thyroid hormones, particularly in women, whilst Aronson et al. [65] found that the addition of T3 in subjects with resistant depression increased the response rate by 23%. These meta-analyses looked at studies during a period prior to the newer agents such as selective serotonin reuptake inhibitors and are therefore less relevant today, given the widespread use of these agents now. A more recent review of studies adding T3 to selective serotonin reuptake inhibitors found 5 randomized controlled trials (RCTs) which were too disparate to meta-analyse, and some open labelled studies. Their conclusion was that the treatment was safe clinically; however, whilst there was some evidence from one RCT and the open-labelled studies of benefit, further studies were required [66]. In terms of larger RCTs, two studies with greater than 100 subjects have shown no response to T3 addition [67,68], whilst another showed an almost trebling of the response rate [69], and bipolar patients with resistant depression also appear to respond [70,71,72]. Some of these studies have also identified baseline thyroid hormone levels which predict response but these are too variable and disparate to be used at this stage. Overall, studies to date have been underpowered, suffered methodological issues and/or not used current depression treatments. One can, therefore, not recommend for or against the use of thyroid hormones in the treatment of depression. Further well-powered, appropriately designed and perhaps genetically based studies are required to identify those patients who are likely to respond.
Genetic Markers to Predict Response to Thyroid Hormone Augmentation
Given what was shown in the above section on genetic predisposition to poor well-being and response to combination therapy, it is not surprising that recent research has examined whether these genes can predict response to thyroid hormone augmentation. This field of research is still in its early days; however, there is some early evidence that genetic markers may provide an answer. Cooper-Kazaz et al. [73] investigated the influence of four polymorphisms in DIO1 and DIO2 on response to T3 supplementation and found that the DIO-1-785T allele, previous shown to be associated with lower D1 function was associated with improved response to T3. There was no association with the other polymorphisms; however, it should be noted that this was a small study (64 subjects, 35 in the treatment group) and needs to be replicated.
Conclusion
Despite what appears to be an abundance of literature, the relationship between hypothyroidism and depression is not clearly defined. Large studies in subjects who do not have thyroid disease do not show an association between mild hypothyroidism and depression, and in fact in males some studies have shown an inverse association (though small). It is not surprising therefore that treatment of subjects with subclinical hypothyroidism has not been clearly shown to improve mental health markers. By contrast, subjects on thyroid hormone replacement have been consistently shown to have poorer well-being, and their well-being appears related to their thyroid function. The reasons for this are not yet defined although several possibilities have been raised in this review. As we enter an age of increasing understanding of genetic basis for disease, genetic variation may identify a population of subjects who may respond better to treatments other than thyroxine. However, how many subjects dissatisfied on thyroxine are a result of this and how many the result of misattribution of symptoms cannot currently be estimated. As yet, studies showing an association between thyroid-related genes and well-being must be considered preliminary. The practice of using thyroid hormones to improve response to antidepressant therapy continues, although there appears to be a lack of clear evidence of benefit, particularly when added to the newer antidepressant agents.
Further studies are required to determine if thyroid-related genes can influence well-being in subjects that are not on thyroid hormone replacement and if thyroid hormones may be beneficial in these subjects if they are not responding to standard antidepressant therapies. Replication of studies which suggest genetic polymorphisms may predict response to combination T4/T3 therapy is also vital. It may be that these genetic studies may finally help us understand the complex relationship between hypothyroidism and depression.
Disclosure Statement
The authors declare no conflict of interests.
References
- 1.Hetzel BS, Chavadej J, Potter BJ. The brain in iodine deficiency. Neuropathol Appl Neurobiol. 1988;14:93–104. doi: 10.1111/j.1365-2990.1988.tb00873.x. [DOI] [PubMed] [Google Scholar]
- 2.Chaouki ML, Maoui R, Benmiloud M. Comparative study of neurological and myxoedematous cretinism associated with severe iodine deficiency. Clin Endocrinol (Oxf) 1988;28:399–408. doi: 10.1111/j.1365-2265.1988.tb03671.x. [DOI] [PubMed] [Google Scholar]
- 3.Friesema EC, Grueters A, Biebermann H, Krude H, von Moers A, Reeser M, Barrett TG, Mancilla EE, Svensson J, Kester MH, Kuiper GG, Balkassmi S, Uitterlinden AG, Koehrle J, Rodien P, Halestrap AP, Visser TJ. Association between mutations in a thyroid hormone transporter and severe X-linked psychomotor retardation. Lancet. 2004;364:1435–1437. doi: 10.1016/S0140-6736(04)17226-7. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz CE, May MM, Carpenter NJ, Rogers RC, Martin J, Bialer MG, Ward J, Sanabria J, Marsa S, Lewis JA, Echeverri R, Lubs HA, Voeller K, Simensen RJ, Stevenson RE. Allan-Herndon-Dudley syndrome and the monocarboxylate transporter 8 (MCT8) gene. Am J Hum Genet. 2005;77:41–53. doi: 10.1086/431313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.LaFranchi S. Congenital hypothyroidism: etiologies, diagnosis, and management. Thyroid. 1999;9:735–740. doi: 10.1089/thy.1999.9.735. [DOI] [PubMed] [Google Scholar]
- 6.Gruters A, Krude H. Detection and treatment of congenital hypothyroidism. Nat Rev. 2012;8:104–113. doi: 10.1038/nrendo.2011.160. [DOI] [PubMed] [Google Scholar]
- 7.Heuer H. The importance of thyroid hormone transporters for brain development and function. Best Pract Res Clin Endocrinol Metab. 2007;21:265–276. doi: 10.1016/j.beem.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Kirkegaard C, Faber J. The role of thyroid hormones in depression. Eur J Endocrinol. 1998;138:1–9. doi: 10.1530/eje.0.1380001. [DOI] [PubMed] [Google Scholar]
- 9.Henley WN, Koehnle TJ. Thyroid hormones and the treatment of depression: An examination of basic hormonal actions in the mature mammalian brain. Synapse. 1997;27:36–44. doi: 10.1002/(SICI)1098-2396(199709)27:1<36::AID-SYN4>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 10.Bauer M, Heinz A, Whybrow PC. Thyroid hormones, serotonin and mood: Of synergy and significance in the adult brain. Mol Psychiatry. 2002;7:140–156. doi: 10.1038/sj.mp.4000963. [DOI] [PubMed] [Google Scholar]
- 11.Whybrow PC, Prange AJ., Jr A hypothesis of thyroid-catecholamine-receptor interaction. Its relevance to affective illness. Arch Gen Psychiatry. 1981;38:106–113. doi: 10.1001/archpsyc.1981.01780260108012. [DOI] [PubMed] [Google Scholar]
- 12.Gordon JT, Kaminski DM, Rozanov CB, Dratman MB. Evidence that 3,3′,5-triiodothyronine is concentrated in and delivered from the locus coeruleus to its noradrenergic targets via anterograde axonal transport. Neuroscience. 1999;93:943–954. doi: 10.1016/s0306-4522(99)00146-3. [DOI] [PubMed] [Google Scholar]
- 13.Mason GA, Bondy SC, Nemeroff CB, Walker CH, Prange AJ., Jr The effects of thyroid state on beta-adrenergic and serotonergic receptors in rat brain. Psychoneuroendocrinology. 1987;12:261–270. doi: 10.1016/0306-4530(87)90050-3. [DOI] [PubMed] [Google Scholar]
- 14.Engum A, Bjoro T, Mykletun A, Dahl AA. An association between depression, anxiety and thyroid function – a clinical fact or an artefact? Acta Psychiatr Scand. 2002;106:27–34. doi: 10.1034/j.1600-0447.2002.01250.x. [DOI] [PubMed] [Google Scholar]
- 15.Panicker V, Evans J, Bjoro T, Asvold BO, Dayan CM, Bjerkeset O. A paradoxical difference in relationship between anxiety, depression and thyroid function in subjects on and not on T4: findings from the Hunt study. Clin Endocrinol (Oxf) 2009;71:574–580. doi: 10.1111/j.1365-2265.2008.03521.x. [DOI] [PubMed] [Google Scholar]
- 16.Forman-Hoffman V, Philibert RA. Lower TSH and higher T4 levels are associated with current depressive syndrome in young adults. Acta Psychiatr Scand. 2006;114:132–139. doi: 10.1111/j.1600-0447.2005.00703.x. [DOI] [PubMed] [Google Scholar]
- 17.Williams MD, Harris R, Dayan CM, Evans J, Gallacher J, Ben-Shlomo Y. Thyroid function and the natural history of depression: findings from the Caerphilly Prospective Study (CAPS) and a meta-analysis. Clin Endocrinol. 2009;70:484–492. doi: 10.1111/j.1365-2265.2008.03352.x. [DOI] [PubMed] [Google Scholar]
- 18.Roberts LM, Pattison H, Roalfe A, Franklyn J, Wilson S, Hobbs FD, Parle JV. Is subclinical thyroid dysfunction in the elderly associated with depression or cognitive dysfunction? Ann Intern Med. 2006;145:573–581. doi: 10.7326/0003-4819-145-8-200610170-00006. [DOI] [PubMed] [Google Scholar]
- 19.Almeida OP, Alfonso H, Flicker L, Hankey G, Chubb SA, Yeap BB. Thyroid hormones and depression: the health in men study. Am J Geriatr Psychiatry. 2011;19:763–770. doi: 10.1097/JGP.0b013e31820dcad5. [DOI] [PubMed] [Google Scholar]
- 20.Guimaraes JM, de Souza Lopes C, Baima J, Sichieri R. Depression symptoms and hypothyroidism in a population-based study of middle-aged Brazilian women. J Affect Disord. 2009;117:120–123. doi: 10.1016/j.jad.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 21.Gulseren S, Gulseren L, Hekimsoy Z, Cetinay P, Ozen C, Tokatlioglu B. Depression, anxiety, health-related quality of life, and disability in patients with overt and subclinical thyroid dysfunction. Arch Med Res. 2006;37:133–139. doi: 10.1016/j.arcmed.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 22.Bono G, Fancellu R, Blandini F, Santoro G, Mauri M. Cognitive and affective status in mild hypothyroidism and interactions with L-thyroxine treatment. Acta Neurol Scand. 2004;110:59–66. doi: 10.1111/j.1600-0404.2004.00262.x. [DOI] [PubMed] [Google Scholar]
- 23.van de Ven AC, Muntjewerff JW, Netea-Maier RT, de Vegt F, Ross HA, Sweep FC, Kiemeney LA, Vos PE, Buitelaar JK, Hermus AR, den Heijer M, Janzing JG. Association between thyroid function, thyroid autoimmunity, and state and trait factors of depression. Acta Psychiatr Scand. 2012;126:377–384. doi: 10.1111/j.1600-0447.2012.01870.x. [DOI] [PubMed] [Google Scholar]
- 24.Philibert RA, Beach SR, Gunter TD, Todorov AA, Brody GH, Vijayendran M, Elliott L, Hollenbeck N, Russell D, Cutrona C. The relationship of deiodinase 1 genotype and thyroid function to lifetime history of major depression in three independent populations. Am J Med Genetics B. 2011;156B:593–599. doi: 10.1002/ajmg.b.31200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pop VJ, Maartens LH, Leusink G, van Son MJ, Knottnerus AA, Ward AM, Metcalfe R, Weetman AP. Are autoimmune thyroid dysfunction and depression related? J Clin Endocrinol Metab. 1998;83:3194–3197. doi: 10.1210/jcem.83.9.5131. [DOI] [PubMed] [Google Scholar]
- 26.Kirim S, Keskek SO, Koksal F, Haydardedeoglu FE, Bozkirli E, Toledano Y. Depression in patients with euthyroid chronic autoimmune thyroiditis. Endocr J. 2012;59:705–708. doi: 10.1507/endocrj.ej12-0035. [DOI] [PubMed] [Google Scholar]
- 27.Engum A, Bjoro T, Mykletun A, Dahl AA. Thyroid autoimmunity, depression and anxiety; are there any connections? An epidemiological study of a large population. J Psychosom Res. 2005;59:263–268. doi: 10.1016/j.jpsychores.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 28.Saravanan P, Chau WF, Roberts N, Vedhara K, Greenwood R, Dayan CM. Psychological well-being in patients on ‘adequate' doses of L-thyroxine: results of a large, controlled community-based questionnaire study. Clin Endocrinol (Oxf) 2002;57:577–585. doi: 10.1046/j.1365-2265.2002.01654.x. [DOI] [PubMed] [Google Scholar]
- 29.Saravanan P, Visser TJ, Dayan CM. Psychological well-being correlates with free thyroxine but not free 3,5,3′-triiodothyronine levels in patients on thyroid hormone replacement. J Clin Endocrinol Metab. 2006;91:3389–3393. doi: 10.1210/jc.2006-0414. [DOI] [PubMed] [Google Scholar]
- 30.Wekking EM, Appelhof BC, Fliers E, Schene AH, Huyser J, Tijssen JG, Wiersinga WM. Cognitive functioning and well-being in euthyroid patients on thyroxine replacement therapy for primary hypothyroidism. Eur J Endocrinol. 2005;153:747–753. doi: 10.1530/eje.1.02025. [DOI] [PubMed] [Google Scholar]
- 31.Walsh JP, Ward LC, Burke V, Bhagat CI, Shiels L, Henley D, Gillett MJ, Gilbert R, Tanner M, Stuckey BG. Small changes in thyroxine dosage do not produce measurable changes in hypothyroid symptoms, well-being, or quality of life: results of a double-blind, randomized clinical trial. J Clin Endocrinol Metab. 2006;91:2624–2630. doi: 10.1210/jc.2006-0099. [DOI] [PubMed] [Google Scholar]
- 32.Samuels MH, Schuff KG, Carlson NE, Carello P, Janowsky JS. Health status, psychological symptoms, mood, and cognition in L-thyroxine-treated hypothyroid subjects. Thyroid. 2007;17:249–258. doi: 10.1089/thy.2006.0252. [DOI] [PubMed] [Google Scholar]
- 33.Kramer CK, von Muhlen D, Kritz-Silverstein D, Barrett-Connor E. Treated hypothyroidism, cognitive function, and depressed mood in old age: The Rancho Bernardo study. Eur J Endocrinol. 2009;161:917–921. doi: 10.1530/EJE-09-0606. [DOI] [PubMed] [Google Scholar]
- 34.Bould H, Panicker V, Kessler D, Durant C, Lewis G, Dayan C, Evans J. Investigation of thyroid dysfunction in general practice is more likely in patients with high psychological morbidity. Fam Pract. 2012;29:163–167. doi: 10.1093/fampra/cmr059. [DOI] [PubMed] [Google Scholar]
- 35.Canaris GJ, Manowitz NR, Mayor G, Ridgway EC. The Colorado Thyroid Disease Prevalence study. Arch Intern Med. 2000;160:526–534. doi: 10.1001/archinte.160.4.526. [DOI] [PubMed] [Google Scholar]
- 36.Leese GP, Flynn RV, Jung RT, Macdonald TM, Murphy MJ, Morris AD. Increasing prevalence and incidence of thyroid disease in Tayside, Scotland: the thyroid epidemiology audit and research study (tears) Clin Endocrinol (Oxf) 2008;68:311–316. doi: 10.1111/j.1365-2265.2007.03051.x. [DOI] [PubMed] [Google Scholar]
- 37.Taylor P, Iqbal A, Minassian C, Sayers A, Draman M, Greenwood R, Hamilton W, Okosieme O, Panicker V, Thomas S, Dayan C. Falling threshold for treatment of borderline elevated TSH levels – balancing benefits and risks: evidence from a large community based study. JAMA Intern Med 2013, in press. [DOI] [PubMed]
- 38.Jorde R, Waterloo K, Storhaug H, Nyrnes A, Sundsfjord J, Jenssen TG. Neuropsychological function and symptoms in subjects with subclinical hypothyroidism and the effect of thyroxine treatment. J Clin Endocrinol Metab. 2006;91:145–153. doi: 10.1210/jc.2005-1775. [DOI] [PubMed] [Google Scholar]
- 39.Jaeschke R, Guyatt G, Gerstein H, Patterson C, Molloy W, Cook D, Harper S, Griffith L, Carbotte R. Does treatment with L-thyroxine influence health status in middle-aged and older adults with subclinical hypothyroidism? J Gen Intern Med. 1996;11:744–749. doi: 10.1007/BF02598988. [DOI] [PubMed] [Google Scholar]
- 40.Kong WM, Sheikh MH, Lumb PJ, Naoumova RP, Freedman DB, Crook M, Dore CJ, Finer N. A 6-month randomized trial of thyroxine treatment in women with mild subclinical hypothyroidism. Am J Med. 2002;112:348–354. doi: 10.1016/s0002-9343(02)01022-7. [DOI] [PubMed] [Google Scholar]
- 41.Meier C, Staub JJ, Roth CB, Guglielmetti M, Kunz M, Miserez AR, Drewe J, Huber P, Herzog R, Muller B. TSH-controlled L-thyroxine therapy reduces cholesterol levels and clinical symptoms in subclinical hypothyroidism: a double blind, placebo-controlled trial (Basel Thyroid study) J Clin Endocrinol Metab. 2001;86:4860–4866. doi: 10.1210/jcem.86.10.7973. [DOI] [PubMed] [Google Scholar]
- 42.Baldini M, Colasanti A, Orsatti A, Airaghi L, Mauri MC, Cappellini MD. Neuropsychological functions and metabolic aspects in subclinical hypothyroidism: the effects of L-thyroxine. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:854–859. doi: 10.1016/j.pnpbp.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 43.Escobar-Morreale HF, Obregon MJ, Escobar del Rey F, Morreale de Escobar G. Replacement therapy for hypothyroidism with thyroxine alone does not ensure euthyroidism in all tissues, as studied in thyroidectomized rats. J Clin Invest. 1995;96:2828–2838. doi: 10.1172/JCI118353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Escobar-Morreale HF, del Rey FE, Obregon MJ, de Escobar GM. Only the combined treatment with thyroxine and triiodothyronine ensures euthyroidism in all tissues of the thyroidectomized rat. Endocrinology. 1996;137:2490–2502. doi: 10.1210/endo.137.6.8641203. [DOI] [PubMed] [Google Scholar]
- 45.Bunevicius R, Kazanavicius G, Zalinkevicius R, Prange AJ., Jr Effects of thyroxine as compared with thyroxine plus triiodothyronine in patients with hypothyroidism. N Engl J Med. 1999;340:424–429. doi: 10.1056/NEJM199902113400603. [DOI] [PubMed] [Google Scholar]
- 46.Bunevicius R, Jakubonien N, Jurkevicius R, Cernicat J, Lasas L, Prange AJ., Jr Thyroxine vs. thyroxine plus triiodothyronine in treatment of hypothyroidism after thyroidectomy for Graves' disease. Endocrine. 2002;18:129–133. doi: 10.1385/ENDO:18:2:129. [DOI] [PubMed] [Google Scholar]
- 47.Sawka AM, Gerstein HC, Marriott MJ, MacQueen GM, Joffe RT. Does a combination regimen of thyroxine (T4) and 3,5,3′-triiodothyronine improve depressive symptoms better than T4 alone in patients with hypothyroidism? Results of a double-blind, randomized, controlled trial. J Clin Endocrinol Metab. 2003;88:4551–4555. doi: 10.1210/jc.2003-030139. [DOI] [PubMed] [Google Scholar]
- 48.Clyde PW, Harari AE, Getka EJ, Shakir KM. Combined levothyroxine plus liothyronine compared with levothyroxine alone in primary hypothyroidism: a randomized controlled trial. Jama. 2003;290:2952–2958. doi: 10.1001/jama.290.22.2952. [DOI] [PubMed] [Google Scholar]
- 49.Walsh JP, Shiels L, Lim EM, Bhagat CI, Ward LC, Stuckey BG, Dhaliwal SS, Chew GT, Bhagat MC, Cussons AJ. Combined thyroxine/liothyronine treatment does not improve well-being, quality of life, or cognitive function compared to thyroxine alone: A randomized controlled trial in patients with primary hypothyroidism. J Clin Endocrinol Metab. 2003;88:4543–4550. doi: 10.1210/jc.2003-030249. [DOI] [PubMed] [Google Scholar]
- 50.Siegmund W, Spieker K, Weike AI, Giessmann T, Modess C, Dabers T, Kirsch G, Sanger E, Engel G, Hamm AO, Nauck M, Meng W. Replacement therapy with levothyroxine plus triiodothyronine (bioavailable molar ratio 14:1) is not superior to thyroxine alone to improve well-being and cognitive performance in hypothyroidism. Clin Endocrinol (Oxf) 2004;60:750–757. doi: 10.1111/j.1365-2265.2004.02050.x. [DOI] [PubMed] [Google Scholar]
- 51.Appelhof BC, Fliers E, Wekking EM, Schene AH, Huyser J, Tijssen JG, Endert E, van Weert HC, Wiersinga WM. Combined therapy with levothyroxine and liothyronine in two ratios, compared with levothyroxine monotherapy in primary hypothyroidism: a double-blind, randomized, controlled clinical trial. J Clin Endocrinol Metab. 2005;90:2666–2674. doi: 10.1210/jc.2004-2111. [DOI] [PubMed] [Google Scholar]
- 52.Escobar-Morreale HF, Botella-Carretero JI, Gomez-Bueno M, Galan JM, Barrios V, Sancho J. Thyroid hormone replacement therapy in primary hypothyroidism: a randomized trial comparing L-thyroxine plus liothyronine with L-thyroxine alone. Ann Intern Med. 2005;142:412–424. doi: 10.7326/0003-4819-142-6-200503150-00007. [DOI] [PubMed] [Google Scholar]
- 53.Levitt JA, Silverberg J. T4 plus T3 for hypothyroidism: a double-blind comparison with usual T4. Proc of the 74th Ann Meet American Thyroid Association, Los Angeles, 2002.
- 54.Rodriguez T, Lavis VR, Meininger JC, Kapadia AS, Stafford LF. Substitution of liothyronine at a 1:5 ratio for a portion of levothyroxine: effect on fatigue, symptoms of depression, and working memory versus treatment with levothyroxine alone. Endocr Pract. 2005;11:223–233. doi: 10.4158/EP.11.4.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saravanan P, Simmons DJ, Greenwood R, Peters TJ, Dayan CM. Partial substitution of thyroxine (T4) with tri-iodothyronine in patients on T4 replacement therapy: results of a large community-based randomized controlled trial. J Clin Endocrinol Metab. 2005;90:805–812. doi: 10.1210/jc.2004-1672. [DOI] [PubMed] [Google Scholar]
- 56.Grozinsky-Glasberg S, Fraser A, Nahshoni E, Weizman A, Leibovici L. Thyroxine-triiodothyronine combination therapy versus thyroxine monotherapy for clinical hypothyroidism: meta-analysis of randomized controlled trials. J Clin Endocrinol Metab. 2006;91:2592–2599. doi: 10.1210/jc.2006-0448. [DOI] [PubMed] [Google Scholar]
- 57.Ma C, Xie J, Huang X, Wang G, Wang Y, Wang X, Zuo S. Thyroxine alone or thyroxine plus triiodothyronine replacement therapy for hypothyroidism. Nucl Med Commun. 2009;30:586–593. doi: 10.1097/MNM.0b013e32832c79e0. [DOI] [PubMed] [Google Scholar]
- 58.Joffe RT, Brimacombe M, Levitt AJ, Stagnaro-Green A. Treatment of clinical hypothyroidism with thyroxine and triiodothyronine: a literature review and metaanalysis. Psychosomatics. 2007;48:379–384. doi: 10.1176/appi.psy.48.5.379. [DOI] [PubMed] [Google Scholar]
- 59.Nygaard B, Jensen EW, Kvetny J, Jarlov A, Faber J. Effect of combination therapy with thyroxine (T4) and 3,5,3′-triiodothyronine versus T4 monotherapy in patients with hypothyroidism, a double-blind, randomised cross-over study. Eur J Endocrinol. 2009;161:895–902. doi: 10.1530/EJE-09-0542. [DOI] [PubMed] [Google Scholar]
- 60.Panicker V, Saravanan P, Vaidya B, Evans J, Hattersley AT, Frayling TM, Dayan CM. Common variation in the DIO2 gene predicts baseline psychological well-being and response to combination thyroxine plus triiodothyronine therapy in hypothyroid patients. J Clin Endocrinol Metab. 2009;94:1623–1629. doi: 10.1210/jc.2008-1301. [DOI] [PubMed] [Google Scholar]
- 61.van der Deure WM, Appelhof BC, Peeters RP, Wiersinga WM, Wekking EM, Huyser J, Schene AH, Tijssen JG, Hoogendijk WJ, Visser TJ, Fliers E. Polymorphisms in the brain-specific thyroid hormone transporter OATP1C1 are associated with fatigue and depression in hypothyroid patients. Clin Endocrinol (Oxf) 2008;69:804–811. doi: 10.1111/j.1365-2265.2008.03267.x. [DOI] [PubMed] [Google Scholar]
- 62.Parle JV, Franklyn JA, Cross KW, Jones SR, Sheppard MC. Thyroxine prescription in the community: serum thyroid stimulating hormone level assays as an indicator of undertreatment or overtreatment. Br J Gen Pract. 1993;43:107–109. [PMC free article] [PubMed] [Google Scholar]
- 63.Flynn RW, Bonellie SR, Jung RT, MacDonald TM, Morris AD, Leese GP. Serum thyroid-stimulating hormone concentration and morbidity from cardiovascular disease and fractures in patients on long-term thyroxine therapy. J Clin Endocrinol Metab. 2010;95:186–193. doi: 10.1210/jc.2009-1625. [DOI] [PubMed] [Google Scholar]
- 64.Altshuler LL, Bauer M, Frye MA, Gitlin MJ, Mintz J, Szuba MP, Leight KL, Whybrow PC. Does thyroid supplementation accelerate tricyclic antidepressant response? A review and meta-analysis of the literature. Am J Psychiatry. 2001;158:1617–1622. doi: 10.1176/appi.ajp.158.10.1617. [DOI] [PubMed] [Google Scholar]
- 65.Aronson R, Offman HJ, Joffe RT, Naylor CD. Triiodothyronine augmentation in the treatment of refractory depression. A meta-analysis. Arch Gen Psychiatry. 1996;53:842–848. doi: 10.1001/archpsyc.1996.01830090090013. [DOI] [PubMed] [Google Scholar]
- 66.Cooper-Kazaz R, Lerer B. Efficacy and safety of triiodothyronine supplementation in patients with major depressive disorder treated with specific serotonin reuptake inhibitors. Int J Neuropsychopharmacol. 2008;11:685–699. doi: 10.1017/S1461145707008206. [DOI] [PubMed] [Google Scholar]
- 67.Garlow SJ, Dunlop BW, Ninan PT, Nemeroff CB. The combination of triiodothyronine (t3) and sertraline is not superior to sertraline monotherapy in the treatment of major depressive disorder. J Psychiatr Res. 2012;46:1406–1413. doi: 10.1016/j.jpsychires.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Appelhof BC, Brouwer JP, van Dyck R, Fliers E, Hoogendijk WJ, Huyser J, Schene AH, Tijssen JG, Wiersinga WM. Triiodothyronine addition to paroxetine in the treatment of major depressive disorder. J Clin Endocrinol Metab. 2004;89:6271–6276. doi: 10.1210/jc.2004-1147. [DOI] [PubMed] [Google Scholar]
- 69.Cooper-Kazaz R, Apter JT, Cohen R, Karagichev L, Muhammed-Moussa S, Grupper D, Drori T, Newman ME, Sackeim HA, Glaser B, Lerer B. Combined treatment with sertraline and liothyronine in major depression: a randomized, double-blind, placebo-controlled trial. Arch Gen Psychiatry. 2007;64:679–688. doi: 10.1001/archpsyc.64.6.679. [DOI] [PubMed] [Google Scholar]
- 70.Kelly T, Lieberman DZ. The use of triiodothyronine as an augmentation agent in treatment-resistant bipolar ii and bipolar disorder nos. J Affect Disord. 2009;116:222–226. doi: 10.1016/j.jad.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 71.Stancer HC, Persad E. Treatment of intractable rapid-cycling manic-depressive disorder with levothyroxine. Clinical observations. Arch Gen Psychiatry. 1982;39:311–312. doi: 10.1001/archpsyc.1982.04290030045008. [DOI] [PubMed] [Google Scholar]
- 72.Bauer M, Berghofer A, Bschor T, Baumgartner A, Kiesslinger U, Hellweg R, Adli M, Baethge C, Muller-Oerlinghausen B. Supraphysiological doses of L-thyroxine in the maintenance treatment of prophylaxis-resistant affective disorders. Neuropsychopharmacology. 2002;27:620–628. doi: 10.1016/S0893-133X(02)00320-2. [DOI] [PubMed] [Google Scholar]
- 73.Cooper-Kazaz R, van der Deure WM, Medici M, Visser TJ, Alkelai A, Glaser B, Peeters RP, Lerer B. Preliminary evidence that a functional polymorphism in type 1 deiodinase is associated with enhanced potentiation of the antidepressant effect of sertraline by triiodothyronine. J Affect Disord. 2009;116:113–116. doi: 10.1016/j.jad.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 74.Grabe HJ, Volzke H, Ludemann J, Wolff B, Schwahn C, John U, Meng W, Freyberger HJ. Mental and physical complaints in thyroid disorders in the general population. Acta Psychiatr Scand. 2005;112:286–293. doi: 10.1111/j.1600-0447.2005.00586.x. [DOI] [PubMed] [Google Scholar]
- 75.de Jongh RT, Lips P, van Schoor NM, Rijs KJ, Deeg DJ, Comijs HC, Kramer MH, Vandenbroucke JP, Dekkers OM. Endogenous subclinical thyroid disorders, physical and cognitive function, depression, and mortality in older individuals. Eur J Endocrinol. 2011;165:545–554. doi: 10.1530/EJE-11-0430. [DOI] [PubMed] [Google Scholar]
- 76.Kritz-Silverstein D, Schultz ST, Palinska LA, Wingard DL, Barrett-Connor E. The association of thyroid stimulating hormone levels with cognitive function and depressed mood: the Rancho Bernardo study. J Nutr Health Aging. 2009;13:317–321. doi: 10.1007/s12603-009-0029-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gussekloo J, van Exel E, de Craen AJ, Meinders AE, Frolich M, Westendorp RG. Thyroid status, disability and cognitive function, and survival in old age. JAMA. 2004;292:2591–2599. doi: 10.1001/jama.292.21.2591. [DOI] [PubMed] [Google Scholar]
- 78.Kim JM, Stewart R, Kim SY, Bae KY, Yang SJ, Kim SW, Shin IS, Yoon JS. Thyroid stimulating hormone, cognitive impairment and depression in an older Korean population. Psychiatry Invest. 2010;7:264–269. doi: 10.4306/pi.2010.7.4.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Frey A, Lampert A, Dietz K, Striebich S, Locher C, Fedorenko O, Mohle R, Gallinat J, Lang F, Lang UE. Thyrotropin serum concentrations in healthy volunteers are associated with depression-related personality traits. Neuropsychobiology. 2007;56:123–126. doi: 10.1159/000112954. [DOI] [PubMed] [Google Scholar]
- 80.van Boxtel MP, Menheere PP, Bekers O, Hogervorst E, Jolles J. Thyroid function, depressed mood, and cognitive performance in older individuals: the Maastricht aging study. Psychoneuroendocrinology. 2004;29:891–898. doi: 10.1016/j.psyneuen.2003.08.002. [DOI] [PubMed] [Google Scholar]