Abstract
Cervical ultrasound scanning (US) is considered a key examination, by all major thyroid and endocrine specialist societies for the postoperative follow-up of thyroid cancer patients to assess the risk of recurrence. Neck US imaging is readily available, non-invasive, relatively easy to perform, cost-effective, and can guide diagnostic and therapeutic procedures with low complication rates. Its main shortcoming is its operator-dependency. Because of the pivotal role of US in the care of thyroid cancer patients, the European Thyroid Association convened a panel of international experts to review technical aspects, indications, results, and limitations of cervical US in the initial staging and follow-up of thyroid cancer patients. The main aim is to establish guidelines for both a cervical US scanning protocol and US-guided diagnostic and therapeutic procedures in patients with thyroid cancer. This report presents (1) standardization of the US scanning procedure, techniques of US-guided fine-needle aspiration, and reporting of findings; (2) definition of criteria for classification of malignancy risk based on cervical US imaging characteristics of neck masses and lymph nodes; (3) indications for US-guided fine-needle aspiration and for biological in situ assessments; (4) proposal of an algorithm for the follow-up of thyroid cancer patients based on risk stratification following histopathological and cervical US findings, and (5) discussion of the potential use of US-guided localization and ablation techniques for locoregional thyroid metastases.
Key Words: Neck ultrasonography, Recurrence risk stratification, Fine-needle aspiration biopsy, Lymph nodes, Thyroglobulin, Laser therapy, Ethanol injection
Background and Objectives
The risk-adapted approach to the treatment of thyroid cancer requires an appropriate staging and risk assessment of the disease. The European and American consensus and guidelines for the diagnosis and treatment of thyroid cancer have defined a framework for the preoperative staging and follow-up of patients with thyroid cancer [1,2]. The most common prognostic scoring system is the American Joint Committee on Cancer/International union against cancer TNM staging system, based mainly on the extent of the tumor and age and advocated by the European Thyroid Association (ETA) [2]. In accordance with the ETA consensus, patients are grouped into three risk categories at the time of initial treatment:
– Very low risk: unifocal T1a (≤1 cm) N0M0 and no extension beyond the thyroid capsule.
– Low risk: T1b (>1 cm) N0M0 or T2N0M0 or multifocal T1N0M0.
– High risk: any T3 and T4 or any T, N1 or any M1.
Recognizing that the commonly used clinicopathologic staging systems were designed to predict death and not recurrence, the updated American Thyroid Association guidelines proposed a novel staging system to predict the risk of recurrent/persistent disease in differentiated thyroid cancer. Microscopic invasion into the perithyroidal soft tissues, cervical lymph node metastases and 131I uptake outside the thyroid bed on the posttreatment scan performed after thyroid remnant ablation are classified as intermediate risk [1,3].
Subsequent application of these staging and risk assessment approaches has shown that the initial preoperative risk assessment is significantly modified by the response to total thyroidectomy and subsequent radioactive iodine remnant ablation [3,4]. According to published guidelines, in the first 1-2 years after initial surgery and radioiodine treatment, risk restratification relies primarily on thyroglobulin (Tg) measurement and neck ultrasound (US).
The usefulness of neck US for the assessment of therapeutic response is based on the fact that recurrences or metastases are generally located in the cervical lymph nodes (LNs) (60-75%), or more rarely in the thyroid bed (20%) [5]. Thus, the majority of recurrences are detected by neck US (94-96%), since recurrences are rarely palpable (18%) and frequently measure <1 cm (29-50%) [6,7,8].
Neck US results are highly dependent on equipment, scanning protocol and training. Some of these issues have been addressed in the technical appendix of the American Association of Clinical Endocrinologists, Associazione Medici Endocrinologi, and ETA thyroid nodule guidelines [9] and in a consensus for good practice guide by the French Society of Endocrinology [10].
Therefore, the aim of the present guidelines is to provide the first evidence-based review of the indications for neck US in the risk stratification of patients with differentiated thyroid cancer, following initial surgery and radioiodine ablation. These guidelines address the following questions:
– What are the standards for US examination, US-guided fine-needle aspiration (US-FNA) techniques, and reports?
– Can US differentiate between benign and pathological cervical LN and lesions in the thyroid bed?
– When should neck US be performed?
– What are the indications for US-FNA and Tg and calcitonin in situ assessments?
– Are there new US methods for the preoperative localization of cervical recurrences or alternatives to the surgical treatment of cervical recurrences?
Working Methods and Grading of Recommendations
Working Methods
The ETA Executive Committee set up a taskforce to develop guidelines for cervical US and US-guided techniques in the postoperative management of patients with thyroid cancer based upon the importance of these technologies in the published European, American and French consensus [1,2,11] and the French good practice guide for cervical US and US-guided techniques in treating differentiated thyroid cancer of follicular origin, published in 2011 [10]. A chairperson was selected to lead the task force (L.L.) and 5 other members were chosen based on clinical expertise, academic record and representation of endocrinology, radiology and nuclear medicine (M.F.E., L.H., S.J.M., R.P., T.R., G.R.). Members of the taskforce were subsequently endorsed by the ETA Guidelines Board and the ETA Executive Committee and each panel member declared whether they had any potential conflict of interest. Relevant articles were identified by searching Medline at PubMed (NLM) using the following search terms ‘ultrasound ’ and ‘thyroid cancer ’ from 1990 to December 2012. Recommendations were developed based on the literature, including published guidelines and expert opinion where appropriate. A preliminary document was generated by the chairperson and critically reviewed by the members of the taskforce. The panel agreed that recommendations would be based on consensus of the panel. Task force deliberations took place via electronic communication and during a meeting that was held in Paris on January 18th, 2013. The draft guidelines were reviewed by the taskforce and the ETA guidelines Board and then posted on the ETA website for all members to review. Suggestions and comments were considered for inclusion into the text.
Grading of Recommendations
The ETA Executive Committee elected to rate the recommendations according to the system developed by the Grading of Recommendations, Assessment, Development, and Evaluation Group. The strength of the recommendation and the quality of the evidence of the grading is reported in table 1[12]. The final document was approved by the ETA in July 2013.
Table 1.
Type of grading and definition of grades
| Grading type | Definition |
| Strength of the recommendation (SOR) | |
| Grade 1 | Strong recommendation (for or against) |
| Applies to most patients in most circumstances | |
| Benefits clearly outweigh the risk (or vice versa) | |
| Grade 2 | Weak recommendation (for or against) |
| Best action may differ depending on circumstances or patient values | |
| Benefits and risks or burdens are closely balanced, or uncertain | |
| Quality of the evidence (QOE) | |
| +++ | High quality; evidence at low risk of bias, such as randomized trials showing consistent results directly applicable to the recommendation |
| ++ | Moderate quality; studies with methodological flaws, showing inconsistent or indirect evidence |
| + | Low quality; case series or unsystematic clinical observations |
The SOR is indicated by the number 1 or 2. Grade 1 indicates a strong recommendation (for or against), while grade 2 indicates a weak recommendation or a suggestion that may not be appropriate for every patient, depending on context, patient values and preferences. Grading the quality of the evidence took into account study design, study quality, consistency of results and directness of the evidence. The QOE is indicated by plus signs at three levels. Each recommendation is preceded by a description of the evidence.
Providing a Standard for US Examination and FNA Techniques and Reports
Background
US imaging of the thyroid bed and cervical LN forms an integral part of the evaluation of patients with thyroid cancer. Optimal imaging requires equipment that meets specific technical standards and a trained operator working within a thyroid team.
Requirements for US Training
US is an operator-dependent imaging technique. In the American Association of Clinical Endocrinologists/Associazione Medici Endocrinologi/ETA thyroid nodules guidelines, the panel of experts insisted on the fact that most countries have no restrictions on who can perform thyroid and neck US and suggested the following minimums for expertise in thyroid US: at least 30 cases of thyroid tumors, metastatic lymph nodes, and local recurrences evaluated per year and at least 150 US-FNA procedures performed per year, with an inadequate sampling rate of less than 10%. However, according to the Bethesda conference, it was judged that it is difficult to stipulate a specific number of samples that should be collected before training is complete. We believe that standardized ultrasonographic characteristics that define lymph nodes as suspicious may minimize differences between operators.
Technical Considerations
To maximize axial resolution, a high-frequency linear probe (at least 12 MHz with a footprint of 3.5 cm or more) should ideally be used. The probe should have variable frequency settings so that lower frequencies (8 or 10 MHz) can be used for deep-field examination. A small sector or convex transducer with variable frequency of up to 8.5 MHz can be valuable for evaluation of the upper mediastinum.
Color flow or power Doppler examination is required. For color flow Doppler, a low pulse repetition frequency of 800 Hz or less and low wall filter settings optimize the detection of low flow vascularity seen both in the periphery of LNs with early metastatic deposits and in the normal hilum.
The patient's neck should be extended. This position maximizes imaging accessibility of the low central and level IV LN fields.
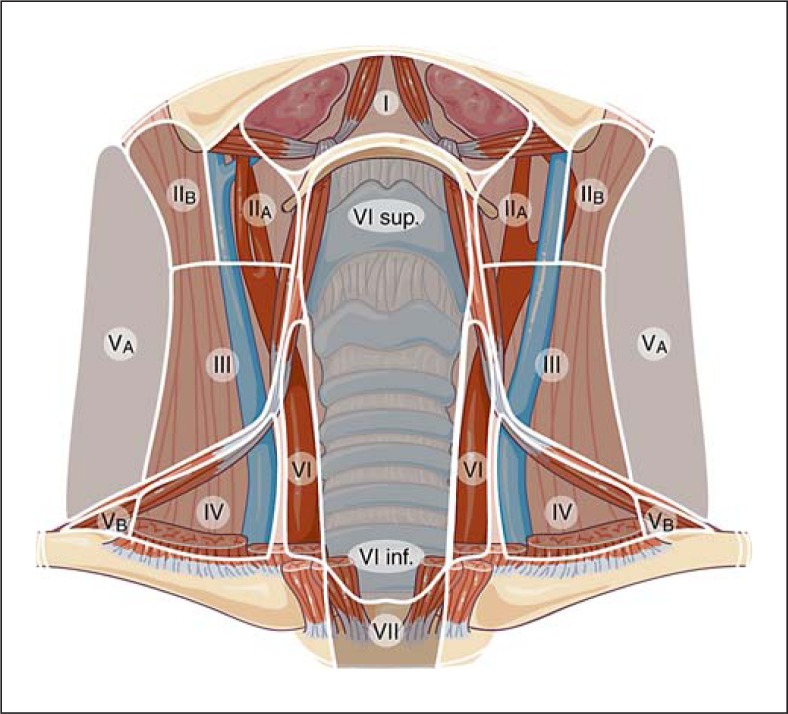
Lateral and central cervical compartments should be evaluated in the transverse plane and any identified abnormalities should be imaged in the longitudinal plane and assessed also by Doppler. A consistent protocol for imaging allows for comprehensive evaluation: scanning in the transverse plane from the upper part of the neck to the clavicles should include levels II to VI. Three different regions need to be scanned separately: central level (VI), levels IIA to IV included, and then levels VA and VB.
The probe can then be angled inferiorly in the sternal notch to assess the upper mediastinum. Some authors describe this region as level VII, ranging from the sternal notch to the innominate (brachiocephalic) artery. Others consider that lymph nodes involving regions not located within the neck should be referred to by the name of their specific nodal group (i.e. superior mediastinum). US cannot adequately image the deeper compartments of the neck, such as the retro- or parapharyngeal regions. Evaluation of the tracheo-esophageal groove can be facilitated if the patient's head is turned to the side. For identification of the anatomic location of LNs, the patient's neck should be in the neutral position.
Standardization of Reporting Results
The US report should document:
– The thyroid bed: presence and size of remnants and description of any potentially abnormal lesions (size in 3 dimensions, shape, borders, echogenicity, internal consistency, and vascularization).
– All LN compartments: description of any indeterminate or suspicious LNs (see definitions below), including anatomic location (level), size in 3 dimensions, gray-scale (internal consistency, echogenicity, presence of calcifications) and Doppler US features. Anatomic location is best described using the terminology and classification used for neck dissection [13]. The location of suspect LNs can be drawn on a diagram indicating levels (fig. 1) [14].
Fig. 1.
Example of diagram useful for marking the location of suspicious and indeterminate lymph nodes after total thyroidectomy. Specific US anatomic landmarks are used: level III LNs lie above the omo-hyoid muscle and level IV LNs below it. Separation between level II and III is the division of the common carotid artery in its two branches. By courtesy of Dr. H. Monpeyssen [14].
Technique of FNA
Needle movements should be visualized in real time. US should document the placement of the needle tip. For LNs with mixed content and a significant solid portion, the needle should target the solid portion for cytology assessment and the fluid can be aspirated for Tg measurement and also for cytological study after centrifugation. A 24- to 27-gauge needle is utilized with or without a syringe holder, depending on the operator's preference. A 21-gauge needle can be used but may cause more pain.
Two methods for US-FNA are employed. With the perpendicular approach, the needle enters perpendicular to the long axis of the transducer. With the parallel approach, the needle approaches the LN just under the transducer and parallel to its long axis; it can be visualized along its length as it enters the LN. A specimen for cytology is obtained either with the capillary technique, without applying suction, or with aspiration. The cytological specimen is prepared by making a slide smear or rinsing the needle in a liquid preparation.
Numerous patients are treated with oral anticoagulant and antiaggregant drugs. LN FNA is considered at low risk of causing bleeding complications [15,16]. These complications are not increased after FNA of neck lesions in patients taking antithrombotic therapy, such as clopidogrel, heparin or warfarin [17]. Data on bleeding complications do not exist for LN FNA. The authors suggest that for patients taking warfarin the INR be <2.5 or <3 if medically acceptable. Antiaggregant drugs, including aspirin, can be maintained.
Thyroglobulin and Calcitonin in situ Assessment
After aspiration, the needle is rinsed using either a physiological saline solution (0.9% NaCl), an assay buffer, or a ‘Tg or calcitonin-free ’ serum pool supplied by the laboratory. Assay of Tg in situ is carried out using the immunometric assay method calibrated in line with the CRM 457 standard (functional sensitivity between 0.1 and 1.0 µg/l) [10,11]. Two methods for expressing the results are used in the literature:
– ng/ml is used by many groups. This method does not take into account dilution due to rinsing but gives the actual concentration of Tg in the LN. A recent report validated 1.0 ng/ml of FNA-Tg as a cutoff value for diagnosing LN metastasis of papillary thyroid cancer [18].
– ng/FNA: a more suitable result which reflects the quantity of Tg in the needle after rinsing (1 ml per rinsed needle), and not the concentration in the LN [19].
– The decision threshold applied varies between studies and must be viewed in conjunction with the functional sensitivity of the assay. The published decision thresholds are [10]:
– Tg <1 ng/FNA: normal.
– Tg between 1 and 10 ng/FNA: to be compared with the results from cytology.
– Tg >10 ng/FNA: suggests the presence of tumor tissue.
Some authors have reported that the FNA-Tg was correlated with serum TSH and serum Tg levels and the comparison of FNA-Tg and serum Tg levels could be worthwhile [18].
Recommendations
(R1) Cervical US for thyroid cancer surveillance should be performed by an experienced operator with the patient's neck extended. A variable frequency probe of at least 12 MHz should be used for gray-scale imaging. Doppler evaluation should be performed. Grade: QOE = +; SOR = grade 1.
(R2) US imaging should include examination of both thyroid bed and LNs located in levels II to VI. LNs can be classified as normal, intermediate or suspicious (see below) and drawn on a diagram indicating levels. Grade: QOE = +; SOR = grade 1.
US-FNA of suspicious LNs should be performed using a small gauge needle (24-27 G). The specimen should be sent for cytological evaluation and for Tg or calcitonin measurement. Grade: QOE = +; SOR = grade 1.
(R3) Antiplatelet agents or oral anticoagulants can be maintained, provided INR is below 2.5-3. The following precautions must be taken: use of small gauge needles (24-27 G) with real time visualization during the procedure, a minimal number of passes, post procedure compression as needed for up to 10 min and sonographic evaluation to search the development of a hematoma [16]. Grade: QOE = +; SOR = grade 1.
Differentiating Benign and Pathological US Patterns of the Thyroid Bed and Cervical LNs
Background
Assessment of the Thyroid Bed
Assessment of the thyroid bed (central compartment, level VI) is difficult during the first 3 postoperative months and should be postponed until after this period. Small remnants can be seen (usually as isoechoic and ovoid lesions). The thyroid bed appears as an inverted triangular hyperechoic area [20]. A hypoechoic lesion in the thyroid bed raises suspicion of persistent/recurrent disease. Hypoechoic lesions may also represent autoimmune thyroiditis, granulomas, reactive benign LNs, or parathyroid adenomas.
Suspicious lesions are typically characterized by [20,21,22,23] (fig. 2):
Fig. 2.
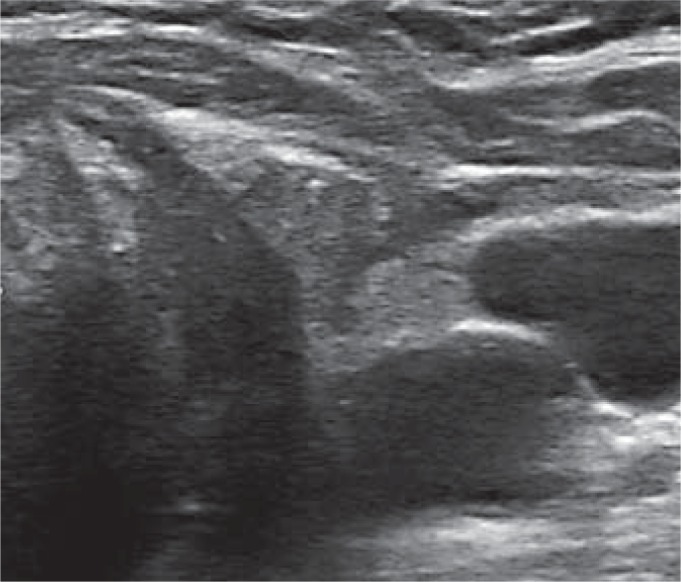
Recurrence located in the thyroid bed.
– Ovoid shape in the longitudinal plane but taller-than-wide in the transverse plane.
– Hypoechogenicity.
– Microcalcifications and cystic components.
– Irregular borders.
– Increased vascularization.
Assessment of Cervical LNs
Normal LNs: the number of sonographically visualized normal LNs varies considerably among individuals and decreases with age. The hilum is seen in 28.6-87% of normal LNs [24,25,26,27]. Hilar vascularization is seen in about two-thirds of normal LNs. The hilum may not be visible with gray-scale imaging and yet may be visualized, due to its vascularization, using color Doppler. Absence of a hilum or of hilar vascularization may be without significance, but presence of a normal hilum virtually eliminates suspicion of malignancy. Peripheral vascularization can be seen in up to 18% of benign LNs [24,25,26,27]. The long axis is of poor diagnostic value and only the short axis or Steinkamp's ratio (L/S, longer to shorter diameters) should be considered [28]. A short axis diameter greater than 8 mm in level II, and 5 mm for levels III, IV and VI, raises the suspicion of malignancy. Normally, LNs are ovoid, but a round shape (L/S ratio below 2) can be seen in up to 36% [24,25,26,27,29].
Abnormal LNs: sensitivity and specificity of abnormal US and Doppler-US patterns are summarized in table 2 [25,26,27,29,30].
Table 2.
Reported range of the diagnostic value of US signs for the detection of cervical metastatic lymph nodes from thyroid carcinoma
| Sign | Sensitivity, % | Specificity, % | NPV, % | PPV, % | Accuracy, % | % of normal LN with the sign |
|---|---|---|---|---|---|---|
| Microcalcifications | 5–69 | 93–100 | 33–60 | 88–100 | 56–72 | 0 |
| Cystic aspect | 10–34 | 91–100 | 30–66 | 77–100 | 48–65 | 0 |
| Peripheral vascularization | 40–86 | 57–93 | 31–70 | 77–80 | 54–71 | 1–18 |
| Hyperechogenicity | 30–87 | 43–95 | 38–84 | 66–96 | 56–90 | 4–17 |
| Round shape | 37 | 70 | 45 | 63 | 4–36 | |
| Hilum present | 0–0.5 | 29–48 | ||||
| Absent vascularization | 0 | 33–36 |
The location of LNs is of particular importance when attempting to identify malignancy. Almost half of metastatic LNs are located in levels III and IV and the other half in level VI [25]. Unilateral cervical metastases are most frequently located on the same side as the initial thyroid tumor, and up to 16% occur bilaterally [31].
Experience with US elastography is limited to two studies providing sensitivities of 83-85% and specificities of 98-100% [32,33].
Based on the data in table 2, cervical LNs can be classified into three groups:
Normal (fig. 3):
Fig. 3.
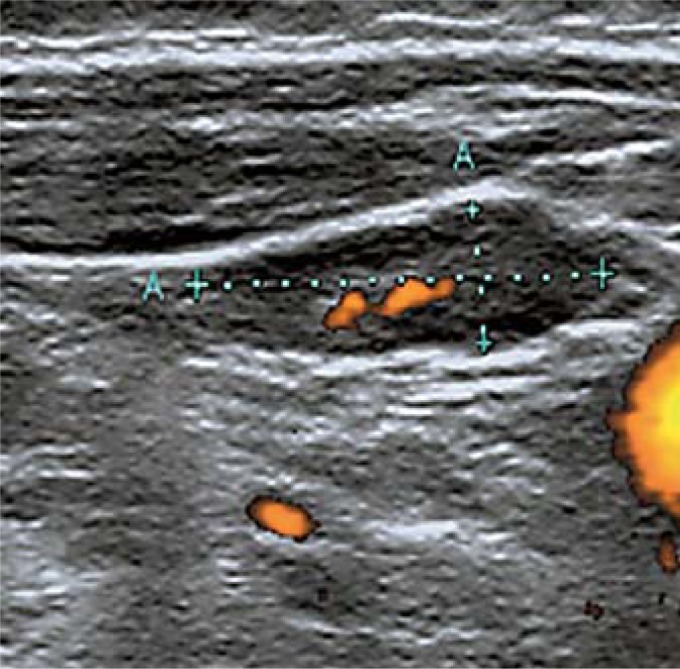
Normal lymph node.
– Hilum preserved.
– Ovoid shape and normal size.
– Absent or hilar vascularization.
– No other suspicious signs.
Indeterminate (fig. 4):
Fig. 4.
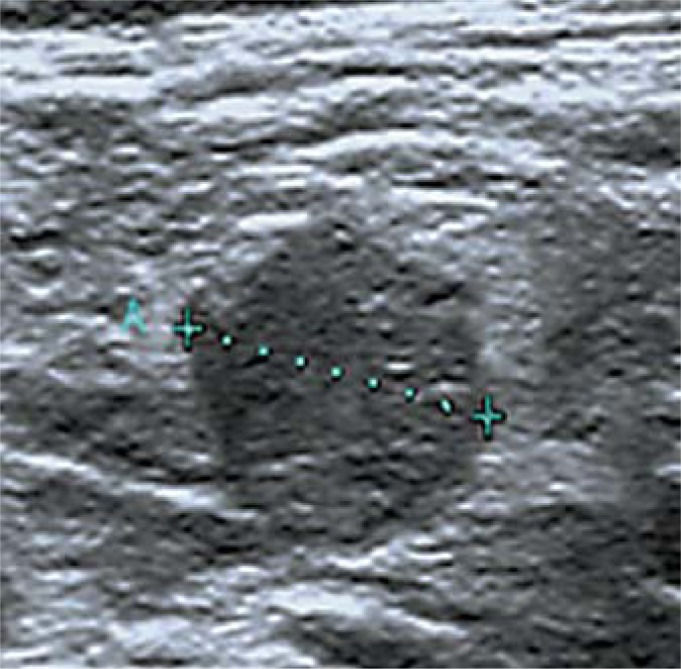
Indeterminate cervical lymph node: absence of hilum and round shape.
– Absence of a hilum and at least one of the following characteristics: round shape; increased short axis, ≥8 mm in level II and ≥5 mm in levels III and IV; increased central vascularization.
Suspicious for malignancy (at least one of the following characteristics) (fig. 5):
Fig. 5.
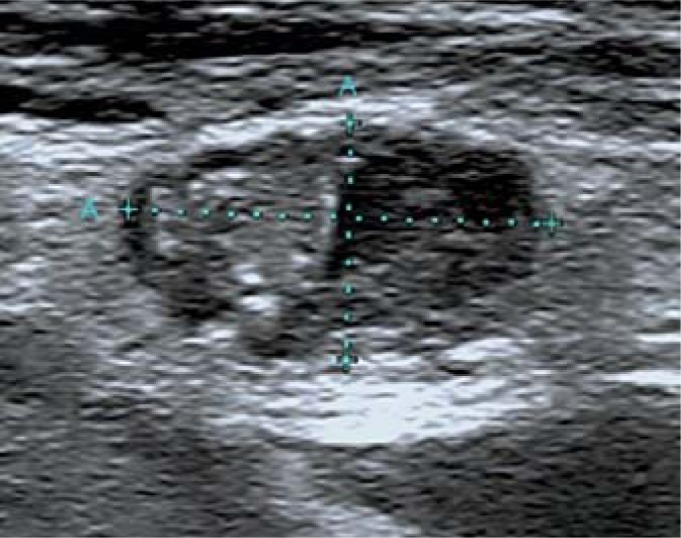
Cervical lymph node metastasis.
– Microcalcifications.
– Partially cystic appearance.
– Peripheral or diffusely increased vascularization.
– Hyperechoic tissue looking like thyroid.
Small LNs are defined by a shorts axis <5-7 mm. Knowing the serum Tg and the individual risk of recurrence helps in the interpretation of US imaging results, especially for small LNs with an indeterminate sonographic appearance.
Recurrence in Veins, Subcutaneous Tissues and/or Muscles
No reports describe the US characteristics of thyroid cancer recurrence in the subcutis and/or muscles. Such lesions appear as solid tissue zones with irregular borders, vascularized using power Doppler.
Tumor thrombus in the thyroid veins or the internal jugular veins may be caused by thyroid malignancy [34,35]. US is a valuable noninvasive method for the characterization of tumor extension into the great veins of the neck and can be recommended for visualizing early venous invasion in cases of suspected thyroid malignancy.
Recommendations
Table 3 summarizes the most important key points for the clinician.
Table 3.
Key points for the clinician
| Recommendations and suggested actions | QOE | SOR grade | |
|---|---|---|---|
| Technical standards and standardization of reporting results | |||
| (R1) US examination | – High-frequency linear probe | + | 1 |
| – Color flow or power Doppler | |||
| – Thyroid bed (VI) and lateral compartments (II–IV, V) analysis and diagram indicating levels | |||
| – Classification of LN | |||
| (R2) FNA | 24 – 27 gauge Tg or calcitonin in situ assessment | + | 1 |
| (R3) Antiplatelet agents or oral anticoagulants | Can be maintained INR <2.5 – 3 | + | 1 |
| (R4) Mass in the thyroid bed | Suspicious if hypoechoic and/or cystic component, calcifications, irregular shape, increased vascularization | + | 1 |
| (R5) Lymph node | – Normal: normal hilum, ovoid shape, absent or hilar vascularization and no suspicious feature | +++ | 1 |
| – Suspicious: cystic areas, microcalcifications, peripheral or diffusely increased vascularization, hyperechoic tissue looking like thyroid | |||
| – Indeterminate: absence of a hilum and at least one of the following signs: round shape, increased short axis, increased central vascularization | |||
| Indications for cervical US | |||
| (R6) After total thyroidectomy, at the time of ablation | |||
| US should be performed to check the lateral compartments if: | – Cancer fortuitously discovered at histology | + | 1 |
| – No available detailed preoperative US | |||
| – Radioiodine activity outside the thyroid bed on post ablation scan | |||
| – High preablation Tg value | |||
| (R7) Three months after ablation, for pT4, R1 | |||
| Reassess tumor extension or persistence with US or other imaging methods | ++ | 1 | |
| (R8) Six to 12 months after total thyroidectomy: reassess the risk of recurrence | |||
| US mandatory + serum Tg level (under LT4 or rhTSH stimulation) | +++ | 1 | |
| (R9) During follow-up (1 – 5years) | |||
| Very-low-risk and low-risk patients | If checkup at 6 months is normal, annual US is not necessary | + | 2 |
| High-risk patients | Annual cervical US is recommended, depending on pTNM staging, serum Tg level and reassessment results | + | 1 |
| (R10) After 5 years | |||
| Very-low-risk and low-risk patients | Regular US is not recommended. A final US scan combined with basal ultrasensitive serum Tg 5 – 7 years after initial treatment may be carried out | + | 1 |
| High-risk patients | – A second risk assessment 5 years postoperatively should be carried out | + | 2 |
| – US combined with basal and/or stimulated serum Tg measurements can be continued annually depending on the results of the risk reassessment | |||
| (R11) After a lobectomy | First US recommended 6 – 12 months after surgery and should, in principle, be regular and at 2- to 3-year intervals | + | 2 |
| Indications for US-FNA and in situ biological marker assessment | |||
| (R12) Suspicious and indeterminate LNs | FNA cytology and FNA-Tg are indicated but should take into account both the stage and histology of the disease as well as the size and location of the LNs and the serum Tg level | +++ | 1 |
| Small LN | <5 – 7 mm in their short axis Conservative approach | + | 1 |
| Suspicious thyroid bed lesions above 10 mm or growing | FNA is recommended | + | 1 |
(R4) A mass in the thyroid bed should be considered as suspicious if it is hypoechoic and/or has a cystic component, calcifications, an irregular shape or borders, or has increased vascularization. Grade: QOE = +; SOR = grade 1.
(R5) An LN with a normal hilum and no suspicious features can be considered normal with a very high probability. Grade: QOE = +++; SOR = grade 1.
LNs harboring cystic areas, microcalcifications or peripheral vascularization should be considered as suspicious. Grade: QOE = +++; SOR = grade 1.
LNs harboring absence of a hilum and round shape or increased short axis or increased central vascularization should be considered as indeterminate. Grade: QOE = +++; SOR = grade 1.
Indications for Cervical Ultrasound
Background
In experienced hands, neck US is the most valuable tool for detecting locoregional and thyroid bed metastases, whether preoperatively or during short- or long-term postoperative follow-up [36,37,38,39,40,41].
The cross analysis of neck US and postablation 131I scintigraphy with planar and/or neck SPECT-CT if the latter is available, enables early assessment of the risk of persistent or recurrent disease [42], especially in the case of low postoperative nonstimulated serum Tg levels in ATA intermediate-risk patients [43]. During follow-up, neck US results must be interpreted in combination with the serum Tg level. Some authors have reported that it is unusual to find any residual disease if the serum Tg, while taking L-thyroxine, is <0.15 or 0.27 ng/ml [44], or if stimulated serum Tg level is ≤1.4 ng/ml [44,45] in TgAb-negative patients. Nevertheless, 10-20% of patients with LN metastases have undetectable serum Tg, especially so in patients with aggressive or poorly differentiated histological subtypes.
US helps to identify structural persistent disease even in low-risk patients [46].
Neck US combined with serum Tg level allows individualized risk re-stratification during postoperative follow-up [3,4].
Neck US is always recommended if the serum Tg level rises and/or in case of clinical cervical abnormalities [1,10].
Recommendations
Table 3 summarizes the most important key points for the clinician concerning indications for US and for US-FNA.
(R6) After Total Thyroidectomy, at the Time of Ablation (1-3 Months after Surgery)
US should be performed to check the lateral compartments in case of
– If the cancer is incidentally discovered at histology.
– If no detailed preoperative US is available to detect the persistence of any LN metastases [47,48,49].
– If a pronounced activity has been detected outside the thyroid bed on post-ablation scans.
– If pre-ablation Tg values are considerably higher than expected, given the volume of residual thyroid tissue seen on the postablation scan (i.e. when TSH >30 mIU/l).
Grade: QOE = +; SOR = grade 1.
(R7) Three months after radioiodine treatment for pT4 tumors extending to the esophagus and/or the trachea, and the R1 stage, it is worthwhile to reassess tumor extension (and/or persistence) with US or other imaging methods (cervicothoracic computerized scan, 18FDG-PET scanning, magnetic resonance imaging) [10]. Grade: QOE = ++; SOR = grade 1.
(R8) Six to 12 Months after Initial Treatment
This evaluation is the most important one and allows the clinician to re-assess the risk of recurrence and evaluate the effect of initial therapy. Ultrasound imaging is mandatory and serum basal (using ultrasensitive assays) or stimulated Tg should be offered to any patient regardless of the previous risk stratification. Grade: QOE = +++; SOR = grade 1.
For very-low-risk patients without radioiodine ablation, cervical US may be carried out during L-thyroxine therapy in order to examine the thyroid bed and the central and lateral cervical LN compartments. Grade: QOE = ++; SOR = grade 2.
For low- and high-risk patients treated by radioiodine, cervical US (with Tg measurement during L-thyroxine therapy or under rhTSH stimulation) should be carried out to examine the thyroid bed and the central and lateral cervical LN compartments [1,8,38]. Grade: QOE = ++; SOR = grade 1.
(R9) During Follow-Up (1-5 Years)
According to risk re-stratification at 6-12 months postoperatively [4], if Tg is undetectable (in TgAb-negative patients) and US is normal, very-low-risk and low-risk patients are at very low risk of persistent/recurrent disease and subsequent annual US is therefore not necessary [50]. Grade: QOE = +; SOR = grade 2.
For high-risk patients, annual cervical US is recommended, depending on individual patient characteristics, pTNM staging, serum Tg level and reassessment results [1]. Grade: QOE = +; SOR = grade 1.
Other imaging modalities should be employed (cervicothoracic computed tomography, magnetic resonance imaging, 18FDG-PET scanning) in case of discrepancy between serum Tg and cervical US results [51]. Grade: QOE = +; SOR = grade 1.
(R10) After 5 Years
For very-low-risk and low-risk patients, regular US is not recommended if the first period of follow-up did not demonstrate any clinical, US, or biochemical abnormalities. A final ultrasound scan 5-7 years combined with basal ultrasensitive serum Tg after initial treatment may be carried out [6,10]. Grade: QOE = +; SOR = grade 1.
For high risk patients, a second risk assessment 5 years postoperatively should be carried out, and US combined with basal and/or stimulated serum Tg measurements can be continued annually, depending on the results of the risk reassessment. Grade: QOE = +; SOR = grade 2.
(R11) After a Lobectomy
Cervical US is the principal monitoring tool since the serum Tg level is of limited usefulness [37]. Grade: QOE = +; SOR = grade 1.
The thyroid bed and the contralateral lobe should be carefully examined for lesions which might justify a FNA. The first US is recommended 6-12 months after surgery and should, in principle, be regular with 2- to 3-year intervals. Grade: QOE = +; SOR = grade 2.
Indications for US-FNA and in situ Biological Marker Assessment
Background
US-FNA, performed to obtain both cytology and Tg determination, is commonly considered the best available technique for the early diagnosis of differentiated thyroid carcinoma neck metastases [6,52]. This technique is hampered by a 6-8% false-negative rate [52].
Sonographically suspicious lesions in the thyroid bed [53] should undergo US-FNA. Nevertheless, caution should be taken in the interpretation of FNA specimens that have low cellularity and lack characteristic cytologic features of thyroid carcinoma [54].
The Dilemma of Small Lesions
Small (short axis <5-7 mm) cervical LNs in the lateral neck can remain stable for long periods of time and have been reported to be safely followed with serial US [1,10,55]; their significance is unclear. Clinicians must consider the dilemma of minimizing explorations for cured patients that may lead to anxiety. Moreover, surgical resection of persistent PTC in cervical LNs achieves biochemical remission, when most stringently defined, in only 27% of patients [56]. Therefore, a wait-and-see approach is acceptable, for small LNs and for thyroid bed lesions below 10 mm [57].
Tg measurement from the FNA washout improves the sensitivity of US-FNA cytology for the identification of metastatic LNs [6,52,58,59,60]. In certain cases, especially when a granuloma located in the thyroid bed is suspected, in situ Tg assessment can provide valuable information: an FNA-Tg level lower than the threshold value has the added value of suggesting a benign lesion rather than tumor recurrence [61].
FNA-Tg measurement is most often accurate even in the presence of serum TgAb [58,62,63]. However, high serum TgAb levels have also been reported to interfere with FNA-Tg measurements and thereby result in falsely low FNA-Tg levels [60]. In medullary thyroid cancer, FNA washout for calcitonin measurement can also be performed for diagnosis of metastatic LNs [64].
Recommendations
(R12) For sonographically suspicious LNs, FNA cytology and FNA-Tg are indicated but should take into account the size of the LNs. Grade: QOE = +++; SOR = grade 1.
For sonographically indeterminate LNs, indications for FNA cytology and FNA-Tg should take into account both the stage and the histology of the disease as well as the size and location of the LNs and the serum Tg level. Grade: QOE = +++; SOR = grade 1.
FNA-Tg measurement is highly reliable in the diagnosis of neck metastases in PTC patients, even in cases with negative stimulated-Tg or positive TgAb. However, high-serum TgAb levels may interfere with FNA-Tg measurements and thereby result in falsely low FNA-Tg levels. Grade: QOE = +++; SOR = grade 1.
LNs <5-7 mm in their short axis can be difficult to evaluate, their clinical significance is limited, and a conservative approach is prudent. Grade: QOE = +; SOR = grade 1.
FNA is recommended for sonographically suspicious thyroid bed lesions above 10 mm or for which growth has been documented, while monitoring can be chosen for smaller or stable lesions. Grade: QOE = +; SOR = grade 1.
New Methods for Preoperative Localization of Cervical Recurrence and Alternatives to Surgical Treatment of Cervical Recurrence
This chapter deals with methods used by specialized teams. The aim of this section is to offer guidance in relation to this topic and not to provide guidelines.
Pre- and Perioperative Localization of Cervical Recurrences
The major concerns in the surgical treatment of extensive or recurrent thyroid cancer include the difficulty of precise intraoperative tumor localization, the possibility of missing small metastatic LNs during the operation, and complications. The usefulness and feasibility of different techniques and intraoperative probes have been reported [65,66,67]. The radioguided localization by Tc-albumin macroaggregates has not been considered by the group as an ultrasound guided technique.
Charcoal Suspension Tattooage
This technique has been described to mark cervical recurrences that might be difficult to localize by the surgeon, either due to small size or deep location. Its main advantage is its persistence for at least 3 months after charcoal injection. The only described complication is a cutaneous spot along the scar (3.6% of the cases). The procedure is similar to US-FNA; a 27-gauge needle is used to inject a sterile solution of charcoal suspension in and around the suspect LN. The optimal quantity of the solution of coal is around 0.3-0.5 ml.
Success rates of the procedure vary from 84 to 96%, with failures being related to LNs located posteriorly to the carotid arteries or jugular veins [68]. The size of the lesions varies from 4 to 18 mm [69]. The drawback of this technique is the limitation to LNs and masses visible by US and accessible without risk with a fine needle.
Charcoal tattoo localization, considered a safe, low-cost technique, could be useful for facilitating surgical procedures.
Methylene blue can be used with the disadvantage of rapid spreading.
Radio-Guided Surgery
To improve the completeness of surgical excision for persistent or recurrent differentiated thyroid carcinoma, an intraoperative 131I probe may be used to localize LN metastases that concentrate 131I [66]. Use of the probe was considered decisive in 20 of 54 patients. However, this study did not compare the value of this probe with that of preoperative US [66].
A pilot study of radioguided surgery, using an intraoperative 18FDG-PET probe for tumor localization, reported complete resection in 12 differentiated thyroid cancer patients [65].
Alternatives to Surgical Treatment for Recurrent Cervical Thyroid Malignancy: Place of Ultrasound-Guided Ablation Techniques
Background
All these methods rely on causing coagulative necrosis and small vessel thrombosis. The best evidence exists for percutaneous ethanol injection therapy (PEIT) [70] in cystic thyroid nodules, with an around 80% remission rate, in contrast to an approximately 50% shrinkage in solid benign nodules, whether functioning or not. Due to a number of side effects of PEIT, and long-term recurrence, the only remaining indication, according to current guidelines [71], is cystic thyroid nodules.
Benign solid thyroid nodules can also be treated with interstitial laser photocoagulation (ILP) [72] or radiofrequency ablation [73]. The same techniques could be applied to locoregional thyroid metastases as proposed more than a decade ago [73]. The main problem with evaluating these US-guided techniques for treating malignancy is the uncontrolled nature of the few available studies, from few centers in highly selected patients. There is an obvious likelihood of treating some but not all affected nodules/nodes, and we lack data on whether quality of life and prognosis are improved compared with observation or standard therapy. With these shortcomings, remarkably favorable results have been obtained using PEIT as well as ILP in patients with LN metastases from papillary thyroid cancer [74,75,76]. In 63 patients, 101 of 109 metastatic LNs responded (92 completely) to PEIT, without any significant side effects [75]. In a feasibility study, in 5 patients with papillary thyroid cancer (8 LNs), ILP has been suggested as an alternative and to perform similarly [76].
In conclusion, locoregional thyroid metastases from papillary thyroid cancer may be considered for treatment by US-guided ablation techniques (PEIT or ILP) in selected inoperable or comorbid patients with limited volume metastases. In this case, the therapy should be performed within a multidisciplinary team in highly specialized centers.
Disclosure Statement
The authors declare that no financial or other conflicts of interest exist in relation to the content of the article.
References
- 1.Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Pacini F, Schlumberger M, Sherman SI, Steward DL, Tuttle RM. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 2.Pacini F, Schlumberger M, Dralle H, Elisei R, Smit JW, Wiersinga W. European consensus for the management of patients with differentiated thyroid carcinoma of the follicular epithelium. Eur J Endocrinol. 2006;154:787–803. doi: 10.1530/eje.1.02158. [DOI] [PubMed] [Google Scholar]
- 3.Tuttle RM, Tala H, Shah J, Leboeuf R, Ghossein R, Gonen M, Brokhin M, Omry G, Fagin JA, Shaha A. Estimating risk of recurrence in differentiated thyroid cancer after total thyroidectomy and radioactive iodine remnant ablation: using response to therapy variables to modify the initial risk estimates predicted by the new American Thyroid Association staging system. Thyroid. 2010;20:1341–1349. doi: 10.1089/thy.2010.0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castagna MG, Maino F, Cipri C, Belardini V, Theodoropoulou A, Cevenini G, Pacini F. Delayed risk stratification, to include the response to initial treatment (surgery and radioiodine ablation), has better outcome predictivity in differentiated thyroid cancer patients. Eur J Endocrinol. 2011;165:441–446. doi: 10.1530/EJE-11-0466. [DOI] [PubMed] [Google Scholar]
- 5.Schlumberger M. Papillary and follicular thyroid carcinoma. N Engl J Med. 1998;338:297–306. doi: 10.1056/NEJM199801293380506. [DOI] [PubMed] [Google Scholar]
- 6.Frasoldati A, Pesenti M, Gallo M, Caroggio A, Salvo D, Valcavi R. Diagnosis of neck recurrences in patients with differentiated thyroid carcinoma. Cancer. 2003;97:90–96. doi: 10.1002/cncr.11031. [DOI] [PubMed] [Google Scholar]
- 7.Rouxel A, Hejblum G, Bernier MO, Boelle PY, Menegaux F, Mansour G, Hoang C, Aurengo A, Leenhardt L. Prognostic factors associated with the survival of patients developing loco-regional recurrences of differentiated thyroid carcinomas. J Clin Endocrinol Metab. 2004;89:5362–5368. doi: 10.1210/jc.2003-032004. [DOI] [PubMed] [Google Scholar]
- 8.Torlontano M, Attard M, Crocetti U, Tumino S, Bruno R, Costante G, D'Azzo G, Meringolo D, Ferretti E, Sacco R, Arturi F, Filetti S. Follow-up of low risk patients with papillary thyroid cancer: role of neck ultrasonography in detecting lymph node metastases. J Clin Endocrinol Metab. 2004;89:3402–3407. doi: 10.1210/jc.2003-031521. [DOI] [PubMed] [Google Scholar]
- 9.Gharib H, Papini E, Paschke R, Duick DS, Valcavi R, Hegedus L, Vitti P. American Association of Clinical Endocrinologists, Associazione Medici Endocrinologi, and European Thyroid Association medical guidelines for clinical practice for the diagnosis and management of thyroid nodules: executive summary of recommendations. J Endocrinol Invest. 2010;33:51–56. [PubMed] [Google Scholar]
- 10.Leenhardt L, Borson-Chazot F, Calzada M, Carnaille B, Charrie A, Cochand-Priollet B, Cao CD, Leboulleux S, Le Clech G, Mansour G, Menegaux F, Monpeyssen H, Orgiazzi J, Rouxel A, Sadoul JL, Schlumberger M, Tramalloni J, Tranquart F, Wemeau JL. Good practice guide for cervical ultrasound scan and echo-guided techniques in treating differentiated thyroid cancer of vesicular origin. Ann Endocrinol (Paris) 2011;72:173–197. doi: 10.1016/j.ando.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 11.Borson-Chazot F, Bardet S, Bournaud C, Conte-Devolx B, Corone C, D'Herbomez M, Henry JF, Leenhardt L, Peix JL, Schlumberger M, Wemeau JL, Baudin E, Berger N, Bernard MH, Calzada-Nocaudie M, Caron P, Catargi B, Chabrier G, Charrie A, Franc B, Hartl D, Helal B, Kerlan V, Kraimps JL, Leboulleux S, Le Clech G, Menegaux F, Orgiazzi J, Perie S, Raingeard I, Rodien P, Rohmer V, Sadoul JL, Schwartz C, Tenenbaum F, Toubert ME, Tramalloni J, Travagli JP, Vaudrey C. Guidelines for the management of differentiated thyroid carcinomas of vesicular origin. Ann Endocrinol (Paris) 2008;69:472–486. doi: 10.1016/j.ando.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 12.Swiglo BA, Murad MH, Schunemann HJ, Kunz R, Vigersky RA, Guyatt GH, Montori VM. A case for clarity, consistency, and helpfulness: state-of-the-art clinical practice guidelines in endocrinology using the grading of recommendations, assessment, development, and evaluation system. J Clin Endocrinol Metab. 2008;93:666–673. doi: 10.1210/jc.2007-1907. [DOI] [PubMed] [Google Scholar]
- 13.Robbins KT, Clayman G, Levine PA, Medina J, Sessions R, Shaha A, Som P, Wolf GT. Neck dissection classification update: revisions proposed by the American Head and Neck Society and the American Academy of Otolaryngology, Head and Neck Surgery. Arch Otolaryngol Head Neck Surg. 2002;128:751–758. doi: 10.1001/archotol.128.7.751. [DOI] [PubMed] [Google Scholar]
- 14.Tramalloni J, Monpeyssen H. Echographie de la thyroïde Paris Elsevier Masson. 2013. p. 191. [Google Scholar]
- 15.Potet J, Weber-Donat G, Thome A, Valbousquet L, Peroux E, Konopacki J, Baccialone J, Teriitehau CA. Periprocedural management of hemostasis risk in interventional radiology. J Radiol. 2011;92:659–670. doi: 10.1016/j.jradio.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 16.Patel IJ, Davidson JC, Nikolic B, Salazar GM, Schwartzberg MS, Walker TG, Saad WA. Consensus guidelines for periprocedural management of coagulation status and hemostasis risk in percutaneous image-guided interventions. J Vasc Interv Radiol. 2012;23:727–736. doi: 10.1016/j.jvir.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 17.Abu-Yousef MM, Larson JH, Kuehn DM, Wu AS, Laroia AT. Safety of ultrasound-guided fine needle aspiration biopsy of neck lesions in patients taking antithrombotic/anticoagulant medications. Ultrasound Q. 2011;27:157–159. doi: 10.1097/RUQ.0b013e31822b5681. [DOI] [PubMed] [Google Scholar]
- 18.Moon JH, Kim YI, Lim JA, Choi HS, Cho SW, Kim KW, Park HJ, Paeng JC, Park YJ, Yi KH, Park do J, Kim SE, Chung JK. Thyroglobulin in washout fluid from lymph node fine-needle aspiration biopsy in papillary thyroid cancer: large-scale validation of the cutoff value to determine malignancy and evaluation of discrepant results. J Clin Endocrinol Metab. 2013;98:1061–1068. doi: 10.1210/jc.2012-3291. [DOI] [PubMed] [Google Scholar]
- 19.Borel AL, Boizel R, Faure P, Barbe G, Boutonnat J, Sturm N, Seigneurin D, Bricault I, Caravel JP, Chaffanjon P, Chabre O. Significance of low levels of thyroglobulin in fine needle aspirates from cervical lymph nodes of patients with a history of differentiated thyroid cancer. Eur J Endocrinol. 2008;158:691–698. doi: 10.1530/EJE-07-0749. [DOI] [PubMed] [Google Scholar]
- 20.Ko MS, Lee JH, Shong YK, Gong GY, Baek JH. Normal and abnormal sonographic findings at the thyroidectomy sites in postoperative patients with thyroid malignancy. AJR Am J Roentgenol. 2010;194:1596–1609. doi: 10.2214/AJR.09.2513. [DOI] [PubMed] [Google Scholar]
- 21.Shin JH, Han BK, Ko EY, Kang SS. Sonographic findings in the surgical bed after thyroidectomy: comparison of recurrent tumors and nonrecurrent lesions. J Ultrasound Med. 2007;26:1359–1366. doi: 10.7863/jum.2007.26.10.1359. [DOI] [PubMed] [Google Scholar]
- 22.Kamaya A, Gross M, Akatsu H, Jeffrey RB. Recurrence in the thyroidectomy bed: sonographic findings. AJR Am J Roentgenol. 2011;196:66–70. doi: 10.2214/AJR.10.4474. [DOI] [PubMed] [Google Scholar]
- 23.Hahn SY, Shin JH, Han BK, Ko EY, Kang SS, Chung JH, Kim JH, Oh YL, Son YI. Predictive factors related to the recurrence at US-guided fine needle aspiration in postoperative patients with differentiated thyroid cancer. Clin Endocrinol (Oxf) 2012;74:270–275. doi: 10.1111/j.1365-2265.2010.03915.x. [DOI] [PubMed] [Google Scholar]
- 24.Kuna SK, Bracic I, Tesic V, Kuna K, Herceg GH, Dodig D. Ultrasonographic differentiation of benign from malignant neck lymphadenopathy in thyroid cancer. J Ultrasound Med. 2006;25:1531–1540. doi: 10.7863/jum.2006.25.12.1531. [DOI] [PubMed] [Google Scholar]
- 25.Leboulleux S, Girard E, Rose M, Travagli JP, Sabbah N, Caillou B, Hartl DM, Lassau N, Baudin E, Schlumberger M. Ultrasound criteria of malignancy for cervical lymph nodes in patients followed up for differentiated thyroid cancer. J Clin Endocrinol Metab. 2007;92:3590–3594. doi: 10.1210/jc.2007-0444. [DOI] [PubMed] [Google Scholar]
- 26.Park JS, Son KR, Na DG, Kim E, Kim S. Performance of preoperative sonographic staging of papillary thyroid carcinoma based on the sixth edition of the AJCC/UICC TNM classification system. AJR Am J Roentgenol. 2009;192:66–72. doi: 10.2214/AJR.07.3731. [DOI] [PubMed] [Google Scholar]
- 27.Sohn YM, Kwak JY, Kim EK, Moon HJ, Kim SJ, Kim MJ. Diagnostic approach for evaluation of lymph node metastasis from thyroid cancer using ultrasound and fine-needle aspiration biopsy. AJR Am J Roentgenol. 2010;194:38–43. doi: 10.2214/AJR.09.3128. [DOI] [PubMed] [Google Scholar]
- 28.Steinkamp HJ, Cornehl M, Hosten N, Pegios W, Vogl T, Felix R. Cervical lymphadenopathy: ratio of long- to short-axis diameter as a predictor of malignancy. Br J Radiol. 1995;68:266–270. doi: 10.1259/0007-1285-68-807-266. [DOI] [PubMed] [Google Scholar]
- 29.do Rosario PW, Fagundes TA, Maia FF, Franco AC, Figueiredo MB, Purisch S. Sonography in the diagnosis of cervical recurrence in patients with differentiated thyroid carcinoma. J Ultrasound Med. 2004;23:915–920. doi: 10.7863/jum.2004.23.7.915. [DOI] [PubMed] [Google Scholar]
- 30.Alzahrani AS, Alsuhaibani H, Salam SA, Al Sifri SN, Mohamed G, Al Sobhi S, Sulaiman O, Akhtar M. Diagnostic accuracy of high-resolution neck ultrasonography in the follow-up of differentiated thyroid cancer: a prospective study. Endocr Pract. 2005;11:165–171. doi: 10.4158/EP.11.3.165. [DOI] [PubMed] [Google Scholar]
- 31.Machens A, Holzhausen HJ, Dralle H. Skip metastases in thyroid cancer leaping the central lymph node compartment. Arch Surg. 2004;139:43–45. doi: 10.1001/archsurg.139.1.43. [DOI] [PubMed] [Google Scholar]
- 32.Lyshchik A, Moses R, Barnes SL, Higashi T, Asato R, Miga MI, Gore JC, Fleischer AC. Quantitative analysis of tumor vascularity in benign and malignant solid thyroid nodules. J Ultrasound Med. 2007;26:837–846. doi: 10.7863/jum.2007.26.6.837. [DOI] [PubMed] [Google Scholar]
- 33.Alam F, Naito K, Horiguchi J, Fukuda H, Tachikake T, Ito K. Accuracy of sonographic elastography in the differential diagnosis of enlarged cervical lymph nodes: comparison with conventional B-mode sonography. AJR Am J Roentgenol. 2008;191:604–610. doi: 10.2214/AJR.07.3401. [DOI] [PubMed] [Google Scholar]
- 34.Kobayashi K, Hirokawa M, Yabuta T, Fukushima M, Kihara M, Higashiyama T, Tomoda C, Takamura Y, Ito Y, Miya A, Amino N, Miyauchi A. Tumor thrombus of thyroid malignancies in veins: importance of detection by ultrasonography. Thyroid. 2011;21:527–531. doi: 10.1089/thy.2010.0099. [DOI] [PubMed] [Google Scholar]
- 35.Marcy PY, Thariat J, Bozec A, Poissonnet G, Benisvy D, Dassonville O. Venous obstruction of thyroid malignancy origin: the Antoine Lacassagne institute experience. World J Surg Oncol. 2009;7:40. doi: 10.1186/1477-7819-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Torlontano M, Crocetti U, Augello G, D'Aloiso L, Bonfitto N, Varraso A, Dicembrino F, Modoni S, Frusciante V, Di Giorgio A, Bruno R, Filetti S, Trischitta V. Comparative evaluation of recombinant human thyrotropin-stimulated thyroglobulin levels, 131I whole-body scintigraphy, and neck ultrasonography in the follow-up of patients with papillary thyroid microcarcinoma who have not undergone radioiodine therapy. J Clin Endocrinol Metab. 2006;91:60–63. doi: 10.1210/jc.2005-1185. [DOI] [PubMed] [Google Scholar]
- 37.Schlumberger M, Pacini F, Wiersinga WM, Toft A, Smit JW, Sanchez Franco F, Lind P, Limbert E, Jarzab B, Jamar F, Duntas L, Cohen O, Berg G. Follow-up and management of differentiated thyroid carcinoma: a European perspective in clinical practice. Eur J Endocrinol. 2004;151:539–548. doi: 10.1530/eje.0.1510539. [DOI] [PubMed] [Google Scholar]
- 38.Stulak JM, Grant CS, Farley DR, Thompson GB, van Heerden JA, Hay ID, Reading CC, Charboneau JW. Value of preoperative ultrasonography in the surgical management of initial and reoperative papillary thyroid cancer. Arch Surg. 2006;141:489–494. doi: 10.1001/archsurg.141.5.489. [DOI] [PubMed] [Google Scholar]
- 39.Pacini F, Molinaro E, Castagna MG, Agate L, Elisei R, Ceccarelli C, Lippi F, Taddei D, Grasso L, Pinchera A. Recombinant human thyrotropin-stimulated serum thyroglobulin combined with neck ultrasonography has the highest sensitivity in monitoring differentiated thyroid carcinoma. J Clin Endocrinol Metab. 2003;88:3668–3673. doi: 10.1210/jc.2002-021925. [DOI] [PubMed] [Google Scholar]
- 40.Baskin HJ. Detection of recurrent papillary thyroid carcinoma by thyroglobulin assessment in the needle washout after fine-needle aspiration of suspicious lymph nodes. Thyroid. 2004;14:959–963. doi: 10.1089/thy.2004.14.959. [DOI] [PubMed] [Google Scholar]
- 41.Lee CY, Snyder SK, Lairmore TC, Dupont SC, Jupiter DC. Utility of surgeon-performed ultrasound assessment of the lateral neck for metastatic papillary thyroid cancer. J Oncol. 2012;2012:973124. doi: 10.1155/2012/973124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ciappuccini R, Heutte N, Trzepla G, Rame JP, Vaur D, Aide N, Bardet S. Postablation (131)I scintigraphy with neck and thorax SPECT-CT and stimulated serum thyroglobulin level predict the outcome of patients with differentiated thyroid cancer. Eur J Endocrinol. 2011;164:961–969. doi: 10.1530/EJE-11-0156. [DOI] [PubMed] [Google Scholar]
- 43.Robenshtok E, Grewal RK, Fish S, Sabra M, Tuttle RM. A low postoperative nonstimulated serum thyroglobulin level does not exclude the presence of radioactive iodine avid metastatic foci in intermediate-risk differentiated thyroid cancer patients. Thyroid. 2013;23:436–442. doi: 10.1089/thy.2012.0352. [DOI] [PubMed] [Google Scholar]
- 44.Brassard M, Borget I, Edet-Sanson A, Giraudet AL, Mundler O, Toubeau M, Bonichon F, Borson-Chazot F, Leenhardt L, Schvartz C, Dejax C, Brenot-Rossi I, Toubert ME, Torlontano M, Benhamou E, Schlumberger M. Long-term follow-up of patients with papillary and follicular thyroid cancer: a prospective study on 715 patients. J Clin Endocrinol Metab. 2011;96:1352–1359. doi: 10.1210/jc.2010-2708. [DOI] [PubMed] [Google Scholar]
- 45.Malandrino P, Latina A, Marescalco S, Spadaro A, Regalbuto C, Fulco RA, Scollo C, Vigneri R, Pellegriti G. Risk-adapted management of differentiated thyroid cancer assessed by a sensitive measurement of basal serum thyroglobulin. J Clin Endocrinol Metab. 2011;96:1703–1709. doi: 10.1210/jc.2010-2695. [DOI] [PubMed] [Google Scholar]
- 46.Pitoia F, Bueno F, Urciuoli C, Abelleira E, Cross G, Tuttle RMM. Outcome of patients with differentiated thyroid cancer risk stratified according to the American Thyroid Association and Latin American Thyroid Society risk of recurrence classification systems. Thyroid 2013, E-pub ahead of print. [DOI] [PubMed]
- 47.Solorzano CC, Carneiro DM, Ramirez M, Lee TM, Irvin GL., 3rd Surgeon-performed ultrasound in the management of thyroid malignancy. Am Surg. 2004;70:576–580. [PubMed] [Google Scholar]
- 48.do Rosario PW, Borges MA, Alves MF, Purisch S, Padrao EL, Rezende LL, Barroso AL. Follow-up of high-risk patients with differentiated thyroid cancer without persistent disease after initial therapy. Arq Bras Endocrinol Metabol. 2006;50:909–913. doi: 10.1590/s0004-27302006000500012. [DOI] [PubMed] [Google Scholar]
- 49.Nascimento C, Borget I, Al Ghuzlan A, Deandreis D, Chami L, Travagli JP, Hartl D, Lumbroso J, Chougnet C, Lacroix L, Baudin E, Schlumberger M, Leboulleux S. Persistent disease and recurrence in differentiated thyroid cancer patients with undetectable postoperative stimulated thyroglobulin level. Endocr Relat Cancer. 2011;18:R29–R40. doi: 10.1677/ERC-10-0292. [DOI] [PubMed] [Google Scholar]
- 50.Vaisman F, Shaha A, Fish S, Tuttle R. Initial therapy with either thyroid lobectomy or total thyroidectomy without radioactive iodine remnant ablation is associated with very low rates of structural disease recurrence in properly selected patients with differentiated thyroid cancer. Clin Endocrinol (Oxf) 2011;75:112–119. doi: 10.1111/j.1365-2265.2011.04002.x. [DOI] [PubMed] [Google Scholar]
- 51.Kloos RT. Approach to the patient with a positive serum thyroglobulin and a negative radioiodine scan after initial therapy for differentiated thyroid cancer. J Clin Endocrinol Metab. 2008;93:1519–1525. doi: 10.1210/jc.2007-2357. [DOI] [PubMed] [Google Scholar]
- 52.Pacini F, Fugazzola L, Lippi F, Ceccarelli C, Centoni R, Miccoli P, Elisei R, Pinchera A. Detection of thyroglobulin in fine needle aspirates of nonthyroidal neck masses: a clue to the diagnosis of metastatic differentiated thyroid cancer. J Clin Endocrinol Metab. 1992;74:1401–1404. doi: 10.1210/jcem.74.6.1592886. [DOI] [PubMed] [Google Scholar]
- 53.Krishnamurthy S, Bedi DG, Caraway NP. Ultrasound-guided fine-needle aspiration biopsy of the thyroid bed. Cancer. 2001;93:199–205. doi: 10.1002/cncr.9029. [DOI] [PubMed] [Google Scholar]
- 54.Zhao L, Gong Y, Wang J, Dawlett M, Huo L, Caraway NP, Guo M. Ultrasound-guided fine-needle aspiration biopsy of thyroid bed lesions from patients with thyroidectomy for thyroid carcinomas. Cancer Cytopathol. 2013;121:101–107. doi: 10.1002/cncy.21202. [DOI] [PubMed] [Google Scholar]
- 55.Robenshtok E, Fish S, Bach A, Dominguez JM, Shaha A, Tuttle RM. Suspicious cervical lymph nodes detected after thyroidectomy for papillary thyroid cancer usually remain stable over years in properly selected patients. J Clin Endocrinol Metab. 2012;97:2706–2713. doi: 10.1210/jc.2012-1553. [DOI] [PubMed] [Google Scholar]
- 56.Al-Saif O, Farrar WB, Bloomston M, Porter K, Ringel MD, Kloos RT. Long-term efficacy of lymph node reoperation for persistent papillary thyroid cancer. J Clin Endocrinol Metab. 2010;95:2187–2194. doi: 10.1210/jc.2010-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rondeau G, Fish S, Hann LE, Fagin JA, Tuttle RM. Ultrasonographically detected small thyroid bed nodules identified after total thyroidectomy for differentiated thyroid cancer seldom show clinically significant structural progression. Thyroid. 2011;21:845–853. doi: 10.1089/thy.2011.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boi F, Baghino G, Atzeni F, Lai ML, Faa G, Mariotti S. The diagnostic value for differentiated thyroid carcinoma metastases of thyroglobulin (Tg) measurement in washout fluid from fine-needle aspiration biopsy of neck lymph nodes is maintained in the presence of circulating anti-Tg antibodies. J Clin Endocrinol Metab. 2006;91:1364–1369. doi: 10.1210/jc.2005-1705. [DOI] [PubMed] [Google Scholar]
- 59.Cignarelli M, Ambrosi A, Marino A, Lamacchia O, Campo M, Picca G, Giorgino F. Diagnostic utility of thyroglobulin detection in fine-needle aspiration of cervical cystic metastatic lymph nodes from papillary thyroid cancer with negative cytology. Thyroid. 2003;13:1163–1167. doi: 10.1089/10507250360731578. [DOI] [PubMed] [Google Scholar]
- 60.Jeon MJ, Park JW, Han JM, Yim JH, Song DE, Gong G, Kim TY, Baek JH, Lee JH, Shong YK, Bae Kim W. Serum antithyroglobulin antibodies interfere with thyroglobulin detection in fine-needle aspirates of metastatic neck nodes in papillary thyroid carcinoma. J Clin Endocrinol Metab. 2013;98:153–160. doi: 10.1210/jc.2012-2369. [DOI] [PubMed] [Google Scholar]
- 61.Suh YJ, Son EJ, Moon HJ, Kim EK, Han KH, Kwak JY. Utility of thyroglobulin measurements in fine-needle aspirates of space occupying lesions in the thyroid bed after thyroid cancer operations. Thyroid. 2013;23:280–288. doi: 10.1089/thy.2011.0303. [DOI] [PubMed] [Google Scholar]
- 62.Spencer CA, Bergoglio LM, Kazarosyan M, Fatemi S, LoPresti JS. Clinical impact of thyroglobulin (Tg) and Tg autoantibody method differences on the management of patients with differentiated thyroid carcinomas. J Clin Endocrinol Metab. 2005;90:5566–5575. doi: 10.1210/jc.2005-0671. [DOI] [PubMed] [Google Scholar]
- 63.Latrofa F, Ricci D, Montanelli L, Rocchi R, Piaggi P, Sisti E, Grasso L, Basolo F, Ugolini C, Pinchera A, Vitti P. Thyroglobulin autoantibodies in patients with papillary thyroid carcinoma: comparison of different assays and evaluation of causes of discrepancies. J Clin Endocrinol Metab. 2012;97:3974–3982. doi: 10.1210/jc.2012-2406. [DOI] [PubMed] [Google Scholar]
- 64.Boi F, Maurelli I, Pinna G, Atzeni F, Piga M, Lai ML, Mariotti S. Calcitonin measurement in wash-out fluid from fine needle aspiration of neck masses in patients with primary and metastatic medullary thyroid carcinoma. J Clin Endocrinol Metab. 2007;92:2115–2118. doi: 10.1210/jc.2007-0326. [DOI] [PubMed] [Google Scholar]
- 65.Kim WW, Kim JS, Hur SM, Kim SH, Lee SK, Choi JH, Kim S, Choi JY, Lee JE, Kim JH, Nam SJ, Yang JH, Choe JH. Radioguided surgery using an intraoperative pet probe for tumor localization and verification of complete resection in differentiated thyroid cancer: a pilot study. Surgery. 2011;149:416–424. doi: 10.1016/j.surg.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 66.Travagli JP, Cailleux AF, Ricard M, Baudin E, Caillou B, Parmentier C, Schlumberger M. Combination of radioiodine (131I) and probe-guided surgery for persistent or recurrent thyroid carcinoma. J Clin Endocrinol Metab. 1998;83:2675–2680. doi: 10.1210/jcem.83.8.5014. [DOI] [PubMed] [Google Scholar]
- 67.Soprani F, Bondi F, Puccetti M, Armaroli V. Charcoal tattoo localization for differentiated thyroid cancer recurrence in the central compartment of the neck. Acta Otorhinolaryngol Ital. 2012;32:87–92. [PMC free article] [PubMed] [Google Scholar]
- 68.Kang TW, Shin JH, Han BK, Ko EY, Kang SS, Hahn SY, Kim JS, Oh YL. Preoperative ultrasound-guided tattooing localization of recurrences after thyroidectomy: safety and effectiveness. Ann Surg Oncol. 2009;16:1655–1659. doi: 10.1245/s10434-009-0431-7. [DOI] [PubMed] [Google Scholar]
- 69.Hartl DM, Chami L, Al Ghuzlan A, Leboulleux S, Baudin E, Schlumberger M, Travagli JP. Charcoal suspension tattoo localization for differentiated thyroid cancer recurrence. Ann Surg Oncol. 2009;16:2602–2608. doi: 10.1245/s10434-009-0572-8. [DOI] [PubMed] [Google Scholar]
- 70.Bennedbaek FN, Hegedus L. Treatment of recurrent thyroid cysts with ethanol: a randomized double-blind controlled trial. J Clin Endocrinol Metab. 2003;88:5773–5777. doi: 10.1210/jc.2003-031000. [DOI] [PubMed] [Google Scholar]
- 71.Paschke R, Hegedus L, Alexander E, Valcavi R, Papini E, Gharib H. Thyroid nodule guidelines: agreement, disagreement and need for future research. Nat Rev Endocrinol. 2011;7:354–361. doi: 10.1038/nrendo.2011.1. [DOI] [PubMed] [Google Scholar]
- 72.Dossing H, Bennedbaek FN, Hegedus L. Long-term outcome following interstitial laser photocoagulation of benign cold thyroid nodules. Eur J Endocrinol. 2011;165:123–128. doi: 10.1530/EJE-11-0220. [DOI] [PubMed] [Google Scholar]
- 73.Faggiano A, Ramundo V, Assanti AP, Fonderico F, Macchia PE, Misso C, Marciello F, Marotta V, Del Prete M, Papini E, Lombardi G, Colao A, Spiezia S. Thyroid nodules treated with percutaneous radiofrequency thermal ablation: a comparative study. J Clin Endocrinol Metab. 2012;97:4439–4445. doi: 10.1210/jc.2012-2251. [DOI] [PubMed] [Google Scholar]
- 74.Hay ID, Charboneau JW. The coming of age of ultrasound-guided percutaneous ethanol ablation of selected neck nodal metastases in well-differentiated thyroid carcinoma. J Clin Endocrinol Metab. 2011;96:2717–2720. doi: 10.1210/jc.2011-2196. [DOI] [PubMed] [Google Scholar]
- 75.Heilo A, Sigstad E, Fagerlid KH, Haskjold OI, Groholt KK, Berner A, Bjoro T, Jorgensen LH. Efficacy of ultrasound-guided percutaneous ethanol injection treatment in patients with a limited number of metastatic cervical lymph nodes from papillary thyroid carcinoma. J Clin Endocrinol Metab. 2011;96:2750–2755. doi: 10.1210/jc.2010-2952. [DOI] [PubMed] [Google Scholar]
- 76.Papini E, Bizzarri G, Bianchini A, Valle D, Misischi I, Guglielmi R, Salvatori M, Solbiati L, Crescenzi A, Pacella CM, Gharib H. Percutaneous ultrasound-guided laser ablation is effective for treating selected nodal metastases in papillary thyroid cancer. J Clin Endocrinol Metab. 2013;98:E92–E97. doi: 10.1210/jc.2012-2991. [DOI] [PubMed] [Google Scholar]