Abstract
Background
It is well known that thyroid hormone withdrawal (THW) in thyroid cancer patients can induce a decrease in quality of life (QOL). Recombinant human thyrotropin (rh-TSH) has been used to avoid this; however, no blinded studies have ever documented the effect.
Objective
To compare QOL in patients with differentiated thyroid cancer (DTC) treated with either rh-TSH or liothyronine (L-T3) THW for 10 days.
Study Design
Double-blind, randomised cross-over.
Patients
Fifty-six patients with DTC treated by total thyroidectomy and indication for postsurgery radioiodine (RI) ablation therapy.
Intervention
Randomisation to either L-T3 and rh-TSH prior to the first RI course and following this to ingest placebo tablets and receive placebo injections before a second RI uptake measurement 4-6 months later, or to receive placebo before the primary RI ablation and active therapy 4-6 months later.
Main Outcome Measures
QOL was measured by SF-36 and 2 visual analogue scale (VAS) scores at baseline and during RI therapy or RI uptake.
Results
A significant difference in QOL was seen in 2 of 4 predefined SF-36 domains (7.2 and 6.6%) and 2 VAS scales (10 and 14%), favouring rh-TSH therapy.
Conclusion
This is the first blinded randomised clinical trial describing the effect of rh-TSH compared to L-T3 THW on QOL in DTC patients. A significant difference was demonstrated, though smaller than described in previous non-blinded studies.
Key Words: Thyroid cancer, Recombinant human thyrotropin, Quality of life, Triiodothyronine
Introduction
The overall prognosis of differentiated thyroid cancer (DTC) is favourable, and previous reports describe a 30-year survival rate of approximately 75% [1,2]. A trend towards even better survival rates has been reported in more recent publications, indicating a life expectancy close to the background population for low-risk patients [3]. These are typically younger patients with jobs and families. Therefore a possible decrease in treatment-related quality of life (QOL) will significantly impact their lives and may, from a patient's point of view, be unacceptable.
TSH-stimulated radioiodine (RI) therapy is an important tool in the treatment of DTC. This requires a high concentration of TSH in serum. Traditionally, this has been achieved by withdrawal of thyroid hormone replacement therapy (THW) to induce an endogenous sensitive TSH (s-TSH) increase. A THW course of levothyroxine (L-T4) for 4-6 weeks induces symptoms of hypothyroidism and a decrease in QOL [4,5,6,7].
To avoid the THW-induced decrease in QOL, the use of recombinant human thyrotropin (rh-TSH) has been suggested, and several studies have confirmed that the use of rh-TSH is comparable to endogenous TSH stimulation induced by L-T4 THW. rh-TSH is therefore used for RI ablation therapy as well as to stimulate thyroglobulin secretion and iodine uptake for diagnostic purposes [8,9,10,11,12,13,14,15,16,17,18,19].
Previous studies have focused on comparing the safety of rh-TSH on the risk of recurrence of cancer, with open design QOL studies carried out concomitantly as a secondary end point. Results from these studies concluded that it was possible to avoid the decrease in THW-related QOL by using rh-TSH.
It is noteworthy, however, that no blinded randomised clinical trials have been carried out to verify the impact on QOL of rh-TSH compared to THW in these patients. Furthermore, no trials have evaluated a possible nocebo effect, which must be expected with THW.
Previous studies have been made on patients with an L-T4 THW of 3-6 weeks or a mixture of L-T4 THW and liothyronine (L-T3) THW for 10-14 days. The advantage of using L-T3 THW is that the half-life is shorter and thereby it is possible to shorten the withdrawal period to 10-14 days instead of 3-6 weeks.
The aim of this study was to conduct the first randomised clinical trial comparing QOL in patients with DTC treated with either rh-TSH or L-T3 THW for 10 days before RI therapy or diagnostic RI uptake measurement.
Materials and Methods
Patients
Consecutive patients with DTC treated by total thyroidectomy and indication of postsurgery RI ablation therapy were included in the study.
Patients with age <18 or >75 years, as well as patients not able to give informed consent, pregnant or lactating patients were excluded from the study.
Patients were asked to participate in the trial at their first visit after surgery. All patients included had a post-RI ablation scintigraphy performed and a second TSH-stimulated RI scintigraphy and thyroglobulin measurement 4-6 months later. All patients received L-T3 substitution after surgery and during the study period.
Design
A double-blinded, placebo-controlled, cross-over study.
The patients were randomised to one of the following two groups:
• To continue with L-T3 and to receive rh-TSH stimulation with 0.9 mg Thyrogen® (Genzyme) × 2 days minus 1 and 2 prior to RI therapy, and following this to have placebo tablets and placebo injections with isotonic NaCl prior to the RI uptake measurement 4-6 months later
• To receive the placebo treatment in connection with the primary RI therapy and the T3 tablets and rh-TSH injections prior to the second RI uptake measurement
Placebo tablets were produced by Glostrup Pharmacy. They were similar to the T3 tablets used – L-T3 20 μg from Nycomed. Both placebo and T3 tablets were packed in identical boxes and labelled for the individual patient. The placebo injections were given by an unblinded nurse, who was not otherwise involved in the clinical trial.
Block randomisation was used: for every 10 patients, 5 were randomised to rh-TSH in the first period and 5 to receive rh-TSH in the second period.
Two departments participated in the study: the Departments of Oncology at Herlev University Hospital and Odense University Hospital.
Methods
QOL was evaluated using a questionnaire, SF-36, according to the Danish version [20] focusing on the following items: general health, vitality, social functioning, and mental health (0-100, 100 = best). Two visual analogue scales (VAS) were used (0-100, 0 = best). One scale evaluated physical and one psychological symptoms. QOL was measured before changes in medication in both periods, in relation to the primary RI ablation therapy and in relation to the iodine uptake 4-6 months later.
s-TSH was measured using Immulite 2500, with normal range 0.4-4.0 mU/l, inter- and intra-assay coefficient of variation approximately 5% (Herlev), or Immulite 2000, with normal range 0.3-4.0 mU/l, inter- and intra-assay coefficient of variation approximately 5% (Odense).
s-TSH was measured concomitantly with the QOL measurements.
Registrations
The study was approved by the Danish Ethical Committee Dathyrca 1, journal No. H-B-2007-043, the National Committee on Biomedical Research Ethics EudraCT No. 2007-002713-39, the Danish Data Protection Agency journal No. 2007-41-1201/HEH.750.86-11 and registered in www.Clinicaltrials.gov under NCT00604318.
Statistical Analyses
Power calculation was based on the following parameters from SF-36: 80% power, α = 0.05, minimal important difference 10 points. To evaluate general health, 43 patients were required, for social functioning 56, for mental health 34 and for vitality 45. It was decided to include 56 patients [20]. Data was compared by t test (for continuous variables) and Wilcoxon rank sum tests (for ordinary variables). Calculations were made using R statistical software version 2.9.0 (R Foundation for Statistical Computing, Vienna, 2009). All p values are two-sided.
The period effect was estimated between intervention period 1 and intervention period 2. The period effect was analysed by paired sample t tests. Calculations of treatment effects were made by a 2-way analysis of variance. Treatment and placebo effects were calculated as post hoc tests and corrected for multiple comparisons with the Bonferroni-Holm method [21]. As the study included several end points, a false discovery rate method was used to correct for multiple tests [22]. The analysis was made as ‘under-treatment ’ analysis, and drop-out/excluded patients during the study were excluded from the final analysis.
Patients
Fifty-six patients (16 men and 40 women) completed the study. The median age was 46 years (range 23-71). Twenty-five patients received rh-TSH in the first period and 31 in the second. The starting dose of T3 was 40-60 μg/day, with the intention of achieving a full suppression of TSH, without major side effects; see figure 1 for consort diagram, and table 1 for baseline characteristics. All patients had postsurgery RI ablation therapy with 131I – 3.7 GBq.
Fig. 1.
Consort diagram showing the flow of participants throughout the trial.
Table 1.
Baseline characteristics of the study participants
| Active therapy in the first period (n = 25) | Active therapy in the second period (n = 31) | |
|---|---|---|
| Males/females | 4/21 | 11/20 |
| Age, years | ||
| Median | 45 | 47 |
| Range | 24 – 69 | 23 – 71 |
| Papillary/follicular cancer T3 dose, µg/day | 19/6 | 27/6 |
| Median | 40 | 40 |
| Quartiles | 40 – 60 | 40 – 60 |
Results
Sensitive TSH
s-TSH values before changing medication and related to THW and rh-TSH are seen in table 2.
Table 2.
s-TSH values (mU/l) related to THW and rh-TSH stimulation
| s-TSH 0 |
s-TSH |
|||
|---|---|---|---|---|
| first period | second period | THW | rh-TSH stimulation | |
| Median | 0.31 | 0.13 a | 56.00 | 88.00b |
| Quartiles | 0.09 – 3.40 | 0.02 – 4.10 | 40.00 – 75.00 | 71.00 – 110.00 |
p = 0.178, s-TSH in the first versus the second period
p < 0.001, s-TSH on THW versus rh-TSH stimulation.
No significant difference was observed comparing s-TSH prior to the first and second RI treatment/uptake. When comparing THW and rh-TSH stimulation, a significantly lower s-TSH was observed in patients on THW (median: 56 mU/l, quartiles 40-75) compared to rh-TSH (median: 88 mU/l, quartiles 71-110; p < 0.001). No difference in s-TSH according to which period the rh-TSH was given (first or second) was observed.
Quality of Life
Table 3 describes the difference between the SF-36 domains and VAS scores related to THW and rh-TSH stimulation.
Table 3.
Changes in SF-36 domains and VAS scores related to THW and rh-TSH stimulation
| Time 0 |
THW | rh-TSH stimulation | Difference between rh-TSH and THW | p value rh-TSH vs. THW | ||
|---|---|---|---|---|---|---|
| period 1 | period 2 | |||||
| SF-36 | ||||||
| General health | 66.5±19.1 | 68.6±20.0 | 65.1±21.2 | 68.0±20.2 | 3.0 | 0.18 |
| Vitality | 54.9±24.4 | 60.9±21.7 | 53.6±24.1 | 60.4±25.8 | 6.6 | 0.06 |
| Social functioning | 77.5±24.0 | 85.7±20.3 | 77.7±22.1 | 85.1±20.6 | 7.2 | 0.008 |
| Mental health | 67.8±21.4 | 75.9±17.8 | 69.7±22.4 | 76.3±16.9 | 6.6 | 0.02 |
| VAS | ||||||
| Physical symptoms | 23.7±27.8 | not measured | 39.0±32.8 | 24.7±26.0 | –14.0 | 0.004 |
| Psychological symptoms | 27.7±26.9 | not measured | 36.3±32.0 | 27.6±29.7 | –10.0 | 0.02 |
SF-36: range 0 – 100, 100 best; VAS: range 0 – 100, 0 best.
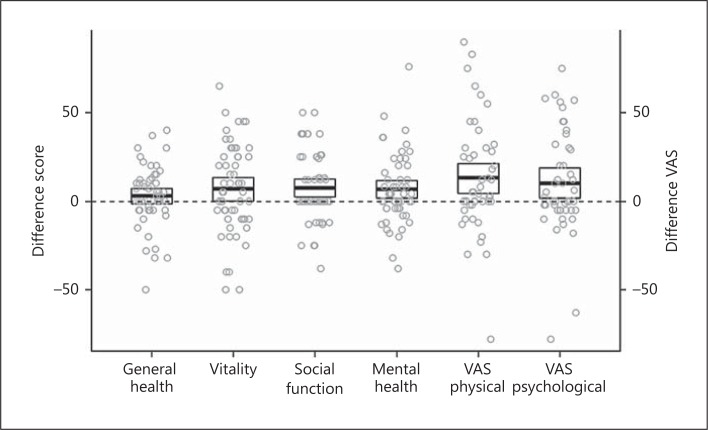
A significant difference in QOL on rh-TSH versus THW was observed in 2 of 4 preselected SF-36 domains (social functioning and mental health) with a mean difference of 7.2 and 6.6 points, respectively (table 3; fig. 2). Evaluated by VAS scales, a similar significant difference was seen for physical symptoms at 14, and of psychologically symptoms at 10 points, indicating a higher QOL following rh-TSH than THW (table 3; fig. 2). However, large individual differences were observed with a range of score from approximately −50 to +50% in all domains (fig. 2).
Fig. 2.
Difference in QOL scores – mean ± SD and individual differences of QOL scores on rh-TSH minus those on T3 THW in 4 preselected SF-36 domains (general health, vitality, social function and mental health) and QOL on T3 THW minus those on rh-TSH in 2 VAS (physical and psychological). Positive values indicate an increase in QOL related to rh-TSH.
A significantly higher QOL was observed in all domains (p < 0.001) before changing medication prior to the second study period compared to the first. However, no period effect could be detected on QOL the day of RI ablation/uptake comparing patients having rh-TSH in the first or second period.
No correlation was observed comparing the pretreatment QOL scores to the effect of rh-TSH.
Discussion
Several studies have been published describing the impact on QOL of rh-TSH compared to THW. In all trials the premise for conducting these studies was that QOL would be preserved when THW was substituted with rh-TSH. The primary objective of these studies was therefore to prove the efficacy and safety of rh-TSH, i.e. the rise in s-TSH induced by rh-TSH would result in an equal rise in s-TSH compared to the endogenous s-TSH rise following THW and ultimately confirmed by equality of disease-free survival [8,10,11,13,16,19].
The effect on QOL has been described in 9 previous trials (table 4) involving more than 2,000 patients: 1 study presents data based on a questionnaire [14], 3 studies are prospective longitudinal open trials [9,10,15] and 5 are randomised open label trials [8,16,17,18,19]. However, none of these trials were blinded and QOL, in most of the studies, was measured as a secondary outcome. Different methods have been used to evaluate QOL: SF-12, SF-36, Billewicz, FACIT, VAS scores and sick leave. However, in all of the trials the authors describe a highly significant improvement in QOL using rh-TSH compared to THW.
Table 4.
Studies comparing QOL in THW compared to rh-TSH stimulation
| Reference | n | QOL test | Method | Results | Type of study |
|---|---|---|---|---|---|
| Borget et al. [14], 2007 | 306 invited, 292 completed | Duration of sick leave Questionnaire concerning sick leave | L-T4 THW for 3 weeks or rh-TSH stimulation Likely to require sick leave primary outcome | Likely to require sick leave 33% on THW vs. 11% on rh-TSH (p = 0.001) Duration of sick leave 11.2 vs. 3 days (p = 0.002) | Retrospective and prospective non-randomised questionnaire |
| Meier et al. [10], 1994 | 19 | Billewicz Mood state comparison | On T3 rh-TSH stimulation L-T3 THW median 19 days (15 – 28) In all patients QOL secondary outcome | Billewicz: 3 of 19 had hypothyroid symptoms at start vs. 15 on THW vs. none on rh-TSH Mood state comparison: 89 – 94% experienced decreased vigour and activity on THW vs. 0 – 5% on rh-TSH |
Prospective longitudinal trial |
| Schroeder et al. [15], 2006 | 225 | SF-36 (scale 0 – 100) Billewicz | On L-T4 L-T4 THW for 4 weeks (74% were changed to L-T3 with THW, 2 weeks before WBS) rh-TSH stimulation In all patients QOL secondary outcome | SF-36: no difference between basic (on L-T4) and rh-TSH; significant difference in all SF-36 domains THW vs. rh-TSH (p < 0.0001) Calculated difference THW vs. rh-TSH was 9.7±3.3 (mean±SD) including 10 SF-36 domains Billewicz: all signs and symptoms were described as significantly increased related to THW No difference of QOL if patients were changed to L-T3 | Prospective longitudinal trial |
| Dueren et al. [9], 2010 | 192, 128 protocol compliant | SF-12 Aspects of daily life | THW T4 4 – 5 weeks or T3 3 weeks (n = 72) 3 – 6 months after surgery rh-TSH stimulation 6 – 12 months later | SF-12: scores 49±23 on THW vs. 74±17 re-TSH Less absent from work: absent 47.8% on THW vs. 4.5% on rh-TSH stimulation | Prospective longitudinal trial |
| Pacini et al. [13], 2006 | 63 | SF-36 (scale 0 – 100) Billewicz | Baseline (within 2 weeks after surgery) THW for 4 – 6 weeks or rh-TSH stimulation QOL secondary outcome of the study | SF-36: significant difference (p < 0.02) in 5 of 8 domains in the THW vs. rh-TSH Calculated difference 16.57±11.1 (mean±SD) including all 10 SF-36 domains Billewicz: scores 27 + 7 after THW vs. 18 + 4 related to rh-TSH (p < 0.0001) | Randomised open labelled trial |
| Taïeb et al. [17], 2009 | 74 | VAS (scale 0 – 100) FACIT-F, FACT-G, FACIT-F TOI BDI CES-D | One week after surgery L-T4 THW for 5 weeks or rh-TSH It is not stated whether QOL is primary or secondary outcome of the study | VAS score: THW vs. rh-TSH; fatigue 20.5±28.0 vs. 56.5±28.4; mood changes 13.5±22.0 vs. 43.8±31.6; general swelling 7.6±18.9 vs. 45.1±33.3; all p < 0.001 Decrease in QOL I THW in FACIT-F, FACIT-F TOI and FACT-G (p < 0.003) Correlation between changes in FACT-G and FACIT-F scores and initial state of anxiety and depression | Randomised open labelled trial |
| Lee et al. [18], 2010 | 291 | Seven-item QOL questionnaire (scale 0 – 31) | After thyroidectomy L-T4 THW 4 weeks or L-T3 THW 2 weeks or rh-TSH QOL at ablation QOL secondary outcome of the study | Total score (scale 0 – 31): 4.2±1.9 rh-TSH vs. 15.1±3.1 L-T4 THW vs. 15.8±4.1 L-T3 THW p < 0.001 L-T4/L-T3 THW vs. rh-TSH p n.s. L-T4 THW vs. L-T3 THW | Randomised open labelled trial |
| Schlumberger et al. [19], 2012 | 752 | SF-36 (scale 0 – 100) Billewicz (percent of patients having symptoms) | THW (L-T4 28 days or L-T3 14 days – the ratio of L-T4/L-T3 THW not given) or rh-TSH QOL secondary outcome of the study | At RI therapy SF-36: described as significant THW vs. rh-TSH (p values not given) Calculated difference 7.0±4.3 (mean±SD) on 10 SF-36 domains Billewicz: described as significant (p values not given) Calculated difference percent of patients with hypothyroid symptoms (13 symptoms) 20±11.6% (mean±SD) | Randomised open labelled trial |
| Mallick et al. [8], 2012 | 438, data analyses on 421 | SF-36 Daily life activity | THW or rh-TSH | SF-36: difference at 30 – 40 in favour of rh-TSH compared to THW Difficulties at work: 9.4% on rh-TSH vs. 22.1% on THW | Randomised open labelled trial |
‘Calculated difference’ calculated by the authors. WBS = Whole body scan.
The nocebo effect (from the Latin nocoebo, ‘to harm ’) is associated with a person's prior expectations regarding adverse effects from treatment, which can induce both somatic and psychological symptoms. The phenomenon is common in clinical practice and has recently become a subject of research and discussion among general scientists as well as clinicians and ethicists [23,24]. Thyroid cancer patients are very aware of their inability to produce thyroid hormone themselves, and being dependent on thyroid hormone medication. Therefore the patients will expect a decrease in QOL when thyroid hormones are withdrawn for several weeks. To prove that the use of rh-TSH actually does prevent a decrease in QOL, a blinded trial was considered pertinent. Therefore, although the use of rh-TSH has been accepted for a decade, we found it relevant to perform a blinded placebo-controlled trial.
The use of L-T3 as bridging has been suggested to reduce the period of THW, and the rationale for this is the shorter half-life of L-T3 – approximately 12 h – compared to 1 week using L-T4. By this method it is possible to reduce the THW period from 3-4 weeks to 10-14 days, and still achieve the intended s-TSH of more than 30 mU/l [12]. Three trials have compared QOL using L-T4 THW alone versus bridging with L-T3 and a shorter THW period [9,15,25]. Dueren et al. [9] compared 72 patients in whom a period of L-T3 bridging was used and a THW of L-T3 for 2-3 weeks was compared with L-T4 THW for 4-5 weeks. At the follow-up no difference in QOL was found. In accordance with this, Schroeder et al. [15] were unable to detect a difference in QOL using a bridging period of L-T3, but did question the length of hypothyroid symptoms; however, the design of the study did not permit them to evaluate this issue. This question, however, was addressed by Leboeuf et al. [25] evaluating QOL in 20 patients in a blinded study using the Billewicz score several times during the period. They demonstrated that bridging with L-T3 and a THW of 3 weeks could not prevent profound hypothyroidism. In our study it was confirmed that 10 days of L-T3 THW were sufficient to increase s-TSH to more than 30 mU/l.
It is important to identify a minimal level of change consistent with real, as opposed to statistically significant, benefits by using a QOL questionnaire [26,27]. In our trial using SF-36, we found a small, but significant difference of 7.2 points in social functioning and 6.6 points in mental health, and one may question whether these differences are actually within the limits of human discriminative ability. It is generally agreed upon that the minimal important difference in analyses of QOL using SF-36 are 5-10 points – or half of the SD, which, in most studies including ours, approximates 10 points. Therefore, the general recommendations are to set the minimal important difference to approximately 10 points, which we used in our power calculation. However, our results showed a minor (7.2 and 6.6 points) but significant difference in 2 of 4 scales and large individual differences. This, combined with a difference of 10-14 points evaluated by VAS scores, indicates a clinically significant difference.
Many factors can potentially affect QOL, and a study by Taïeb et al. [17] described that changes in QOL were dependent on initial scores. In our study, large individual changes were observed and no correlation was found between initial levels of QOL and the effect of rh-TSH versus THW. Impaired physical performance is another important factor to be considered, and in our study this parameter (on a VAS scale) had the highest difference of 14% in this group of younger patients with a median age of 46 (range 23-71). Previous studies by Borget et al. [14] and Dueren et al. [9] have described a significant difference in sick leave comparing withdrawal with rh-TSH stimulation, however in an unblinded design. This could indicate that impaired working ability had a significant effect on QOL and could also have an overall social economic impact.
The impaired physical performance could be related to adverse cardiac effects and a risk of heart failure. Several studies have confirmed the adverse cardiac effects due to overt or subclinical hypothyroidism (see Biondi [28] for a comprehensive review). However, exact data on the acute effect of hypothyroidism on the heart is lacking. Our study could have improved by a registration during hypothyroidism of sick leave and an evaluation of the acute effects on the heart by e.g. a myocardial scintigraphy or echocardiography
Several possible advantages can be gained by using rh-TSH:
A decreased residence time of RI in the remnant thyroid cells and hereby reduced exposure of various organs, including the bone marrow, to RI [29,30]
Avoidance of even short-term hypothyroidism, which is particularly desirable in older patients suffering from heart disease and hypertension [31]
Better compliance, due to a reduction in the patient's fear of decreased QOL related to THW [32]
A simpler regime using L-T4 throughout the follow-up without any interruption of the medical substitution treatment
Several weaknesses in our study should be mentioned:
A comparison with THW of L-T4 would have been of interest; however, comparing both regimes was not feasible
The s-TSH levels were significantly different comparing the two periods, with an s-TSH at a median 56 mU/l on L-T3 THW compared to a median 88 mU/l on the rh-TSH stimulation; the significance of this is unknown
A relatively small number of patients were included in the study, although a significant result was observed
Conclusion
This is the first blinded randomised clinical trial which describes the effect of rh-TSH compared to L-T3 THW on QOL in DTC patients, and we demonstrated a significant difference in 2 out of 4 SF-36 domains and 2 VAS scores. The differences were, however, smaller than described in previous non-blinded studies and the cumulative effect of avoiding THW and a possible nocebo effect must be taken into account. Our overall recommendation is to use rh-TSH as a standard treatment instead of L-T3 THW due to the possible advantages of using the simpler regime by continuing the L-T4 therapy. However, in countries/hospitals with a limited budget the use of rh-TSH may be preserved for older and more fragile patients.
Disclosure Statement
The authors declare that no financial or other conflicts of interest exist in relation to the content of the article.
References
- 1.Schlumberger MJ. Papillary and follicular thyroid carcinoma. N Engl J Med. 1998;338:297–306. doi: 10.1056/NEJM199801293380506. [DOI] [PubMed] [Google Scholar]
- 2.Mazzaferri EL, Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med. 1994;97:418–428. doi: 10.1016/0002-9343(94)90321-2. [DOI] [PubMed] [Google Scholar]
- 3.Schlumberger M, Hitzel A, Toubert ME, et al. Comparison of seven serum thyroglobulin assays in the follow-up of papillary and follicular thyroid cancer patients. J Clin Endocrinol Metab. 2007;92:2487–2495. doi: 10.1210/jc.2006-0723. [DOI] [PubMed] [Google Scholar]
- 4.Luster M, Felbinger R, Dietlein M, Reiners C. Thyroid hormone withdrawal in patients with differentiated thyroid carcinoma: a one hundred thirty-patient pilot survey on consequences of hypothyroidism and a pharmacoeconomic comparison to recombinant thyrotropin administration. Thyroid. 2005;15:1147–1155. doi: 10.1089/thy.2005.15.1147. [DOI] [PubMed] [Google Scholar]
- 5.Dow KH, Ferrell BR, Anello C. Quality-of-life changes in patients with thyroid cancer after withdrawal of thyroid hormone therapy. Thyroid. 1997;7:613–619. doi: 10.1089/thy.1997.7.613. [DOI] [PubMed] [Google Scholar]
- 6.Chow SM, Au KH, Choy TS, et al. Health-related quality-of-life study in patients with carcinoma of the thyroid after thyroxine withdrawal for whole body scanning. Laryngoscope. 2006;116:2060–2066. doi: 10.1097/01.mlg.0000240287.57704.01. [DOI] [PubMed] [Google Scholar]
- 7.Tagay S, Herpertz S, Langkafel M, et al. Health-related quality of life, depression and anxiety in thyroid cancer patients. Qual Life Res. 2006;15:695–703. doi: 10.1007/s11136-005-3689-7. [DOI] [PubMed] [Google Scholar]
- 8.Mallick U, Harmer C, Yap B, et al. Ablation with low-dose radioiodine and thyrotropin alfa in thyroid cancer. N Engl J Med. 2012;366:1674–1685. doi: 10.1056/NEJMoa1109589. [DOI] [PubMed] [Google Scholar]
- 9.Dueren C, Dietlein M, Luster M, et al. The use of thyrogen in the treatment of differentiated thyroid carcinoma: an intraindividual comparison of clinical effects and implications of daily life. Exp Clin Endocrinol Diabetes. 2010;118:513–519. doi: 10.1055/s-0029-1234086. [DOI] [PubMed] [Google Scholar]
- 10.Meier CA, Braverman LE, Ebner SA, et al. Diagnostic use of recombinant human thyrotropin in patients with thyroid carcinoma (phase I/II study) J Clin Endocrinol Metab. 1994;78:188–196. doi: 10.1210/jcem.78.1.8288703. [DOI] [PubMed] [Google Scholar]
- 11.Haugen BR, Pacini F, Reiners C, et al. A comparison of recombinant human thyrotropin and thyroid hormone withdrawal for the detection of thyroid remnant or cancer. J Clin Endocrinol Metab. 1999;84:3877–3885. doi: 10.1210/jcem.84.11.6094. [DOI] [PubMed] [Google Scholar]
- 12.Cooper DS, Doherty GM, Haugen BR, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 13.Pacini F, Schlumberger M, Dralle H, Elisei R, Smit JW, Wiersinga W. European consensus for the management of patients with differentiated thyroid carcinoma of the follicular epithelium. Eur J Endocrinol. 2006;154:787–803. doi: 10.1530/eje.1.02158. [DOI] [PubMed] [Google Scholar]
- 14.Borget I, Corone C, Nocaudie M, et al. Sick leave for follow-up control in thyroid cancer patients: comparison between stimulation with thyrogen and thyroid hormone withdrawal. Eur J Endocrinol. 2007;156:531–538. doi: 10.1530/EJE-06-0724. [DOI] [PubMed] [Google Scholar]
- 15.Schroeder PR, Haugen BR, Pacini F, et al. A comparison of short-term changes in health-related quality of life in thyroid carcinoma patients undergoing diagnostic evaluation with recombinant human thyrotropin compared with thyroid hormone withdrawal. J Clin Endocrinol Metab. 2006;91:878–884. doi: 10.1210/jc.2005-2064. [DOI] [PubMed] [Google Scholar]
- 16.Pacini F, Ladenson PW, Schlumberger M, et al. Radioiodine ablation of thyroid remnants after preparation with recombinant human thyrotropin in differentiated thyroid carcinoma: results of an international, randomized, controlled study. J Clin Endocrinol Metab. 2006;91:926–932. doi: 10.1210/jc.2005-1651. [DOI] [PubMed] [Google Scholar]
- 17.Taïeb D, Sebag F, Cherenko M, et al. Quality of life changes and clinical outcomes in thyroid cancer patients undergoing radioiodine remnant ablation (RRA) with recombinant human TSH (rhTSH): a randomized controlled study. Clin Endocrinol (Oxf) 2009;71:115–123. doi: 10.1111/j.1365-2265.2008.03424.x. [DOI] [PubMed] [Google Scholar]
- 18.Lee J, Yun MJ, Nam KH, Chung WY, Soh EY, Park CS. Quality of life and effectiveness comparisons of thyroxine withdrawal, triiodothyronine withdrawal, and recombinant thyroid-stimulating hormone administration for low-dose radioiodine remnant ablation of differentiated thyroid carcinoma. Thyroid. 2010;20:173–179. doi: 10.1089/thy.2009.0187. [DOI] [PubMed] [Google Scholar]
- 19.Schlumberger M, Catargi B, Borget I, et al. Strategies of radioiodine ablation in patients with low-risk thyroid cancer. N Engl J Med. 2012;366:1663–1673. doi: 10.1056/NEJMoa1108586. [DOI] [PubMed] [Google Scholar]
- 20.Bjorner JB, Damsgaard MT, Watt T, Groenvold M. Tests of data quality, scaling assumptions, and reliability of the Danish SF-36. J Clin Epidemiol. 1998;51:1001–1011. doi: 10.1016/s0895-4356(98)00092-4. [DOI] [PubMed] [Google Scholar]
- 21.Holm H. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6:65–70. [Google Scholar]
- 22.Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I. Controlling the false discovery rate in behavior genetics research. Behav Brain Res. 2001;125:279–284. doi: 10.1016/s0166-4328(01)00297-2. [DOI] [PubMed] [Google Scholar]
- 23.Hauser W, Hansen E, Enck P. Nocebo phenomena in medicine: their relevance in everyday clinical practice. Dtsch Ärztebl Int. 2012;109:459–465. doi: 10.3238/arztebl.2012.0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brody H, Colloca L, Miller FG. The placebo phenomenon: implications for the ethics of shared decision-making. J Gen Intern Med. 2012;27:739–742. doi: 10.1007/s11606-011-1977-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leboeuf R, Perron P, Carpentier AC, Verreault J, Langlois MF. L-T3 preparation for whole-body scintigraphy: a randomized-controlled trial. Clin Endocrinol (Oxf) 2007;67:839–844. doi: 10.1111/j.1365-2265.2007.02972.x. [DOI] [PubMed] [Google Scholar]
- 26.Bjorner JB, Wallenstein GV, Martin MC, et al. Interpreting score differences in the SF-36 vitality scale: using clinical conditions and functional outcomes to define the minimally important difference. Curr Med Res Opin. 2007;23:731–739. doi: 10.1185/030079907x178757. [DOI] [PubMed] [Google Scholar]
- 27.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-realted quality of life: the remarkable universality of half a standard deviation. Med Care. 2003;41:582–592. doi: 10.1097/01.MLR.0000062554.74615.4C. [DOI] [PubMed] [Google Scholar]
- 28.Biondi B. Heart failure and thyroid dysfunction. Eur J Endocrinol. 2012;167:609–618. doi: 10.1530/EJE-12-0627. [DOI] [PubMed] [Google Scholar]
- 29.Luster M, Sherman SI, Skarulis MC, et al. Comparison of radioiodine biokinetics following the administration of recombinant human thyroid stimulating hormone and after thyroid hormone withdrawal in thyroid carcinoma. Eur J Nucl Med Mol Imaging. 2003;30:1371–1377. doi: 10.1007/s00259-003-1230-1. [DOI] [PubMed] [Google Scholar]
- 30.De Kelzer B, Brans B, Hoekstra A, et al. Tumour dosimetry and response in patients with metastatic differentiated thyroid cancer using recombinant human thyrotropin before radioiodine therapy. Eur J Nucl Med Mol Imaging. 2003;30:367–373. doi: 10.1007/s00259-002-1076-y. [DOI] [PubMed] [Google Scholar]
- 31.Duntas LH, Biondi B. Short-term hypothyroidism after levothyroxine-withdrawal in patients with differentiated thyroid cancer: clinical and quality of life consequences. Eur J Endocrinol. 2007;156:13–19. doi: 10.1530/eje.1.02310. [DOI] [PubMed] [Google Scholar]
- 32.Cohen O, Dabhi S, Karasik A, Zila ZS. Compliance with follow-up and the informative value of diagnostic whole-body scan in patients with differentiated thyroid carcinoma given recombinant human TSH. Eur J Endocrinol. 2004;150:285–290. doi: 10.1530/eje.0.1500285. [DOI] [PubMed] [Google Scholar]