Abstract
In this issue of Neuron, Tani et al. (2014) revisit a disputed issue where biochemical and physiological data have provided conflicting results. Using a novel stimulation protocol, the authors isolate the contribution of the glutamate-glutamine cycle to excitatory synaptic transmission.
Keywords: astrocyte, glutamate-glutamine cycle
The classic role of astrocytes within the nervous system is one of housekeeper, maintaining the necessary supplies that enable neurons, the stars of the show, to mediate information processing. This neurocentric view has dramatically changed over the last 20 years with evidence that astrocytes directly participate in synaptic transmission by sensing and responding to a myriad of activity-dependent regimes (Perea et al., 2009). As the excitement of active participation of astrocytes in the “tripartite synapse” has increased, the perceived importance of astrocytes in conventional housekeeping functions has dwindled. In fact, there is direct evidence against one major housekeeping function, that of providing sufficient levels of glutamate for neurons to maintain excitatory synaptic transmission (Masson et al., 2006; Kam and Nicoll, 2007). The conclusions from these electrophysiological studies question the functional significance of canonical biochemical pathways that indicate astrocytes to provide glutamate for neurons via the glutamate-glutamine cycle (Figure 1; Hertz, 1979; Kandel et al., 2000).
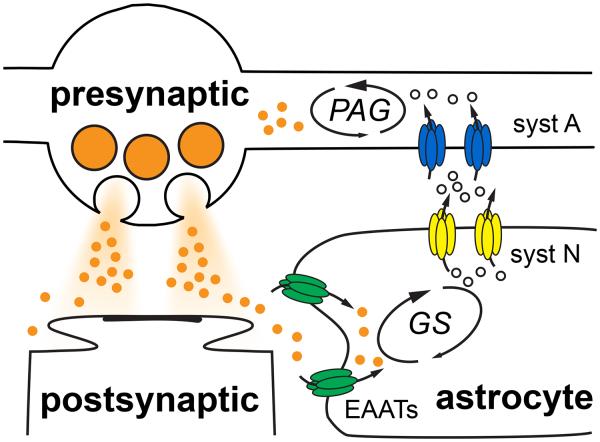
Figure 1. The canonical glutamate-glutamine cycle.
Following synaptic vesicle fusion from presynaptic boutons and receptor activation at postsynaptic membranes, glutamate (orange circles) is taken up by excitatory amino acid transporters (EAATs; green) located on astrocytes. Glutamine synthetase (GS) rapidly converts glutamate to glutamine (open circles) that is then exported by system N transporters (syst N; yellow). System A transporters (syst A; blue) localized to neurons then take up glutamine that is converted back to glutamate by phosphate-activated glutaminase (PAG).
Following vesicular release, glutamate rapidly diffuses away from active zones and is subsequently bound for uptake by excitatory amino acid transporters (EAATs; Tzingounis and Wadiche, 2007). Astrocytic membranes near synapses are enriched with EAATs that take up the majority of synaptically-released glutamate. Rather than translocate potentially excitotoxic glutamate back to neurons, the glutamate-glutamine cycle posits that the high glutamine synthetase (GS) activity in astrocytes readily converts glutamate to glutamine. Glutamine is then shuttled to the extracellular milieu by system N transporters and into neurons by system A transporters. The cycle is completed when neuronal phosphate-activated glutaminase (PAG) hydrolyses glutamine back to glutamate that can be used to refill synaptic vesicles (Figure 1). The metabolic compartmentation suggests that a majority of synaptically-released glutamate originates from astrocytic-derived glutamine (Laake et al., 1995); however, pharmacological and genetic inhibition of key cycle components failed to suppress glutamatergic synaptic transmission (Masson et al., 2006; Kam and Nicoll, 2007). Although Kam and Nicoll (2007) demonstrated that exogenous glutamine application can augment releasable glutamate, the persistence of glutamatergic transmission in the absence of glutamine, glutaminase and even astrocytes called into question the necessity of the glutamate-glutamine cycle for neurotransmission (Masson et al., 2006; Kam and Nicoll, 2007).
Now Tani et al. (2014) revisit the role of astrocytic glutamine production in the maintenance of excitatory synaptic transmission, using pharmacology and a clever paradigm to dissect the contribution of neurotransmitter versus vesicle availability. Similar to prior findings, the authors show that low frequency excitatory transmission at Schaffer collateral-CA1 synapses in hippocampal slices is unaffected by inhibition of astrocytic glutamine synthetase with the irreversible inhibitor MSO. However, prolonged stimulation at 2 or 20 Hz after MSO treatment reduces fEPSPs in a manner that is rescued with exogenous glutamine application. Importantly, pre-treatment of slices with MSO avoided its known non-specific acute effects (Kam and Nicoll, 2007). Glutamine application rescued a component of the fEPSP depression after ~1000 stimuli, suggesting the existence of a reservoir of transmitter that is depleted only following robust activity. To isolate this latent component of transmission, the authors devised a stimulation protocol that interleaves a burst of high frequency stimulation (HFS; 50 sec of 20 Hz = 1000 pulses) with a recovery period of low frequency stimulation (LFS; 200 sec at 0.2 Hz stimulation). This protocol allows identification of activity-dependent reduction in glutamate availability during the LFS in the absence of other changes in vesicle availability that can occur during HFS. This pattern of stimulation may also have physiological relevance since bursts of 20-30 Hz spike trains that last for over a minute have been recorded in mouse hippocampus during exploration (Berke et al., 2008). Indeed, this intermittent pattern of HFS (iHFS) generates a progressive and persistent decrement in fEPSPs during the LFS periods between each successive HFS that is completely rescued by exogenous glutamine. The acute reduction of fEPSPs within each burst of HFS, however, is insensitive to glutamine and therefore reflects other presynaptic mechanisms.
The authors perform several control experiments to assure that the glutamine-sensitive loss of fEPSPs revealed during iHFS results from reduced glutamate release rather than other potential mechanisms. They show that the reduction in fEPSPs persists without changes to the fiber volley amplitude, thus the loss of transmission does not result from fewer stimulated axons. This is an important issue because small changes to the fiber volley can dramatically alter the postsynaptic response. They also use a two-pathway experiment to show that the depression of fEPSPs is synapse specific. That is, monitoring transmission in a second pathway with LFS revealed that decreased transmission was limited to synapses subjected to iHFS and did not result from a general reduction in efficacy throughout the slice. As an alternative approach, the authors use a fluorescent biosensor to image decreased extracellular glutamate with iHFS as well its rescue by glutamine application. Finally, the crucial role of glutamine production to maintenance of transmission was further confirmed by the total absence of synaptic recovery in MSO-treated slices whereas exogenous glutamine allowed rapid recovery of transmission in MSO-treated slices even during subsequent HFS. Together, these data argue that extracellular glutamine and astrocytic GS are necessary to support glutamatergic transmission during periods of increased neurotransmitter release.
To further validate the potential role of the glutamate-glutamine cycle in maintaining synaptic transmission during physiologically-relevant patterns of neural activity, the authors tested naturally occurring low and high frequency patterns of CA3 pyramidal cell spiking acquired from freely-moving rats. The high frequency natural pattern caused depression of fEPSPs dependent on glutamine, mimicking the results from continuous HFS or iHFS stimulation, whereas the natural low frequency pattern did not alter fEPSPs. Since the patterns used in the slice experiments were from rodents running in a familiar environment and were delivered for shorter durations than might occur with novel cues (Berke et al., 2008), the authors suggest that the results likely underestimate the contribution of glutamine-dependent excitatory transmission in vivo.
Together the findings of Tani et al. (2014) point to a critical role of astrocytes in maintaining synaptic glutamate release during periods of robust neuronal activity, an idea that is consistent with several prior studies (Bacci et al., 2002; Masson et al., 2006; Kam and Nicoll, 2007; Tani et al., 2010; Billups et al., 2013). Despite the consensus, there are questions that remain unanswered. Tani et al. (2014) performed the majority of experiments in slices with transected axons to exclude somatic sources of glutamate that could confound the contribution of the glutamate-glutamine cycle. Non-transected slices unexpectedly displayed the same glutamine-dependent depression by iHFS confirming that the components of the glutamate-glutamine cycle are peri-synaptic. Yet the known molecular components (SNAT1 and SNAT2) of system A transporters, which take up ~90% glutamine into neurons in vivo (Kanamori and Ross, 2006), are rarely expressed in presynaptic terminals (Conti and Melone, 2006). Indeed, Tani et al., (2010) found little evidence for the involvement of system A transporters in maintaining glutamine-dependent epileptiform activity and instead postulated that yet-unidentified transporters were necessary for the synthesis of glutamate destined for synaptic release. In contrast, Billups et al., (2013) provides evidence for functional presynaptic glutamine uptake with pharmacology consistent with system A in brainstem. Identifying the molecular components of the glutamate-glutamine cycle that sustain synaptic glutamate release in hippocampus and cortex is crucial to fully understand role of the glutamine-glutamate cycle in synaptic transmission.
Second, it is not clear how neurotransmitter depletion generates a loss of synaptic efficacy. Tani et al., (2014) show that depression following iHFS increases sensitivity of fEPSPs to blockade by a low affinity antagonist, suggesting that the average concentration of glutamate per synapse is reduced. This could occur by either a reduction in the concentration of transmitter per vesicle (quantal content) or by a reduction in multivesicular release that is known to occur at CA3-CA1 synapses. In addition, the authors also report changes in the paired-pulse ratio suggestive of altered release probability. The mechanisms underlying how manipulations of neurotransmitter content alters synaptic vesicular release are disputed, and may be best addressed by examining transmission at single release sites.
Finally, Tani et al. (2014) clearly delineate the presence of a glutamate reservoir by establishing that extracellular glutamine is required only after ~1000 stimuli. This reservoir enables basal transmission to be unaffected by inhibition of the glutamate-glutamine cycle components for long periods as with low frequency stimulation (Masson et al., 2006; Kam and Nicoll, 2007). Identifying the source of glutamate that sustains transmission, whether it is glutamate or glutamine derived from other sources, or whether 1000 stimuli is simply needed to release all of the filled vesicles at low release probability sites, will be a question for future studies. Regardless of the remaining unknowns, Tani et al. (2014) provide definitive evidence for the necessity of the glutamate-glutamine cycle in excitatory transmission and thus the importance of conventional housekeeping functions charged to glia.
Acknowledgments
This work was supported by NIH NS064025 (L.O.-W.) and NS065920 (J.I.W.). We would like to thank Anastassios Tzingounis for comments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement:
The authors have no conflicts of interest associated with this manuscript.
References
- Bacci A, Sancini G, Verderio C, Armano S, Pravettoni E, Fesce R, Franceschetti S, Matteoli M. Block of glutamate-glutamine cycle between astrocytes and neurons inhibits epileptiform activity in hippocampus. J Neurophysiol. 2002;88:2302–2310. doi: 10.1152/jn.00665.2001. [DOI] [PubMed] [Google Scholar]
- Berke JD, Hetrick V, Breck J, Greene RW. Transient 23-30 Hz oscillations in mouse hippocampus during exploration of novel environments. Hippocampus. 2008;18:519–529. doi: 10.1002/hipo.20435. [DOI] [PubMed] [Google Scholar]
- Billups D, Marx MC, Mela I, Billups B. Inducible presynaptic glutamine transport supports glutamatergic transmission at the calyx of Held synapse. J Neurosci. 2013;33:17429–17434. doi: 10.1523/JNEUROSCI.1466-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti F, Melone M. The glutamine commute: lost in the tube? Neurochem Int. 2006;48:459–464. doi: 10.1016/j.neuint.2005.11.016. [DOI] [PubMed] [Google Scholar]
- Hertz L. Functional interactions between neurons and astrocytes I. Turnover and metabolism of putative amino acid transmitters. Prog Neurobiol. 1979;13:277–323. doi: 10.1016/0301-0082(79)90018-2. [DOI] [PubMed] [Google Scholar]
- Kam K, Nicoll R. Excitatory synaptic transmission persists independently of the glutamate-glutamine cycle. J Neurosci. 2007;27:9192–9200. doi: 10.1523/JNEUROSCI.1198-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanamori K, Ross BD. Kinetics of glial glutamine efflux and the mechanism of neuronal uptake studied in vivo in mildly hyperammonemic rat brain. J Neurochem. 2006;99:1103–1113. doi: 10.1111/j.1471-4159.2006.04152.x. [DOI] [PubMed] [Google Scholar]
- Kandel ER, Schwartz JH, Jessell TM. Principles of neural science. 4th McGraw-Hill, Health Professions Division; New York: 2000. [Google Scholar]
- Laake JH, Slyngstad TA, Haug FM, Ottersen OP. Glutamine from glial cells is essential for the maintenance of the nerve terminal pool of glutamate: immunogold evidence from hippocampal slice cultures. J Neurochem. 1995;65:871–881. doi: 10.1046/j.1471-4159.1995.65020871.x. [DOI] [PubMed] [Google Scholar]
- Masson J, Darmon M, Conjard A, Chuhma N, Ropert N, Thoby-Brisson M, Foutz AS, Parrot S, Miller GM, Jorisch R, et al. Mice lacking brain/kidney phosphate-activated glutaminase have impaired glutamatergic synaptic transmission, altered breathing, disorganized goal-directed behavior and die shortly after birth. J Neurosci. 2006;26:4660–4671. doi: 10.1523/JNEUROSCI.4241-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perea G, Navarrete M, Araque A. Tripartite synapses: astrocytes process and control synaptic information. Trends Neurosci. 2009;32:421–431. doi: 10.1016/j.tins.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Tani H, Dulla CG, Farzampour Z, Taylor-Weiner A, Huguenard J, Reimer RJ. A local glutamate-glutamine cycle sustains excitatory transmitter release. Neuron This issue. 2014 doi: 10.1016/j.neuron.2013.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tani H, Dulla CG, Huguenard JR, Reimer RJ. Glutamine is required for persistent epileptiform activity in the disinhibited neocortical brain slice. J Neurosci. 2010;30:1288–1300. doi: 10.1523/JNEUROSCI.0106-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzingounis AV, Wadiche JI. Glutamate transporters: confining runaway excitation by shaping synaptic transmission. Nat Rev Neurosci. 2007;8:935–947. doi: 10.1038/nrn2274. [DOI] [PubMed] [Google Scholar]