Abstract
Objective
Aim of this study was to report clinical outcomes of cervical cancer patients treated with weekly cisplatin chemo-radiation therapy (chemoRT) stratified by pre-treatment cisplatin in vitro chemosensitivity.
Methods
This was a retrospective analysis of patients with cervical cancer seen at our institution between May 2009 and August 2011. Patients underwent pre-treatment in vitro chemoresponse testing (Precision Therapeutics, Inc.) and were treated with concurrent weekly cisplatin chemoRT. The study consisted of 33 patients with FIGO tumor stages Ib2 to IIIb. Pre-treatment cisplatin chemoresponse of individual patient tumors was determined from chemoresponse dose response curves and scored as responsive (R), intermediate response (IR), or nonresponsive (NR).
Results
There were 28 patients with squamous cell carcinoma and 5 with adenocarcinoma. Cisplatin chemosensitivity was R and IR in 18 patient specimens and NR in 15. The 2-year recurrence-free survivals (RFS) were 87% for patients whose specimens tested R+ IR to cisplatin compared to 58% for those whose specimens were NR (p = 0.036). The 2-year RFS was 86% for the R + IR group compared to 46% for the NR group for patients with tumors that were squamous cell histology (p = 0.009). Stepwise proportional hazards modeling for RFS demonstrated that chemoresponsiveness to cisplatin (p = 0.029) and FDG-PET lymph node status (p = 0.011) were the only independent predictors of RFS for patients with squamous cell histology.
Conclusion
Pre-treatment in vitro cisplatin chemoresponse testing of cervix cancer biopsies was technically feasible and prognostic of RFS in patients treated with weekly cisplatin chemoRT.
Keywords: Cisplatin, ChemoRT, Chemoresponse
Introduction
Concurrent chemo-radiation (chemoRT) became the standard of care for patients with advanced cervical cancer following the NIH Clinical Alert announcement in 1999.1 Weekly cisplatin chemoRT is the most commonly used regimen. It is thought that concurrent cisplatin sensitizes tumor cells to irradiation and that this mechanism2 is responsible for the improved therapeutic results demonstrated in prospective clinical trials. Cisplatin has also been shown to have direct cytotoxic effects.3–6 It is not known if radiosensitization, direct toxicity, or both are responsible for improved results of cisplatin chemoRT compared to RT alone. A meta-analysis suggests that the improved results of chemoRT are due to a decrease in the distant metastatic rate. This suggesting an important role for direct cisplatin cytotoxicity.7
In vitro chemotherapy testing of pretreatment tumor specimens is a logical approach to determine drug cytotoxicity before initiating therapy. The development of an in vitro RT assay with the addition of cisplatin to evaluate clinical outcomes has been reported for 17 patients with advanced cervical cancer.8 No relationship between the addition of cisplatin to the RT assay and clinical outcomes was demonstrated. The results of a recent study of in vitro chemoresponse in 273 cervix cancer patient specimens demonstrated the feasibility of performing the in vitro chemoresponsiveness assay and that there is variability in chemoresponse among patients.9
The aim of this current study was to report the clinical outcomes of cervical cancer patients treated with weekly cisplatin chemoRT based on pretreatment cisplatin in vitro chemoresponse testing. The hypothesis of this study was that clinical outcome would vary based on pretreatment in vitro sensitivity to cisplatin.
Materials and Methods
Between May 2009 and August 2011 a consecutive group of 75 patients with a new diagnosis of cervical cancer underwent routine pretreatment chemoresponse testing with the commercial ChemoFx® test (Precision Therapeutics, Inc.; Pittsburgh, PA). From this group of 75 patients the assay did not grow in vitro in 31 patients and grew successfully in 44 (59%). The prescribed ChemoRT was not completed in 8 patients (8/44) due to patient noncompliance and 3 tumors (3/44) were of uncommon histology. The remaining 33 patients (33/44) are the subject of this report. They had squamous cell carcinoma or adenocarcinoma and completed treatment with curative weekly cisplatin chemoRT per our institutional guidelines.10 Briefly, patient treatment consisted of weekly external irradiation, weekly brachytherapy, and weekly chemotherapy with Cisplatin. Data collection was performed prospectively into an institutional cervix cancer database. This retrospective study was approved by the Washington University Human Research Protection Office with waiver of informed consent.
All patients underwent a pretreatment staging workup including history and physical examination, examination under anesthesia, and a whole-body FDG-PET/CT. Cervix biopsies were obtained at the time of examination under anesthesia for surgical pathologic evaluation and chemoresponse assay testing. Patients were staged using International Federation of Gynecology and Obstetrics (FIGO) clinical staging. A repeat FDG-PET/CT was performed 3 months after completing chemoRT to evaluate response to treatment.
Chemoresponse Assay
Fresh tumor specimens obtained at the time of examination under anesthesia were placed in McCoy’s medium on ice and sent to the commercial laboratory. ChemoFx methods have been previously reported.11 Briefly, tumor specimens were mechanically disrupted to release and establish malignant epithelial cells as monolayer cultures. The cultures were then tested against a series of ten serial dilutions of cisplatin with a range of drug concentrations of 0.1 to 100uM.
Following 72 hours of drug treatment, surviving cells were fixed, stained, and counted using automated microscopy and cell-counting software. Three replicates at each drug concentration were performed, and the average cell counts from each drug dose of cisplatin were divided by the average cell counts from the corresponding control wells. The resultant survival fraction (SF) was calculated, and represents the percentage of cells surviving (i.e. remaining in the well post-treatment) at each drug dose. The SF (y-axis) was plotted against the cisplatin dose (x-axis) to generate a dose-response curve (Figure 1) and to determine the associated response category: responsive (R), intermediate response (IR), or non-responsive (NR).11
Figure 1.
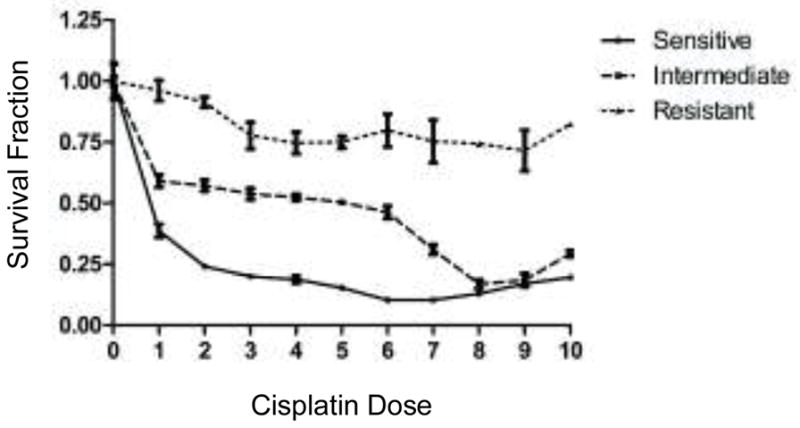
Sample dose response curves for cisplatin displaying R, IR, and NR results.
Statistical Analysis
The patient and tumor characteristics for the two groups were compared using a Chi-square or Fishers exact test. Tumor recurrence and survival times were measured from the completion of chemoRT. StatView Version 5.0.1 software (SAS Institute Cary, NC) was used for the analysis. A value of p < 0.05 was set as the threshold for significance for all study outcomes. The Kaplan-Meier (product-limit) method was used to derive estimates of survival based on total sample size. The test of equivalence of estimates of survival was performed by the generalized Wilcoxon test. A post-hoc power calculation was performed for Figure 2 and was 0.63 (18 versus 15 patients) and for Figure 3 the power was 0.79 (17 versus 11 patients). The Cox proportional hazards model was used for the multivariate analysis.
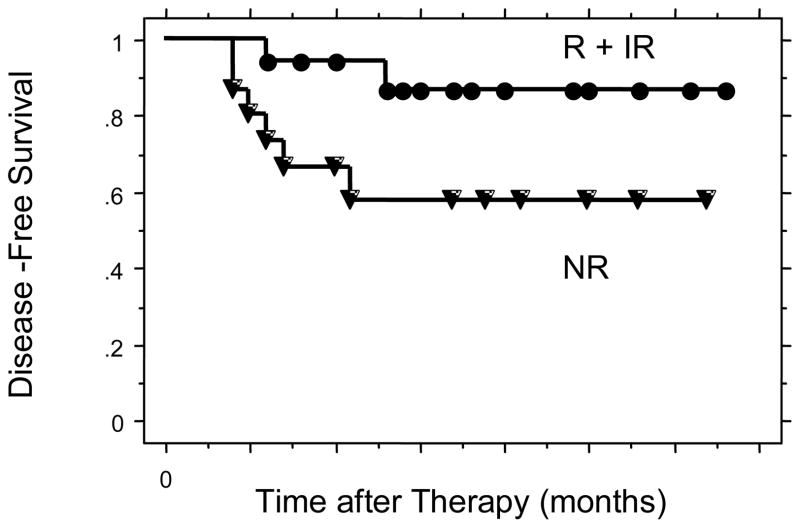
Figure 2.
Disease-Free survival for all patients comparing R + IR to NR.
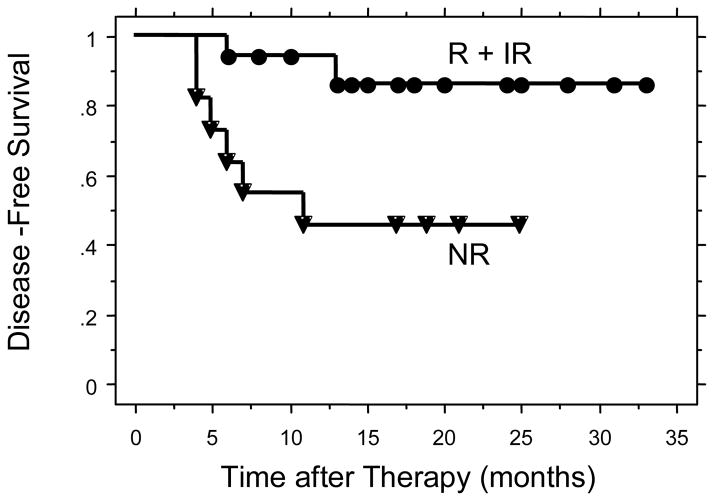
Figure 3.
Disease-Free survival for patients with tumors of squamous cell histology comparing R + IR to NR.
Results
Patient Characteristics
The mean age at diagnosis, FIGO clinical stage, tumor histology, rates of lymph node involvement, and number of cycles of weekly cisplatin were similar for the two groups (Table 1). All patients completed their prescribed external irradiation and intracavitary irradiation per our institutional policy. The mean survival time for those alive at the time of last follow-up was 19 months (range 6 to 33 months). At last follow-up 24 patients were without evidence of disease (NED), 4 were alive with disease, and 5 were dead due to progressive cervical cancer.
Table 1.
Patient and Tumor Characteristics
| Characteristic | Cisplatin Responsive | Cisplatin Non-responsive | p value |
|---|---|---|---|
| Mean age at diagnosis (yr) | 52 | 49 | 0.49 |
| FIGO Stage | 0.31 | ||
| Ib2 | 3 | 3 | |
| IIa | 0 | 2 | |
| IIb | 9 | 4 | |
| IIIa | 0 | 1 | |
| IIIb | 6 | 5 | |
| Histology | 0.15 | ||
| Squamous | 17 | 11 | |
| Adenocarcinoma | 1 | 4 | |
| Lymph Nodes | 0.28 | ||
| Name | 12 | 8 | |
| Pelvic only | 3 | 6 | |
| Pelvic & Para-aortic | 3 | 1 | |
| Cycles Weekly Cisplatin | 0.53 | ||
| Six | 13 | 11 | |
| ≤ 5 | 5 | 4 |
Survival
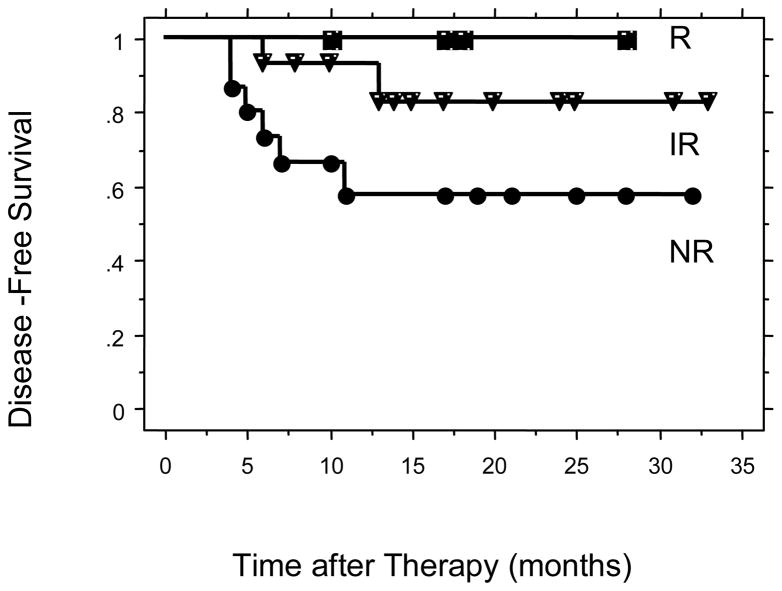
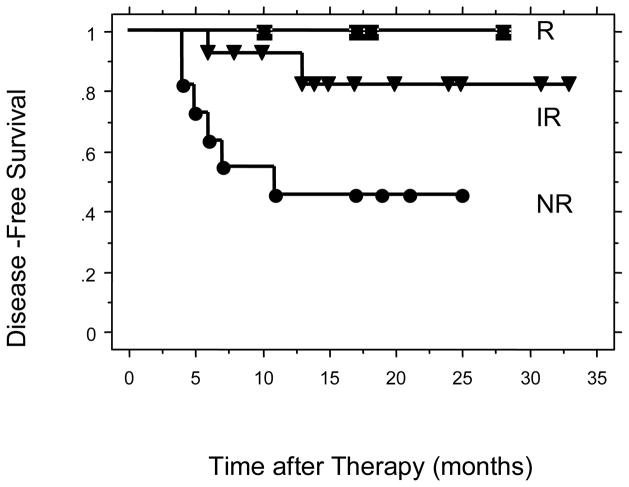
The two year disease-free survivals were 87% for the cisplatin R + IR group and 58% for the cisplatin NR group (p = 0.036) (Figure 2). There were 28 patients in the study with squamous cell carcinoma. The 2-year disease free survivals for these patients were 86% for the cisplatin R + IR group and 46% for the cisplatin NR group (p = 0.01) (Figure 3). The 2-year disease free survivals for all patients by level of cisplatin response was 100% for cisplatin responsive, 83% for cisplatin intermediate response, and 58% for cisplatin non-responsive (p = 0.10) (Figure 4). The 2-year disease-free survivals for those with squamous cell carcinoma were 100% for cisplatin responsive, 82% for cisplatin intermediate response, and 46% for cisplatin non-responsive (p = 0.03) (Figure 5).
Figure 4.
Disease-Free survival for all patients comparing R, IR, and NR.
Figure 5.
Disease-Free survival for patients with tumors of squamous cell histology comparing R, IR, and NR.
A Cox Proportional Hazards multivariate analysis for disease-free survival was performed. Included in the model were age at diagnosis, FIGO clinical stage, FDG-PET lymph node status at diagnosis, and cisplatin chemosensitivity. FDG-PET lymph node status (p = 0.0263) and cisplatin chemosensitivity (p = 0.0254) were the only statistically significant independent predictors of disease-free survival.
Discussion
In a previous report we demonstrated that the use of chemoresponse testing in patients with cervical cancer was feasible in a large series of specimens submitted for commercial testing from multiple institutions across the United States.9 Multiple chemotherapy agents were tested and reported in that initial study, but there was no data presented regarding patient treatment or outcome. The study did demonstrate that the in vitro response rates of primary cervix cancer biopsy specimens to single agent cisplatin were 48% for adenocarcinoma and 53% for squamous cell cancer. In our current study the overall response rate was 55% for squamous cell and adenocarcinoma.
The aim of our current study was to report the clinical outcomes of cervical cancer patients treated with weekly cisplatin chemoRT based on pretreatment cisplatin in vitro chemoresponse testing. We found that cisplatin chemosensitivity testing of pre-treatment cervical biopsies correlated with clinical response and disease-free outcomes for patients with advanced stage cervical cancer treated with weekly cisplatin chemoRT. Chemoresponse testing was reported as responsive, intermediate response, and non-responsive. Our data indicate that the clinical outcomes of patients was best for those whose tumor tested responsive to cisplatin, intermediate for those testing intermediate, and worst for those testing non-responsive.
The traditional test of chemotherapeutic agent effectiveness for patient outcomes for chemotherapy alone or for chemoRT is to perform a prospective clinical trial utilizing the drugs in question and subsequently evaluate the patient’s response to treatment. Clinical responses to cisplatin in patients with advanced and recurrent cervical cancer range from 13 to 40%.3,4,12–17 Despite recent guidelines advising against the use of chemosensitivity assays for individual patient management, in vitro pre-treatment testing of human cervical cancer specimens for chemoresponse could become a new paradigm to improve patient outcomes.18 Weekly cisplatin chemoRT is the standard of care treatment for patients with advanced stage cervical cancer. Cisplatin chemoresponse testing in our study showed that 45% of the specimens tested were non-responsive and that the 2-year disease-free survival was significantly diminished for these patients compared to the outcome for patients whose pre-treatment biopsy specimens tested responsive to cisplatin.
This study is a retrospective report of patients treated with weekly cisplatin and their outcomes are correlated to the pretreatment cisplatin chemoresponse, which emphasizes the importance of cytoxic chemotherapy in the control of subclinical disease. Alternative chemotherapy regimens for cervical cancer, both singly and in combination do exist, and can be tested with this assay. Standard weekly cisplatin could be replaced with other chemotherapy regimens for appropriately selected patients and, if validated, the results of this assay could be used in the future to select patients for alternative regimens. Recent data has shown that PI3K/Akt pathway may be implicated in resistance to standard chemoradiation.19,20 This assay could be modified in the future to include sensitivity testing for biologically targeted reagents.
The major limitation of this study was that it was not a prospective treatment study. However, the study did include all patients seen at our institution during the study period and they were treated per established institutional guidelines.10 All data were collected in a prospective database.
Our data suggest that in vitro testing of cervical cancer biopsy specimens for cisplatin chemosensitivity is feasible and prognostic of outcome for patients with advanced stage cervical cancer treated with weekly cisplatin chemoRT. These data provide compelling evidence that a multi-institutional study of chemosensitivity testing of cervical cancer specimens, standardized treatment, and evaluation of outcomes should be performed.
Footnotes
Conflict of Interest: None
References
- 1.National Cancer Institute (NCI) NCI issues clinical announcement on cervical cancer: chemotherapy plus radiation improves survival: National Institutes of Health (press release) NCI Press; Bethesda (MD): 1999. [Google Scholar]
- 2.Ziegler W, Kopp JM. The effect of combined treatment of HeLa cells with cisplatin andirradiation upon survival and recovery from radiation damage. Radiotherapy and Oncology. 1987;8:71–78. doi: 10.1016/s0167-8140(87)80024-5. [DOI] [PubMed] [Google Scholar]
- 3.Bonomi P, Blessing J. Randomized trial of three cisplatin dose schedules in squamous-cell carcinoma of the cerivix. A Gynecologic Oncology Group study. Journal of Clinical Oncology. 1985;3:1079–1085. doi: 10.1200/JCO.1985.3.8.1079. [DOI] [PubMed] [Google Scholar]
- 4.Thigpen JT, Shingleton H, Homesley H, et al. Cis-platinum in treatment of advanced or recurrent squamous cell carcinoma of the cervix: A phase II study of the Gynecologic Oncology Group. Cancer. 1981;48:899–903. doi: 10.1002/1097-0142(19810815)48:4<899::aid-cncr2820480406>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 5.Xu Y, Yu H, Qin H, et al. Inhibition of autophagy enhances cisplatin cytotoxicity through endoplasmic reticulum stress in human cervical cancer cells. Cancer Letters. 2012;314:232–243. doi: 10.1016/j.canlet.2011.09.034. [DOI] [PubMed] [Google Scholar]
- 6.Schloffer D, Horky M, Kotala V, et al. Induction of cell cycle arrest and apoptosis in human cervix carcinoma cells during therapy by cisplatin. Cancer Detection and Prevention. 2003;27:481–493. doi: 10.1016/j.cdp.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Green JA, Kirwan JM, Tierney JF, et al. Survival and recurrence after concomitant chemotherapy and radiotherapy for cancer of the uterine cervix: a systematic review and meta-analysis. The Lancet. 2001;358:781–786. doi: 10.1016/S0140-6736(01)05965-7. [DOI] [PubMed] [Google Scholar]
- 8.Randall LM, Monk BJ, Moon J, et al. Prospective evaluation of an in vitro radiation resistance assay in locally advanced cancer of the uterine cervix: A Southwest Oncology Group Study. Gynecologic Oncology. 2010;119:417–421. doi: 10.1016/j.ygyno.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grigsby PW, Gan CM, Tillmanns TD, et al. The Oncologist. 2012. In Vitro Chemoresponse Analysis of Cervical Cancer Patient Specimens. Submitted. [DOI] [PubMed] [Google Scholar]
- 10.Kidd EA, Siegel BA, Dehdashti F, et al. Clinical Outcomes of Definitive Intensity-Modulated Radiation Therapy With Fluorodeoxyglucose-Positron Emission Tomography Simulation in Patients With Locally Advanced Cervical Cancer. International Journal of Radiation Oncology*Biology*Physics. 2010;77:1085–1091. doi: 10.1016/j.ijrobp.2009.06.041. [DOI] [PubMed] [Google Scholar]
- 11.Brower SL, Fensterer JE, Bush JE. The ChemoFx assay: an ex vivo chemosensitivity and resistance assay for predicting patient response to cancer chemotherapy. Methods in Molecular Biology. 2008;414:57–78. doi: 10.1007/978-1-59745-339-4_6. [DOI] [PubMed] [Google Scholar]
- 12.Long HJ, 3rd, Bundy BN, Grendys EC, Jr, et al. Randomized phase III trial of cisplatin with or without topotecan in carcinoma of the uterine cervix: a Gynecologic Oncology Group Study. Journal of Clinical Oncology. 2005;23:4626–33. doi: 10.1200/JCO.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 13.Moore DH, Blessing JA, McQuellon RP, et al. Phase III Study of Cisplatin With or Without Paclitaxel in Stage IVB, Recurrent, or Persistent Squamous Cell Carcinoma of the Cervix: A Gynecologic Oncology Group Study. Journal of Clinical Oncology. 2004;22:3113–3119. doi: 10.1200/JCO.2004.04.170. [DOI] [PubMed] [Google Scholar]
- 14.Thigpen JT, Blessing JA, DiSaia PJ, et al. A randomized comparison of a rapid versus prolonged (24 hr) infusion of cisplatin in therapy of squamous cell carcinoma of the uterine cervix: a Gynecologic Oncology Group study. Gynecologic Oncology. 1989;32:198–202. doi: 10.1016/s0090-8258(89)80033-2. [DOI] [PubMed] [Google Scholar]
- 15.Potter ME, Hatch KD, Potter MY, et al. Factors affecting the response of recurrent squamous cell carcinoma of the cervix to cisplatin. Cancer. 1989;63:1283–6. doi: 10.1002/1097-0142(19890401)63:7<1283::aid-cncr2820630709>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 16.Lele SB, Piver MS. Weekly cisplatin induction chemotherapy in the treatment of recurrent cervical carcinoma. Gynecologic Oncology. 1989;33:6–8. doi: 10.1016/0090-8258(89)90594-5. [DOI] [PubMed] [Google Scholar]
- 17.Omura GA, Blessing JA, Vaccarello L, et al. Randomized trial of cisplatin versus cisplatin plus mitolactol versus cisplatin plus ifosfamide in advanced squamous carcinoma of the cervix: a Gynecologic Oncology Group study. Journal of Clinical Oncology. 1997;15:165–71. doi: 10.1200/JCO.1997.15.1.165. [DOI] [PubMed] [Google Scholar]
- 18.Burstein HJ, Mangu PB, Somerfield MR, et al. American Society of Clinical Oncology Clinical Practice Guideline Update on the Use of Chemotherapy Sensitivity and Resistance Assays. Journal of Clinical Oncology. 2011;29:3328–3330. doi: 10.1200/JCO.2011.36.0354. [DOI] [PubMed] [Google Scholar]
- 19.Schwarz JK, Payton JE, Rashmi R, et al. Pathway-specific analysis of gene expression data identifies the PI3K/Akt pathway as a novel therapeutic target in cervical cancer. Clinical Cancer Research. 2012;18:1464–71. doi: 10.1158/1078-0432.CCR-11-2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McIntyre JB, Wu JS, Craighead PS, et al. PIK3CA mutational status and overall survival in patients with cervical cancer treated with radical chemoradiotherapy. Gynecologic Oncology. 2013;128:409–14. doi: 10.1016/j.ygyno.2012.12.019. [DOI] [PubMed] [Google Scholar]