SUMMARY
Background
The beta-2 adrenergic receptor (ADRB2) is an important target for epinephrine, a neurotransmitter in pain signalling. ADRB2 haplotypes affect receptor expression and ligand response, and have been linked to painful non-GI disorders.
Aims
To assess whether ADRB2 polymorphisms (rs1042713, rs1042714) are risk alleles for functional GI (FGID) and extraintestinal functional (EIFD) diagnoses, and whether ADRB2 predicts GI symptoms and health-related quality of life (HRQOL).
Methods
Of 398 subjects (49.6 ± 2.9 years, 68.0% female), 170 (42.5%) met Rome III criteria for ≥1 FGID [IBS (n = 139, 34.9%); functional dyspepsia (FD, n = 136, 34.1%), functional chest pain (FCP, n = 25, 6.2%)], while 228 were healthy controls. FGID subjects reported on bowel symptom severity and burden (10-cm VAS), frequency (days/last 2 weeks), EIFD, psychiatric diagnoses and HRQOL (SF 36). Multivariable models determined the contribution of ADRB2 polymorphisms to HRQOL, and mediational analyses assessed functional diagnoses as potential intermediates.
Results
rs1042714 minor G alleles were associated with FGID diagnoses (OR 1.8; 95% CI 1.2–2.7; P = 0.009), particularly FD (OR 2.1, 95% CI 1.3–3.3), with trends towards IBS (P = 0.19) and FCP (P = 0.06) diagnoses. Within IBS, G allele carriers had more severe bowel symptoms (P = 0.025), and symptomatic days (P = 0.009). G allele carriers had greater numbers of EIFD (1.0 ± 0.1 vs. 0.4 ± 0.07, P < 0.001) and poorer HRQOL. The effect of ADRB2 on HRQOL was partially mediated by FGID, EIFD and psychiatric diagnoses.
Conclusions
ADRB2 minor alleles at rs1042714 predict FGID and EIFD, and may influence bowel symptom severity and HRQOL. These findings provide indirect evidence of sympathetic nervous system role in FGID pathophysiology.
INTRODUCTION
Functional gastrointestinal disorders (FGID) consist of a heterogeneous group of conditions with chronic, painful symptoms not explained by structural abnormalities.1 Pathogenesis of FGIDs is incompletely understood, although genetic factors, low-grade inflammation, intestinal dysbiosis, visceral hypersensitivity and brain–gut axis dysfunction all have been postulated to contribute.2, 3 Development of effective FGID management strategies have been hampered by a poor understanding of precise pathophysiological mechanisms; therefore, identification of genetic risk factors might be an important step towards recognition of phenotypically distinct IBS subgroups. The genetic subtyping of IBS phenotypes ultimately could facilitate the implementation of symptom-and pattern-specific treatment trials.4, 5
Recent preclinical and clinical studies demonstrate evidence linking stress-related neuroendocrine factors to aberrations within the brain–gut axis.4, 6 An abnormal stress response has also been implicated in the pathogenesis of painful extraintestinal functional disorders (EIF-Ds), such as fibromyalgia and chronic fatigue syndrome, which can overlap with FGID in perhaps 50% of cases.7 In the setting of stress, the central nervous system (CNS) augments sympathetic nervous system (SNS) activity, with parallel suppression of parasympathetic nervous system (PNS) activity. The prime mediator of this stress response is the autonomic nervous system (ANS), which, via regions including the locus coeruleus in the brainstem, also is an integral component of the brain–gut axis. The SNS and PNS modulate gastrointestinal function via a third branch of the ANS, the enteric nervous plexus. ANS deregulation, with increased sympathetic and decreased parasympathetic activity similar to the stress response, is known to occur in FGID.6, 8 Catecholamines, the primary neurotransmitters of the SNS, are elevated in both FGID and EIFD,8, 9 and have been shown to augment pain signalling in both the ANS and CNS.8, 10 Therefore, there is evidence supporting aberrant ANS activity and abnormal stress response in both FGID and EIFD, and some symptoms, particularly pain, could be mediated by excess catecholamine signalling.
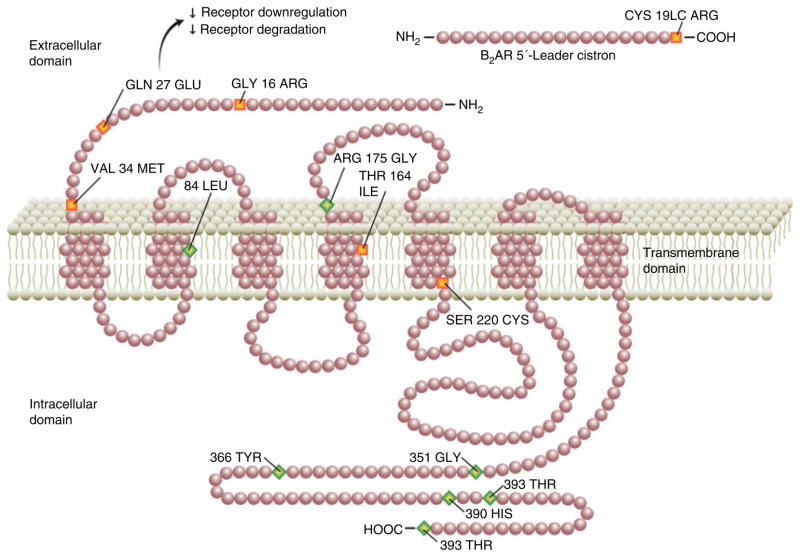
The beta-2-adrenergic receptor (ADRB2) is the main target of the catecholamine epinephrine, and a primary mediator of the stress response. Blocking ADRB2 decreases pain sensitivity in both humans and animals.11, 12 ADRB2 is widely expressed both in the gastrointestinal tract and in the CNS.13 Recently, adrenergic receptor gene polymorphisms (e.g. rs1042713, rs1383914) have been linked to fibromyalgia and temporomandibular joint disorder, two EIFDs that are frequently encountered in FGID patients.13–15 Single-nucleotide polymorphisms (SNPs) located in the coding region of the ADRB2 gene have been shown to be associated with increased altered receptor response to catecholamines as well as altered receptor expression, mediated by catechol-amine-induced receptor internalisation (Figure 1).13 It is unknown, however, whether ADRB2 polymorphisms are present at a higher frequency in FGID.
Figure 1.
Beta-2 adrenergic receptor (ADRB2) polymorphisms. The ADRB2 extracellular domain contains two polymorphisms, Arg16Gly (rs1042713; B16) and Glu27Gln (rs1042714; B27). Both are missense polymorphisms (orange), which lead to alterations in amino acid sequence. In the case of rs1042714, this may lead to decreased receptor degradation and down-regulation, in turn enhancing adrenergic response. The silent polymorphisms (green) have no known effect on amino acid sequence.
In this study, we hypothesised that ADRB2 receptor polymorphisms are associated with both FGID and EIFD. Our primary aim was to determine whether common variants within the ADRB2 gene serve as risk factors for FGIDs. Additionally, we sought to evaluate whether these polymorphisms may influence (i) bowel symptom severity; (ii) health-related quality of life (HRQOL); and (iii) burden of comorbid EIFDs and psychiatric diagnoses in an FGID population.
METHODS
Subjects and clinical characteristics
The subjects in this report were identified from a prospectively maintained databank and tissue repository at our institution from 2006 to 2010, yielding a total of 170 FGID patients and 228 healthy controls to study. All participants completed a comprehensive multidimensional symptom, affective state and quality-of-life questionnaire as part of the study, including the Rome III diagnostic questionnaire.16 Sputum and/or blood samples were collected for DNA analysis at the time of enrolment. Blood was collected in the instances where the patient was to undergo phlebotomy for clinical purposes; if a blood specimen was not required for other laboratory testing, a sputum sample was collected. In cases where blood was available, this sample was analysed preferentially, given the overall higher DNA yields with this specimen. All participants provided written informed consent prior to collection of biological specimens and clinical data. The study protocol was approved by the Human Research Protection Office (institutional review board) at Washington University School of Medicine and Barnes-Jewish Hospital, St. Louis, Missouri.
The FGID group was composed of adult subjects (≥18 years old) who carried a clinical FGID diagnosis, and met Rome III criteria for at least one FGID. Prior to enrolment in the study, all FGID subjects had organic bowel disease excluded with a comprehensive evaluation, including, but not limited to, laboratory, endoscopic and radiographic testing, performed at the discretion of the treating gastroenterologist. The control group comprised patients undergoing screening endoscopy in the absence of ongoing gastrointestinal diagnoses or symptoms at the time of enrolment, and without evidence for FGID using Rome III criteria. With the exception of three subjects (one mother and two daughters in the FGID group), to our knowledge, all of the participants were genetically unrelated.
Instruments
Bowel symptom assessment
The burden of gastrointestinal symptoms within the 2 weeks proceeding enrolment in the study was evaluated in terms of symptom severity, bother and frequency. The severity and bother of gastrointestinal symptoms were assessed with 10-cm visual analogue scales (VAS), using previously described methods.17 Symptom frequency (number of symptomatic days) within the proceeding 2 weeks also was quantified (0–14 days).
Extraintestinal functional and mood disorders
As part of the multidimensional questionnaire, the prior diagnoses of EIFDs were quantified. Subjects were asked to indicate whether they had past diagnoses, and a single, blinded investigator (BC) reviewed medical records to confirm the presence of the following disorders: chronic pelvic pain, interstitial cystitis (spastic bladder), chronic fatigue syndrome, fibromyalgia, migraine headache, chronic headache (other than migraine), chronic back pain, palpitations, premenstrual syndrome, dysmenorrhoeal, dyspareunia and temporomandibular joint disorder. Similarly, historical psychiatric diagnoses were determined by subject self-report and medical record review (depression, generalised anxiety, bipolar disorder, panic disorder and somatisation disorder).
The short form health survey (SF 36)
The SF 36 was used as a validated assessment of health status and its impact on health-related quality of life (HRQOL).17 The SF 36 is divided into physical and mental domains and assesses the role that medical conditions and pain on physical and emotional well-being and limitation of day-to-day and pleasurable activities. Given the mechanism of subject recruitment, this information was collected only in a subset (n = 71) of the healthy controls.
DNA sample collection and genotyping
Sputum or blood samples were collected for DNA extraction and were frozen and banked by the Biobank Core of the Washington University Digestive Diseases Research Center. DNA was extracted from these samples using Qiagen Gentra Puregene Reagents (Qiagen Inc., Valencia, CA, USA). Two SNPs associated with ADRB2 were analysed (rs1042713, rs1042714). These SNPs were chosen on the basis of prior studies in which they have been associated with altered ADRB2 function as well as findings that these SNPs are as risk alleles for EIFDs.13–15 Genotyping was performed using Sequenom MassARRAY technology (Sequenom Inc., San Diego, CA, USA) in a single batch run containing all of the study samples (cases and controls). The laboratory staff was blinded to both the study subject group assignment and the study hypotheses.
Statistical analysis
Chi-squared testing was used to calculate Hardy–Wein-berg equilibrium for each polymorphism using previously described methods.18 Grouped values are reported as mean, standard error of mean and 95% confidence intervals (CI) unless otherwise indicated. Between-group comparisons were performed using Student’s t-tests for continuous variables, and chi-squared analyses were performed for all genotype frequency data, and for binomial data as appropriate. One-way ANOVA analyses allowed for the determination of between-group differences in measures based on ADRB2 genotype. In each case, P < 0.05 was required for statistical significance. Statistical analysis was carried out using SPSS Statistics v.20 (IBM, Armonk, NY, USA). Multivariate linear regression models were developed using an ‘enter method’ to establish the independent contributions of ROME III diagnoses, EIFD and psychiatric diagnoses, demographics (age, gender) and ADRB2 minor allele carrier status on health-related quality of life in the FGID patient subgroup. These models were then incorporated into separate Sobel mediational analyses, which included EIFD, psychiatric, and FGID diagnoses to explore whether any effect of ADRB2 genotype on HRQOL in FGID patients might be partially mediated by the presence of EIFD or psychiatric diagnoses. Preconditionally, all of the variables to be included in the analysis were required to be significantly correlated using Pearson correlations, and linear regression models were developed in including the independent (ADRB2 minor allele carrier) and dependent variables (HRQOL) of interest, followed by second order models, which included the mediational variables of interest (e.g. FGID, EIFD, psychiatric diagnoses) to determine the unstandardised regression coefficients and their standard errors. Ratios of model estimated indirect: total effects of the independent variables on HRQOL were determined as estimates of the amount of the correlative relationship between ADRB2 genotype and HRQOL accounted for via the mediating diagnoses.
RESULTS
Subject demographic and clinical characteristics
Over a 5-year period, 170 subjects with FGID (mean age 43.9 ± 6.3 years) were identified, reporting an average of 1.6 ± 0.04 FGIDs per subject using Rome III criteria (Table 1). During the same period, 228 controls were enrolled (mean age 54.2 ± 1.1 years), none with FGID according to our inclusion criteria. Gender proportions reflected the female predisposition typically seen with FGIDs (79.4% in FGID group vs. 60.5% in control group, P < 0.001). Furthermore, EIFDs were more common in the FGID group (1.5 ± 0.2 EIFDs per subject) than in the control group (0.7 ± 0.02, P < 0.001). Within the FGID group, Rome III criteria for IBS were met by 139 (34.9%) subjects, while criteria for functional dyspepsia (FD) and functional chest pain (FCP) were met by 136 (34.1%) and 25 (6.2%) subjects respectively. One-fourth of the subjects had multiple FGIDs: 92 (23.1%) subjects had 2 FGIDs and 9 (2.3%) had ≥3 FGID.
Table 1.
Baseline demographics, clinical and ADRB2 genotype characteristics
| FGID (n = 170) | Healthy controls (n = 228) | P value | |
|---|---|---|---|
| Age, mean ± S.E.M. | 43.9 ± 6.3 | 54.2 ± 1.1 | 0.08 |
| Female gender (%) | 135 (79.4) | 138 (60.5) | <0.001 |
| Total no. Rome III diagnoses | 1.6 ± 0.04 | N/A | |
| Irritable bowel syndrome (IBS)* | 139 (34.9) | ||
| IBS-D | 44 (11.0) | ||
| IBS-C | 18 (4.5) | ||
| IBS-M | 77 (19.3) | ||
| Functional dyspepsia (FD) | 136 (34.1) | ||
| Postprandial distress (PDS) | 85 (21.3) | ||
| Epigastric pain syndrome (EPS) | 92 (23.1) | ||
| Functional chest pain | 25 (6.2) | ||
| GI symptom severity [VAS] | 7.2 ± 0.1 | ||
| GI Symptom bother [VAS] | 7.1 ± 0.2 | ||
| GI Symptom frequency [days/last 2 weeks] | 9.7 ± 0.3 | ||
| SF36 total† | 49.0 ± 1.7 | 70.0 ± 2.3 | <0.001 |
| SF36 physical | 42.8 ± 1.8 | 63.9 ± 2.6 | <0.001 |
| SF36 mental | 50.7 ± 1.8 | 70.4 ± 1.8 | <0.001 |
| Any extraintestinal functional diagnoses (EIFD)‡ | 100 (59.9) | 45 (19.9) | <0.001 |
| No. extraintestinal functional diagnoses (EIFDs), mean | 1.5 ± 0.2 | 0.7 ± 0.2 | <0.001 |
| Any psychiatric diagnosis‡ | 90 (53.9) | 60 (26.5) | <0.001 |
| Depression | 70 (41.9) | 44 (19.5) | <0.001 |
| Anxiety disorder | 55 (32.9) | 27 (11.9) | <0.001 |
| ADRB2 genotypes§ | |||
| rs1042713 | |||
| AA | 24 (14.9) | 44 (20.8) | 0.15 |
| AG | 82 (50.9) | 105 (49.5) | |
| GG | 55 (34.2) | 63 (29.7) | |
| rs1042714 | |||
| GG | 29 (18.0) | 24 (10.8) | 0.004 |
| CG | 81 (50.3) | 99 (44.6) | |
| CC | 51 (31.7) | 99 (44.6) | |
Percentages listed for individual FGIDs reflect an overall percentage within the entire study cohort.
SF36 data were available in most FGID subjects (n = 165), but only in a subset (n = 71) of healthy controls.
EIFD and psychiatric diagnosis data were unavailable in 5 individuals (3 healthy controls, and 2 FGID subjects).
ADRB2 genotyping was unsuccessful for rs1042714 in 15 subjects (9 FGID, 6 controls) and for rs1042713 in 25 subjects (9 FGID and 16 controls). Percentages shown apply the total number of individuals successfully genotyped as the denominator.
In the FGID cohort, subjects reported 9.7 ± 0.3 symptomatic days in the previous 2 weeks. Symptom severity was rated 7.1 ± 0.2 and symptom bother 7.2 ± 0.2 on 10-cm VAS scales. These values were similar within FGID subgroups (e.g. IBS vs. FD, P > 0.05 for each).
Genotype data
The observed and expected frequencies of the two SNPs (rs1042713, rs1042714) associated with ADRB2 were in Hardy–Weinberg equilibrium with a χ2 P > 0.05 for each. Genotyping was not successful in 15 (5.0%) subjects for rs1042714, and in 25 (8.3%) subjects for rs1042713. The failure of genotyping was likely a result of one of two issues: insufficient DNA quality/quantity or failure of PCR amplification. In 7 of the 15 cases of failed rs1042714 genotyping, the subject also failed to genotype for rs1042713 (presumably as a result of issues with DNA quality/quantity). In the remaining eight cases, interpretable genotypes for rs1042713 were obtained, suggesting PCR amplification failure in these cases. The genotype differences between FGID and control subjects for rs1042714 remained significant when missing cases were imputed as having a major allele homozygote (CC) genotype (F = 6.28, P = 0.013). Duplicate analyses were not performed and, as such, inconsistency rates are not reported.
When allele frequencies were analysed, G allele carrier status within rs1042714 was significantly associated with the presence of FGIDs (P = 0.009) with an odds ratio (OR) of 1.8 (95% CI 1.2–2.7). Within the rs1042714 genotype, both GG homozygotes and CG heterozygotes demonstrated higher burdens of FGID compared with CC homozygotes (Figure S1).
When FGIDs were analysed individually, rs1042714 G allele carrier status was significantly associated with FD, [OR 2.1, 95% CI 1.3–3.3; P = 0.001]. Furthermore, there was a trend towards higher rates of IBS [OR 1.3, 95% CI 0.9–2.1; P = 0.19] and FCP [OR 2.6, 95% CI 0.9–7.1; P = 0.06] in G allele carriers (Figure S2). In contrast, with rs1042713, the A allele was not associated with an increased rate of FGID as a whole, or with individual functional diagnoses. IBS [OR 0.9, 95% CI 0.6–1.4, P = 0.64], FD [OR 0.7, 0.5–1.1, P = 0.17] or FCP [OR 0.6, 0.3–1.5, P = 0.27].
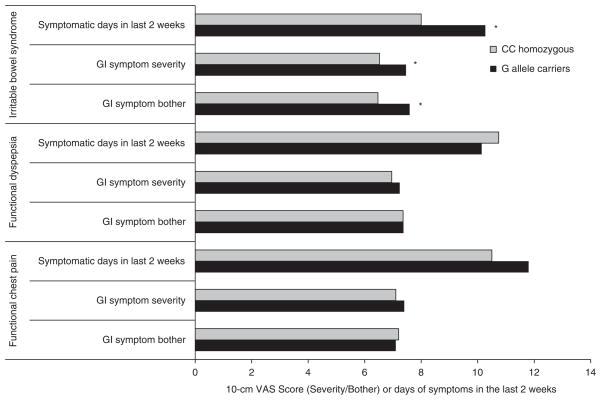
Symptom burden and rs1042714
As a significant association was found between rs1042714 and FGIDs, further analysis was undertaken to evaluate the relationship between this SNP and symptom burden. In the IBS group as a whole, G allele carriers had a higher gastrointestinal symptom severity, bother and frequency when compared with CC homozygotes (Figure 2). In contrast, within the FCP and FD subgroups, the G allele did not conspicuously affect gastrointestinal symptom burden.
Figure 2.
Gastrointestinal symptom burden among functional GI disorder (FGID) subgroups by rs1042714 genotype. *P < 0.025 for each.
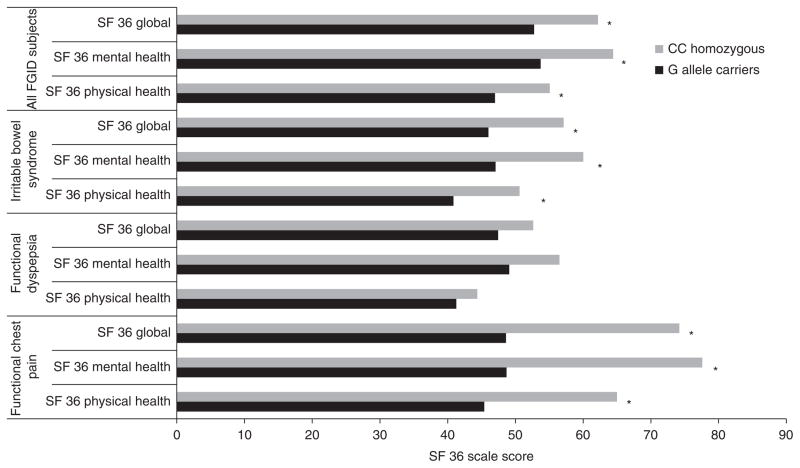
Health-related quality of life (HRQOL) and rs1042714
The influence of rs1042714 on HRQOL was assessed within the patient cohort. In the FGID group as a whole, G allele carrier status was associated with lower HRQOL within the mental health, physical health and global domains (Figure 3). When individual FGIDs were analysed, lower HRQOL within all dimensions of the SF 36 were noted to be associated with rs1042714 in the IBS (P < 0.03 for each) and FCP subgroups (P < 0.001 for each). A similar trend was seen in the FD group, although these observations did not reach statistical significance (P > 0.05 for each). A ‘dose effect’ was observed, wherein CC homozygotes had the highest SF 36 scores across all three domains, and further decremental loss of HRQOL in individuals with additional ADRB2 minor alleles (Figure S3).
Figure 3.
Health-related quality of life (HRQOL) in functional GI disorder (FGID) by rs1042714 genotype. *P ≤ 0.03 for each.
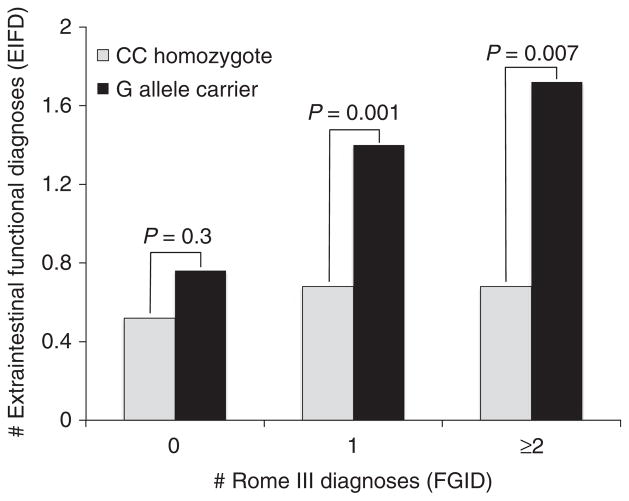
Extraintestinal functional disorders and ADRB2 polymorphisms
In the study population as a whole, rs1042714 G allele carriers had a higher burden of EIFDs regardless of FGID status, with G allele carriers having an average of 1.0 ± 0.1 EIFD per subject, compared to 0.4 ± 0.07 EIFD per CC homozygous subject (P < 0.001). Overall, the most common EIFDs endorsed by participants were chronic back pain (17.8%), migraine headache (16.1%), fibromyalgia (7.5%) and chronic pelvic pain (6.3%). While the FGID subjects as a whole had greater numbers of EIFDs compared with controls subjects, G allele carriers meeting Rome III criteria for at least one FGID had nearly twice as many EIFD diagnoses (1.5 ± 0.2 EIFDs, compared to 0.7 ± 0.2 EIFD with CC homozygosity, P = 0.001) (Figure 4). FGID carriers of the A allele at rs1042713, had a similar rate of EIFDs when compared to GG homozygotes (P = 0.6); given this, no further analysis of the impact of this single nucleotide polymorphism was pursued.
Figure 4.
Extraintestinal functional disorders (EIFDs) and Beta-2 adrenergic receptor (ADRB2) rs1042714 polymorphisms. Minor G allele carriers were at an increased risk for EIFDs, with the number of EIFDs increased in parallel with the number of FGIDs detected in the individual.
Psychiatric diagnoses and ADRB2 polymorphism
Study participants with an rs1042714 G allele were significantly more likely to have a historical psychiatric diagnosis (OR 1.8, 95% CI 1.1–2.8; P = 0.01) compared with their major allele homozygous counterparts. Anxiety accounted for a substantial portion of this observation, with ADRB2 minor allele carriers having more than twice the rates of anxiety (25.0% vs. 11.9%, P = 0.002); modestly higher rates of depression (30.6% vs. 23.8%, P = 0.15) also were noted those with at least one G allele.
Multivariate regression analysis and mediational analysis
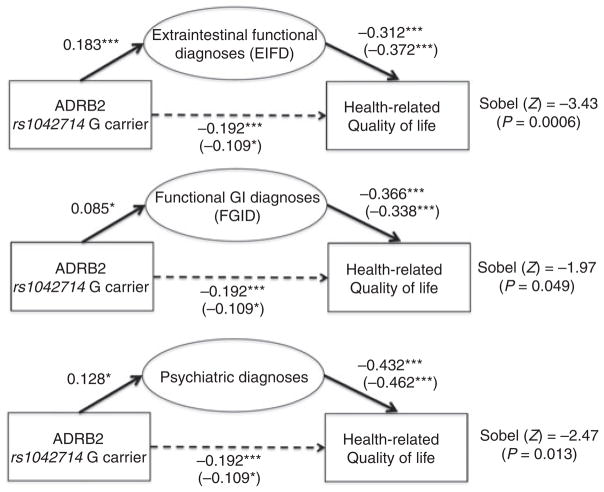
Multivariate regression analysis performed to assess factors influencing HRQOL in the FGID cohort led to the creation of three linear regression models, all with SF 36 as the dependent variable. In the first model, variables included age, gender, rs1042714 G allele carrier status (present or absent) and the number of ROME III FGID diagnoses satisfied. Here, only the presence of FGID diagnoses (B = −8.5, P < 0.001) and carrier status of the G allele at rs1042714 (B = −9.6, P = 0.003) were significantly associated with lower HRQOL. The second model expanded upon the first, but now also included the number of endorsed EIFDs; age and gender again were not predictive of significant impact with HRQOL. However, FGIDs (B = −6.9, P < 0.001), EIFDs (B = −4.8, P = 0.001) and carrier status of the G allele at rs1042714 (B = −6.2, P = 0.045) all were associated with poorer HRQOL. A final model now adding psychiatric diagnoses again found that mood disorders (B = −8.0, P < 0.001), FGID diagnoses (B = −5.9, P < 0.001) and EIFD (B = −12.9, P = 0.002) independently predicted HRQOL, while ADRB2 G allele status no longer retained statistical significance (B = −5.5, P = 0.056). Mediational analyses demonstrated that the effect of ADRB2 G allele positivity on HRQOL was indirectly mediated by the presence of FGIDs (13.5% effect), EIFDs (43.2% effect) and psychiatric comorbidity (33.3% effect). These models suggest a partial mediation of the direct effect of ADRB2 on HRQOL via the presence of psychiatric and/or functional comorbidity (Figure 5).
Figure 5.
Mediational analyses. The effect of Beta-2 adrenergic receptor (ADRB2) rs1042714 genotype on health-related quality of life (HRQOL). Standard correlation coefficients for each of the variables are shown, with unstandardised regression coefficients reported in parentheses. Sobel tests provide the overall significance of the putative mediational model (*P < 0.05; **P < 0.01; ***P < 0.001).
DISCUSSION
The sympathetic branch of the autonomic nervous system and its catecholaminergic neurotransmission increasingly is gaining appreciation as an important mechanistic factor in the development of functional pain disorders, and is an attractive putative target for future FGID therapeutic strategies. This study is the first to identify a functional polymorphism within the beta-2-adrenergic receptor gene as a risk factor for FGID. Our results suggest that G allele carrier status portends a more severe phenotype in subjects with IBS. Furthermore, this study confirms a link between a specific ADRB2 polymorphism and EIFD, with greater number of EIFD in minor allele carriers within the rs1042714 polymorphism. This SNP also was associated with poorer HRQOL, an effect that appears to be at least partially mediated by the presence of a greater burden of EIFD and psychiatric comorbidity.1
This work provides further evidence supporting the role of the ANS, and more particularly the sympathetic branch, in symptom origination and presentation in both FGID and EIFD. Prior studies evaluating cardioautonomic function in subjects with IBS subjects have demonstrated that lower heart rate variability, a marker of increased sympathetic activity, correlates with increased pain severity.19, 20 Moreover, elevated basal levels of nor-epinephrine have been found in the plasma of IBS patients when compared with controls.21 Similar alterations in ANS activity, specifically an increased SNS activity, have been observed in individuals with EIFDs.9, 22 We speculate that due to increases in ADRB2 receptor affinity for norepinephrine resulting from the SNP examined in this study, minor (G) allele carriers are more sensitive to endogenous catecholamines.13 As a result, minor allele carriers may have greater susceptibility to stress-induced augmentation of visceral and somatic sensory function. This enhanced sensory function might result from up-regulation of afferent pain neurotransmission in the periphery, as well as greater central responsiveness to these signals.
Still, this observation explains a small portion of the complex pathogenesis of FGID. Low-grade inflammation, intestinal dysbiosis, visceral hypersensitivity and brain–gut axis dysfunction all may be relevant to pathogenesis and/or symptomatic presentations.2, 3 The contribution of genetic variation to these factors to this process has been a focus of considerable research interest, first described in epidemiological observations demonstrating a familial clustering of FGIDs.23, 24 This has led to studies involving over 50 SNPs for links to FGIDs,5 many of which have focused on the serotonin neurotransmission or gut-related immune pathways. Individual studies have had variable results, however, and associations between these genetic loci and FGIDs have been inconsistent.5, 25–28 However, a limited number of these studies have been hypothesis driven, and fewer still explored the association of gene variants with bowel symptom expression, extraintestinal and psychiatric comorbidity and HRQOL.
In recent years, efforts to gain insight into FGID pathophysiology have shifted partially to sympathetic nervous system dysfunction, and several investigators have focused on the role of polymorphisms within the adrenergic pathway. For instance, Kim et al. identified a polymorphism within the alpha 2 adrenergic receptor (ADRA2A) in constipation-predominant IBS and demonstrated an association between this polymorphism and somatic symptom burden.25 However, the relationships between polymorphisms within the ADRB2 have not been explored and, several factors make the examined polymorphisms within the coding region of the ADRB2 gene (rs1042713, rs1042714) attractive targets for investigation in FGID subjects: firstly, ADRB2 is widely expressed both in the gastrointestinal tract and in the central nervous system; second, blockade of this receptor decreases sensitivity to painful stimuli11, 12; furthermore, the ADRB2 polymorphisms examined appear to be functional polymorphisms, which alter ligand responsiveness.13 Finally, there is evidence that these polymorphisms are associated with fibromyalgia and temporomandibular joint disorder, both of which are EIFDs frequently encountered in FGID patients.13–15
Our results indicate that ADRB2 polymorphisms, in particular rs1042714, may have a role in the pathogenesis of FGIDs. Clinically, this polymorphism was associated with higher FGID and EIFD burden, especially in IBS patients. Importantly, the presence of a minor G allele in the FGID cohort translated into significantly greater IBS symptom severity, bother and more symptomatic days. These findings indirectly suggest that changes in sympathetic tone related to this polymorphism may have a role in heightened awareness of both gastrointestinal and extraintestinal symptoms in FGID patients. Alterations in ANS function have been implicated in somatisation states and traits, which too may be associated with both gastrointestinal (FGID) and extraintestinal (EIFD) diagnoses. Furthermore, conditions such as fibromyalgia and chronic fatigue (which fall into the broad category of EIFD) often are seen often in FGID patients.27, 28 Our results implicate genetic variations in autonomic response as a potential link between FGIDs and somatic extraintestinal disorders. The relationship between FGIDs and EIFDs is further supported by our observations regarding HRQOL with respect to ADRB2 polymorphisms. Univariate and multivariate analysis revealed a strong influence of the rs1042714 polymorphism on HRQOL in FGID subjects. However, a multivariate model that included EIFDs and psychiatric comorbidity no longer showed a similar relationship between this ADRB2 polymorphism and HRQOL. This observation was informed by our mediational analyses, which suggest that the effect of this ADRB2 polymorphism on HRQOL is partially mediated by its independent influence on several clinical features, including FGIDs, EIFDs and psychiatric comorbidity. Lending further indirect support of this hypothesis is our finding of parallel rises in the burden of EIFDs and FGIDs among carriers of the rs1042714 risk allele.
Our study has several notable limitations. As with most genetic studies of a similar design using a modest sample size, a false-positive association is always a consideration. This is somewhat mitigated by the fact that we only evaluated two SNPs, which have both been associated with other functional disorders in observational studies as well as alterations in ADRB2 function at a cellular level. It has been suggested that genetic association studies in common disorders, such as FGIDs, should have a sample size well over 1000 patients to fully evaluate individual SNPs, emphasising the need for these results to be confirmed in larger, multisite population-based studies. However, our results underscore the importance of continued hypothesis-driven genetic work, which will both inform and inspire future prospective studies. Second, the control group was composed of a convenience sample rather than age- and gender-matched controls. This potentially introduces confounding demographic variables within our control group; we feel that the likelihood of this is low, as, on multivariable analysis, neither age nor gender was found to influence our findings. Also, we did not collect data on specific FGID treatments at the time of GI symptom assessments; this could have confounded the patient symptom ratings. Furthermore, it should be emphasised that this cohort has been derived from a tertiary referral centre where subjects generally demonstrate a high degree of both FGID and EIFD comorbidity. This may limit the generalisability of our findings, and it may be that the observations relating to ADRB2 may be particularly relevant to this highly comorbid subset of FGID patients.
It might be anticipated based on the observations in the current work that the use of beta-antagonist pharmacotherapies could be useful in the management of IBS-associated abdominal pain. Indeed, such approaches have been shown to be effective in preventing and improving pain symptoms among other chronic nongastrointestinal pain conditions, such as fibromyalgia, temporomandibular joint disorder and migraine headaches.29, 30 The results in using beta-blockers to treat IBS are mixed, albeit in small trials and case reports using both selective and nonselective beta-antagonists.31, 32 Additional work is needed to clarify whether beta-blockade is more effective in patients with enhanced susceptibility (e.g. those with up-regulated adrenergic responses resulting from genetic variations in receptor regulation and configuration).
In conclusion, the results of our study implicate the rs1042714 ADRB2 polymorphism as a risk factor for the development of FGIDs and EIFDs. Furthermore, this ADRB2 polymorphism portends a more symptomatic FGID phenotype, which reports poorer HRQOL. These findings lend further evidence of a role of genetic factors, and more specifically the key role of autonomic nervous system dysfunction in the pathogenesis, symptom experiences and quality of life in FGID patients.
Supplementary Material
Acknowledgments
Declaration of personal interests: Dr Gyawali has served as a consultant for Given Imaging, Abbott Labs, and ProStrakan, has received research funding from Given Imaging, and has served as a speaker for Ironwood/Forest Labs. Dr Sayuk has served as a speaker for Takeda and Ironwood/Forest Labs.
Declaration of funding interests: Supported in part by (NIHMS320979) VMK, (R01DK064798) RDN and (K23 DK84113) GSS. The Washington University Digestive Disease Research Center Cores are supported by NIH grant P30DK052574.
Footnotes
AUTHORSHIP
Guarantor of the article: Dr Sayuk.
Author contributions: GS Sayuk, VM Kushnir, CP Gyawali: study concept and design; data analysis and interpretation; drafting and revision of manuscript; study supervision; RD Newberry: study concept and design; data analysis and interpretation; drafting and revision of manuscript; P Kibe, A Sabzpoushan, N Kanuri, B Cassell, B Nix: data collection and analysis. All authors approved the final version of the manuscript.
Additional Supporting Information may be found in the online version of this article:
Figure S1. Number of recorded functional GI disorder (FGID) diagnoses by rs1042714 haplotype. F = 5.08, P = 0.007 across groups.
Figure S2. Odds ratios for functional GI (FGID) and extraintestinal functional disorder (EIFD) diagnoses in rs1042714 G allele carriers.
Figure S3. Health-related quality of life (HRQOL) on SF 36 by rs1042714 ADRB2 genotype. Minor allele homozygotes reported worse QOL than ADRB2 heterozygotes on total SF 36 (P = 0.009), mental (P = 0.003) and physical (P = 0.04) scores.
References
- 1.Longstreth G, Thompson W, Chey W, Houghton L, Mearin F, Spiller R. Functional bowel disorders. In: Drossman D, Corazziari E, Delvaux M, Spiller R, Talley N, Thompson W, Whitehead W, editors. Rome III: The Functional Gastrointestinal Disorders. McLean, VA: Degnon; 2006. pp. 487–555. [Google Scholar]
- 2.Mayer EA. Clinical practice. Irritable bowel syndrome. N Engl J Med. 2008;358:1692–9. doi: 10.1056/NEJMcp0801447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ohman L, Simren M. Pathogenesis of IBS: role of inflammation, immunity and neuroimmune interactions. Nat Rev Gastroenterol Hepatol. 2010;7:163–73. doi: 10.1038/nrgastro.2010.4. [DOI] [PubMed] [Google Scholar]
- 4.Chang L. The role of stress on physiologic responses and clinical symptoms in irritable bowel syndrome. Gastroenterology. 2011;140:761–5. doi: 10.1053/j.gastro.2011.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saito YA, Mitra N, Mayer EA. Genetic approaches to functional gastrointestinal disorders. Gastroenterology. 2010;138:1276–85. doi: 10.1053/j.gastro.2010.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta V, Sheffield D, Verne GN. Evidence for autonomic dysregulation in the irritable bowel syndrome. Dig Dis Sci. 2002;47:1716–22. doi: 10.1023/a:1016424007454. [DOI] [PubMed] [Google Scholar]
- 7.Whitehead WE, Palsson O, Jones KR. Systematic review of the comorbidity of irritable bowel syndrome with other disorders: what are the causes and implications? Gastroenterology. 2002;122:1140–56. doi: 10.1053/gast.2002.32392. [DOI] [PubMed] [Google Scholar]
- 8.Tsigos C, Chrousos GP. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J Psychosom Res. 2002;53:865–71. doi: 10.1016/s0022-3999(02)00429-4. [DOI] [PubMed] [Google Scholar]
- 9.Martinez-Lavin M. Biology and therapy of fibromyalgia. Stress, the stress response system, and fibromyalgia. Arthritis Res Ther. 2007;9:216. doi: 10.1186/ar2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Argoff C. Mechanisms of pain transmission and pharmacologic management. Curr Med Res Opin. 2011;27:2019–31. doi: 10.1185/03007995.2011.614934. [DOI] [PubMed] [Google Scholar]
- 11.Tchivileva IE, Lim PF, Smith SB, et al. Effect of catechol-O-methyltransferase polymorphism on response to propranolol therapy in chronic musculoskeletal pain: a randomized, double-blind, placebo-controlled, crossover pilot study. Pharmacogenet Genomics. 2010;20:239–48. doi: 10.1097/FPC.0b013e328337f9ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nackley AG, Tan KS, Fecho K, Flood P, Diatchenko L, Maixner W. Catechol-O-methyltransferase inhibition increases pain sensitivity through activation of both beta2- and beta3-adrenergic receptors. Pain. 2007;128:199–208. doi: 10.1016/j.pain.2006.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diatchenko L, Anderson AD, Slade GD, et al. Three major haplotypes of the beta2 adrenergic receptor define psychological profile, blood pressure, and the risk for development of a common musculoskeletal pain disorder. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:449–62. doi: 10.1002/ajmg.b.30324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hocking LJ, Smith BH, Jones GT, Reid DM, Strachan DP, Macfarlane GJ. Genetic variation in the beta2-adrenergic receptor but not catecholamine-O-methyltransferase predisposes to chronic pain: results from the 1958 British Birth Cohort Study. Pain. 2010;149:143–51. doi: 10.1016/j.pain.2010.01.023. [DOI] [PubMed] [Google Scholar]
- 15.Vargas-Alarcon G, Fragoso JM, Cruz-Robles D, et al. Association of adrenergic receptor gene polymorphisms with different fibromyalgia syndrome domains. Arthritis Rheum. 2009;60:2169–73. doi: 10.1002/art.24655. [DOI] [PubMed] [Google Scholar]
- 16.Drossman DACE, Delvaux M, Spiller RC, Talley NJ, Thompson WG, Whitehead W, editors. Rome III: the Functional Gastrointestinal Disorders. McLean: Degnon Associates, Inc; 2006. [Google Scholar]
- 17.Winslow ER, Clouse RE, Desai KM, et al. Influence of spastic motor disorders of the esophageal body on outcomes from laparoscopic antireflux surgery. Surg Endosc. 2003;17:738–45. doi: 10.1007/s00464-002-8538-y. [DOI] [PubMed] [Google Scholar]
- 18.Edwards AWF. Fundamentals of Mathematical Genetics. 2. Cambridge, U.K: Cambridge University Press; 2000. [Google Scholar]
- 19.Cain KC, Jarrett ME, Burr RL, Hertig VL, Heitkemper MM. Heart rate variability is related to pain severity and predominant bowel pattern in women with irritable bowel syndrome. Neurogastroenterol Motil. 2007;19:110–8. doi: 10.1111/j.1365-2982.2006.00877.x. [DOI] [PubMed] [Google Scholar]
- 20.Burr RL, Heitkemper M, Jarrett M, Cain KC. Comparison of autonomic nervous system indices based on abdominal pain reports in women with irritable bowel syndrome. Biol Res Nurs. 2000;2:97–106. doi: 10.1177/109980040000200203. [DOI] [PubMed] [Google Scholar]
- 21.Posserud I, Agerforz P, Ekman R, Bjornsson ES, Abrahamsson H, Simren M. Altered visceral perceptual and neuroendocrine response in patients with irritable bowel syndrome during mental stress. Gut. 2004;53:1102–8. doi: 10.1136/gut.2003.017962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen H, Neumann L, Shore M, Amir M, Cassuto Y, Buskila D. Autonomic dysfunction in patients with fibromyalgia: application of power spectral analysis of heart rate variability. Semin Arthritis Rheum. 2000;29:217–27. doi: 10.1016/s0049-0172(00)80010-4. [DOI] [PubMed] [Google Scholar]
- 23.Levy RL, Jones KR, Whitehead WE, Feld SI, Talley NJ, Corey LA. Irritable bowel syndrome in twins: heredity and social learning both contribute to etiology. Gastroenterology. 2001;121:799–804. doi: 10.1053/gast.2001.27995. [DOI] [PubMed] [Google Scholar]
- 24.Morris-Yates A, Talley NJ, Boyce PM, Nandurkar S, Andrews G. Evidence of a genetic contribution to functional bowel disorder. Am J Gastroenterol. 1998;93:1311–7. doi: 10.1111/j.1572-0241.1998.440_j.x. [DOI] [PubMed] [Google Scholar]
- 25.Kim HJ, Camilleri M, Carlson PJ, et al. Association of distinct alpha(2) adrenoceptor and serotonin transporter polymorphisms with constipation and somatic symptoms in functional gastrointestinal disorders. Gut. 2004;53:829–37. doi: 10.1136/gut.2003.030882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gonsalkorale WM, Perrey C, Pravica V, Whorwell PJ, Hutchinson IV. Interleukin 10 genotypes in irritable bowel syndrome: evidence for an inflammatory component? Gut. 2003;52:91–3. doi: 10.1136/gut.52.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bradley LA. Pathophysiology of fibromyalgia. Am J Med. 2009;122(12 Suppl):S22–30. doi: 10.1016/j.amjmed.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frissora CL, Koch KL. Symptom overlap and comorbidity of irritable bowel syndrome with other conditions. Curr Gastroenterol Rep. 2005;7:264–71. doi: 10.1007/s11894-005-0018-9. [DOI] [PubMed] [Google Scholar]
- 29.Light KC, Bragdon EE, Grewen KM, Brownley KA, Girdler SS, Maixner W. Adrenergic dysregulation and pain with and without acute beta-blockade in women with fibromyalgia and temporomandibular disorder. J Pain. 2009;10:542–52. doi: 10.1016/j.jpain.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silberstein SD, Holland S, Freitag F, Dodick DW, Argoff C, Ashman E. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1337–45. doi: 10.1212/WNL.0b013e3182535d20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fielding JF. Timolol treatment in the irritable bowel syndrome. Digestion. 1981;22:155–8. doi: 10.1159/000198627. [DOI] [PubMed] [Google Scholar]
- 32.McIntyre AS, Burnham WR, Thompson DG. Atenolol in irritable bowel syndrome. Lancet. 1988;1:67. doi: 10.1016/s0140-6736(88)91056-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.