Abstract
Cytomegalovirus (CMV) infection and delayed immune reconstitution (IR) remain serious obstacles for successful haploidentical stem cell transplantation (haplo-SCT). CMV-specific IR varied according to whether patients received manipulated/unmanipulated grafts or myeloablative/reduced intensity conditioning. CMV infection commonly occurs following impaired IR of T cell and its subsets. Here, we discuss the factors that influence IR based on currently available evidence. Adoptive transfer of donor T cells to improve CMV-specific IR is discussed. One should choose grafts from CMV-positive donors for transplant into CMV-positive recipients (D+/R+) because this will result in better IR than would grafts from CMV-negative donors (D−/R+). Stem cell source and donor age are other important factors. Posttransplant complications, including graft-versus-host disease and CMV infection, as well as their associated treatments, should also be considered. The effects of varying degrees of HLA disparity and conditioning regimens are more controversial. As many of these factors and strategies are considered in the setting of haplo-SCT, it is anticipated that haplo-SCT will continue to advance, further expanding our understanding of IR and CMV infection.
1. Introduction
Haploidentical stem cell transplantation (haplo-SCT) is an alternative treatment for transplant candidates lacking a human leukocyte antigen- (HLA-) matched related or appropriate unrelated donor. After hematopoietic stem cell transplantation (HSCT), T cells are regenerated through thymic and peripheral pathways, with the thymus generating a more diverse T cell repertoire. Because thymic function is poor in adults, posttransplantation immune reconstitution (IR) in the months following transplant depends on the peripheral expansion of mature T lymphocytes in the allograft. Impaired recovery of adaptive immunity following haplo-SCT remains an outstanding issue and is associated with increased risk of infection, including bacterial, fungal, and cytomegalovirus (CMV) infections. CMV infection after haplo-SCT continues to adversely affect transplant outcomes [1–4] despite the use of prophylactic or preemptive treatment [5]. Lack of CMV-specific immune recovery has been reported as consistently associated with relapses of CMV infection and the development of CMV disease after allogeneic stem cell transplantation [6–9]. Therefore, this review summarizes the kinetics of CMV-specific T cell recovery and its association with CMV infection after haplo-SCT. Strategies to improve CMV-specific IR are also discussed.
2. Cytomegalovirus-Specific T Cell Immune Reconstitution after Haplo-SCT (Table 1)
Table 1.
CMV-specific immune recovery after haploidentical stem cell transplantation.
| Group/Reference | Number | Disease | Graft manipulation | Conditioning | NRM or TRM | CMV infection | Immune reconstitution (IR) | Comments |
|---|---|---|---|---|---|---|---|---|
| TCD haplo-SCT | ||||||||
| Perugia; [2] | 17 | End-stage chemoresistant leukemia | Extensively TCD | TBI + ATG + Cy + Thio | 40% NRM; mainly CMV and Aspergillus infection | NR | NR | |
| Perugia; [11] | 43 | Acute leukemia | Extensively TCD | TBI + ATG + Flu + Thio | The infection-related mortality rate 25–35% | NR | CD4+ >0.1 × 109/L at day 60 and >0.3 × 109/L at day 180 | |
| Lilleri et al.; [12] | 48 | Malignant or nonmalignant hematological diseases | T cell-depleted peripheral blood CD34+ progenitor cells | ATG + TBI or chemotherapy | 9% in R+ or 8% in R− (1-year) | 4% in R− and 83% in R+ | 61% recipients reconstituting CMV-CTL within the first 3 months | Young patients |
| Chen et al.; [13] | 22 | Refractory hematological malignancies | Mobilized peripheral blood stem cells depleted of CD3+ cells | Flu + Thio + Mel + OKT3 | NR | 1/22 patients developed CMV infection | The median number of CD4+ and CD8+ T cells was about 0.2 × 109/L and above 0.1 × 109/L at 3 months | Pediatric recipients |
| Federmann et al.; [14] | 28 | Hematological malignancies | CD3/CD19-depleted grafts | Flu or (Clo) + Thio + Mel + OKT-3 | NR | Eight of 28 patients had cytomegalovirus reactivation | A median of 205 CD3+ cells/μL, 70 CD3+ CD4+ cells/μL, and 66 CD3+ CD8+ cells/μL on day 100 | |
| Pérez-Martínez et al.; [16] | 30 | Acute leukemia | CD3/CD19-depleted | Flu + Bu + Thio + mP | 23% NRM (7/30) | Two of 30 patients have died because of CMV pneumonia | A median of 167 ± 64/μL CD4+ cells versus 364 ± 174/μL CD8+ cells on day 30, 155 ± 47/μL versus 410 ± 119/μL on day 60, and 217 ± 72/μL versus 537 ± 192/μL on day 90. | Children |
| Unmanipulated haplo-SCT | ||||||||
| Peking University; [21] | 50 | Hematological diseases | G-CSF-primed bone marrow and unmanipulated PBSCs | Ara-C + Bu + Cy + simustine + ATG | 19.5 ± 6.0% NRM (2-year) | The cumulative incidence of CMV antigenemia in the early posttransplant phase was 49.9 ± 7.2% | CD4+ T cells at 152.91 (13.29–579.63)/μL on day 90, 163.28 (23.29–875.60)/μL on day 180, and 277.49 (16.91–579.48)/μL on day 365; CD8+ T cells at 672.79 (48.23–2,556.01) on day 90, 918.42 (115.00–4,047.91)/μL on day 180, and 884.16 (175.84–2,441.58)/μL on day 365 | |
| Peking University; [22] | 42 | Malignant hematological disorders | G-CSF-primed bone marrow and unmanipulated PBSCs | Ara-C + Bu + Cy + simustine + ATG | 24% NRM (10/42) | The cumulative incidence of CMV reactivation was 87.67% (75.70–95.48%); 5 of them had CMV disease (day 22–50). | The CD8+ T cell count equaled that of controls at day 60, and the median number of CMV-CTL cells was comparable to that of controls from day 30 to day 360 | |
| Kurokawa et al.; [24] | 66 | Hematologic malignancies | Unmanipulated PBSCs and/or bone marrow | ATG + BU + Mel with TBI or Flu | 11% NRM (7/66) | CMV antigenemia occurred in 45 of 57 evaluable patients | CD3+ >1600/μL at day 180 and CD8+ >1200/μL at day 180. The lowest numbers of CD3+, CD4+, and CD8+ T cells were seen at 1 month after transplantation but all continued to rise until 6 months after transplantation. | |
| Lee et al.; [25] | 83 | Acute leukemia and myelodysplastic syndrome | Unmanipulated PBSCs | BU + Flu + ATG | 18% (95% CI, 12%–29%) TRM | Fifty-eight of 72 evaluated patients (81%) had CMV pp65 antigenemia. | CD8+ lymphocyte counts exceeded pretransplantation levels at 2 months, and >90% of patients maintained counts >200/μL at 3 months after transplantation. 12 months after transplantation, 24 of 27 patients (89%) had CD4+ lymphocyte counts more than 200/μL. | |
| Kanda et al.; [26] | 12 | Hematologic malignancies | Unmanipulated PBSCs | Alemtuzumab + TBI or Flu based | 17% NRM (2/12) | Ten of the 12 patients experienced CMV reactivation, and CMV disease was observed in three patients | CD3+/CD4+ and CD3+/CD8+ T cells were strongly suppressed within 2 months after haploidentical peripheral blood SCT but recovered on day 90. CMV-CTLs were detected on day 90 at 0.03–0.25% of CD8+ T cells | |
| Rizzieri et al.; [27] | 49 | Hematologic malignancies or marrow failure | Unmanipulated PBSCs | Flu + Cy + Alemtuzumab | 31% NRM (15/49) | Twenty-five percent of patients experienced a severe infection, whereas 86% experienced reactivated CMV | The median number of CD4+ and CD8+ T cells was about 100/μL and 400/μL at 3 months for transplant recipients without GVHD. |
TCD: T cell-depleted; PBSC: peripheral blood stem cell; NRM: nonrelapse mortality; TRM: treatment-related mortality; CMV: cytomegalovirus; R−: CMV-negative recipients; R+: CMV-positive recipients; CMV-CTL: cytomegalovirus-specific T cells; NR: not reported; TBI: total body irradiation; ATG: antithymocyte globulin; Cy: cyclophosphamide; Thio: thiotepa; Flu: fludarabine; Mel: melphalan; Clo: clofarabine; Bu: busulfan; mP: methylprednisolone; Ara-C: cytosine arabinoside.
2.1. Manipulated (T Cell Depleted, TCD) Haplo-SCT
Using a megadose of extensively T cell depleted, G-CSF-mobilized stem cells and a fludarabine-based conditioning protocol [10], the Perugia group demonstrated that haplo-SCT could be successful in patients with acute leukemia. Early results [2] showed a nonrelapse mortality rate of 40%, with infection as the leading cause of death, mainly CMV and Aspergillus. Additionally, improved IR and fewer deaths secondary to infection occurred when G-CSF was eliminated from the regimen [11]. The results showed that in patients not treated with G-CSF, CD4+ cell counts were greater than 0.1 × 109/L 60 days after transplantation and greater than 0.3 × 109/L at 180 days. Subsequently, Lilleri et al. [12] performed a study with 48 young patients who received a TCD, allogeneic myeloablative HSCT from an HLA-disparate relative. The 1-year cumulative incidence of both CMV infection and specific T cell reconstitution was 83% among the 23 CMV-seropositive patients, and these incidences were 4% and 8%, respectively, among the 25 CMV-seronegative patients [12]. CMV-specific T cell (CMV-CTL) reconstitution was significantly delayed in patients receiving TCD grafts compared with patients receiving unmanipulated HSCTs [12].
Reduced intensity conditioning (RIC) is used to minimize toxicity while allowing rapid engraftment and expediting immune reconstitution during the early posttransplant period, thereby protecting the host from infection. Data showed that IR was rapid in 22 pediatric recipients after RIC and CD3-depleted haplo-SCT and was similar to, if not better than, outcomes obtained after myeloablative haploidentical transplantation [13]. CMV was detected in only one patient in this group, and no patient had died of viremia. In an attempt to reduce the risk of graft-versus-host disease (GVHD) and Epstein-Barr virus-related lymphoproliferative disease, Federmann et al. used CD3/CD19-depleted grafts with RIC and observed that T cell reconstitution after haplo-SCT was delayed with a median of 205 CD3+ cells/μL, 70 CD3+ CD4+ cells/ul, and 66 CD3+ CD8+ cells/μL on day 100, respectively [14]. Eight of the 28 patients had CMV reactivation, and no deaths due to infections were observed. Bader et al. reported their experience of CD3/CD19-depleted haplo-SCT for 22 children with acute leukemia [15]. Reconstitution with T cells can start on day 30 and the early T cell regeneration following transplantation results from the expansion of T precursor cells contained in the stem cell transplant. Thymus-dependent T cell regeneration only begins on day 100. In contrast to these published data, reports from Pérez-Martínez et al. using allogeneic CD3/CD19-depleted grafts showed that T cell recovery achieved normal values within the first 60 days after transplantation [16]. And up to 2 years, 2 of the 30 patients had died because of CMV pneumonia. Similar results were reported in patients with acquired severe aplastic anemia [17].
2.2. Unmanipulated Haplo-SCT
Although extensive depletion of T cells or selective depletion of alloreactive T cell subsets improves engraftment and reduces GVHD, this manipulation is associated with prolonged immune deficiencies and increased risk of infection. In an attempt to perform haplo-SCT without T cell depletion, Peking University researchers developed the GIAC protocol for haplo-SCT by combining G-CSF-primed bone marrow and unmanipulated PBSCs [18–22] (Figure 1). Using this protocol [23], we previously observed that patients undergoing haplo-SCT had a higher 100-day cumulative incidence of CMV antigenemia compared with a matched group (65% versus 39%), whereas the incidence of CMV-associated interstitial pneumonia was the same between the two groups (17% versus 17%). We investigated IR in patients with hematological malignancies after haploidentical transplantation and HLA-matched transplant [21]. Compared with those from HLA-matched recipients, T cell subset cell counts in the first 90 days after grafting were lower in haploidentical recipients. The difference was most striking for CD4+ and CD4+ naïve T cells. T cells appeared equally functional among patients without GVHD from both groups. Furthermore, we prospectively investigated CMV-CTL IR in 42 recipients after haplo-SCT [22]. CMV reactivation occurred in 36 of the 42 patients, but only 5 had CMV disease. The CD8+ T cell count in transplant recipients was equal to that of controls at day 60 after transplantation. The median number of CMV-specific T cells and the subsets to which they belonged was comparable to those of the controls from day 30 to day 360. Furthermore, CMV-CTLs from transplant recipients were found at high frequencies and demonstrated robust proliferation capacities and interferon-γ responses at 1 year after transplantation.
Figure 1.
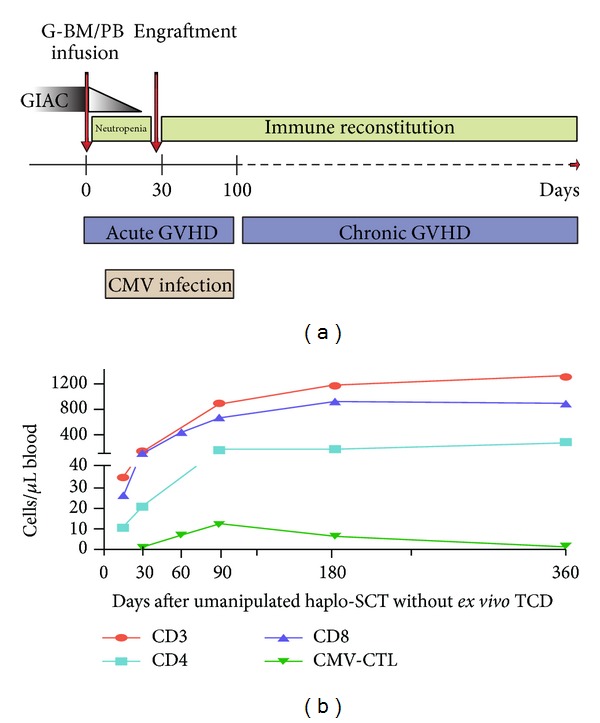
T cell immune reconstitution and CMV infection following unmanipulated haplo-SCT without ex vivo TCD (GIAC transplant protocol, Peking University Institute of Hematology). CMV, cytomegalovirus; GVHD, graft-versus-host disease; CMV-CTL, CMV-specific CTL; TCD, T cell depleted; G-BM/PB, combining G-CSF-primed bone marrow (G-BM) and peripheral blood (G-PB) harvests.
Recent reports showed that it is feasible to perform haplo-SCT without ex vivo TCD after RIC. Kurokawa et al. from Japan [24] conducted haplo-SCT on 66 adults with hematologic malignancies using RIC without TCD. CMV antigenemia occurred in 45 of 57 evaluable patients at a median of 19 days after transplantation. CMV-related diseases were diagnosed in 3 patients, and one patient died of CMV-colitis. The lowest numbers of CD3+, CD4+, and CD8+ T cells were observed at 1 month after transplantation, but all values continued to increase until 6 months after transplantation and remained stable thereafter [24]. Data from a Korean study [25] showed a RIC therapy with busulfan, fludarabine, and antithymocyte globulin (ATG) for haplo-SCT in acute leukemia and myelodysplastic syndrome. Fifty-eight of 72 evaluated patients (81%) had at least 1 positive assay result for CMV pp65 antigenemia. Four patients developed CMV disease, and 3 of them died of CMV-colitis per se or of other causes. Despite the use of ATG, CD8+ lymphocyte counts exceeded pretransplantation levels at 2 months, whereas CD4+ lymphocyte counts recovered more slowly, with only approximately half of all patients showing CD4+ lymphocyte counts > 200/μL at 2 to 6 months after transplantation [25].
Alemtuzumab, which has a strong lympholytic effect, is usually used against GVHD in a reduced intensity conditioning regimen. Using an in vivo alemtuzumab-based regimen, Kanda et al. [26] reported that CD3+/CD4+ and CD3+/CD8+ T cells were strongly suppressed within 2 months after haploidentical peripheral blood SCT but recovered on day 90. CMV-specific cytotoxic T lymphocytes were detected on day 90 after transplantation in two patients and represented 0.03% and 0.25% of CD8+ T cells, respectively, for each patient. Ten of the 12 patients experienced CMV reactivation, and CMV disease was observed in three patients but was not fatal. Rizzieri et al. [27] extended the prior work and reported the large series assessing outcomes and immune reconstitution in nonmyeloablative haplo-SCT for 49 patients with alemtuzumab-based regimen. Twenty-five percent of the patients experienced a severe infection, whereas 86% experienced reactivated CMV with CMV disease in seven patients. Quantitative lymphocyte recovery through expansion of transplanted T cells was noted by 3 to 6 months [27]. Recently, Kanda et al. [28] updated their transplant data with continued use of in vivo T cell depletion with alemtuzumab. Nine patients experienced positive CMV antigenemia with CMV disease in three patients, none of which was fatal. The numbers of CD4+ and CD8+ T-cells remained low within one year after HSCT. The median quantities of CMV-specific CD8+ T lymphocytes as measured by the tetramer-based assay were 0.05%, 0.01%, and 1.83% at 90, 180, and 365 days after HSCT, respectively.
3. Cytomegalovirus Infection Associated with T Cell Immune Reconstitution
IR of the immune subsets is likely to have the greatest impact on clinical outcomes after haplo-SCT [29]. In healthy CMV-seropositive individuals, high frequencies of CMV-specific CD4+ and CD8+ T cells that mediate control of viral reactivation can be detected [30, 31]. Both the quantity and quality of CMV-specific T cell recovery are essential for immune control of CMV infection following HSCT. A strategy of deferred antiviral therapy based on the presence of a detectable functional CMV-specific T cell response at the time of documentation of CMV DNAemia was clinically administered and allowed for the sparing of antiviral treatment in transplant patients [32, 33]. A recent phase II study by Blyth et al. showed that donor-derived CMV-specific T cells reduce the requirement for CMV-directed pharmacotherapy without increased GVHD after allo-HSCT [34].
In immunocompromised HSCT recipients, few patients with levels of CMV-specific CD8+ lymphocytes > 2 × 106–10 × 106/L developed CMV disease [35–37]. Both CD4+ and CD8+ CMV-specific IR are required for protection from recurrent activation [38–40], and an absolute CD4+ and CD8+ T cell response at day 60 may confer protection against viremia in young patients [41]. Borchers et al. [42] reported that the presence of CMV-CTL before day 50 and their expansion after reactivation appear to protect against recurrent CMV reactivation. In patients with uncontrolled reactivation, differentiated CMV-specific T cells of the late differentiation phenotype CD45RA+CD27−CD28− did not develop [37]. Furthermore, Lilleri et al. [12] found that detection of CMV-specific T cells also correlated with control of CMV infection after T cell depleted haplo-SCT.
In our own analysis [43], high CMV-CTL with terminally differentiated effector CD45RO−CD62L− (TEMRA) phenotype in the allografts was associated with reduced risk of CMV reactivation when sufficient CD45RO+CD62L− cells (TEM) were provided by infusion (≥0.208 × 106/kg). Early after transplantation, there was significant expansion of CMV-CTL with central memory CD45RO+CD62L+ (TCM) phenotype when CMV was reactivated [23, 43]. We further investigated CMV-CTL in bone marrow (BM) after haplo-SCT. BM-resident CMV-CTLs displayed distinct phenotypes when CMV was reactivated [23], as there are more TEMRA in the BM at day 360 after SCT and relatively higher TNaive cells (CD45RO−CD62L+) in the BM at day 90 in patients with infection compared with those without infection. This result suggested that CTL in BM may play an important role in controlling CMV infection, as mature T cells in the BM play an essential role in maintaining normal peripheral T cell numbers, and CMV-CTL could therefore be more efficiently derived from the BM than from the PB [44, 45].
4. Factors Influencing CMV-Specific IR
The process of IR is influenced by patient- and transplant-related factors, such as donor and patient ages, primary disease, transplant type, conditioning regimen, stem cell source, HLA disparity, GVHD, and infection [46]. Not surprisingly, the intensity of immunosuppression and the degree of T cell depletion in transplant protocols, such as ATG or alemtuzumab, both critically affect the risk of CMV reactivation [47]. As for CMV-specific IR after haplo-SCT, there are several influences, except graft manipulation described above.
4.1. Donor and Recipient CMV Serostatus
CMV-negative recipients of grafts from CMV negative donors (D−/R−) rarely develop CMV infection and D− should be chosen when possible. Ljungman et al. reported that only acute GVHD grade II–IV and D−/R+ were significant risk factors for CMV disease after multivariate analysis [48]. D+/R+ transplants, on average, generate higher levels of multifunctional CMV-specific T cells and require less antiviral therapy compared with D−/R+ transplants [49]. D+/R+ patients had a lower cumulative incidence of CMV reactivation, recurrent reactivation, CMV disease, and mortality compared with D−/R+ patients [50]. Pretransplant human CMV infection of the recipient is a major factor driving human CMV-specific immune reconstitution [12]. Our previous data also suggested protective immunity could be transferred by infusion of CMV-CTL within allografts [43]. Nevertheless, Pietersma et al. found that reactivation of CMV infection occurred more frequently in patients receiving a CMV-positive graft but was less severe than in patients receiving a CMV-negative graft [51]. These data suggest roles for both virus and CMV-specific immunity present in the graft. Based on current knowledge, the use of D+/R+ transplant is preferred for improved IR, and D−/R− is preferred for decreased risk of CMV infection. Other donor/recipient combinations remain to be confirmed in clinical trials. Determining CMV serostatus and levels of CMV-CTL in the donor grafts may help in controlling CMV reactivation, which is closely correlated with immune reconstitution and differentiation of CMV-CTL subsets.
4.2. Stem Cell Source and Graft Composition
Numerous studies have compared IR during SCT using different stem cell sources. IR after peripheral blood stem cell transplantation (PBSCT) is generally characterized by faster myeloid and lymphoid recovery versus BMT [52–54]. Along with accelerated and sustained naïve CD4+ recovery, improved in vitro proliferative responses have been measured following PBSCT [52–54]. Hakki et al. suggested that BM as the source of stem cells resulted in delayed recovery of functional T cell immunity at 3 months after transplantation [39]. In the setting of HLA-matched sibling transplantation, recipients receiving PBSCT had lower risks of documented bacterial, fungal, and viral infection, including CMV viremia [52]. These data clearly indicate rapid T cell reconstitution and a lower incidence of CMV infection when PBSCT is used.
Transplantation using PBSCs with ex vivo TCD is the most common HLA-mismatched/haploidentical transplant approach in Europe and the United States [55]. In China, Peking University researchers combined G-BM and G-PB harvests (G-BM/PB) without ex vivo TCD for the GIAC protocol and achieved encouraging results [18–21]. Recently, unmanipulated PBSCT [56] and unmanipulated G-BM [57] have been accomplished in haplo-SCT settings with very encouraging results. However, limited data are available concerning CMV-specific IR after haplo-SCT. Lilleri et al. reported that children receiving T cell depleted transplants exhibited significantly delayed CMV-specific T cell reconstitution, and only D− and BM as a stem cell source were found to significantly delay CMV-specific T cell reconstitution [12]. A small comparative series showed better survival among patients who received T cell-replete transplants, with less viral infections, including CMV reactivation, and better immune reconstitution of T cell subsets compared with T cell-depleted haplo-SCT [58]. Reconstitution of CMV-specific T cell immunity may have proceeded at a faster rate in patients treated with our GIAC protocol than in patients described in other reports of haplo-SCT [23]. A differential pattern of T cell reconstitution is expected after in vivo TCD and ex vivo TCD haplo-SCT. In TCD haplo-SCT, the time lapse during IR, even in the absence of GVHD, is most likely lengthened by extensive ex vivo T cell depletion itself, while greater numbers of donor T cells cotransfused with allografts are not immediately eradicated by in vivo TCD. The effect of in vivo T cell depletion could be balanced by graft infusion at the time of transplantation [43, 59]. Therefore, using PBSCT or G-BM/PB is preferred for IR to CMV.
4.3. Conditioning Regimens
Although limited, studies comparing IR following myeloablative and nonmyeloablative regimens have been insightful. Maris et al. compared IR for one year after transplantation in 51 patients receiving HLA-matched PBSCT following nonmyeloablative conditioning with a reference group of 67 myeloablative recipients [60]. Both regimens demonstrated similar levels of total and subset-specific lymphocyte recovery, lymphoproliferative responses to viral stimulants, and in total and pathogen-specific antibody levels. Overall infection rates were significantly lower in nonmyeloablated patients, who also had lower rates of CMV infection coinciding with greater numbers of CMV-specific T cells at days 30 and 90. Data from Nakamae and colleagues showed [61] that residual host cells after nonmyeloablative SCT reduce progression to higher CMV viral load in transplant recipients; however, this effect does not appear to protect against serious complications of CMV. Recent results [62] showed that CMV reactivation was less common in the RIC group during the midrecovery period, while there was no difference during the late-recovery period. CMV disease is as much of a problem following nonmyeloablative transplantation as it is following myeloablative transplantation [61, 63].
4.4. GVHD and Steroid Administration
The deleterious effects of acute GVHD on T cell function are well established. GVHD inhibits T cell recovery through T cell apoptosis via activation-induced cell death, immunosuppressive cytokine production by regulatory cell populations, and direct damage to thymic epithelium and stroma [64, 65]. GVHD appears to adversely affect all levels of T cell function, from delaying T cell ontogeny and limiting TCR diversity to impairing cytokine production in recovered T cells. Multivariable analysis showed that patients receiving methylprednisolone had a 1.5 times higher risk of infection, with acute GVHD being another independent risk factor for infections after transplantation [66]. Steroids can suppress immune function by promoting the development of high IL-10-producing regulatory T cells and inhibiting GATA-3 phosphorylation [67, 68]. High-dose steroid use (≥2 mg/kg/d) predicts delayed recovery of functional T cell immunity at 3 months after transplantation [39]. Özdemir et al. [69] showed that steroid administration resulted in a significant impairment in CD8+ tumor necrosis factor α (TNFα) production but not a decrease in the frequency of CMV-specific CD8+ T cells. Corticosteroid treatment may favor active viral replication even in patients with CMV-specific T cells [12]. These findings have implications regarding the tapering of steroids in patients with active infections normally controlled by T cell responses, such as CMV disease.
4.5. Subclinical CMV Reactivation
It is known that CMV infection drives T cells to an effector phenotype in healthy individuals [70]. Subclinical CMV reactivation while on ganciclovir appears to be a potent stimulator of T-cell function [39]. Among patients who received ganciclovir at engraftment, those who had breakthrough antigenemia had significantly better recovery of T cell function at 3 months compared with patients who remained antigenemia negative [39]. In the setting of HSCT and the absence of high-dose steroids, low-level, short-term antigenemia may, in fact, have a protective effect by enhancing late immune function. CMV infection is required for the generation and/or maintenance of the CMV-specific T cell pool, and reactivation of latent virus was identified as the main factor leading to immune reconstitution [12, 41]. Our data also showed that CMV-CTLs with a central memory CD45RO+CD62L+ phenotype significantly expanded when CMV was reactivated [23, 43]. However, prolonged CMV reactivation may lead to exhaustion of T cells, as has been suggested for other antigens [71]. These studies suggested that subclinical CMV reactivation, but not persistent CMV reactivation, may be required for the reconstitution of CMV-specific T cell responses.
4.6. Age and Degree of HLA Disparity
Children may have a better capacity than adults to develop anti-CMV primary immune responses after HSC transplantation [41]. Patients <8 years of age demonstrate improved IR, with a probability ratio of 4.57, and this likely results in better reconstitution of CMV-specific CD4+ and CD8+ T cells [12]. Increased thymic function might be responsible for better immune reconstitution in younger children [72], especially when compared with adult patients in whom naive thymic emigrants have been reported to appear in the circulation only 6 months after receipt of a T cell depleted HSCT [73]. Recently, Azevedo et al. [74] investigated long-term IR after RIC based haplo-SCT with TCD, which followed by preemptive donor lymphocyte infusions (DLI). They found the proportion of naive and memory subsets in the recipients, both within CD8+ and CD4+ T cells, more closely resembled that observed in age-matched control subjects than in the donors. Their data [74] suggested that long-term IR was successfully achieved after haploidentical HSCT, a process that appears to have largely relied on de novo T cell production. IR after haplo-SCT is usually slower than that after matched-sibling donor or matched-unrelated donor transplants [75]; however, the impact of HLA disparity on CMV-specific IR after haplo-SCT remains uncertain.
5. Adoptive Immunotherapy to Accelerate CMV-Specific T Cell Immune Reconstitution
Any further reduction in CMV infection after haplo-SCT will only be achieved by hastening posttransplant IR. To improve posttransplant IR, various strategies of adoptive donor T cell immunotherapy have been investigated over the past years. However, T cell-based adoptive therapy is problematic in the adult haploidentical transplant setting, for alloreactivity still exists. Research is focusing on strategies to hasten IR by adding back broad-repertoire or pathogen-specific mature donor T lymphocytes after ex vivo depletion of antidonor alloreactivity [76, 77].
Amrolia et al. demonstrated an accelerated immune reconstitution in 16 patients who received adoptively transferred T cells that were allodepleted in vitro [78]. After 2 to 4 months, CMV-specific T cells and a broad Vβ T cell receptor repertoire could be observed, while the incidence of GVHD was low. Posttransplantation CD8-depleted DLI can also contribute to improved T cell recovery after haplo-SCT for the treatment of advanced hematologic malignancies, while reducing the incidence and severity of acute GVHD [79]. Despite the high incidence of CMV reactivation (82%), no patients developed CMV disease. Circulating CD3+/CD4+ T cells significantly increased at day 120 after DLI, while the expansion of CD3+/CD8+ was at a median value of 23/μL. Preliminary studies using gene engineering of donor lymphocytes to deplete alloreactive T cells appear to be promising [80, 81], but larger-scale investigations are warranted to confirm the results.
Given high degree of mismatching makes cell immunotherapy impossible, Perruccio et al. [76] improved the immune recovery after myeloablative haploidentical SCT by the infusion of nonalloreactive clones specific for CMV and Aspergillus. Within 3 weeks of the immunotherapy infusion, CMV-specific CD4+ T cell clones were 404 ± 124 per 106 cells, and IFN-γ-producing CMV-specific CD8+ cells were detected in normal frequencies. Of the 25 patients who received CMV-specific adoptive therapy, CMV reactivation was observed in only 7 patients, while thirty of the 33 control patients experienced repeated CMV reactivation. More recently, Perruccio et al. [82, 83] tested a photodynamic approach to purge DLI of alloreactive, but not pathogen-specific, donor T cells. Pathogen-specific responses to CMV were retained, although with a 19 ± 9.7 time reduction in frequency [83]. Not only did the researchers achieve the success of described prophylactic infusion, but Feuchtinger et al. [84] also treated 18 patients with refractory CMV infection after allo-SCT using polyclonal CMV-specific T cells. In 83% of cases, CMV infection was cleared or viral burden was significantly reduced. Viral control was associated with the in vivo expansion of CMV-specific T lymphocytes in 12 of 16 evaluable cases, without GVHD induction or acute side effects.
These manipulated DLI approaches are effective but expensive and labor intensive, and the effect on global IR is unclear. For a long time following transplant, allogeneic DLI can accelerate IR, treat infections, and provide a graft-versus-malignancy effect [85, 86]. Currently, we focus mainly on the infusion of G-CSF-mobilized peripheral blood progenitor cells. Previous studies have shown the multiple biological effects of G-CSF on peripheral blood stem cells, such as the ability to polarize T cells from Th1 to Th2 and the promotion of regulatory T cell and tolerogenic dendritic cell differentiation [87, 88]. Huang et al. [89] reported that G-CSF-mobilized peripheral blood progenitor cell infusion produces superior disease-free survival in patients who received unprimed lymphocytes for relapse after allo-HSCT, although the difference in the incidence of severe GVHD was not significant. We extended the use of DLI for the treatment of infections. Our preliminary data showed that DLI is an effective and safe therapy for EBV-associated PTLD after mismatched/haploidentical SCT [90]. Investigation of DLI for CMV infection and other opportunistic infections is underway. Until pathogen-specific T cells and/or alloreactive-depleted T cells are more readily available, unmanipulated, nonspecific DLI will continue to play a role in the treatment of uncontrolled infections and improvement of IR following haplo-SCT.
6. Conclusions
The current options for haplo-SCT present intrinsic challenges. In T cell depleted haplo-SCT, the minimal residual T lymphocytes in the grafts successfully prevent lethal GVHD without any posttransplantation immunosuppression, but the small number of T cells infused leads to delayed IR. In unmanipulated haplo-SCT, although the high T cell content of the graft potentially enhances the graft-versus-leukemia effect, recipients of unmanipulated grafts from alternative donors remain at risk of TRM for months/years after transplantation because of GVHD and its immunosuppressive treatments that antagonize T cell expansion and function. Delayed IR and increased risk of CMV infection remain critical problems early after transplantation, although long-term IR can successfully be achieved after haplo-SCT [74, 91].
To address these shortcomings, several factors identified to affect IR to CMV should be considered for better outcome (Figure 2). Our data indicate that selection of D+ for R+, young donor, stem cells derived from PBSC or G-BM/PB, subclinical CMV reactivation while on antiviral therapy, avoidance of GVHD, and decreased steroid dose can improve CMV-specific IR. The effects of varying degrees of HLA disparity and conditioning regimens are uncertain. Therefore, more in-depth preclinical and clinical studies in this area are needed, both in terms of reconstitution of normal immune cell function and their effectiveness in anti-CMV cell activity.
Figure 2.
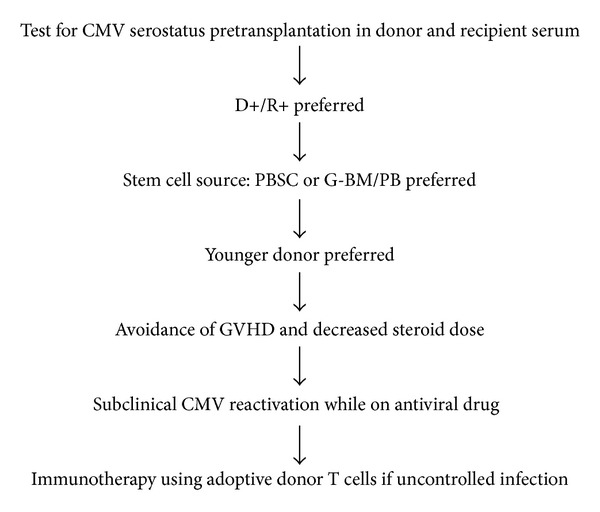
Proposed algorithm for improving CMV-specific IR following haplo-SCT. CMV, cytomegalovirus; D+/R+, CMV-positive recipients of grafts from CMV-positive donors; PBSC, peripheral blood stem cell; G-BM/PB, combining G-CSF-primed bone marrow (G-BM) and peripheral blood (G-PB) harvests; GVHD, graft-versus-host disease.
Acknowledgment
This work was supported by the Key Program of the National Natural Science Foundation of China (Grant 81230013), the Chang Jiang Scholars Program, and the National Natural Science Foundation of China (Grant 81100388).
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Rizzieri DA, Liang PK, Long GD, et al. Partially matched, nonmyeloablative allogeneic transplantation: clinical outcomes and immune reconstitution. Journal of Clinical Oncology. 2007;25(6):690–697. doi: 10.1200/JCO.2006.07.0953. [DOI] [PubMed] [Google Scholar]
- 2.Aversa F, Tabilio A, Terenzi A, et al. Successful engraftment of T-cell-depleted haploidentical ’three-loci’ incompatible transplants in leukemia patients by addition of recombinant human granulocyte colony-stimulating factor-mobilized peripheral blood progenitor cells to bone marrow inoculum. Blood. 1994;84(11):3948–3955. [PubMed] [Google Scholar]
- 3.Aversa F, Terenzi A, Tabilio A, et al. Full haplotype-mismatched hematopoietic stem-cell transplantation: a phase II study in patients with acute leukemia at high risk of relapse. Journal of Clinical Oncology. 2005;23(15):3447–3454. doi: 10.1200/JCO.2005.09.117. [DOI] [PubMed] [Google Scholar]
- 4.George B, Pati N, Gilroy N, et al. Pre-transplant cytomegalovirus (CMV) serostatus remains the most important determinant of CMV reactivation after allogeneic hematopoietic stem cell transplantation in the era of surveillance and preemptive therapy. Transplant Infectious Disease. 2010;12(4):322–329. doi: 10.1111/j.1399-3062.2010.00504.x. [DOI] [PubMed] [Google Scholar]
- 5.Reusser P, Einsele H, Lee J, et al. Randomized multicenter trial of foscarnet versus ganciclovir for preemptive therapy of cytomegalovirus infection after allogeneic stem cell transplantation. Blood. 2002;99(4):1159–1164. doi: 10.1182/blood.v99.4.1159. [DOI] [PubMed] [Google Scholar]
- 6.Boeckh M, Leisenring W, Riddell SR, et al. Late cytomegalovirus disease and mortality in recipients of allogeneic hematopoietic stem cell transplants: importance of viral load and T-cell immunity. Blood. 2003;101(2):407–414. doi: 10.1182/blood-2002-03-0993. [DOI] [PubMed] [Google Scholar]
- 7.Moins-Teisserenc H, Busson M, Scieux C, et al. Patterns of cytomegalovirus reactivation are associated with distinct evolutive profiles of immune reconstitution after allogeneic hematopoietic stem cell transplantation. The Journal of Infectious Diseases. 2008;198(6):818–826. doi: 10.1086/591185. [DOI] [PubMed] [Google Scholar]
- 8.Gratama JW, Boeckh M, Nakamura R, et al. Immune monitoring with iTAg MHCTetramers for prediction of recurrent or persistent cytomegalovirus infection or disease in allogeneic hematopoietic stem cell transplant recipients: a prospective multicenter study. Blood. 2010;116(10):1655–1662. doi: 10.1182/blood-2010-03-273508. [DOI] [PubMed] [Google Scholar]
- 9.Borchers S, Luther S, Lips U, et al. Tetramer monitoring to assess risk factors for recurrent cytomegalovirus reactivation and reconstitution of antiviral immunity post allogeneic hematopoietic stem cell transplantation. Transplant Infectious Disease. 2011;13(3):222–236. doi: 10.1111/j.1399-3062.2011.00626.x. [DOI] [PubMed] [Google Scholar]
- 10.Aversa F. Haploidentical haematopoietic stem cell transplantation for acute leukaemia in adults: experience in Europe and the United States. Bone Marrow Transplantation. 2008;41(5):473–481. doi: 10.1038/sj.bmt.1705966. [DOI] [PubMed] [Google Scholar]
- 11.Volpi I, Perruccio K, Tosti A, et al. Postgrafting administration of granulocyte colony-stimulating factor impairs functional immune recovery in recipients of human leukocyte antigen haplotype—mismatched hematopoietic transplants. Blood. 2001;97(8):2514–2521. doi: 10.1182/blood.v97.8.2514. [DOI] [PubMed] [Google Scholar]
- 12.Lilleri D, Gerna G, Fornara C, et al. Human cytomegalovirus-specific T cell reconstitution in young patients receiving T cell-depleted, allogeneic hematopoietic stem cell transplantation. Journal of Infectious Diseases. 2009;199(6):829–836. doi: 10.1086/597123. [DOI] [PubMed] [Google Scholar]
- 13.Chen X, Hale GA, Barfield R, et al. Rapid immune reconstitution after a reduced-intensity conditioning regimen and a CD3-depleted haploidentical stem cell graft for paediatric refractory haematological malignancies. British Journal of Haematology. 2006;135(4):524–532. doi: 10.1111/j.1365-2141.2006.06330.x. [DOI] [PubMed] [Google Scholar]
- 14.Federmann B, Hägele M, Pfeiffer M, et al. Immune reconstitution after haploidentical hematopoietic cell transplantation: impact of reduced intensity conditioning and CD3/CD19 depleted grafts. Leukemia. 2011;25(1):121–129. doi: 10.1038/leu.2010.235. [DOI] [PubMed] [Google Scholar]
- 15.Bader P, Soerensen J, Jarisch A, et al. Rapid immune recovery and low TRM in haploidentical stem cell transplantation in children and adolescence using CD3/CD19-depleted stem cells. Best Practice and Research: Clinical Haematology. 2011;24(3):331–337. doi: 10.1016/j.beha.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 16.Pérez-Martínez A, González-Vicent M, Valentín J, et al. Early evaluation of immune reconstitution following allogeneic CD3/CD19-depleted grafts from alternative donors in childhood acute leukemia. Bone Marrow Transplantation. 2012;47(11):1419–1427. doi: 10.1038/bmt.2012.43. [DOI] [PubMed] [Google Scholar]
- 17.Koh K-N, Im HJ, Kim BE, et al. Haploidentical haematopoietic stem cell transplantation using CD3 or CD3/CD19 depletion and conditioning with fludarabine, cyclophosphamide and antithymocyte globulin for acquired severe aplastic anaemia. British Journal of Haematology. 2012;157(1):139–142. doi: 10.1111/j.1365-2141.2011.08924.x. [DOI] [PubMed] [Google Scholar]
- 18.Huang X-J, Liu D-H, Liu K-Y, et al. Haploidentical hematopoietic stem cell transplantation without in vitro T-cell depletion for the treatment of hematological malignancies. Bone Marrow Transplantation. 2006;38(4):291–297. doi: 10.1038/sj.bmt.1705445. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, Liu D-H, Xu L-P, et al. Superior graft-versus-leukemia effect associated with transplantation of haploidentical compared with HLA-identical sibling donor grafts for high-risk acute leukemia: an historic comparison. Biology of Blood and Marrow Transplantation. 2011;17(6):821–830. doi: 10.1016/j.bbmt.2010.08.023. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y, Liu D-H, Xu L-P, et al. Haploidentical/mismatched hematopoietic stem cell transplantation without in vitro T cell depletion for T cell acute lymphoblastic leukemia. Biology of Blood and Marrow Transplantation. 2012;18(5):716–721. doi: 10.1016/j.bbmt.2011.08.024. [DOI] [PubMed] [Google Scholar]
- 21.Chang Y-J, Zhao X-Y, Huo M-R, et al. Immune reconstitution following unmanipulated HLA-mismatched/haploidentical transplantation compared with HLA-identical sibling transplantation. Journal of Clinical Immunology. 2012;32(2):268–280. doi: 10.1007/s10875-011-9630-7. [DOI] [PubMed] [Google Scholar]
- 22.Luo X-H, Huang X-J, Li D, Liu K-Y, Xu L-P, Liu D-H. Immune reconstitution to cytomegalovirus following partially matched-related donor transplantation: impact of in vivo T-cell depletion and granulocyte colony-stimulating factor-primed peripheral blood/bone marrow mixed grafts. Transplant Infectious Disease. 2013;15(1):22–33. doi: 10.1111/j.1399-3062.2012.00722.x. [DOI] [PubMed] [Google Scholar]
- 23.Lu D-P, Dong L, Wu T, et al. Conditioning including antithymocyte globulin followed by unmanipulated HLA-mismatched/haploidentical blood and marrow transplantation can achieve comparable outcomes with HLA-identical sibling transplantation. Blood. 2006;107(8):3065–3073. doi: 10.1182/blood-2005-05-2146. [DOI] [PubMed] [Google Scholar]
- 24.Kurokawa T, Ishiyama K, Ozaki J, et al. Haploidentical hematopoietic stem cell transplantation to adults with hematologic malignancies: analysis of 66 cases at a single Japanese center. International Journal of Hematology. 2010;91(4):661–669. doi: 10.1007/s12185-010-0561-5. [DOI] [PubMed] [Google Scholar]
- 25.Lee K-H, Lee J-H, Lee J-H, et al. Reduced-intensity conditioning therapy with busulfan, fludarabine, and antithymocyte globulin for HLA-haploidentical hematopoietic cell transplantation in acute leukemia and myelodysplastic syndrome. Blood. 2011;118(9):2609–2617. doi: 10.1182/blood-2011-02-339838. [DOI] [PubMed] [Google Scholar]
- 26.Kanda Y, Oshima K, Asano-Mori Y, et al. In vivo alemtuzumab enables haploidentical human leukocyte antigen-mismatched hematopoietic stem-cell transplantation without ex vivo graft manipulation. Transplantation. 2005;79(10):1351–1357. doi: 10.1097/01.tp.0000158718.49286.14. [DOI] [PubMed] [Google Scholar]
- 27.Rizzieri DA, Liang PK, Long GD, et al. Partially matched, nonmyeloablative allogeneic transplantation: clinical outcomes and immune reconstitution. Journal of Clinical Oncology. 2007;25(6):690–697. doi: 10.1200/JCO.2006.07.0953. [DOI] [PubMed] [Google Scholar]
- 28.Kanda Y, Oshima K, Kako S, et al. In vivo T-cell depletion with alemtuzumab in allogeneic hematopoietic stem cell transplantation: combined results of two studies on aplastic anemia and HLA-mismatched haploidentical transplantation. American Journal of Hematology. 2013;88(4):294–300. doi: 10.1002/ajh.23392. [DOI] [PubMed] [Google Scholar]
- 29.Chang Y-J, Zhao X-Y, Huang X-J. Immune reconstitution after haploidentical hematopoietic stem cell transplantation. Biology of Blood and Marrow Transplantation. 2014;20(4):440–449. doi: 10.1016/j.bbmt.2013.11.028. [DOI] [PubMed] [Google Scholar]
- 30.Kern F, Bunde T, Faulhaber N, et al. Cytomegalovirus (CMV) phosphoprotein 65 makes a large contribution to shaping the T cell repertoire in CMV-exposed individuals. Journal of Infectious Diseases. 2002;185(12):1709–1716. doi: 10.1086/340637. [DOI] [PubMed] [Google Scholar]
- 31.Waldrop SL, Pitcher CJ, Peterson DM, Maino VC, Picker LJ. Determination of antigen-specific memory/effector CD4+ T cell frequencies by flow cytometry. Evidence for a novel, antigen-specific homeostatic mechanism in HIV-associated immunodeficiency. Journal of Clinical Investigation. 1997;99(7):1739–1750. doi: 10.1172/JCI119338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Avetisyan G, Aschan J, Hägglund H, Ringdén O, Ljungman P. Evaluation of intervention strategy based on CMV-specific immune responses after allogeneic SCT. Bone Marrow Transplantation. 2007;40(9):865–869. doi: 10.1038/sj.bmt.1705825. [DOI] [PubMed] [Google Scholar]
- 33.Solano C, Benet I, José Remigia M, et al. Immunological monitoring for guidance of preemptive antiviral therapy for active cytomegalovirus infection in allogeneic stem-cell transplant recipients: a pilot experience. Transplantation. 2011;92(4):e17–e20. doi: 10.1097/TP.0b013e318224f263. [DOI] [PubMed] [Google Scholar]
- 34.Blyth E, Clancy L, Simms R, et al. Donor-derived CMV-specific T cells reduce the requirement for CMV-directed pharmacotherapy after allogeneic stem cell transplantation. Blood. 2013;121(18):3745–3758. doi: 10.1182/blood-2012-08-448977. [DOI] [PubMed] [Google Scholar]
- 35.Gratama JW, van Esser JWJ, Lamers CHJ, et al. Tetramer-based quantification of cytomegalovirus (CMV)-specific CD8+ T lymphocytes in T-cell-depleted stem cell grafts and after transplantation may identify patients at risk for progressive CMV infection. Blood. 2001;98(5):1358–1364. doi: 10.1182/blood.v98.5.1358. [DOI] [PubMed] [Google Scholar]
- 36.Cwynarski K, Ainsworth J, Cobbold M, et al. Direct visualization of cytomegalovirus-specific T-cell reconstitution after allogeneic stem cell transplantation. Blood. 2001;97(5):1232–1240. doi: 10.1182/blood.v97.5.1232. [DOI] [PubMed] [Google Scholar]
- 37.Moins-Teisserenc H, Busson M, Scieux C, et al. Patterns of cytomegalovirus reactivation are associated with distinct evolutive profiles of immune reconstitution after allogeneic hematopoietic stem cell transplantation. Journal of Infectious Diseases. 2008;198(6):818–826. doi: 10.1086/591185. [DOI] [PubMed] [Google Scholar]
- 38.Pourgheysari B, Piper KP, McLarnon A, et al. Early reconstitution of effector memory CD4+ CMV-specific T cells protects against CMV reactivation following allogeneic SCT. Bone Marrow Transplantation. 2009;43(11):853–861. doi: 10.1038/bmt.2008.403. [DOI] [PubMed] [Google Scholar]
- 39.Hakki M, Riddell SR, Storek J, et al. Immune reconstitution to cytomegalovirus after allogeneic hematopoietic stem cell transplantation: impact of host factors, drug therapy, and subclinical reactivation. Blood. 2003;102(8):3060–3067. doi: 10.1182/blood-2002-11-3472. [DOI] [PubMed] [Google Scholar]
- 40.Hoegh-Petersen M, Roa L, Liu Y, et al. Low cytomegalovirus-specific T-cell counts at reactivation are associated with progression to high-level viremia or disease in seropositive recipients of hematopoietic cell grafts from seropositive but not seronegative donors. Cytotherapy. 2012;14(2):194–204. doi: 10.3109/14653249.2011.634402. [DOI] [PubMed] [Google Scholar]
- 41.Lilleri D, Gerna G, Fornara C, Lozza L, Maccario R, Locatelli F. Prospective simultaneous quantification of human cytomegalovirus-specific CD4+ and CD8+ T-cell reconstitution in young recipients of allogeneic hematopoietic stem cell transplants. Blood. 2006;108(4):1406–1412. doi: 10.1182/blood-2005-11-012864. [DOI] [PubMed] [Google Scholar]
- 42.Borchers S, Luther S, Lips U, et al. Tetramer monitoring to assess risk factors for recurrent cytomegalovirus reactivation and reconstitution of antiviral immunity post allogeneic hematopoietic stem cell transplantation. Transplant Infectious Disease. 2011;13(3):222–236. doi: 10.1111/j.1399-3062.2011.00626.x. [DOI] [PubMed] [Google Scholar]
- 43.Luo X-H, Huang X-J, Liu K-Y, Xu L-P, Liu D-H. Protective immunity transferred by infusion of cytomegalovirus-specific CD8+ T cells within donor grafts: its associations with cytomegalovirus reactivation following unmanipulated allogeneic hematopoietic stem cell transplantation. Biology of Blood and Marrow Transplantation. 2010;16(7):994–1004. doi: 10.1016/j.bbmt.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 44.Di Rosa F, Pabst R. The bone marrow: a nest for migratory memory T cells. Trends in Immunology. 2005;26(7):360–366. doi: 10.1016/j.it.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 45.Na I-K, Letsch A, Guerreiro M, et al. Human bone marrow as a source to generate CMV-specific CD4+ t cells with multifunctional capacity. Journal of Immunotherapy. 2009;32(9):907–913. doi: 10.1097/CJI.0b013e3181b7be60. [DOI] [PubMed] [Google Scholar]
- 46.Auletta JJ, Lazarus HM. Immune restoration following hematopoietic stem cell transplantation: an evolving target. Bone Marrow Transplantation. 2005;35(9):835–857. doi: 10.1038/sj.bmt.1704966. [DOI] [PubMed] [Google Scholar]
- 47.Schmidt-Hieber M, Schwarck S, Stroux A, et al. Immune reconstitution and cytomegalovirus infection after allogeneic stem cell transplantation: the important impact of in vivo T cell depletion. International Journal of Hematology. 2010;91(5):877–885. doi: 10.1007/s12185-010-0597-6. [DOI] [PubMed] [Google Scholar]
- 48.Ljungman P, Perez-Bercoff L, Jonsson J, et al. Risk factors for the development of cytomegalovirus disease after allogeneic stem cell transplantation. Haematologica. 2006;91(1):78–83. [PubMed] [Google Scholar]
- 49.Zhou W, Longmate J, Lacey SF, et al. Impact of donor CMV status on viral infection and reconstitution of multifunction CMV-specific T cells in CMV-positive transplant recipients. Blood. 2009;113(25):6465–6476. doi: 10.1182/blood-2009-02-203307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ugarte-Torres A, Hoegh-Petersen M, Liu Y, et al. Donor serostatus has an impact on cytomegalovirus-specific immunity, cytomegaloviral disease incidence, and survival in seropositive hematopoietic cell transplant recipients. Biology of Blood and Marrow Transplantation. 2011;17(4):574–585. doi: 10.1016/j.bbmt.2010.07.020. [DOI] [PubMed] [Google Scholar]
- 51.Pietersma FL, van Dorp S, Minnema MC, et al. Influence of donor cytomegalovirus (CMV) status on severity of viral reactivation after allogeneic stem cell transplantation in CMV-seropositive recipients. Clinical Infectious Diseases. 2011;52(7):e144–e148. doi: 10.1093/cid/cir002. [DOI] [PubMed] [Google Scholar]
- 52.Storek J, Dawson MA, Storer B, et al. Immune reconstitution after allogeneic marrow transplantation compared with blood stem cell transplantation. Blood. 2001;97(11):3380–3389. doi: 10.1182/blood.v97.11.3380. [DOI] [PubMed] [Google Scholar]
- 53.Talmadge JE, Reed E, Ino K, et al. Rapid immunologic reconstitution following transplantation with mobilized peripheral blood stem cells as compared to bone marrow. Bone Marrow Transplantation. 1997;19(2):161–172. doi: 10.1038/sj.bmt.1700626. [DOI] [PubMed] [Google Scholar]
- 54.Ottinger HD, Beelen DW, Scheulen B, Schaefer UW, Grosse-Wilde H. Improved immune reconstitution after allotransplantation of peripheral blood stem cells instead of bone marrow. Blood. 1996;88(7):2775–2779. [PubMed] [Google Scholar]
- 55.Aversa F. Haploidentical haematopoietic stem cell transplantation for acute leukaemia in adults: experience in Europe and the United States. Bone Marrow Transplantation. 2008;41(5):473–481. doi: 10.1038/sj.bmt.1705966. [DOI] [PubMed] [Google Scholar]
- 56.Huang W, Li H, Gao C, et al. Unmanipulated HLA-mismatched/haploidentical peripheral blood stem cell transplantation for high-risk hematologic malignancies. Transfusion. 2012;52(6):1354–1362. doi: 10.1111/j.1537-2995.2011.03478.x. [DOI] [PubMed] [Google Scholar]
- 57.Di Bartolomeo P, Santarone S, De Angelis G, et al. Haploidentical, unmanipulated, G-CSF-primed bone marrow transplantation for patients with high-risk hematologic malignancies. Blood. 2013;121(5):849–857. doi: 10.1182/blood-2012-08-453399. [DOI] [PubMed] [Google Scholar]
- 58.Ciurea SO, Mulanovich V, Saliba RM, et al. Improved early outcomes using a T-cell replete graft compared with T-cell depleted haploidentical hematopoietic stem-cell transplantation. Biology of Blood and Marrow Transplantation. 2012;18(12):1835–1844. doi: 10.1016/j.bbmt.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mohty M. Mechanisms of action of antithymocyte globulin: T-cell depletion and beyond. Leukemia. 2007;21(7):1387–1394. doi: 10.1038/sj.leu.2404683. [DOI] [PubMed] [Google Scholar]
- 60.Maris M, Boeckh M, Storer B, et al. Immunologic recovery after hematopoietic cell transplantation with nonmyeloablative conditioning. Experimental Hematology. 2003;31(10):941–952. doi: 10.1016/s0301-472x(03)00201-7. [DOI] [PubMed] [Google Scholar]
- 61.Nakamae H, Kirby KA, Sandmaier BM, et al. Effect of conditioning regimen intensity on CMV infection in allogeneic hematopoietic cell transplantation. Biology of Blood and Marrow Transplantation. 2009;15(6):694–703. doi: 10.1016/j.bbmt.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim SH, Kee SY, Lee DG, et al. Infectious complications following allogeneic stem cell transplantation: reduced-intensity vs. myeloablative conditioning regimens. Transplant Infectious Disease. 2013;15(1):49–59. doi: 10.1111/tid.12003. [DOI] [PubMed] [Google Scholar]
- 63.George B, Kerridge I, Gilroy N, et al. Fludarabine-based reduced intensity conditioning transplants have a higher incidence of cytomegalovirus reactivation compared with myeloablative transplants. Bone Marrow Transplantation. 2010;45(5):849–855. doi: 10.1038/bmt.2009.273. [DOI] [PubMed] [Google Scholar]
- 64.Lin M-T, Tseng L-H, Frangoul H, et al. Increased apoptosis of peripheral blood T cells following allogeneic hematopoietic cell transplantation. Blood. 2000;95(12):3832–3839. [PubMed] [Google Scholar]
- 65.Clave E, Busson M, Douay C, et al. Acute graft-versus-host disease transiently impairs thymic output in young patients after allogeneic hematopoietic stem cell transplantation. Blood. 2009;113(25):6477–6484. doi: 10.1182/blood-2008-09-176594. [DOI] [PubMed] [Google Scholar]
- 66.Sayer HG, Longton G, Bowden R, Pepe M, Storb R. Increased risk of infection in marrow transplant patients receiving methylprednisolone for graft-versus-host disease prevention. Blood. 1994;84(4):1328–1332. [PubMed] [Google Scholar]
- 67.Liberman AC, Druker J, Refojo D, Holsboer F, Arzt E. Glucocorticoids inhibit GATA-3 phosphorylation and activity in T cells. The FASEB Journal. 2009;23(5):1558–1571. doi: 10.1096/fj.08-121236. [DOI] [PubMed] [Google Scholar]
- 68.Franchimont D. Overview of the actions of glucocorticoids on the immune response: a good model to characterize new pathways of immunosuppression for new treatment strategies. Annals of the New York Academy of Sciences. 2004;1024:124–137. doi: 10.1196/annals.1321.009. [DOI] [PubMed] [Google Scholar]
- 69.Özdemir E, St John LS, Gillespie G, et al. Cytomegalovirus reactivation following allogeneic stem cell transplantation is associated with the presence of dysfunctional antigen-specific CD8+ T cells. Blood. 2002;100(10):3690–3697. doi: 10.1182/blood-2002-05-1387. [DOI] [PubMed] [Google Scholar]
- 70.Van Lier RAW, Ten Berge IJM, Gamadia LE. Human CD8+ T-cell differentiation in response to viruses. Nature Reviews Immunology. 2003;3(12):931–938. doi: 10.1038/nri1254. [DOI] [PubMed] [Google Scholar]
- 71.Sester U, Presser D, Dirks J, Gärtner BC, Köhler H, Sester M. PD-1 expression and IL-2 loss of cytomegalovirus- specific t cells correlates with viremia and reversible functional anergy. American Journal of Transplantation. 2008;8(7):1486–1497. doi: 10.1111/j.1600-6143.2008.02279.x. [DOI] [PubMed] [Google Scholar]
- 72.Mackall CL, Fleisher TA, Brown MR, et al. Age, thymopoiesis, and CD4+ T-lymphocyte regeneration after intensive chemotherapy. The New England Journal of Medicine. 1995;332(3):143–149. doi: 10.1056/NEJM199501193320303. [DOI] [PubMed] [Google Scholar]
- 73.Roux E, Dumont-Girard F, Starobinski M, et al. Recovery of immune reactivity after T-cell-depleted bone marrow transplantation depends on thymic activity. Blood. 2000;96(6):2299–2303. [PubMed] [Google Scholar]
- 74.Azevedo RI, Soares MV, Albuquerque AS, et al. Long-term immune reconstitution of naive and memory T cell pools after haploidentical hematopoietic stem cell transplantation. Biology of Blood and Marrow Transplantation. 2013;19(5):703–712. doi: 10.1016/j.bbmt.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 75.Fu YW, Wu DP, Cen JN, et al. Patterns of T-cell reconstitution by assessment of T-cell receptor excision circle and T-cell receptor clonal repertoire after allogeneic hematopoietic stem cell transplantation in leukemia patients—a study in Chinese patients. European Journal of Haematology. 2007;79(2):138–145. doi: 10.1111/j.1600-0609.2007.00885.x. [DOI] [PubMed] [Google Scholar]
- 76.Perruccio K, Tosti A, Burchielli E, et al. Transferring functional immune responses to pathogens after haploidentical hematopoietic transplantation. Blood. 2005;106(13):4397–4406. doi: 10.1182/blood-2005-05-1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Waller EK, Giver CR, Rosenthal H, et al. Facilitating T-cell immune reconstitution after haploidentical transplantation in adults. Blood Cells, Molecules, and Diseases. 2004;33(3):233–237. doi: 10.1016/j.bcmd.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 78.Amrolia PJ, Muccioli-Casadei G, Huls H, et al. Adoptive immunotherapy with allodepleted donor T-cells improves immune reconstitution after haploidentical stem cell transplantation. Blood. 2006;108(6):1797–1808. doi: 10.1182/blood-2006-02-001909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dodero A, Carniti C, Raganato A, et al. Haploidentical stem cell transplantation after a reduced-intensity conditioning regimen for the treatment of advanced hematologic malignancies: posttransplantation CD8-depleted donor lymphocyte infusions contribute to improve T-cell recovery. Blood. 2009;113(19):4771–4779. doi: 10.1182/blood-2008-10-183723. [DOI] [PubMed] [Google Scholar]
- 80.Traversari C, Marktel S, Magnani Z, et al. The potential immunogenicity of the TK suicide gene does not prevent full clinical benefit associated with the use of TK-transduced donor lymphocytes in HSCT for hematologic malignancies. Blood. 2007;109(11):4708–4715. doi: 10.1182/blood-2006-04-015230. [DOI] [PubMed] [Google Scholar]
- 81.Tey S-K, Dotti G, Rooney CM, Heslop HE, Brenner MK. Inducible caspase 9 suicide gene to improve the safety of allodepleted T cells after haploidentical stem cell transplantation. Biology of Blood and Marrow Transplantation. 2007;13(8):913–924. doi: 10.1016/j.bbmt.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Perruccio K, Topini F, Tosti A, et al. Photodynamic purging of alloreactive T cells for adoptive immunotherapy after haploidentical stem cell transplantation. Blood Cells, Molecules, and Diseases. 2008;40(1):76–83. doi: 10.1016/j.bcmd.2007.06.022. [DOI] [PubMed] [Google Scholar]
- 83.Perruccio K, Topini F, Tosti A, et al. Optimizing a photoallodepletion protocol for adoptive immunotherapy after haploidentical SCT. Bone Marrow Transplantation. 2012;47(9):1196–200. doi: 10.1038/bmt.2011.237. [DOI] [PubMed] [Google Scholar]
- 84.Feuchtinger T, Opherk K, Bethge WA, et al. Adoptive transfer of pp65-specific T cells for the treatment of chemorefractory cytomegalovirus disease or reactivation after haploidentical and matched unrelated stem cell transplantation. Blood. 2010;116(20):4360–4367. doi: 10.1182/blood-2010-01-262089. [DOI] [PubMed] [Google Scholar]
- 85.Smith FO, Thomson B. T-cell recovery following marrow transplant: experience with delayed lymphocyte infusions to accelerate immune recovery or treat infectious problems. Pediatric Transplantation. 1999;3(1):59–64. doi: 10.1034/j.1399-3046.1999.00072.x. [DOI] [PubMed] [Google Scholar]
- 86.Loren AW, Porter DL. Donor leukocyte infusions after unrelated donor hematopoietic stem cell transplantation. Current Opinion in Oncology. 2006;18(2):107–114. doi: 10.1097/01.cco.0000208781.61452.d3. [DOI] [PubMed] [Google Scholar]
- 87.Rutella S, Zavala F, Danese S, Kared H, Leone G. Granulocyte colony-stimulating factor: a novel mediator of T cell tolerance. The Journal of Immunology. 2005;175(11):7085–7091. doi: 10.4049/jimmunol.175.11.7085. [DOI] [PubMed] [Google Scholar]
- 88.Anderlini P, Champlin RE. Biologic and molecular effects of granulocyte colony-stimulating factor in healthy individuals: recent findings and current challenges. Blood. 2008;111(4):1767–1772. doi: 10.1182/blood-2007-07-097543. [DOI] [PubMed] [Google Scholar]
- 89.Huang X, Guo N, Ren H, Zhang Y, Gao Z, Lu D. An improved anti-leukemic effect achieved with donor progenitor cell infusion for relapse patients after allogeneic bone marrow transplantation. Chinese Medical Journal. 2003;116(5):736–741. [PubMed] [Google Scholar]
- 90.Xu L-P, Liu D-H, Liu K-Y, et al. The efficacy and safety of donor lymphocyte infusion to treat Epstein-Barr virus associated lymphoproliferative diseases after allogeneic hematopoietic stem cell transplantation. Chinese Journal of Internal Medicine. 2010;49(11):955–958. [PubMed] [Google Scholar]
- 91.Slatter MA, Brigham K, Dickinson AM, et al. Long-term immune reconstitution after anti-CD52-treated or anti-CD34-treated hematopoietic stem cell transplantation for severe T-lymphocyte immunodeficiency. Journal of Allergy and Clinical Immunology. 2008;121(2):361–367. doi: 10.1016/j.jaci.2007.10.035. [DOI] [PubMed] [Google Scholar]


