Abstract
Root knot nematodes (RKN) can infect most of the world’s agricultural crop species and are among the most important of all plant pathogens. As yet however we have little understanding of their origins or the genomic basis of their extreme polyphagy. The most damaging pathogens reproduce by obligatory mitotic parthenogenesis and it has been suggested that these species originated from interspecific hybridizations between unknown parental taxa. We have sequenced the genome of the diploid meiotic parthenogen Meloidogyne floridensis, and use a comparative genomic approach to test the hypothesis that this species was involved in the hybrid origin of the tropical mitotic parthenogen Meloidogyne incognita. Phylogenomic analysis of gene families from M. floridensis, M. incognita and an outgroup species Meloidogyne hapla was carried out to trace the evolutionary history of these species’ genomes, and we demonstrate that M. floridensis was one of the parental species in the hybrid origins of M. incognita. Analysis of the M. floridensis genome itself revealed many gene loci present in divergent copies, as they are in M. incognita, indicating that it too had a hybrid origin. The triploid M. incognita is shown to be a complex double-hybrid between M. floridensis and a third, unidentified, parent. The agriculturally important RKN have very complex origins involving the mixing of several parental genomes by hybridization and their extreme polyphagy and success in agricultural environments may be related to this hybridization, producing transgressive variation on which natural selection can act. It is now clear that studying RKN variation via individual marker loci may fail due to the species’ convoluted origins, and multi-species population genomics is essential to understand the hybrid diversity and adaptive variation of this important species complex. This comparative genomic analysis provides a compelling example of the importance and complexity of hybridization in generating animal species diversity more generally.
Keywords: Genome sequencing, Phylogenomics, Meloidogyne incognita, Meloidogyne hapla, Meloidogyne floridensis, Comparative genomics, Hybrid speciation
Introduction
Root-knot nematodes (RKN) belong to the genus Meloidogyne, contain approximately 100 described species, and are globally important crop pathogens (Moens, Perry & Starr, 2009). The most frequent, widespread, and damaging species (M. incognita, M. arenaria, and M. javanica) are tropical RKN that are highly polyphagous, infecting crop species producing the majority of the world’s food supply, with the damage attributable to RKN ∼5% of world agriculture (Taylor & Sasser, 1978; Trudgill & Blok, 2001; Sasser & Carter, 1985). The adaptive phenotypic diversity of these pathogens is also remarkable, with great variability observed both within and between species with respect to host range and isolate-specific vulnerability to control measures (Trudgill & Blok, 2001; Castagnone-Sereno, 2006). The tropical RKN typically reproduce by obligatory mitotic parthenogenesis and possess aneuploid genomes (Triantaphyllou, 1982; Triantaphyllou, 1985). These species have previously been suggested to be hybrid taxa, and phylogenetic analysis of nuclear loci supports this conclusion (Dalmasso & Berge, 1983; Triantaphyllou, 1985; Hugall, Stanton & Moritz, 1999; Castagnone-Sereno, 2006; Lunt, 2008).
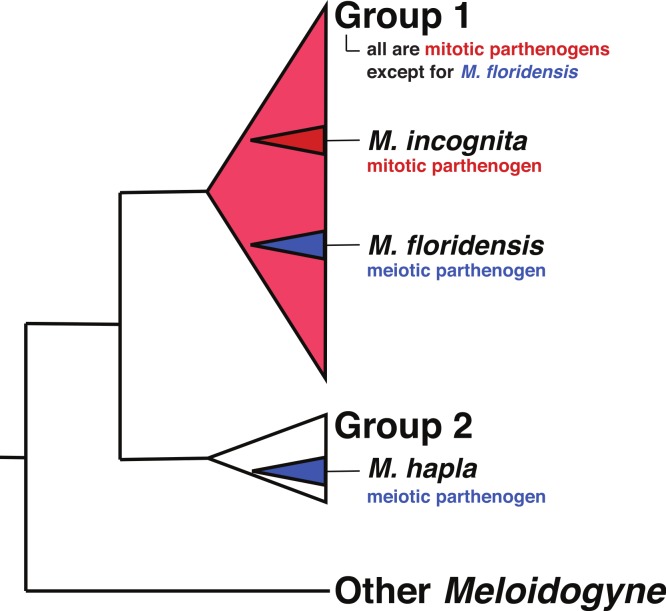
Hybrid speciation has a long history of study in plants, with hybrid species formation having had a very significant influence on our understanding of species formation, diversity, and adaptation (Arnold, 1997; Soltis & Soltis, 2009). By contrast hybridization has been thought to be much less common in animals, though the utilization of multilocus genetics, and more recently genomics, has increased interest in the consequences of animal hybridization and several reviews suggest that it is much more common and important than previously thought (Mallet, 2007; Mallet, 2005; Bullini, 1994; Nolte & Tautz, 2010; Schwenk, Brede & Streit, 2008; Seehausen, 2006). Although there have been repeated suggestions that the tropical (“Group 1”) RKN might have hybrid origins, the parental species involved have never been identified. The phylogenies in Hugall, Stanton & Moritz (1999) and Lunt (2008) indicate that these parents (as represented by divergent sequence clusters within the apomictic RKN) are more closely related to each other than either is to M. hapla, though neither had a parental species within their sampling schemes. Meloidogyne floridensis is a plant pathogenic root knot nematode that was originally characterized as M. incognita, but has since been described as a separate species on the basis of its morphology and a unique esterase isozyme pattern (Jeyaprakash et al., 2006; Handoo et al., 2004). Despite both nuclear rRNA and mtDNA sequences placing it within the phylogenetic diversity of the tropical mitotic parthenogen (apomict) species (Tigano et al., 2005; Holterman et al., 2009) (Fig. 1), M. floridensis is a meiotic parthenogen (automict) with the standard chromosome count of the meiotically reproducing RKN species (n = 18), has bivalent chromosomes, and an observable meiotic division (Handoo et al., 2004). M. floridensis appears to suppress the second meiotic division which is a known form of automictic reproduction called first-division restitution and a pathway by which parental heterozygosity can be maintained (Bell, 1982, p. 40). With the exception of M. floridensis, all of the Group 1 RKN (De Ley et al., 2002; Holterman et al., 2009) are apomicts, unable to reproduce by meiosis, lacking bivalents, and exhibiting extensive aneuploidy. This phylogenetic distribution of reproductive modes, with M. floridensis phylogenetically nested within the diversity of the apomict RKN (Fig. 1), is unanticipated as it implies the physiologically unlikely route of re-emergence of meiosis from within the obligate mitotic parthenogens. An alternative explanation for these observations is that the observed phylogenetic relationships have not arisen from a typical ancestor-descendent bifurcating process, but instead have been shaped by reticulate evolution and transfer of genes by interspecific hybridization with M. floridensis a parent of the tropical apomict species.
Figure 1. The relationships of tropical apomict Meloidogyne.
This image summarizes the relationships of the tropical apomict Meloidogyne root knot nematodes (“Group 1”) to other Meloidogyne. Meloidogyne floridensis is a Group 1 species that can reproduce by meiotic parthenogenesis (blue colouration) while all other Group 1 species are obligate mitotic parthenogens (red colouration). Meloidogyne hapla is a meiotic parthenogenic species in Group 2. We have not used bifurcating trees to represent the relationships within the Group 1 and 2 species because of issues (highlighted in this paper) concerning possible hybrid origins of some taxa.
The origins of Meloidogyne incognita genomic duplicates
The M. incognita genome revealed that many of the genes of this species are present as highly divergent copies (Abad et al., 2008), a situation that seems to apply to the other tropical apomicts too (Lunt, 2008), though the origin of these divergent copies is controversial. One possible way to account for the high divergence between alleles is that they have originated by a process of ‘endoduplication’ (Fig. 2A). Here we use endoduplication to refer to two distinct processes, although their genomic outcomes are similar. Firstly, the entire M. incognita genome might have doubled to become tetraploid. The homologous chromosomes may have then diverged, and the extant pattern of partial retention of duplicated loci could be the result of gene loss. This process would leave many areas of the genome possessing divergent copies. Second, an alternative mechanism possible in apomictic species such as M. incognita, is that former alleles that are released from the homogenizing effects of recombination, can independently accumulate mutations over long periods of time resulting in highly divergent homologous loci (‘alleles’) within a diploid genome (White, 1945, pg. 283, Judson & Normark, 1996).
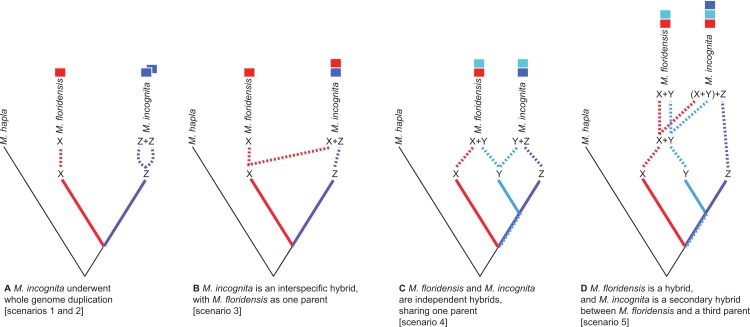
Figure 2. Scenarios of the possible relationships between Meloidogyne floridensis, Meloidogyne incognita and Meloidogyne hapla, and the origins of duplicated gene copies.
M. hapla is a diploid species in a different sub-generic group to that of M. incognita and M. floridensis. Species “X”, “Y” and “Z” are postulated ancestral parents that could have given rise to M. incognita and M. floridensis. (A) Scenarios 1 and 2: Here M. floridensis is a diploid sister species to M. incognita and possesses the “X” genome. Scenario 1 postulates reacquisition of apomixis in M. floridensis from an apomict ancestor, while Scenario 2 postulates that the apomicts repeatedly lost meiosis independently. Under both these scenarios, the presence of significant duplications in M. incognita suggests that it has undergone whole genome endoduplication. The duplicated genomes (“Z + Z”) in M. incognita are diverging under Muller’s ratchet. (B) Scenario 3: Ancestor “X” gave rise to the diploid species M. floridensis, and also interbred with “Z” to yield M. incognita, which thus carries two divergent copies of each gene (“X + Z”). In this model only M. incognita, not M. floridensis, is predicted to carry two homeologues of many genes. (C) Scenario 4: Both M. floridensis (“X + Y”) and M. incognita (“Y + Z”) are hybrid species, and share one parent (“Y”). In this model both M. incognita and M. floridensis are predicted to carry two homeologues of many genes. (D) Scenario 5: Both M. floridensis (“X + Y”) and M. incognita (“X + Y + Z”) are hybrid species, but M. incognita is a triploid hybrid between the hybrid M. floridensis ancestor (“X + Y”) and another species (“Z”). In this model M. incognita is predicted to carry three, and M. floridensis is predicted to carry two, homeologues of many genes.
Another possible explanation for a genome containing divergent homologous copies of many genes is interspecific hybridization. One (homeologous) copy is inherited from each parental species and the divergence between them derives from the divergence between the hybridizing taxa. It is likely here that all genes would be present as divergent copies, although gene conversion and related processes could homogenize some copies. If it originated by this second mechanism the resulting M. incognita genome would be a mosaic with genomic regions derived from both its parents.
There are several ways in which M. incognita and M. floridensis might be related through hybridization. M. floridensis might be one of the two parental species which hybridized to form the tropical apomicts, including M. incognita (Fig. 2B). Alternatively, M. floridensis might be an independent hybrid that shares one parental taxon with M. incognita, and thus represents a ‘sibling’ hybrid taxon (Fig. 2C). Finally, M. floridensis may itself be a hybrid, but still have played a role as a parent of M. incognita by a subsequent hybridization event (Fig. 2D). This last option predicts three gene copies in M. incognita and two in M. floridensis.
The nuclear gene phylogenies of Lunt (2008) indicate that the parental taxa of the apomict RKN were closely related and derived from within the cluster of Group 1 Meloidogyne species after the divergence of M. enterolobii (=M. mayaguensis). Since this closely matches the phylogenetic position of M. floridensis, which is known to reproduce via sexual recombination as the parental species also must have done, we set out to test by comparative genome sequencing and analysis if M. floridensis was one of the progenitors of the tropical apomicts.
Reproductive mode and Meloidogyne evolutionary history
Given the unexpected distribution of meiosis across Group 1 Meloidogyne species described above (Fig. 1), there are several possible evolutionary pathways for the evolution of reproductive modes (Fig. 2): In scenario 1, M. floridensis has regained meiosis from an apomict state. Alternatively (scenario 2), the numerous apomict species could have lost meiosis many times independently. There are several additional scenarios involving hybrid origins. In scenario 3, the apomicts have hybrid origins with the automict M. floridensis as a putative parent, while in scenario 4 both M. floridensis and the apomicts have independent hybrid origins. In scenario 5, a hybrid M. floridensisis in turn parental to a complex hybrid apomict.
Scenario 1 is very unlikely. Meiosis is an exceptionally complex system to re-evolve once it has been lost (Dollo’s law), and the only suggested example we are aware of in the literature is not supported by robust reanalysis (see Goldberg & Igić, 2008). In addition, the extant apomicts are highly aneuploid, making it necessary for M. floridensis to have re-evolved 18 homologous chromosome pairs, which again suggests that cytologically this route is highly unlikely. Scenario 2 is also not parsimonious, potentially implying very many independent major reproductive transitions. Since there are already genetic data indicating that the apomicts may have hybrid genomes (Lunt, 2008), we focused our analyses on the much more biologically plausible scenarios 3, 4 and 5 that propose hybridization drove the evolution of the apomictic RKN.
Scenario 3 restricts the hybrid taxa to the apomict Group 1 species, and places M. floridensis as one of the hybridizing parental species (Fig. 2B). This model makes predictions that, where divergent homeologous sequences are detected in the M. incognita genome, M. floridensis would possess two alleles closely related to one of these homeologues. The M. floridensis genome itself would also be substantially different from that of M. incognita, not possessing divergent homeologous blocks but rather displaying normal allelic variation, perhaps more similar to that observed in the M. hapla genome.
In scenarios 4 (Fig. 2C) and 5 (Fig. 2D) M. floridensis would also be a product of an interspecific hybridization, as are the apomicts. Both these scenarios predict that the M. floridensis genome will, like M. incognita, show substantial sequence divergence between homeologues throughout its genome, although it may also possess some regions where one parental copy has been eliminated, and remaining diversity is simple allelism. In scenario 4, the parents of M. incognita need not be the same as those of the apomicts, although the phylogenetic position of M. floridensis implies that at least one of them may have been identical or very closely related. The different putative hybrid origins of M. incognita predict two (scenario 4, Fig. 2C) or three (scenario 5, Fig. 2D) homeologous copies, potentially modified by subsequent loss events.
Here, we generate a de novo assembled genome for M. floridensis, identify and analyse a large number of sets of homologous sequences in M. floridensis, M. incognita and M. hapla, and use both gene copy number distributions and gene phylogenies to test the predictions of the different scenarios outlined in Fig. 2.
Materials and Methods
Nematode materials
DNA from female egg mass cultures of Meloidogyne floridensis isolate 5 was generously sourced and provided from culture by Dr. Tom Powers (University of Lincoln, Nebraska, USA) and Dr. Janete Brito (Florida Department of Agriculture and Consumer Services, Gainesville, USA).
Sequencing and draft genome assembly
Meloidogyne floridensis DNA was prepared for sequencing using standard Illumina protocols by the GenePool Genomics Facility of the University of Edinburgh. A 260 bp insert library was sequenced using one lane of an Illumina HiSeq2000 (v2 reagents) with 101 base paired-end sequencing. 14.5 gigabases (Gb) of raw sequence data were adapter trimmed and quality filtered using perl and bash scripts to yield 70.2 M pairs totaling 13.2 Gb.
The genomic DNA sample derived from nematodes isolated from plant roots, and surrounded, therefore, by the bacterial communities of the rhizosphere. Egg masses of RKN are known to be associated with microbial taxa. To identify potential contaminants, we performed a preliminary assembly of all the trimmed reads ignoring pairing information. We then estimated read coverage of each assembled contig by mapping all reads back to the assembly, and annotated 10,000 randomly sampled contigs with the taxonomic order of their best megablast (BLAST+ version 2.2.25+ (Zhang et al., 2000)) match to the NCBI nt database (Benson et al., 2011). A taxon-annotated scatter plot of the GC% and coverage of each contig was used to visualize the contaminants present in the data (Fig. S1) (Kumar & Blaxter, 2012). Distinct GC%-coverage clusters in this plot were annotated with distinct taxonomic matches. A major cluster annotated as nematode was clearly dominant. Additional minor clusters were annotated as deriving from the bacterial orders Bacillales, Burkholderiales, Pseudomonadales and Rhizobiales. These all either had much lower coverage or much higher GC content than the nematode cluster. We conservatively removed contigs that matched the GC content and coverage of the identified contaminant blobs. To ensure optimal contamination removal, a second round of megablast searches was performed and any contigs that matched Bacterial databases were removed. Only reads mapping to the remaining, putatively nematode contigs and their pairs were retained for the next step. The true insert size distribution of these reads was also estimated by mapping the pairs back to the preliminary assembly.
A stringent reassembly of the cleaned read set (11.1 Gb) was performed using reliable coverage information estimated from the preliminary assembly GC%-coverage plot. Velvet v1.1.04 (Zerbino, 2010; Zerbino & Birney, 2008) was used with a k-mer value of 55 and the parameters -exp_cov 45, -cov_cutoff 4.5, and -ins_length 260. Other parameters and assemblers were also tried but this assembly had the best contig length optimality scores (e.g., N50, the contig length at which 50% of the assembly is in contigs of that length or greater) and the highest CEGMA values (using CEGMA version 2.3, Parra, Bradnam & Korf, 2007). Redundant contigs likely to derive from independent assembly of allelic copies were removed using CD-HIT-EST (version 4.5.5, Li & Godzik, 2006) with -c 0.97 (removing all contigs that were more than 97% identical over their entire length to another, longer contig).
Protein predictions and comparisons
A full annotation of the M. floridensis draft genome was not carried out, because no transcriptome data for the species was available. Instead, because we were interested in comparing coding sequences conserved with M. hapla and M. incognita, we used the protein2genome model in exonerate v2.2.0, (Slater & Birney, 2005) to align all M. hapla and M. incognita proteins, derived from the published genome sequences, to the M. floridensis draft genome. We extracted coding sequences (CDSs) that aligned to at least 50% of the length of the query protein sequences. If multiple M. hapla or M. incognita query protein sequences aligned to overlapping loci on the M. floridensis genome, only the longest locus was chosen as a putative M. floridensis CDS. The CDSs for all three species were trimmed after the first stop codon, and only sequences with a minimum of 50 amino acids were retained for further analysis.
To assess the level of self-identity among CDSs in each species, a BLASTn (version 2.2.25 + Altschul et al., 1990) search (with a sensitive E-value cutoff of 1e-5) was performed and the top scoring hit for each sequence to a CDS (other than itself) was selected if the length of the alignment was longer than 70% of the query sequence. The transcriipts of M. incognita were compared to the genomes of M. floridensis and M. hapla to identify levels of between species similarity using the same strategy.
Clustering
We used Inparanoid (version 4.1, Ostlund et al., 2010) and QuickParanoid (Kim & Park) with default settings to assign proteins from the three Meloidogyne species to orthology groups. While assessing the level of duplication within the CDS sets (Fig. 3), we noted that several M. incognita CDS sequences were identical or nearly identical (>98% identity). These are most likely derived from allelic variants rather than gene duplications (which show a separate peak between 95 and 97% identity). To simplify the construction of orthologous gene clusters, we reduced these near identical sequences in each species using CD-HIT-EST, removing any CDSs that were at least 98% identical across their whole length to another CDS.
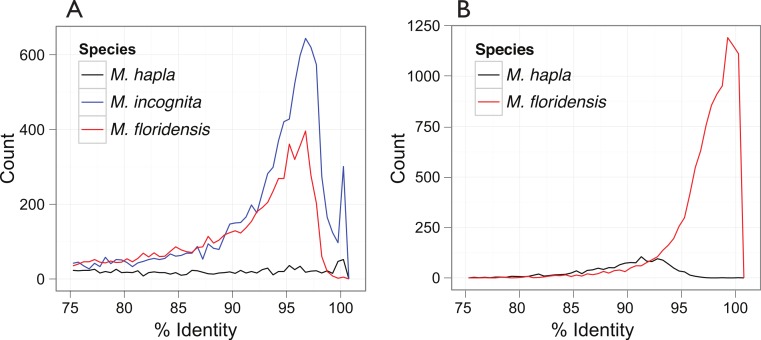
Figure 3. Inter- and intra-genomic identification of duplicated protein-coding regions.
(A) Each coding sequence from each of the three target genomes (M. hapla, M. incognita and M. floridensis) was compared to the set of genes from the same species. The percent identity of the best matching (non-self) coding sequence was calculated, and is plotted as a frequency histogram. Both M. incognita and M. floridensis show evidence of the presence of many duplicates, while M. hapla does not. (B) The M. incognita gene predictions were compared to the M. floridensis genome and the M. hapla gene set. For each M. incognita gene, the similarity of the top matches in each genome was assessed. M. incognita has many genes that are highly similar to those of M. floridensis (similarity >98%). This contrasts with the matches to M. hapla, where the modal similarity is ∼92%, and there is no peak of high-similarity matches.
Phylogenetic analyses
For each InParanoid cluster, Clustal Omega v1.0.3, (Sievers et al., 2011) was first used to align the protein sequences. Tranalign (from the Emboss suite, v6.2.0, Rice, Longden & Bleasby, 2000) was then used along with the protein alignment as a guide to align the nucleotide CDS sequences. Finally, RAxML v7.2.8, (Stamatakis, 2006) was used to create maximum likelihood trees for each set of aligned CDS sequences in three steps: (i) finding the best ML tree by running the GTRGAMMA model for 10 runs using the command “raxmlHPC-PTHREADS-SSE3 -m GTRGAMMA -s $a -# 10 -n $a -T 2”; (ii) getting the bootstrap support values for this tree by running the same model until the autoMRE convergence criterion was satisfied employed the command “raxmlHPC-PTHREADS-SSE3 -m GTRGAMMA -s $a -# autoMRE -n $a.b -T 2 -b 12345”; (iii) using the bootstrap trees to draw bipartitions on the best ML tree used the command “raxmlHPC-PTHREADS-SSE3 -m GTRCAT -f b -t RAxML_bestTree. $a -z RAxML_bootstrap. $a.b -n $a.l -T 2 -o mh”. Gene trees with a BP support of 70% or more were included in the analysis. The resulting trees were imported into the R Ape package v2.8, (Paradis, Claude & Strimmer, 2004) to count the number of trees with the same topology. Datafiles, treefiles, and scripts for processing the trees and other data can be obtained from FigShare 10.6084/m9.figshare.978784.
Results
The genome of Meloidogyne floridensis
The M. floridensis genome was assembled using 11.1 Gb of cleaned data (see Table 1, Fig. S1) from 116 M reads (an estimated ∼100X coverage), using Illumina HiSeq2000 100 base paired-end sequencing of 250 bp fragments. The raw read data have been submitted to the Short Read Archive as accession ERP001338, the final assembly file is available at EMBL, and a blast database, CDS download, and other resources are available at http://brock.bio.ed.ac.uk/M_floridensis/ and http://nematodes.org/genomes/meloidogyne_floridensis.
Table 1. Summary statistics describing genome assemblies of Meloidogyne.
| Species | Meloidogyne hapla | Meloidogyne incognita | Meloidogyne floridensis |
|---|---|---|---|
| Source | NCSU/WormBase WS227 | INRA/WormBase WS227 | 959 Nematode Genomes Project |
| Data URL | ftp://ftp.wormbase.org/pub/wormbase/species/m_hapla/ | ftp://ftp.wormbase.org/pub/wormbase/species/m_incognita/ | http://downloads.nematodegenomes.org |
| Citation | Opperman et al. (2008) | Abad et al. (2008) | This work |
| Maximum scaffold length | 360,446 | 154,116 | 40,762 |
| Number of scaffolds | 3,452 | 9,538 | 81,111 |
| Assembled size (bp) | 53,017,507 | 82,095,019 | 99,886,934 |
| Scaffold N50* (bp) | 37,608 | 12,786 | 3,516 |
| GC% | 27.4 | 31.4 | 29.7 |
| CEGMA** completeness Full/Partial |
92.74/94.35 | 75.00/77.82 | 60.08/72.18 |
| Predicted proteins (used for clustering***) |
13,072 (12,229) | 20,359 (17,999) | 15,327 (15,121) |
Notes.
N50, weighted median contig length; the contig length at which 50% of the assembled genome is present in contigs of that or greater length.
CEGMA, Core Eukaryotic Genes Mapping Approach (Parra, Bradnam & Korf, 2007).
Predicted proteins used for clustering and inferring phylogenies after filtering for length >50 amino acids (see Methods).
Intra-genomic comparisons reveal high numbers of duplicate genes in M. incognita and M. floridensis
Analysis of the distribution of within-genome CDS matches (Fig. 3A) identified an unexpected excess of apparent duplication in M. floridensis. While the CDS set of M. hapla had a relatively low rate of duplication, and no excess of duplicates of any particular divergence level, both M. incognita and M. floridensis had many more duplicates and a peak of divergence between duplicates at 95 to 97% identity. M. incognita showed an additional peak at ∼100% identity likely due to a failure to collapse allelic copies of some genes by the original authors (Abad et al., 2008). Because of the way we constructed our draft genome assembly, collapsing high-identity assembly fragments before analysis, M. floridensis lacked a near complete identity peak. To test for divergent copies of mtDNA within M. floridensis we searched the genomic contigs with a M. floridensis 16S mitochondrial rRNA gene from the international sequence databases (Genbank accession: AY635609.1) using blastn. Different regions of this query matched to two contigs comprising 1087 nucleotides in total and we observed a divergence between query and contig of 4/1087 nucleotides or 0.37%.
The very high frequency of intragenomic duplicate copies with a consistent divergence level strongly suggest that either M. floridensis, like M. incognita, is a hybrid species, with contributions from two distinct parental genomes, or that it has undergone a whole genome duplication. These distinct possibilities are addressed below. Comparing CDS between species we identified a high frequency of near-100% identity between M. incognita and its best match in the M. floridensis genome (Fig. 3B). This pattern was not evident when M. incognita was compared to M. hapla.
Distinguishing sibling from parent–child species relationships
We identified several models that might explain the observed levels of within-genome divergent duplicates in M. incognita and M. floridensis (Fig. 3A). Expectations of relative numbers of (homeologous) gene copies per species, and the phylogenetic relationships of these homeologue sets differ and allow us to distinguish between the models. Thus for example under scenario 3 (Fig. 2B) we test to determine if M. incognita has two divergent homeologous gene copies, one of which is phylogenetically very closely related to the (collapsed) allelic copies in M. floridensis. We therefore clustered the CDS of the three species using InParanoid, after removing all CDS encoding peptides less than 50 amino acids in length.
We defined 11,587 clusters that contained CDS from more than one species, and 4,018 that had representatives from all three species (Fig. S2) These represent a number and proportion similar to comparisons between other nematode species with complete genomes (e.g., 2501 clusters were previously identified containing representatives from four nematode genomes Mitreva et al., 2011). As M. hapla is not expected to have undergone whole genome duplication, and we find no evidence of an excess of diverged duplicates in the M. hapla genome, we selected homologous gene sets where the ancestral gene was likely to have been single-copy by excluding clusters with more than one M. hapla member, and those lacking M. hapla members. We classified these clusters by the numbers of M. incognita and M. floridensis genes they contained (Table 2; Fig. 4). The trees generated and the scripts used to parse them into the categories represented in Fig. 4 are available through FigShare 10.6084/m9.figshare.978784.
Table 2. Numbers of Meloidogyne floridensis and Meloidoigyne incognita members in homeologue gene sets that have one Meloidogyne hapla member.
| 0 M. incognita members | 1 M. incognita member | 2 M. incognita members | 3 M. incognita members | >3 M. incognita members | |
|---|---|---|---|---|---|
| 0 M. floridensis members | 0 | 907 | 327 | 44 | 17 |
| 1 M. floridensis member | 2196 | 2189 | 920 | 102 | 40 |
| 2 M. floridensis members | 226 | 257 | 156 | 36 | 21 |
| 3 M. floridensis members | 17 | 17 | 20 | 7 | 14 |
| >3 M. floridensis members | 8 | 11 | 6 | 4 | 21 |
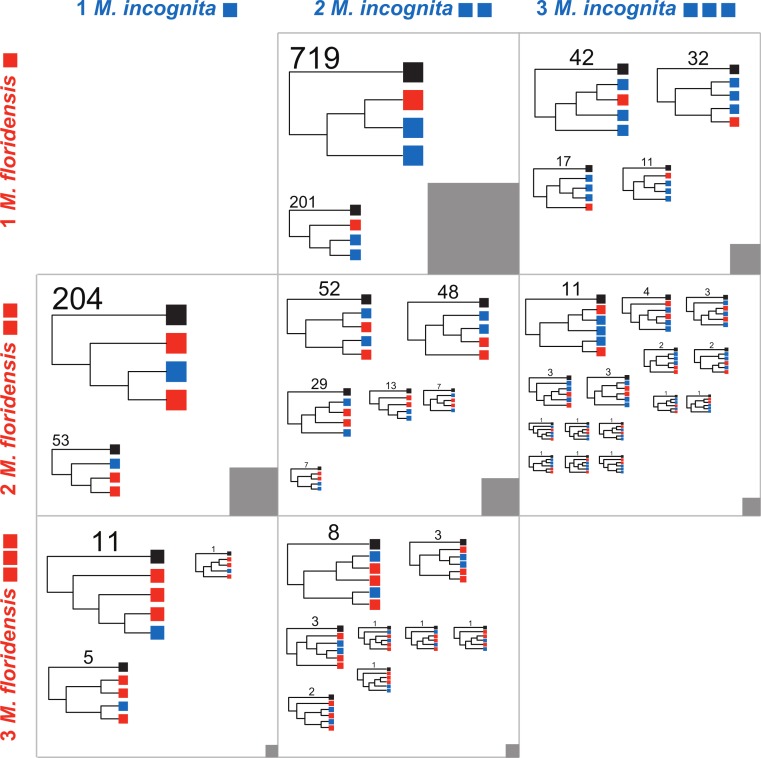
Figure 4. Phylogenomic analyses of clustered gene sets.
For cluster sets represented in Table 2 that had representation of both M. floridensis and M. incognita, more than three members (i.e., where there was more than one possible topology), and fewer than five total members (i.e., where the number of possible topologies was still reasonably low and close to the number of clusters to be analyzed), we generated an estimate of the relationships between the sequences using RAxML. The resultant trees were bootstrapped, and rooted using the M. hapla representative. For each cluster set, the topologies were summarized by the different unique patterns possible. Within each figure cell, each cladogram in the figure is scaled by the number of clusters that returned that topology, with terminal nodes coloured by the origin of the sequences (black representing M. hapla, blue M. incognita, and red M. floridensis). The number of clusters congruent with each cladogram is given above the trees. The numbers of clusters contributing to each cell in the figure is represented by the grey box, which is scaled by the number of clusters summarized (e.g., the box in the central cell represents 902 trees, while the box in the bottom left cell represents 17 trees).
The process of idiosyncratic gene loss (or failure to capture a gene in the draft sequencing and assembly) is evident in the numbers of genes that have one M. hapla representative and no members from either M. incognita (column 1 of Table 2) or M. floridensis (row 1 of Table 2). Here it is striking that the clusters that contain only one M. hapla and one M. floridensis member (Mh1:Mf1:Mi0) outnumber by approximately two to one clusters that have one M. hapla and one M. incognita member (Mh1:Mf0:Mi1). This suggests that the M. floridensis genome draft is a good substrate for these analyses (it contains homologues of many conserved genes apparently lost from, or missing in the draft assembly of, the M. incognita genome), and that the M. incognita draft is either incomplete or has experienced greater rates of gene loss.
The numbers of genes present in clusters that have two or more members, but lack one of M. floridensis or M. incognita (for example the 226 Mh1:Mf2:Mi0 clusters) reveal the potential extent of within-lineage duplication and divergence (and a component of stochastic loss of several homeologues in the missing species). There is no excess of these classes of cluster in M. incognita, arguing against a within-lineage, whole-genome duplication (i.e., against scenarios 1 or 2; Fig. 2A).
The striking feature of the membership of clusters (Table 2) is the number of cases where M. incognita has more cluster members than does M. floridensis. Thus there are 920 clusters in the class Mh1:Mf1:Mi2, but only 257 in the class Mh1:Mf2:Mi1, and 102 clusters in the class Mh1:Mf1:Mi3 compared to 17 in the class Mh1:Mf3:Mi1. This finding argues for the presence in M. incognita of at least one more genome copy than in M. floridensis, i.e., that M. incognita is likely to be a degenerate triploid hybrid (scenario 5, Fig. 2D). It is possible that some of the clusters in the Mh1:Mf1:Mi0 and Mh1:Mf0:Mi1 sets arise from M. floridensis and M. incognita being derived from different, divergent parents.
Phylogenomic analysis of homologue relationships
A second set of predictions from the models in Fig. 2 concerns the phylogenetic relationships of the resulting sets of homologous gene sequences. Each model predicts a particular set of relationships between gene copies in each species. We therefore analyzed each informative set of clusters represented in Table 2 to identify which alternate topology was supported, assuming in each case that the single M. hapla representative was the outgroup. These phylogenomic results are summarized in Fig. 4. For each informative set of clusters, the majority topology supported scenario 5 (Fig. 2D), i.e., that M. floridensis is a hybrid, and was one of the parent species in a hybridization event that gave rise to a triploid M. incognita. Thus for the 902 Mh1:Mf1:Mi2 clusters, the topology in which one M. incognita CDS groups with the M. floridensis CDS to the exclusion of the other M. incognita sequence was favoured in 79% of the clusters, while in only 201 clusters (21%) the two M. incognita genes instead appeared to have arisen by duplication within M. incognita. In the Mh1:Mf2:Mi2 cluster set, one third of the clusters supported the topology where there were two independent sister relationships between M. incognita and M. floridensis genes. A further 48% of the trees were congruent with a triploid status for M. incognita where gene loss (or lack of prediction) had removed one M. incognita representative. The other classes of clusters could be interpreted in the same manner, and displayed trends that supported scenario 5.
Discussion
The genome structure and content of tropical Meloidogyne is revealed by our analyses to have had complex origins. It is likely that hybridization, ploidy change, and subsequent aneuploidy have all played a role in the evolution of the diversity in this genus. The molecular evolutionary patterns revealed by comparative genomics however give us tools to conduct detailed analysis of these histories. This approach allows us to interpret the evolution of different reproductive strategies in terms of genome change, and better understand the evolution of these polyphagous pathogens.
The M. floridensis genome reveals its hybrid origins
Our draft assembly of the genome of M. floridensis reveals a relatively typical nematode genome. The base haploid genome size for Meloidogyninae is likely to be ∼50 Mb. Both the sequenced genome of M. hapla (Opperman et al., 2008), and independent measurement of its genome size from densitometry (Pableo & Triantaphyllou, 1989), yield estimates of 50–54 Mb. The sequenced genome estimate is unlikely to be inflated through issues of uncollapsed haploid contigs, as M. hapla is expected to have reduced heterozygosity through its automictic reproductive mode (Liu, Thomas & Williamson, 2007), and the sequenced strain was inbred (Opperman et al., 2008). Hybrid taxa, containing homeologous chromosomes from more than one parental lineage, would be expected to have genome assembly sizes that are the sum of the parental genomes, albeit modified by idiosyncratic post-hybridization gene loss and repeat copy change. Thus the ∼100 Mb genome size estimated for M. floridensis is in keeping with a base Meloidogyninae genome of ∼50 Mb, with homeologous sequences assembled independently. The divergence between inferred homeologous genes in our genome (∼4–8%) would preclude coassembly of homeologous coding sequences, and the higher divergence found in intergenic and intronic sequences would make them even less likely to be coassembled. The published M. incognita genome is 86 Mb, but ongoing revision of the assembly suggests a true value of ∼130 Mb (E Danchin, pers. comm., 2014), as might be expected for a hypo-triploid species.
The M. floridensis genome assembly is less contiguous than those of M. hapla and M. incognita (reflected in the lower contiguity and content of conserved eukaryotic genes). Such fragmentation is a known limitation of using a single small-insert paired-end library, and refinement of the assembly using larger-insert mate pair, or long single molecule reads, would undoubtedly improve the biological completeness of the product. Our primary aim however was not to produce a highly contiguous assembly, but rather to identify protein-coding sequences (CDS) for use in comparative genomic analyses. Despite the fragmentation we were able to identify over 15,000 CDS segments to address the possible hybrid status of M. floridensis and M. incognita, making it more than sufficient for this study.
We note that both the M. incognita and the M. floridensis genomes have low scores (60–75%) when assessed by the Core Eukaryotic Genes Mapping Approach (CEGMA), compared to the 94% scored by the M. hapla assembly (and assemblies of other nematode genomes). However, the published M. incognita genome, while having much better assembly statistics (only 9,538 scaffolds, and a contiguity ∼4 times that achieved for M. floridensis), has similarly poor scores in CEGMA analysis. Whether this is a reflection of shared divergent biology, or, as we suspect, a poor fragmented assembly, will require additional sequencing data, reassembly and reassessment.
The phylogenetic position of the automictic M. floridensis suggest that this species, or an immediate ancestor, was parental to the tropical apomicts, i.e., being one partner in the hybrid origins of the group (scenarios 3 and 5, Figs. 2B and 2D). It is also possible however that M. floridensis is not directly parental to the apomicts, but rather a hybrid sibling, also arising by interspecific hybridization (scenario 4, Fig. 2C). In this case one parent of M. floridensis is very likely to also have been involved in the hybrid origins of M. incognita as very many loci were found to be nearly identical between M. incognita and M. floridensis (Fig. 3B). In order to distinguish between scenario 3 (diploid parent), scenario 4 (hybrid sibling) and scenario 5 (hybrid parent) we examined the sequence diversity within each species’ genome.
Intra-genomic divergence of coding loci
Information concerning the hybrid status of M. floridensis can be gained from comparing the pattern of gene duplication within its genome to that of other RKN species, since Meloidogyne incognita has been suggested previously to have hybrid origins (Dalmasso & Berge, 1983; Triantaphyllou, 1985; Hugall, Stanton & Moritz, 1999; Castagnone-Sereno, 2006; Lunt, 2008) whereas M. hapla never has. An interspecific hybrid would be expected to have an excess of divergent intra-genomic duplicates compared to a non-hybrid, due to its homeologous chromosome pairs. The genome of M. hapla, a closely related species without a hybrid origin, represents the normal intra-genomic duplication pattern without homeologous chromosomes. In M. hapla there was a very much lower number of divergent duplicates compared to the other species, and these had a wide range of divergences rather than a frequency peak at any divergence value. While there was a slight excess of duplicates with high identity in M. hapla, the distribution overall is consistent with an ongoing rare process of stochastic duplication followed by gradual divergence (Fig. 3A).
In contrast to the pattern observed in M. hapla, the intra-genomic comparisons of both M. incognita and M. floridensis revealed many more divergent duplicated CDS (Fig. 3A). We observed a peak of high-identity duplicates in M. incognita that was absent in M. floridensis. This is most likely because we stringently collapsed high identity segments (as putative allelic copies) during assembly of M. floridensis whereas the M. incognita genome assembly may still contain some of these alleles. Most striking however was the presence in both species of a frequency peak of more diverged duplicates showing ∼96% identity. Such duplicates have been described in M. incognita (Abad et al., 2008; Lunt, 2008) although the scale of these diverged loci and their presence in M. floridensis has not been reported previously.
If the M. floridensis divergent copies were the product of a mixed sample, and thus represented polymorphism rather than homeologs, we would predict that mitochondrial DNA would also display this pattern. Our BLAST search however did not find any divergent contigs matching to our mtDNA genbank query. The query sequence differed by only 0.37% and this divergence between the genbank sequence and our strain is typical of intraspecific polymorphism levels in other nematodes but is approximately ten-fold less than we observe between the nuclear divergent copies in the M. floridensis genome. We therefore do not consider that, even if our starting material had been contaminated with a second M. floridensis strain, such intraspecific polymorphism could account for our much more diverged genomic copies.
Ongoing individual gene duplication events—which we propose has generated the M. hapla distribution—could not have produced these patterns. Instead, the distributions are congruent with either a single major past event of genome duplication followed by divergence, or else hybridization to bring together pre-diverged homeologous chromosome copies that had been evolving independently since the last common ancestor of the parental species. On top of these processes differences in the rates of evolution of individual loci has resulted in variation in observed identity in the extant genomes, producing a distribution around a single peak of divergence. While these two alternative scenarios (endoduplication and homeologous chromosomes) cannot be distinguished on the basis of duplicate divergence data alone, the analysis does suggest that the genome content of both M. floridensis and M. incognita have been shaped in very similar ways by major duplication or divergence events.
Integrating phylogenomic analyses
To distinguish between endoduplication and hybrid origins of these CDS divergences, we examined the phylogenetic histories of sets of homologous loci from the three Meloidogyne genomes. By selecting CDS clusters with only a single member from the M. hapla genome we have likely restricted our analyses to loci that were single copy in the last common ancestor of the three species, and thus do not show the complexities of turnover in large multigene families.
We compared support on a gene-by-gene basis for tree topologies that would support or refute the hybrid versus endoduplication scenarios (Fig. 2, Table 2, Fig. 4). Using this approach we could robustly exclude scenario 1, endoduplication of the M. incognita genome, as a source of duplicate CDS since we frequently observed that these M. incognita sequences were not monophyletic with respect to M. floridensis. If M. incognita had duplicated its own genome we would expect these duplicate CDS to share a recent origin and be each other’s closest relatives. We could similarly exclude scenarios 2 and 3, since intra-genomic comparisons of CDS in the M. floridensis genome revealed that it also possesses divergent duplicates, and phylogenetic analyses indicated that these, just like the M. incognita sequences, are not monophyletic by species.
Thus we suggest that the most parsimonious explanation of the duplicate divergence and phylogenetic data is that both M. floridensis and M. incognita are hybrid species, and the duplicate CDS are homeologues rather than within-species paralogues. We can distinguish between scenario 4 (independent hybrid origins: the two species are step-sisters) and scenario 5 (M. floridensis represents one of the parents of a triploid hybrid M. incognita) by phylogenetic analyses of the clustered CDS. We observed an excess of clusters where there were more M. incognita members than there were M. floridensis members, as would be expected from a triploid species, whether or not it was now losing duplicated genes stochastically. In these clusters, the extra M. incognita CDS was less likely to be sister to one of the other M. incognita CDS than it was to be a sister to a M. incognita–M. floridensis pair. Based on these data we suggest that the triplicate loci in M. incognita are the three homeologues that have resulted from a hybridization event between the hybrid M. floridensis and an unidentified second, likely non-hybrid, parent (scenario 5, Fig. 2D). For clusters containing two M. floridensis homeologues and two M. incognita homeologues, the topology supporting shared hybrid ancestry was again more frequently recovered than topologies supporting independent hybridization events.
Handoo et al. (2004) described the meiosis of M. floridensis as lacking a second maturation division and being ‘intermediate’ between meiotic and mitotic forms of reproduction. The division observed by Handoo et al. (2004) is in fact likely a long-known form of purely meiotic automixis called “first division restitution”. Bell (1982, p. 40), in his classic review of the evolution of mating systems, describes one of the three primary types of automixis as involving the suppression of the second meiotic division, exactly as described by Handoo et al. (2004) for M. floridensis. The maintenance of both homeologs in the M. floridensis genome through meiotic divisions, as we report here, may seem more challenging than in M. incognita, which reproduces only by mitosis. Automixis that maintains the parental heterozygosity is however well described in other animals and we assume a very similar mechanism occurs in M. floridensis (Bell, 1982, p. 40; Smith, 1978, p. 44; Hood & Antonovics, 2004 and refs therein).
Hybrid speciation and adaptive novelty
Animal hybrids have been characterized as rare, unfit, and adversely affected by both competition and gene flow from their parents (Mayr, 1963; Barton, 2001). There is now an increasing awareness in the literature however of animal hybridization as both a speciation mechanism and a route to the generation of novel phenotypic diversity on which natural selection may act (Bullini, 1994; Arnold, 1997; Mavarez & Linares, 2008; Soltis & Soltis, 2009; Abbott et al., 2013). There are a growing number of cases in which animal species have a hybrid origin, i.e.: it is known that all vertebrate constitutive parthenogens, and gynogenetic species have hybrid origins (Avise, 2008); the Italian sparrow (Passer italiae) has been shown to be a nascent hybrid species (Hermansen et al., 2011); hybridization between two species of Rhagoletis tephritid fruitflies has led to expansion into a novel ecological niche (host plant) in the hybrid, and also reproductive isolation from both parents since mating is confined to the host plant (Schwarz et al., 2005). The genetic basis of hybridization in generating adaptive diversity has been revealed in a number of studies: the Heliconius melpomene genome demonstrates that hybridization and introgression has been important for the adaptive radiation of these butterflies, by sharing protective colour-pattern genes among co-mimics (Heliconius Genome Consortium, 2012); the Northern European freshwater ‘invasive sculpin’ fish are hybrids between two geographically isolated Cottus species and they have colonized a novel niche consisting of the extensively human-altered lower reaches of the rivers Rhine and Scheldt (Czypionka et al., 2012). The cichlid adaptive radiation in Lake Malawi involves the evolution of more than 400 species, over a period of only 4.6 million years (Genner et al., 2007), which have colonized and adapted to many diverse lacustrine habitats. Recent genetic studies indicate that this radiation, and cichlid diversification in general, has been strongly influenced by interspecific hybridization (Joyce et al., 2011; Schwarzer et al., 2012; Loh et al., 2012; Genner & Turner, 2012).
It has been suggested that hybrid animal taxa are most likely to succeed where new habitats open up, and such events may have played a significant role in several classic examples of adaptive radiation (Seehausen, 2006; Abbott et al., 2013; Seehausen, 2013; Kearney, 2005). The tropical RKN are exceptionally successful globally-distributed pathogens of diverse agricultural crops (Moens, Perry & Starr, 2009; Trudgill & Blok, 2001). These species have colonized a novel habitat, show extensive functional diversity, and have adapted to crop host-plants in the very brief evolutionary timeframe that agriculture has existed (a few thousand years). This is a situation similar to other animal adaptive radiations where hybridization may also have played a significant role (Seehausen, 2006; Seehausen, 2013; Abbott et al., 2013).
Although the adaptive consequences of hybridization are being increasingly recognized as important for biodiversity, ecology and evolution, the origin of novel traits, colonization of new ecological niches, and adaptive evolution can lead to serious problems if the organisms concerned are pathogens of humans, livestock, or crops (Bisharat et al., 2005; Brasier, 2001; Stukenbrock et al., 2012; Inderbitzin et al., 2011; Goss et al., 2011). It is particularly important therefore to understand the genetic basis of adaptive diversification in relation to existing or emerging pathogens.
The tropical apomictic RKN, exemplified by M. incognita, M. arenaria and M. javanica, possess host ranges that may include practically all agriculturally important species overlapping their distribution, causing M. incognita to be described as the “single most damaging crop pathogen in the world” (Trudgill & Blok, 2001). Such extreme polyphagy is not typically encountered in Meloidogyne species outside of the radiation of tropical apomicts, although some do exploit multiple hosts. The origins and mechanisms of this greatly expanded host range are not only interesting from an evolutionary genomics perspective but also important to our understanding of the mode of action of these globally important crop pathogens. The demonstration of the hybrid origins of M. incognita and M. floridensis, and by implication M. javanica and M. arenaria also, suggests transgressive segregation of adaptive variation might have played an important role in determining host range. Transgressive segregation is when the absolute values of traits in some hybrids exceed the trait variation shown by either parental lineage. Such transgressive phenotypes are common in hybrid offspring in both animals and plants, and particularly so where the parents derive from inbred but divergent lineages (Rieseberg, Archer & Wayne, 1999). Transgressive phenotypes have played a significant role in plant breeding, where crossing of inbred parental lineages can lead to extreme offspring variation onto which artificial selection is imposed, and similar processes are likely to act on hybrid swarms resulting from natural selection acting on inter-species crosses in the wild (Rieseberg, Archer & Wayne, 1999; Stelkens & Seehausen, 2009; Genner & Turner, 2012). We do not yet know whether transgressive phenotypes in hybrid apomict RKN have been shaped by natural selection, but given our increasing awareness of its importance in adaptive radiations, and the frequency with which hybrid plant pathogens are detected in other systems (Stukenbrock et al., 2012; Stukenbrock & McDonald, 2008; Inderbitzin et al., 2011; Brasier, 2001), it may be an important direction for future research allowing us to detect likely pathogens at early stages.
Although we have not yet identified the parental taxa of M. floridensis, or the second parent of the tropical apomict RKN, it is likely that they were facultatively sexual meiotic parthenogens, as this is the most common reproductive mode within Meloidogyne (Triantaphyllou, 1982; Triantaphyllou, 1985; Chitwood & Perry, 2009). This breeding system can fuse the products of a single meiotic division in order to regain diploidy, making these taxa more similar to the inbred lineages of plants highlighted as frequent sources of transgressive segregation and extreme phenotypes (Rieseberg, Whitton & Gardner, 1999) than to the typical (amphimictic) species of hybridizing animals. If this “polyphagy as transgressive segregation” hypothesis were correct then we would predict that the parents of the polyphagous RKN would most likely be automicts with considerably smaller host ranges.
Hybridization and molecular genetic approaches to Meloidogyne diversity
Molecular approaches to understanding the diversity of apomictic RKN have a long history and include studies of isozymes, mitochondrial DNA (mtDNA), ribosomal internal transcribed spacer (ITS), ribosomal RNA genes (rDNA), random amplified polymorphic DNA markers (RAPDs), amplified fragment length polymorphisms (AFLPs), and other marker systems (see Blok & Powers, 2009 for a review). However, if some Meloidogyne species are in fact hybrids, this presents particular problems for the standard molecular approaches used to characterize diversity. These typically assume that species or isolates have diverged following a bifurcating, tree-like, evolutionary pathway. Hybridization violates this assumption and produces more complex evolutionary histories that can either be misrepresented by single locus markers, or else produce intermediate or equivocal signal from multi-locus approaches. For example, a major reason that mtDNA and rDNA sequencing have been useful in evolutionary ecology is that they are effectively haploid, and hybrid taxa, which often retain just one of their parental species’ genotypes at these loci, present particular problems for these approaches (Seehausen, 2006; Hailer et al., 2012; Meyer et al., 2012). While carefully benchmarked marker approaches may still have utility in diagnostics, they will not be able to accurately reflect the complex evolutionary pathway of hybrid Meloidogyne species where different loci are likely to have experienced very different histories. Incongruence between markers is therefore to be expected as a true reflection of history, rather than due to a lack of analytical power. We are currently in the early stages of Meloidogyne comparative genomics and current estimates of the complex phylogenetic relationships between hybrid taxa will need to be constantly refined as more species are added.
Genomic approaches to the RKN system hold many advantages, including documenting the genomic changes associated with host-specialization, extreme polyphagy, and interaction with plant defense systems. An interesting and important question now is whether the main apomictic RKN species have a single origin, with species divergence perhaps related to aneuploidy, or are instead the result of repeated hybridizations of the same or similar parental lineages. Different patterns of origin may determine the extent to which control strategies may be broadly or only locally applicable. Finally, if transgressive segregation is a cause of extreme and unique diversity, including polyphagy and novel resistance breaking isolates, then monitoring of new hybrid lineages may be an agricultural necessity. We are now close to the time where RKN isolates can be characterized not only with a trivial name (e.g., M. incognita race X) but instead a detailed list of genome wide variants and their known association with the environment, response to nematicides, and virulence against a range of plant host species and genotypes—an approach that will surely be extremely valuable in optimizing agricultural success. We caution therefore that although traditional genetic approaches may be valuable for rapid diagnostics, population genomics must be embraced in order to really advance our understanding of these important pathogens and maximize our ability to successfully intervene.
Conclusions
Here we have used whole genome sequencing and evolutionary comparative genomics to demonstrate the complex hybrid origins of key Root Knot Nematode species. Understanding the evolutionary history of Meloidogyne species is a priority since only by this route can the evolution of pathogenicity and resistance, the emergence of new pathogenic strains, horizontal transfer of genes, and geographic spread of one of the world’s most important crop pathogens be properly understood. The importance of animal hybridization to speciation and adaptation is being increasingly recognized, driven by new insights from genome sequencing. Meloidogyne incognita is shown to be an unusual double-hybrid, suggesting that hybridization may be a common and complex process in the history of this group. The Meloidogyne system, with its very recent expansion to fill numerous agricultural ecological niches, shows interesting parallels to natural adaptive radiations that may also have been greatly influenced by hybridization. Further work elucidating whether hybridization contributes adaptively to polyphagy will be important not just in the context of root knot nematodes, but also in determining the interplay of evolutionary forces generating organismal adaptive divergence more generally.
Supplemental information
The contigs from primary assembly of the raw data from Meloidogyne floridensis were annotated with their GC% and their read coverage (estimated by mapping back all the reads used to the contig set). Ten thousand contigs >1000 bp were chosen randomly and compared, using BLASTN, to the GenBank nt nucleotide database. Any contig with a match in the database was further annotated with the taxonomic assignment (at phylum level) of the matched sequence. The contig GC% and coverage values were used to create a scatter plot in R, and contigs with taxonomic assignments were coloured according to the phylum of the best match. This plot was used to devise a data cleaning strategy prior to rigorous assembly.
The complete proteomes of the three Meloidogyne species were clustered using InParanoid. This Venn diagram shows the numbers of clusters that had multiple species membership, and the numbers of proteins that were unique to each species (numbers marked with *). The total number of proteins input for each species are given under the species’ name.
Acknowledgments
We thank Tom Powers and Janete Brito for sourcing and supplying M. floridensis materials, Etienne Danchin for access to M. incognita genome data and Marian Thomson and members of the GenePool Genomics Facility for sequencing support. We thank Africa Gómez, Amir Szitenberg, Steve Moss, Richard Ennos and Karim Gharbi for comments on the manuscript and the project.
Funding Statement
SK was supported by an overseas Research Studentship award of the School of Biological Sciences, University of Edinburgh, and GK by a BBSRC Research Studentship and an ORS award. The GenePool has core support from the NERC (award R8/H10/56) and MRC (G0900740). DL and MB are supported in part by NERC award NE/J011355/1. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
David H. Lunt and Mark L. Blaxter conceived and designed the experiments, analyzed the data, contributed reagents/materials/analysis tools, wrote the paper, prepared figures and/or tables, reviewed drafts of the paper.
Sujai Kumar and Georgios Koutsovoulos performed the experiments, analyzed the data, prepared figures and/or tables, reviewed drafts of the paper.
DNA Deposition
The following information was supplied regarding the deposition of DNA sequences:
NCBI Short Read Archive accession ERP001338.
Data Deposition
References
- Abad et al. (2008).Abad P, Gouzy J, Aury J-M, Castagnone-Sereno P, Danchin EGJ, Deleury E, Perfus-Barbeoch L, Anthouard V, Artiguenave F, Blok VC, Caillaud MC, Coutinho PM, Dasilva C, De Luca F, Deau F, Esquibet M, Flutre T, Goldstone JV, Hamamouch N, Hewezi T, Jaillon O, Jubin C, Leonetti P, Magliano M, Maier TR, Markov GV, McVeigh P, Pesole G, Poulain J, Robinson-Rechavi M, Sallet E, Ségurens B, Steinbach D, Tytgat T, Ugarte E, van Ghelder C, Veronico P, Baum TJ, Blaxter M, Bleve-Zacheo T, Davis EL, Ewbank JJ, Favery B, Grenier E, Henrissat B, Jones JT, Laudet V, Maule AG, Quesneville H, Rosso MN, Schiex T, Smant G, Weissenbach J, Wincker P. Genome sequence of the metazoan plant-parasitic nematode Meloidogyne incognita. Nature Biotechnology. 2008;26(8):909–915. doi: 10.1038/nbt.1482. [DOI] [PubMed] [Google Scholar]
- Abbott et al. (2013).Abbott R, Albach D, Ansell S, Arntzen JW, Baird SJE, Bierne N, Boughman J, Brelsford A, Buerkle CA, Buggs R, Butlin RK, Dieckmann U, Eroukhmanoff F, Grill A, Cahan SH, Hermansen JS, Hewitt G, Hudson AG, Jiggins C, Jones J, Keller B, Marczewski T, Mallet J, Martinez-Rodriguez P, Möst M, Mullen S, Nichols R, Nolte AW, Parisod C, Pfennig K, Rice AM, Ritchie MG, Seifert B, Smadja CM, Stelkens R, Szymura JM, Väinölä R, Wolf JBW, Zinner D. Hybridization and speciation. Journal of Evolutionary Biology. 2013;26(2):229–246. doi: 10.1111/j.1420-9101.2012.02599.x. [DOI] [PubMed] [Google Scholar]
- Altschul et al. (1990).Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. Journal of Molecular Biology. 1990;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Arnold (1997).Arnold ML. Natural hybridization and evolution. New York: Oxford University Press; 1997. [Google Scholar]
- Avise (2008).Avise JC. Clonality: the genetics, ecology, and evolution of sexual abstinence in vertebrate animals. Oxford University Press; 2008. [Google Scholar]
- Barton (2001).Barton NH. The role of hybridization in evolution. Molecular Ecology. 2001;10(3):551–568. doi: 10.1046/j.1365-294x.2001.01216.x. [DOI] [PubMed] [Google Scholar]
- Bell (1982).Bell G. The masterpiece of nature: the evolution and genetics of sexuality. Berkeley: University of California Press; 1982. [Google Scholar]
- Benson et al. (2011).Benson DA, Karsch-Mizrachi I, Lipman DJ, Ostell J, Sayers EW. GenBank. Nucleic Acids Research. 2011;39(Database issue):D32–D37. doi: 10.1093/nar/gkq1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisharat et al. (2005).Bisharat N, Cohen DI, Harding RM, Falush D, Crook DW, Peto T, Maiden MC. Hybrid Vibrio vulnificus. Emerging Infectious Diseases. 2005;11(1):30–35. doi: 10.3201/eid1101.040440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blok & Powers (2009).Blok VC, Powers TO. Root-knot nematodes. Wallingford: CABI Publishing; 2009. Biochemical and molecular identification; pp. 98–118. [Google Scholar]
- Brasier (2001).Brasier CM. Rapid evolution of introduced plant pathogens via interspecific hybridization. Bioscience. 2001;51(2):123–133. doi: 10.1641/0006-3568(2001)051[0123:REOIPP]2.0.CO;2. [DOI] [Google Scholar]
- Bullini (1994).Bullini L. Origin and evolution of animal hybrid species. Trends In Ecology & Evolution. 1994;9(11):422–426. doi: 10.1016/0169-5347(94)90124-4. [DOI] [PubMed] [Google Scholar]
- Castagnone-Sereno (2006).Castagnone-Sereno P. Genetic variability and adaptive evolution in parthenogenetic root-knot nematodes. Heredity. 2006;96(4):282–289. doi: 10.1038/sj.hdy.6800794. [DOI] [PubMed] [Google Scholar]
- Chitwood & Perry (2009).Chitwood DJ, Perry RN. Reproduction, Physiology and Biochemistry. In: Perry RN, Moens M, Starr JL, editors. Root-knot nematodes. CAB International; 2009. pp. 182–200. [Google Scholar]
- Czypionka et al. (2012).Czypionka T, Cheng J, Pozhitkov A, Nolte AW. Transcriptome changes after genome-wide admixture in invasive sculpins (Cottus) Molecular Ecology. 2012;21(19):4797–4810. doi: 10.1111/j.1365-294X.2012.05645.x. [DOI] [PubMed] [Google Scholar]
- Dalmasso & Berge (1983).Dalmasso A, Berge JB. Enzyme polymorphism and the concept of parthenogenetic species, exemplified by Meloidogyne. In: Stone AR, Platt HM, Khalil LF, editors. Concepts in nematode systmatics. London: Academic Press; 1983. pp. 187–196. [Google Scholar]
- De Ley et al. (2002).De Ley IT, De Ley P, Vierstraete A, Karssen G, Moens M, Vanfleteren J. Phylogenetic analyses of Meloidogyne small subunit rDNA. Journal of Nematology. 2002;34(4):319–327. [PMC free article] [PubMed] [Google Scholar]
- Genner & Turner (2012).Genner MJ, Turner GF. Ancient hybridization and phenotypic novelty within Lake Malawi’s cichlid fish radiation. Molecular Biology and Evolution. 2012;29(1):195–206. doi: 10.1093/molbev/msr183. [DOI] [PubMed] [Google Scholar]
- Genner et al. (2007).Genner MJ, Seehausen O, Lunt DH, Joyce DA, Shaw PW, Carvalho GR, Turner GF. Age of cichlids: new dates for ancient lake fish radiations. Molecular Biology and Evolution. 2007;24(5):1269–1282. doi: 10.1093/molbev/msm050. [DOI] [PubMed] [Google Scholar]
- Goldberg & Igić (2008).Goldberg EE, Igić B. On phylogenetic tests of irreversible evolution. Evolution; International Journal of Organic Evolution. 2008;62(11):2727–2741. doi: 10.1111/j.1558-5646.2008.00505.x. [DOI] [PubMed] [Google Scholar]
- Goss et al. (2011).Goss EM, Cardenas ME, Myers K, Forbes GA, Fry WE, Restrepo S, Grünwald NJ. The plant pathogen Phytophthora andina emerged via hybridization of an unknown Phytophthora species and the Irish potato famine pathogen, P. infestans. PLoS ONE. 2011;6(9):e356. doi: 10.1371/journal.pone.0024543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hailer et al. (2012).Hailer F, Kutschera VE, Hallstrom BM, Klassert D, Fain SR, Leonard JA, Arnason U, Janke A. Nuclear genomic sequences reveal that polar bears are an old and distinct bear lineage. Science. 2012;336(6079):344–347. doi: 10.1126/science.1216424. [DOI] [PubMed] [Google Scholar]
- Handoo et al. (2004).Handoo ZA, Nyczepir AP, Esmenjaud D, van der Beek JG, Castagnone-Sereno P, Carta LK, Skantar AM, Higgins JA. Morphological, molecular, and differential-host characterization of Meloidogyne floridensis n. sp. (Nematoda: Meloidogynidae), a root-knot nematode parasitizing peach in Florida. Journal of Nematology. 2004;36(1):20–35. [PMC free article] [PubMed] [Google Scholar]
- Heliconius Genome Consortium (2012).Heliconius Genome Consortium Butterfly genome reveals promiscuous exchange of mimicry adaptations among species. Nature. 2012;487:94–98. doi: 10.1038/nature11041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermansen et al. (2011).Hermansen JS, Saether SA, Elgvin TO, Borge T, Hjelle E, Saetre G-P. Hybrid speciation in sparrows I: phenotypic intermediacy, genetic admixture and barriers to gene flow. Molecular Ecology. 2011;20(18):3812–3822. doi: 10.1111/j.1365-294X.2011.05183.x. [DOI] [PubMed] [Google Scholar]
- Holterman et al. (2009).Holterman M, Karssen G, van den Elsen S, van Megen H, Bakker J, Helder J. Small subunit rDNA-based phylogeny of the tylenchida sheds light on relationships among some high-impact plant-parasitic nematodes and the evolution of plant feeding. Phytopathology. 2009;99(3):227–235. doi: 10.1094/PHYTO-99-3-0227. [DOI] [PubMed] [Google Scholar]
- Hood & Antonovics (2004).Hood ME, Antonovics J. Mating within the meiotic tetrad and the maintenance of genomic heterozygosity. Genetics. 2004;166(4):1751–1759. doi: 10.1534/genetics.166.4.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugall, Stanton & Moritz (1999).Hugall A, Stanton J, Moritz C. Reticulate evolution and the origins of ribosomal internal transcribed spacer diversity in apomictic Meloidogyne. Molecular Biology and Evolution. 1999;16(2):157–164. doi: 10.1093/oxfordjournals.molbev.a026098. [DOI] [PubMed] [Google Scholar]
- Inderbitzin et al. (2011).Inderbitzin P, Davis RM, Bostock RM, Subbarao KV. The ascomycete Verticillium longisporum is a hybrid and a plant pathogen with an expanded host range. PLoS ONE. 2011;6(3):e356. doi: 10.1371/journal.pone.0018260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeyaprakash et al. (2006).Jeyaprakash A, Tigano MS, Brito J, Carneiro RMDG, Dickson DW. Differentiation of Meloidogyne floridensis from M. arenaria using high-fidelity PCR amplified mitochondrial AT-rich sequences. Nematropica. 2006;36(1):1–12. [Google Scholar]
- Joyce et al. (2011).Joyce DA, Lunt DH, Genner MJ, Turner GF, Bills R, Seehausen O. Repeated colonization and hybridization in Lake Malawi cichlids. Current Biology. 2011;21(3):R108–R109. doi: 10.1016/j.cub.2010.11.029. [DOI] [PubMed] [Google Scholar]
- Judson & Normark (1996).Judson OP, Normark BB. Ancient asexual scandals. Trends In Ecology & Evolution. 1996;11(2):41–46. doi: 10.1016/0169-5347(96)81040-8. [DOI] [PubMed] [Google Scholar]
- Kearney (2005).Kearney M. Hybridization, glaciation and geographical parthenogenesis. Trends In Ecology & Evolution. 2005;20(9):495–502. doi: 10.1016/j.tree.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Kim & Park (0000).Kim T, Park S. QuickParanoid—a tool for ortholog clustering. Available at http://pl.postech.ac.kr/QuickParanoid .
- Kumar & Blaxter (2012).Kumar S, Blaxter ML. Simultaneous genome sequencing of symbionts and their hosts. Symbiosis. 2012;55(3):119–126. doi: 10.1007/s13199-012-0154-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li & Godzik (2006).Li W, Godzik A. Cd-hit: a fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics. 2006;22(13):1658–1659. doi: 10.1093/bioinformatics/btl158. [DOI] [PubMed] [Google Scholar]
- Liu, Thomas & Williamson (2007).Liu QL, Thomas VP, Williamson VM. Meiotic parthenogenesis in a root-knot nematode results in rapid genomic homozygosity. Genetics. 2007;176(3):1483–1490. doi: 10.1534/genetics.107.071134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh et al. (2012).Loh Y-HE, Bezault E, Muenzel FM, Roberts RB, Swofford R, Barluenga M, Kidd CE, Howe AE, Di Palma F, Lindblad-Toh K, Hey J, Seehausen O, Salzburger W, Kocher TD, Streelman JT. Origins of shared genetic variation in African cichlids. Molecular Biology and Evolution. 2012;30:906–917. doi: 10.1093/molbev/mss326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunt (2008).Lunt DH. Genetic tests of ancient asexuality in root knot nematodes reveal recent hybrid origins. BMC Evolutionary Biology. 2008;8(1):194. doi: 10.1186/1471-2148-8-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet (2007).Mallet J. Hybrid speciation. Nature. 2007;446(7133):279–283. doi: 10.1038/nature05706. [DOI] [PubMed] [Google Scholar]
- Mallet (2005).Mallet J. Hybridization as an invasion of the genome. Trends In Ecology & Evolution. 2005;20(5):229–237. doi: 10.1016/j.tree.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Mavarez & Linares (2008).Mavarez J, Linares M. Homoploid hybrid speciation in animals. Molecular Ecology. 2008;17(19):4181–4185. doi: 10.1111/j.1365-294X.2008.03898.x. [DOI] [PubMed] [Google Scholar]
- Mayr (1963).Mayr E. Animal species and evolution. Harvard University Press; 1963. [Google Scholar]
- Meyer et al. (2012).Meyer M, Kircher M, Gansauge M-T, Li H, Racimo F, Mallick S, Schraiber JG, Jay F, Prufer K, de Filippo C, Sudmant PH, Alkan C, Fu Q, Do R, Rohland N, Tandon A, Siebauer M, Green RE, Bryc K, Briggs AW, Stenzel U, Dabney J, Shendure J, Kitzman J, Hammer MF, Shunkov MV, Derevianko AP, Patterson N, Andres AM, Eichler EE, Slatkin M, Reich D, Kelso J, Paabo S. A high-coverage genome sequence from an archaic denisovan individual. Science. 2012;338(6104):222–226. doi: 10.1126/science.1224344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitreva et al. (2011).Mitreva M, Jasmer DP, Zarlenga DS, Wang Z, Abubucker S, Martin J, Taylor CM, Yin Y, Fulton L, Minx P, Yang S-P, Warren WC, Fulton RS, Bhonagiri V, Zhang X, Hallsworth-Pepin K, Clifton SW, McCarter JP, Appleton J, Mardis ER, Wilson RK. The draft genome of the parasitic nematode Trichinella spiralis. Nature Genetics. 2011;43(3):228–235. doi: 10.1038/ng.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moens, Perry & Starr (2009).Moens M, Perry RN, Starr JL. Root-knot nematodes. CAB International; 2009. Meloidogyne species—a diverse group of novel and important plant parasites; pp. 1–17. [Google Scholar]
- Nolte & Tautz (2010).Nolte AW, Tautz D. Understanding the onset of hybrid speciation. Trends in Genetics. 2010;26(2):54–58. doi: 10.1016/j.tig.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Opperman et al. (2008).Opperman CH, Bird DM, Williamson VM, Rokhsar DS, Burke M, Cohn J, Cromer J, Diener S, Gajan J, Graham S, Houfek TD, Liu Q, Mitros T, Schaff J, Schaffer R, Scholl E, Sosinski BR, Thomas VP, Windham E. Sequence and genetic map of Meloidogyne hapla: a compact nematode genome for plant parasitism. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(39):14802–14807. doi: 10.1073/pnas.0805946105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostlund et al. (2010).Ostlund G, Schmitt T, Forslund K, Kostler T, Messina DN, Roopra S, Frings O, Sonnhammer ELL. InParanoid 7: new algorithms and tools for eukaryotic orthology analysis. Nucleic Acids Research. 2010;38(Database issue):D196–D203. doi: 10.1093/nar/gkp931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pableo & Triantaphyllou (1989).Pableo EC, Triantaphyllou AC. DNA complexity of the root-knot nematode (Meloidgyne spp.) genome. Journal of Nematology. 1989;21:260–263. [PMC free article] [PubMed] [Google Scholar]
- Paradis, Claude & Strimmer (2004).Paradis E, Claude J, Strimmer K. APE: analyses of phylogenetics and evolution in R language. Bioinformatics. 2004;20(2):289–290. doi: 10.1093/bioinformatics/btg412. [DOI] [PubMed] [Google Scholar]
- Parra, Bradnam & Korf (2007).Parra G, Bradnam K, Korf I. CEGMA: a pipeline to accurately annotate core genes in eukaryotic genomes. Bioinformatics. 2007;23(9):1061–1067. doi: 10.1093/bioinformatics/btm071. [DOI] [PubMed] [Google Scholar]
- Rice, Longden & Bleasby (2000).Rice P, Longden I, Bleasby A. EMBOSS: the European molecular biology open software suite. Trends in Genetics. 2000;16(6):276–277. doi: 10.1016/S0168-9525(00)02024-2. [DOI] [PubMed] [Google Scholar]
- Rieseberg, Archer & Wayne (1999).Rieseberg LH, Archer MA, Wayne RK. Transgressive segregation, adaptation and speciation. Heredity. 1999;83(Pt 4):363–372. doi: 10.1038/sj.hdy.6886170. [DOI] [PubMed] [Google Scholar]
- Rieseberg, Whitton & Gardner (1999).Rieseberg LH, Whitton J, Gardner K. Hybrid zones and the genetic architecture of a barrier to gene flow between two sunflower species. Genetics. 1999;152(2):713–727. doi: 10.1093/genetics/152.2.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasser & Carter (1985).Sasser JN, Carter CC. Overview of the international meloidogyne project 1975–1984. In: Barker KR, Carter CC, Sasser JN, editors. An advanced treatise on Meloidogyne. Raleigh, NC: North Carolina State University; 1985. [Google Scholar]
- Schwarz et al. (2005).Schwarz D, Matta BM, Shakir-Botteri NL, McPheron BA. Host shift to an invasive plant triggers rapid animal hybrid speciation. Nature. 2005;436(7050):546–549. doi: 10.1038/nature03800. [DOI] [PubMed] [Google Scholar]
- Schwarzer et al. (2012).Schwarzer J, Swartz ER, Vreven E, Snoeks J, Cotterill FPD, Misof B, Schliewen UK. Repeated trans-watershed hybridization among haplochromine cichlids (Cichlidae) was triggered by Neogene landscape evolution. Proceedings of the Royal Society B: Biological Sciences. 2012;279(1746):4389–4398. doi: 10.1098/rspb.2012.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwenk, Brede & Streit (2008).Schwenk K, Brede N, Streit B. Introduction. Extent, processes and evolutionary impact of interspecific hybridization in animals. Philosophical Transactions of the Royal Society B: Biological Sciences. 2008;363(1505):2805–2811. doi: 10.1098/rstb.2008.0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seehausen (2006).Seehausen O. Hybridization and adaptive radiation. Trends In Ecology & Evolution. 2006;19(4):198–207. doi: 10.1016/j.tree.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Seehausen (2013).Seehausen O. Conditions when hybridization might predispose populations for adaptive radiation. Journal of Evolutionary Biology. 2013;26(2):279–281. doi: 10.1111/jeb.12026. [DOI] [PubMed] [Google Scholar]
- Sievers et al. (2011).Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, Lopez R, McWilliam H, Remmert M, Söding J, Thompson JD, Higgins DG. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Molecular Systems Biology. 2011;7:539. doi: 10.1038/msb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater & Birney (2005).Slater GSC, Birney E. Automated generation of heuristics for biological sequence comparison. BMC Bioinformatics. 2005;6:31. doi: 10.1186/1471-2105-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith (1978).Smith J. The evolution of sex. Cambridge University Press; 1978. [Google Scholar]
- Soltis & Soltis (2009).Soltis PS, Soltis DE. The role of hybridization in plant speciation. Annual Review of Plant Biology. 2009;60:561–588. doi: 10.1146/annurev.arplant.043008.092039. [DOI] [PubMed] [Google Scholar]
- Stamatakis (2006).Stamatakis A. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 2006;22(21):2688–2690. doi: 10.1093/bioinformatics/btl446. [DOI] [PubMed] [Google Scholar]
- Stelkens & Seehausen (2009).Stelkens R, Seehausen O. Genetic distance between species predicts novel trait expression in their hybrids. Evolution. 2009;63(4):884–897. doi: 10.1111/j.1558-5646.2008.00599.x. [DOI] [PubMed] [Google Scholar]
- Stukenbrock & McDonald (2008).Stukenbrock EH, McDonald BA. The origins of plant pathogens in agro-ecosystems. Annual Review of Phytopathology. 2008;46:75–100. doi: 10.1146/annurev.phyto.010708.154114. [DOI] [PubMed] [Google Scholar]
- Stukenbrock et al. (2012).Stukenbrock EH, Christiansen FB, Hansen TT, Dutheil JY, Schierup MH. Fusion of two divergent fungal individuals led to the recent emergence of a unique widespread pathogen species. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(27):10954–10959. doi: 10.1073/pnas.1201403109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor & Sasser (1978).Taylor AL, Sasser JN. Biology, identification and control of root-knot nematodes (Meloidogyne species) Raleigh, NC: North Carolina State University; 1978. [Google Scholar]
- Tigano et al. (2005).Tigano MS, Dickson D, Tigano M, Jeyaprakash A, Adams B. Phylogeny of Meloidogyne spp. based on 18S rDNA and the intergenic region of mitochondrial DNA sequences. Nematology. 2005;7(6):851–862. doi: 10.1163/156854105776186325. [DOI] [Google Scholar]
- Triantaphyllou (1982).Triantaphyllou AC. Cytogenetics and sexuality of root-knot and cyst nematodes. In: Riggs RD, editor. Nematology in the southern region of the United States. Arkansas Agricultural Experiment Station, Fayetteville, AK: Southern Cooperative Series Bulletin; 1982. pp. pp. 71–76. [Google Scholar]
- Triantaphyllou (1985).Triantaphyllou AC. Cytogenetics, cytotaxonomy and phylogeny of root-knot nematodes. In: Sasser JN, Carter CC, editors. An advanced treatise on meloidogyne. Raleigh: North Carolina State University; 1985. [Google Scholar]
- Trudgill & Blok (2001).Trudgill DL, Blok VC. Apomictic, polyphagous root-knot nematodes: exceptionally successful and damaging biotrophic root pathogens. Annual Review of Phytopathology. 2001;39:53–77. doi: 10.1146/annurev.phyto.39.1.53. [DOI] [PubMed] [Google Scholar]
- White (1945).White MJD. Animal cytology & evolution. 1st edition. Cambridge University Press; 1945. [Google Scholar]
- Zerbino (2010).Zerbino DR. Current protocols in bioinformatics. 2010. Using the Velvet de novo assembler for short-read sequencing technologies; Chapter 11; p. p. Unit 11.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerbino & Birney (2008).Zerbino DR, Birney E. Velvet: algorithms for de novo short read assembly using de Bruijn graphs. Genome Research. 2008;18(5):821–829. doi: 10.1101/gr.074492.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al. (2000).Zhang Z, Schwartz S, Wagner L, Miller W. A greedy algorithm for aligning DNA sequences. Journal of Computational Biology. 2000;7(1–2):203–214. doi: 10.1089/10665270050081478. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The contigs from primary assembly of the raw data from Meloidogyne floridensis were annotated with their GC% and their read coverage (estimated by mapping back all the reads used to the contig set). Ten thousand contigs >1000 bp were chosen randomly and compared, using BLASTN, to the GenBank nt nucleotide database. Any contig with a match in the database was further annotated with the taxonomic assignment (at phylum level) of the matched sequence. The contig GC% and coverage values were used to create a scatter plot in R, and contigs with taxonomic assignments were coloured according to the phylum of the best match. This plot was used to devise a data cleaning strategy prior to rigorous assembly.
The complete proteomes of the three Meloidogyne species were clustered using InParanoid. This Venn diagram shows the numbers of clusters that had multiple species membership, and the numbers of proteins that were unique to each species (numbers marked with *). The total number of proteins input for each species are given under the species’ name.