Abstract
The identification of multipotent adipose-derived stromal cells (ASC) has raised hope that tissue regeneration approaches established with bone-marrow-derived stromal cells (BMSC) can be reproduced with a cell-type that is far more accessible in large quantities. Recent detailed comparisons, however, revealed subtle functional differences between ASC and BMSC, stressing the concept of a common mesenchymal progenitor existing in a perivascular niche across all tissues. Focussing on bone and cartilage repair, this review summarises recent in vitro and in vivo studies aiming towards tissue regeneration with ASC. Advantages of good accessibility, high yield and superior growth properties are counterbalanced by an inferiority of ASC to form ectopic bone and stimulate long-bone healing along with their less pronounced osteogenic and angiogenic gene expression signature. Hence, particular emphasis is placed on establishing whether stem cell activity of ASC is so far proven and relevant for successful osteochondral regeneration, or whether trophic activity may largely determine therapeutic outcome.
Introduction
Established strategies for cartilage and bone repair, such as autologous chondrocyte transplantation (ACT) (Ref. 1) and bone grafting (Ref. 2), have reached broad clinical application and yield satisfactory results due to continuous improvement. These therapies, however, require the excision of healthy tissue from a nonlesioned site, necessarily incorporating the disadvantages of additional medical procedures, donor site morbidity and further rehabilitative burden on the patient (Ref. 3). Repair strategies that are based on autologous bone-marrow-derived stromal cells (BMSC) do not circumvent these problems, but harvesting bone marrow from the iliac crest is generally judged as less invasive (Ref. 4). The discovery that multipotent stromal cells can be isolated from lipoaspirates (Ref. 5) and that the number of adherent cells in an equal volume of adipose tissue exceeds the content of bone marrow aspirate by about 300-fold (Refs 6, 7, 8) challenged the assumption that bone marrow would be the most appropriate source for cell-based therapies of skeletal injuries and diseases.
In order to verify whether adipose-derived stromal cells (ASC) represent an easily accessible cell type that may substitute for BMSC completely in cell-based approaches for osteochondral regeneration, they were characterised in terms of in vitro performance (Refs 9, 10), in vivo localisation (Refs 11, 12) and their ability to differentiate into various mesenchymal cell types (Refs 13, 14, 15, 16). This review summarises current knowledge of ASC and BMSC plasticity and in vivo function, describing similarities and differences between both cell types that have been determined upon expansion. Furthermore, an overview is provided on osteoarticular regenerative approaches that have thus far been conducted using ASC. In summary, data on ASC-based osteoarticular repair strategies indicate that ASC do not possess intrinsic osteochondral potential, such as BMSC, but require reprogramming for in vivo development towards the osteochondral lineage. These observations stress the concept of equivalent mesenchymal progenitors in bone marrow and adipose tissue (Ref. 8). In view of a long list of successful experimental intervention studies in distinct models, trophic functions of ASC may be more relevant than stem cell potential in mediating osteoarticular repair.
Stemness of BMSC and ASC
Criteria for stem cell definition
Thus far absent from the literature is a comprehensive, general convention that defines intrinsic properties for stem cells of any given tissue (Ref. 17). From a functional point of view, a well-accepted interpretation would be that a single stem cell possesses the capacity to build up a physiological, multicellular tissue that is capable of autonomous regeneration in vivo. Specific cellular functions such as asymmetric cell division, prolonged self-renewal and differentiation capacities are needed to fulfil this requirement. Most importantly, in vitro detection of these properties in a particular cell type alone, however, does not necessarily prove stemness. It is self-explanatory that a stem cell only deserves this designation if the observed fundamental capacities represent intrinsic features of the native cell in vivo, rather than being achieved by artificial treatments or molecular reprogramming. These stringent criteria for stem cell definition (Ref. 18) are met by haematopoietic stem cells (HSC), which reconstitute bone marrow when clonally derived HSC are transplanted into lethally irradiated mice (Ref. 19). In the context of osteoarticular repair, BMSC are so far the only entity representing skeletal stem cells, according to this stringent definition. Sacchetti et al. established that clonal BMSC populations are self-renewing and can form an ectopic bone organ after subcutaneous transplantation into immunocompromised mice (Ref. 20). This result was further refined by Chan et al. by demonstrating the formation of multicellular bone tissue at other ectopic sites and by unravelling routes of differentiation from a discrete progenitor subpopulation to cell types that either contribute to bone, cartilage or haematopoiesis-supportive stroma within the new bone organ (Ref. 21). Besides BMSC and HSC, a plethora of other cell types is commonly designated as stem cells, although evidence for clonal in vivo organ formation without pre-induction is missing. This circumstance also holds true for ASC that, nevertheless, are often referred to as adipose-derived mesenchymal stem cells, despite the fact that the capacity of a single ASC to build up a functional mesenchymal tissue has not been shown to date. Thus, the idea of universal mesenchymal stem cells in a perivascular niche (Ref. 22) with an intrinsic capacity to build up and maintain multiple mesenchymal tissues (Ref. 8) by a common mesengenic in vivo process is still an unconfirmed hypothesis. Conclusively, BMSC are the only perivascular cells with proven skeletal stem cell characteristics.
Lack of evidence for stem cell characteristics of ASC
Current data do not exclude that ASC may possess stem cell characteristics, according to the stringent criteria outlined above, and results are encouraging that future experiments may support adipose tissue-specific stem cell properties. In vitro characterisation of isolated ASC demonstrated extensive proliferative potential (Ref. 5). The application of standard in vitro differentiation protocols to ASC reflected possession of osteogenic, adipogenic and chondrogenic differentiation capacity (Ref. 5), although the physiological relevance of these assays has been questioned (Ref. 18). Notably, clonal analyses revealed that >2% of cells within expanded ASC cultures exhibit tri-lineage potential in vitro, indicating that typical isolation protocols lead only to a small fraction of ASC with in vitro multilineage potential after artificial treatment (Ref. 10).
Like the most mesenchymal cells (Ref. 23), ASC express a cell surface marker profile that is comparable to BMSC (Refs 9, 24), fulfilling all requirements that have been suggested as the minimal criteria for defining multipotent mesenchymal stromal cells (Ref. 25). Although most mesenchymal cells also meet these standards (Refs 23, 26), a functional equivalence of ASC and BMSC was construed from this classification and subsequent studies focussed on the question of whether ASC exhibit analogous cartilage and bone regeneration capacities. For this purpose, ASC were directly tested for their application to osteochondral in vivo repair approaches; however, the question whether ASC are skeletal stem cells functionally equivalent to BMSC attracted little interest.
From a retrospective point of view, it seems inconsistent that ASC were first assessed for their cartilage and bone regeneration potential before their capacity to build up and maintain a physiological adipose tissue environment was investigated. Although in vitro engineered adipose tissue would have a promising potential for surgical soft tissue reconstruction (Ref. 27), strategies using ASC for that purpose are instead clearly outnumbered by approaches that use (pre-)adipocytes (Refs 28, 29, 30, 31) or even BMSC (Refs 32, 33, 34). Beside studies that address in vitro engineering of adipose tissue from ASC (Ref. 35), ASC were seeded in fibrin (Ref. 36), alginate (Ref. 37) or collagen scaffolds (Ref. 38) and subjected to an adipogenic pre-induction protocol prior to subcutaneous implantation. As expected after pre-induction, in vivo adipose tissue formation was reported in these studies. Evidence that transplanted clonal ASC can generate adipose tissue in vivo without such a pre-induction is still missing, but such a demonstration would not only be encouraging for their use in adipose tissue engineering but would also further clarify if ASC may indeed represent tissue-specific stem cells distinct from BMSC.
In vitro characteristics of expanded ASC and BMSC
Similar morphological features but different growth behaviour
A thorough review of the literature on in vitro performance of culture-expanded ASC and BMSC reveals strong similarities between stromal cells of both sources, factually overweighing the differences. For instance, no morphological differences were reported to date, and the same spindle-shaped phenotype was frequently described (Refs 39, 40, 41). Upon isolation, adherent human and mouse ASC seem to exhibit a higher proliferation rate (Refs 41, 42, 43, 44, 45), but equal growth behaviour compared to BMSC has also been reported (Refs 40, 46). In an extended comparison of growth kinetics, Dmitrieva et al. included the fact that the amount of colony-forming cells in the adipose-derived stromal vascular fraction (SVF) exceeds that of the bone marrow nucleated fraction (BM-NC) by at least two orders of magnitude (Refs 42, 43, 47). This leads to the result that ASC underwent significantly less population doublings up to the first passage, even if, as usual, more primary BM-NC were initially plated. It could therefore be speculated that signs of senescence only occur later in ASC (Ref. 43) because BMSC underwent more cell divisions in the same passage number. However, Dmitrieva et al. showed that ASC indeed possess extended proliferative potential, since more population doublings were observed before cells acquired a senescent phenotype (Ref. 42).
Dissimilar expression of cell surface markers CD106, CD146 and CD34
Extensive analyses have been conducted to map differences between cultured ASC and BMSC with regard to surface marker expression, leading to a reliable picture in which markers distinguish expanded stromal cells of both sources. Again, it is striking that, despite the application of large panels of antibodies (Refs 48, 49, 50), just a few CD markers are differently expressed between ASC and BMSC. Multiple reports describe higher expression of CD106 in BMSC (Refs 40, 43, 47, 48, 50, 51, 52, 53), whereas there exist some studies in which no difference was detected (Refs 49, 54). Analogous data exist for CD146, a cell surface marker of pericytes (Ref. 55) that has been used to enrich for multipotent cells (Refs 56, 57) and to identify the localisation of multipotent stromal cells in various tissues (Refs 12, 20, 22, 58). Adherent stromal cells from bone marrow and adipose tissue both contain a CD146-positive population, but this fraction is about twofold larger in early passage BMSC (Refs 42, 50, 53, 59).
By means of a quality control check that is commonly performed at the beginning of studies, ASC were frequently analysed for CD34-negativity, since the absence of this marker is a prerequisite to meet the minimal criteria for multipotent mesenchymal stromal cells (Refs 25, 47). However, several reports that dealt with a comparison of ASC and BMSC described that adherent ASC included a substantial CD34-positive fraction, whereas BMSC that were analysed in parallel were completely CD34-negative (Refs 48, 50, 52, 60, 61). In these studies, the selection of adherent cells from the adipose tissue-derived SVF at first led to a twofold enrichment of CD34-positive cells (Ref. 61), followed by a gradual decrease in subsequent passages (Ref. 24). Nevertheless, a considerable number of CD34-positive cells was still detected in passage 4 (Refs 48, 52), and Yoshimura et al. even described that after 20 weeks of cultivation, almost 20% of the ASC population was still CD34-positive. These inconsistent data on CD34 expression in ASC cultures may simply reflect that, depending on tissue source and isolation protocol, CD34-positive endothelial cells were occasionally included in primary isolates and gradually disappeared with culture time, due to unfavoured growth conditions. It remains to be determined, however, if this hypothesis or the choice of an antibody of the appropriate subclass (Ref. 47) accounts for the conflicting results. In any case, the general absence of CD34 in BMSC cultures represents another noticeable difference compared to ASC preparations.
Compared to the CD markers that were discussed above, considerably less experimental data indicate differential expression of CD10 (Ref. 47), CD133 (Ref. 40), CD54 (Ref. 48), HLA-ABC (Ref. 53) and CD49d/f (Refs 48, 50) between BMSC and ASC. In summary, the most substantiated differences regarding cell surface proteins are lower expression levels of CD106 (VCAM-1) and CD146 (MCAM) in ASC versus BMSC, both of which point to a less angiogenic signature of ASC that reoccurs in their gene expression profile as discussed below.
Reduced osteogenic gene expression signature in ASC
In terms of global gene and protein expression profiling, comparisons using cDNA microarrays and two-dimensional electrophoresis revealed high consistencies between multiclonal ASC and BMSC cultures (Refs 52, 62, 63). Nevertheless, hierarchical clustering of protein and gene expression data allowed for a separation of ASC and BMSC specimen, possibly due to the observation that Wnt-signalling-associated genes are more abundant in BMSC (Ref. 52). Increased expression levels of genes that are associated with osteogenesis were detected in BMSC (Refs 62, 64), arguing for a higher degree of osteochondral commitment (Ref. 59). A similar finding was made by mRNA representational difference analysis, where higher ITM2A expression in ASC could be attributed to a lower chondrogenic potential (Ref. 65). An enrichment of genes that are involved in angiogenic signalling pathways was reported for BMSC (Ref. 66) and confirmed by higher expression of angiogenic markers, such as angiopoietin and vascular endothelial growth factor (VEGF) in comparison to ASC (Ref. 59). On the other hand, ASC were shown to exhibit a more adipogenic gene expression pattern (Ref. 64), illustrated by higher expression levels of adiponectin and visfatin (Ref. 59). In line with the higher proliferation rate of ASC, genes involved in mitosis and DNA replication are also up-regulated compared to BMSC (Ref. 67).
Reduced performance of ASC in osteochondral in vitro differentiation assays
In line with indications of an intrinsic osteogenic potential of BMSC, exposure to common osteogenic differentiation media induced more mineralisation (Refs 40, 50, 68, 69), higher alkaline phosphatase activity (Refs 40, 44, 68) and stronger gene expression of osteogenic markers, such as runx2, osteocalcin, osterix, alkaline phosphatase and collagen-1 (Refs 40, 44, 52), compared to ASC. In turn, and corresponding to their physiological origin, ASC seem to exhibit a higher affinity to adipogenic differentiation, since inclusion of lipid droplets (Refs 44, 50, 53) and expression of the adipogenic marker gene peroxisome proliferator-activated receptor (PPARγ) (Refs 44, 53) were more intense than in BMSC upon induction. However, similar adipogenic in vitro differentiation capacities of adipose and bone marrow-derived cells were also reported (Refs 52, 69, 70), but no study described a higher adipogenic potential for BMSC. In line with better in vitro osteogenesis, BMSC also showed better performance in common chondrogenesis assays. In vitro differentiation of BMSC in 3D-pellet culture under treatment with TGF-β resulted in more intense collagen-II staining (Refs 39, 46, 62, 68, 69, 70), proteoglycan deposition (Refs 39, 46, 53, 62, 68, 70) and gene expression of Sox-9 (Ref. 53), compared to ASC. Interestingly, the inferior chondrogenic capacity of ASC can be augmented to BMSC levels when BMP-6 is added to the differentiation medium (Refs 62, 71), an observation with obvious relevance for future in vivo applications of ASC.
Comparison of trophic activity
One main path to tissue reconstruction by cell-based therapeutic strategies involves stem cell activity to establish and build new tissue by proliferating and differentiating cells, which are progeny of the implanted cells. A second way to regeneration is the stimulation of endogenous healing capacity by trophic activity of implanted cells, which attract host progenitor cells and organise repair by local and invading cells. Implanted cells may even disappear after this task has been successfully fulfilled. In this second scenario, even transient stem cell activity or differentiation capacity within target tissues may be dispensable as long as trophic activity is high.
Similar to the established trophic role of BMSC (Refs 72, 73), cultured ASC were shown to secrete a wide range of proteins (Ref. 74) into conditioned media that predominantly exert anti-apoptotic (Refs 75, 76, 77), immunomodulatory (Refs 78, 79, 80) and angiogenic (Refs 77, 81, 82) effects on co-cultured cell types. Extracellular matrix components and secreted enzymes comprised the largest fraction of the secretome, according to mass spectrometry (Refs 83, 84, 85), but these molecules are unlikely to be heavily involved in the observed paracrine signalling. These effects are instead mediated by secreted cytokines that typically appear in nano- or picomolar concentrations. Corresponding ELISA and multiplex approaches primarily identified VEGF (Refs 86, 87, 88), hepatocyte growth factor (HGF) (Refs 82, 89) and insulin-like growth factor-1 (IGF-1) (Refs 77, 90) as factors that are responsible for the described intercellular communication. According to this repertoire, both ASC and BMSC can be expected to display trophic functions (Refs 74, 91), delineating their potential to stimulate bone and cartilage regeneration solely by trophic mechanisms.
In vivo comparison of ASC and BMSC
The extent of the described in vitro differences between ASC and BMSC gives the impression that cells of both sources may fundamentally differ from each other. This point of view must be carefully considered, since in vitro variances may stem from dissimilar donor tissue processing, cell isolation protocols, cell yield and culture methods. In the context of osteochondral regeneration, the proof of in vivo exchangeability of ASC and BMSC is far more important, and aspects of in vivo stem cell activity like trophic activity should be considered, as long as precise healing mechanisms are unclear for the diverse application settings.
Untreated ASC do not form ectopic bone
Ectopic bone formation is a standard activity of human BMSC on calcium phosphate ceramics such as β-tricalcium phosphate (β-TCP) and hydroxyapatite (HA)/TCP in immunodeficient mice (Refs 20, 92, 93, 94, 95, 96, 97) with no osteogenic pre-induction protocols required, in line with skeletal stem cell activity of BMSC. Ectopic transplantation of ASC reliably led to de novo generation of bone when cells were subjected to osteogenic pre-induction before implantation (Refs 45, 98, 99, 100, 101). Overexpression of BMP-2/RUNX2 (Ref. 102) or BMP-7 (Ref. 103) in ASC allowed the omission of the pre-differentiation step.
Whether ASC possess the same intrinsic ability to form ectopic bone without any of these pre-treatments in standard assays, and to what extent they build up new bone themselves, largely remained elusive until the issue was recently addressed by Brocher et al. In this first standardised comparison of BMSC and ASC on multiple donors, ASC generated no ectopic bone on osteoconductive scaffolds, while samples from all BMSC donors formed ossicles under identical conditions (Ref. 59). The additional value of this ASC study was the reliable identification of donor as well as host cells within the ectopic setting via a recently established in situ hybridisation technique, specifically marking highly repetitive DNA sequences of human as well as mouse origin (Ref. 104). This demonstrated that ectopic bone was formed by ASC when they were subjected to chondrogenic pre-differentiation before transplantation. Although, in noninduced samples, ASC survived at the ectopic site for more than 8 weeks and they did not form bone, as seen in previous studies (Refs 45, 99, 100, 102, 105) (Table 1). Only Zannettino et al. have provided convincing data of ectopic bone formation after ASC implantation on HA/TCP in NOD/SCID mice, when CD146-pre-sorted cells were transplanted; however, the origin of bone from donor or host remained unclear (Ref. 12).
Table 1.
Summary of ectopic bone formation studies with ASC
| Pre-induction | ASC donor species | Acceptor species | Scaffold | Author |
|---|---|---|---|---|
| Yes | Human | Mouse | β-TCP | Brocher et al. (Ref. 59) |
| Yes | Human | Mouse | Healos© | Niemeyer et al. (Ref. 149) |
| Yes | Human | Mouse | Collagen- HA/TCP | Hicok et al. (Ref. 150) |
| Yes | Human | Mouse | β-TCP | Hattori et al. (Ref. 99) |
| Yes | Human | Mouse | ACHMS | Hattori et al. (Ref. 100) |
| Yes | Pig | Rat | BA | Schubert et al. (Ref. 45) |
| Yes | Rabbita | Rabbit | COL/PLGA–β-TCP | Hao et al. (Ref. 98) |
| Yes | Rat | Mouse | NanoBCP | Lin et al. (Ref. 101) |
| Yesb | Human | Mouse | PLGA | Lee et al. (Ref. 102) |
| Yesb | Rat | Rat | Collagen | Yang et al. (Ref. 103) |
| No | Human | Mouse | HA/TCP | Zannettino et al. (Ref. 12) |
| No | Human | Mouse | ENGIpore© | Scherberich et al. (Ref. 105) |
| No | Human | Rat | DBM | Supronowicz et al. (Ref. 151) |
| No | Doga | Dog | BCP | Yao et al. (Ref. 152) |
aAutologous setting.
bTransgene expression.
Abbreviations: ACHMS, atelocollagen honeycomb-shaped scaffold with membrane seal; BA, bone allograft; BCP, biphasic calcium phosphate; COL, collagen; DBM, demineralised bone matrix; HA, hydroxyapatite; PLGA, polylactic acid/polyglycolic acid co-polymer; β-TCP, β-tricalcium phosphate.
All in all, beyond their reduced performance in osteochondral in vitro differentiation assays, ASC showed no intrinsic osteochondral in vivo differentiation potential and, thus, seem to possess no skeletal stem cell properties as seen with BMSC, providing a strong argument for fundamental functional differences regarding their use for in vivo osteochondral repair. Since nonclonal cells are widely used for tissue regeneration, the benefit of enhanced availability of ASC, therefore, appears currently to be balanced by an enhanced need for inductive conditions via timely and intensive in vitro culture efforts, if their physical contribution to the new skeletal tissue is desired.
ASC and BMSC require pre-differentiation for ectopic cartilage formation
The most convincing demonstration of spontaneous chondrogenic in vivo potential of ASC and BMSC derives from observations of ectopic cartilage deposits in assays, in which articular chondrocytes form cartilaginous nodules at ectopic sites in the absence of chondrogenic inducers. Importantly, such activity has so far neither been demonstrated in human BMSC nor ASC, and an apparent necessary aspect of common strategies for successful ectopic cartilage formation includes chondrogenic pre-differentiation. Several studies have been performed in which human ASC were either used without scaffolds (Refs 62, 106) or seeded on hydrogels (Refs 106, 107, 108) or glycolic acid/lactic acid copolymer (PLGA) (Refs 109, 110, 111). In addition, different strategies were used for chondrogenic pre-differentiation of ASC (Table 2). All included TGF-β treatment either during 3D pellet culture (Refs 62, 107, 108), in vitro cultivation of ASC in the scaffold (Refs 110, 111) or by adenoviral TGF-β overexpression (Ref. 109) before subcutaneous implantation into immunocompromised mice. Ectopic cartilage composed of implanted cells was observed in all cases, but chondrocyte hypertrophy and matrix calcification were unwanted side effects reminiscent of growth plate chondrocytes (Refs 62, 112). In conclusion, analogous studies without chondrogenic (pre-) induction have remained unsuccessful, with neither BMSC nor ASC displaying intrinsic chondrogenic potential nor trophic activity leading to generation of ectopic cartilage of donor or host origin, compared to chondroprogenitors from cartilage which do display such activity (Ref. 113).
Table 2.
Summary of ectopic cartilage formation studies with ASC
| Pre-induction | ASC donor species | Acceptor species | Scaffold | Author |
|---|---|---|---|---|
| Yes | Human | Mouse | PLGA | Mehlhorn et al. (Ref. 111) |
| Yes | Human | Mouse | fibrin | Yoon et al. (Ref. 108) |
| Yes | Human | Mouse | PLCL/fibrin | Jung et al. (Ref. 110) |
| Yesa | Human | Mouse | PLGA/alginate | Jin et al. (Ref. 109) |
aTransgene expression.
Abbreviations: PLCL, polylactic acid/polycaprolactone co-polymer; PLGA, polylactic acid/polyglycolic acid co-polymer.
Missing evidence for physical ASC contribution to the repair of damaged cartilage
The most direct and least invasive approach to use ASC for the treatment of cartilage defects is by intra-articular injection of cells. Studies that started with an induction of osteoarthritis (OA) by anterior cruciate ligament transection (ACLT) or collagenase treatment, followed by intra-articular injection of autologous ASC, have been conducted in mouse (Ref. 114) and rabbit (Refs 115, 116) (Table 3). Different histological evaluations and OA scoring scales were used to measure OA progression, but in all cases, positive effects of ASC compared to the injection of cell-free solvent were reported. Labelled ASC were detectable in the synovial membrane and medial meniscus 20 days after injection (Ref. 115) and at the synovial lining and cruciate ligaments up to 5 days after injection (Ref. 114). Human ASC injected into unimpaired mouse knee joints showed long-term persistence in joint tissue in 60% of all mice up to 186 days after injection, but a substantial fraction of ASC seemed to have migrated to the bone marrow, adipose tissue and muscle. Thus, while a certain degree of persistence of injected cells can therefore be assumed, evidence for in vivo differentiation of donor ASC or long-term integration into articular cartilage tissue is missing, and contributions by trophic activity cannot be judged.
Table 3.
Summary of orthotopic cartilage formation studies with ASC
| Defect | ASC donor species | Acceptor species | Scaffold | Pre-induction | Long-term engraftment | Author |
|---|---|---|---|---|---|---|
| Femur | Piga | Pig | PGA/PLA | Yes | n.d. | Cui et al. (Ref. 119) |
| Femur | Rabbita | Rabbit | PLGA | Yes | n.d. | Im et al. (Ref. 118) |
| Femur | Rabbita | Rabbit | Fibrin | Yes | >8 weeks | Dragoo et al. (Ref. 117) |
| OA | Rabbita | Rabbit | (i.a. injection) | No | Nonlesional regions | Desando et al. (Ref. 115) |
| OA | Rabbit | Rabbit | (i.a. injection) | No | n.d. | Toghraie et al. (Ref. 116) |
| OA | Mouse | Mouse | (i.a. injection) | No | Nonlesional regions | ter Huurne et al. (Ref. 114) |
aAutologous setting.
Abbreviations: i.a., intraarticular; n.d., not determined; OA, osteoarthritis model; PGA, polyglycolic acid; PLA, polylactic acid; PLGA, polylactic acid/polyglycolic acid co-polymer; β-TCP, β-tricalcium phosphate.
Besides artificial OA induction, the capacity of ASC to repair surgical cartilage incisions has been investigated (Table 3). In a scheme similar to ACT, ASC were first harvested, expanded and subjected to chondrogenic pre-induction for 2–3 weeks in vitro. Cells were then loaded on diverse scaffolds and re-implanted into the lesion site. Corresponding studies were conducted in rabbit (Refs 117, 118) and pig (Ref. 119) and differ in pre-induction methods and scaffold composition, but improved healing was consistently reported compared to cell-free matrix baseline conditions. Among these studies, only Dragoo et al. analysed the persistence of donor cells, making use of ASC expressing a LacZ reporter gene (Ref. 117). All explants showed ASC remaining 8 weeks after transplantation, but their direct contribution to cartilage tissue was not investigated. Notably, approaches for cartilage regeneration without in vitro pre-induction of ASC have so far not been described, although BMSC were successfully used in such a setting (Refs 120, 121, 122). Thus, the question of functional equivalence of BMSC and ASC in cartilage repair studies cannot be judged from the current literature, and a direct comparison of both cell sources under identical conditions is highly desired. Importantly, a lack of osteochondral commitment of expanded ASC, although negating their skeletal stem cell activity, may not be a major disadvantage if high trophic activity is paramount for therapeutic action for tissue regeneration and can be achieved with this cell type.
Site-dependant bone repair capacity of ASC
The majority of ASC-based tissue engineering approaches are directed at orthotopic in vivo formation of bone (Table 4). Across all of these studies, a well agreed upon point is that repair of defective bone by ASC can be achieved when transplantation is preceded by extensive pre-differentiation protocols (Refs 101, 123, 124, 125, 126, 127, 128) or genetic manipulation with genes encoding for bone inducers (Refs 129, 130, 131, 132, 133). Overexpression of BMP-2 represents the most common strategy for the latter technique, using transgenic ASC for local growth factor delivery, rather than expecting spontaneous differentiation into osteoblasts. BMP-2 overexpression in ASC has also been used to substitute strategies that include immobilisation of recombinant BMP-2 protein to scaffolds prior to implantation (Refs 134, 135, 136). Although these cell-free approaches reproducibly led to good healing efficiency, combinations of ASC and recombinant BMP-2 were also used (Refs 137, 138, 139, 140, 141, 142) (not listed in Table 4), but only Levi et al. described that ASC improved defect repair in comparison to the BMP-loaded scaffold alone (Ref. 142).
Table 4.
Summary of orthotopic bone formation studies with ASC
| Pre-induction | Defect | ASC donor species | Acceptor species | Scaffold | Long-term engraftment | Author |
|---|---|---|---|---|---|---|
| Cranium | ||||||
| Yes | Calvaria | Rat | Rat | NanoBCP | n.d. | Lin et al. (Ref. 101) |
| Yes | Palate | Rat | Rat | PLA | n.d. | Conejero et al. (Ref. 123) |
| Yes | Parietal | Human | Rat | PCL/PLGA/TCP | n.d. | Kim et al. (Ref. 127) |
| Yes | Parietal | Human | Rat | PLGA | n.d. | Yoon et al. (Ref. 128) |
| Yes | Parietal | Doga | Dog | Coral scaffold | n.d. | Cui et al. (Ref. 124) |
| Yes | Parietal | Rabbita | Rabbit | PLA | n.d. | Di Bella et al. (Ref. 125) |
| Yes | Parietalb | Rabbita | Rabbit | Gelatin foam | n.d. | Dudas et al. (Ref. 126) |
| Long bone | ||||||
| Yesc | Femur | Human | Rat | CP-collagen | n.d. | Peterson et al. (Ref. 132) |
| Yesc | Femur | Rabbit | Rabbit | PLGA | 4 weeks | Lin et al. (Ref. 131) |
| Yesc | Ulna | Minipig | Minipig | ABM | n.d. | Chen et al. (Ref. 129) |
| Yesc | Radius | Rabbita | Rabbit | PLA/PCL | n.d. | Han and Li (Ref. 130) |
| Vertebral | ||||||
| Yesc | Coccyxb | Pig | Rat | Fibrin | >12 weeks | Sheyn et al. (Ref. 133) |
| Cranium | ||||||
| No | Calvaria | Human, mouse, dog | Mouse | Apatite-PLGA | n.d. | Levi et al. (Ref. 145) |
| No | Mandible | Human | Rat | Fibrin | n.d. | Streckbein et al. (Ref. 146) |
| No | Mandibulab | Pig | Pig | (i.v., i.d.) | n.d. | Wilson et al. (Ref. 147) |
| No | Palate | Rat | Rat | PLA | n.d. | Conejero et al. (Ref. 123) |
| No | Parietal | Mouse | Mouse | Apatite-PLGA | >12 weeks | Cowan et al. (Ref. 144) |
| No | Parietal | Human | Mouse | Apatite-PLGA | 2 weeks | Levi et al. (Ref. 142) |
| No | Parietal | Rabbita | Rabbit | PLA | n.d. | Di Bella et al. (Ref. 125) |
| No | Parietal | Human | Rat | PLGA | n.d. | Yoon et al. (Ref. 128) |
| No | Parietalb | Rabbita | Rabbit | Gelatin foam | n.d. | Dudas et al. (Ref. 126) |
| Long bone | ||||||
| Nod | Femur | Human | Rat | CP-collagen | n.d. | Peterson et al. (Ref. 132) |
| Nod | Femur | Rabbit | Rabbit | PLGA | 4 weeks | Lin et al. (Ref. 131) |
| Nod | Ulna | Minipig | Minipig | ABM | n.d. | Chen et al. (Ref. 129) |
| Nod | Radius | Rabbita | Rabbit | PLA/PCL | n.d. | Han and Li (Ref. 130) |
| No | Tibia | Sheepa | Sheep | Healos© | n.d. | Niemeyer et al. (Ref. 143) |
aAutologous setting.
bNoncritical size.
cTransgene expression.
dControl group of the study.
Abbreviations: ABM, acellular bone matrix; BCP, biphasic calcium phosphate; CP, calcium phosphate ceramic; i.a., intraarticular; i.d., intra defect; n.d., not determined; PCL, polycaprolactone; PLA, polylactic acid; PLGA, polylactic acid/polyglycolic acid co-polymer; β-TCP, β-tricalcium phosphate.
Controversial data exist regarding the performance of expanded but otherwise untreated ASC in the context of long bone repair, although only a limited number of studies is available (Table 4). When nontransgenic or mock-transduced ASC were loaded on carrier matrices and transplanted into long-bone defects, no bone formation was observed (Refs 129, 131, 132). In a sheep long-bone model, ASC were unable to induce defect bridging, while BMSC facilitated defect regeneration in the same setting (Ref. 143). A closer look at the ASC control groups of the above studies further confirms the impression that pre-differentiation or genetic manipulation of ASC is a prerequisite for stimulation of bone formation. This applies even for orthotopic sites in long bones, where the microenvironment is rich in osteoinductive proteins which are released from the defect endings. To our knowledge, the only exception when nontransduced ASC led to substantial bone formation in the context of long-bone repair is a study by Han and Li, in which ASC were used as a control to Runx2-overexpressing cells. Possibly, the surgical connection of the implant to the vascular network was the key to the positive results of this study (Ref. 130).
Dissimilar to long-bone repair, healing of critical size defects in the cranium appears to be less challenging with untreated ASC, since bone formation without any in vitro pre-differentiation was reported in at least four studies (Refs 144, 145, 146, 147). Furthermore, untreated ASC that were transplanted as controls for newly established repair strategies also generated considerable amounts of bone (Refs 125, 128, 142), although complete absence of defect repair by ASC control groups has also been described (Refs 123, 126). Thus, orthotopic bone formation by uninduced ASC appears to be site-dependent and favoured by characteristics of the cranial microenvironment that are not present in long bones. Origin from the ectodermal germ layer, development via the intramembranous pathway and enhanced blood supply differentiates bone in the cranium from long bones. Thus, it is tempting to speculate that one major advantage in the cranium may be the denser vascular network of skull bones, which is especially interesting in the context that, beyond an absent osteochondral commitment, a lower angiogenic signature was noted for ASC (Refs 42, 59, 66, 148) and orthotopic bone formation by ACS can be triggered by co-transplantation of endothelial cells (Ref. 127).
If the trophic activity of ASC is the most crucial for stimulation of bone repair, a lower requirement for attraction and stimulation of endothelial progenitors could explain the better performance of ASC in the cranium. In line with this, a persistence of donor ASC could not be detected for more than 2 to 4 weeks after transplantation, even in settings where complete cranial defect repair was observed (Refs 131, 142). In sharp contrast, a single study by Cowan et al. reported that transplantation of uninduced ASC led to stable engraftment of the cells in a cranial defect and over 95% of nuclei in the newly formed bone were donor-derived after 12 weeks (Ref. 144). As it is not apparent which specific experimental parameters have enabled this exceptional engraftment, analogous success is waiting for repetition. Overall, particular success of ASC in cranial but not long-bone defects suggests that, in view of their low osteochondral and angiogenic signature, ASC affect bone regeneration most probably via their trophic activity than by in situ differentiation to osteoblasts with long-term persistence. Additional precise studies must unravel the contribution of host and donor cells to tissue repair as well as the influence of scaffolds, pre-cultivation, species and defect site in order to reach consensus on the main mechanisms driving ASC-dependent promotion of osteoarticular repair despite lower osteogenic and angiogenic signatures and an apparent lack of skeletal stem cell properties.
Conclusion
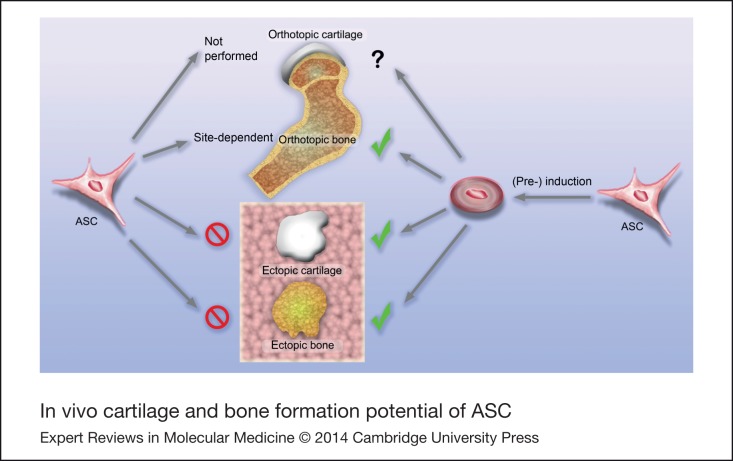
More than 50 in vivo studies have been performed to date in order to verify the potential of ASC to be used for osteoarticular regeneration. In each of the quite heterogeneous experimental setups, specific protocols were established that either enabled chondrogenic or osteogenic differentiation of the cells or that resulted in positive effects on defect healing. Regarding the greater accessibility of ASC compared to BMSC, these data are entirely encouraging for the future use of ACS in skeletal regenerative medicine. However, it is now clear that ASC do not exhibit the same degree of osteoarticular predetermination as BMSC and more manipulation is required to drive ASC into the chondrogenic or osteogenic lineage (Fig. 1). The observations that the spontaneous formation of an ectopic bone organ by BMSC cannot be reproduced with ASC and that orthotopic bone formation is only stimulated at favoured sites confirm this issue and thereby exclude a skeletal stem cell identity for ASC. Altogether, a review of the literature suggests that mainly trophic functions determine the therapeutic outcome after ASC application. Future research is needed on a direct comparison of BMSC and ASC in osteoarticular therapy to decide where and how successful BMSC protocols have to be modified to achieve promising results with ASC.
Figure 1.
In vivo cartilage and bone formation potential of ASC: site dependence and requirement for pre-differentiation.
Financial Support
This work was supported by grants from the German Research Foundation, reference numbers RI707/7-1, RI707/8-1 and BO3661/1-1.
Conflicts of Interest
None.
References
- 1.Brittberg M. et al. (1994) Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. New England Journal of Medicine 331, 889-895 [DOI] [PubMed] [Google Scholar]
- 2.Heslop B.F., Zeiss I.M. and Nisbet N.W. (1960) Studies on transference of bone. I. A comparison of autologous and homologous bone implants with reference to osteocyte survival, osteogenesis and host reaction. British Journal of Experimental Pathology 41, 269-287 [PMC free article] [PubMed] [Google Scholar]
- 3.Marlovits S. et al. (2006) Cartilage repair: generations of autologous chondrocyte transplantation. European Journal of Radiology 57, 24-31 [DOI] [PubMed] [Google Scholar]
- 4.Nejadnik H. et al. (2010) Autologous bone marrow-derived mesenchymal stem cells versus autologous chondrocyte implantation: an observational cohort study. American Journal of Sports Medicine 38, 1110-1116 [DOI] [PubMed] [Google Scholar]
- 5.Zuk P.A. et al. (2001) Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Engineering 7, 211-228 [DOI] [PubMed] [Google Scholar]
- 6.Estes B.T. et al. (2010) Isolation of adipose-derived stem cells and their induction to a chondrogenic phenotype. Nature Protocols 5, 1294-1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oedayrajsingh-Varma M.J. et al. (2006) Adipose tissue-derived mesenchymal stem cell yield and growth characteristics are affected by the tissue-harvesting procedure. Cytotherapy 8, 166-177 [DOI] [PubMed] [Google Scholar]
- 8.Pittenger M.F. et al. (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284, 143-147 [DOI] [PubMed] [Google Scholar]
- 9.Gronthos S. et al. (2001) Surface protein characterization of human adipose tissue-derived stromal cells. Journal of Cellular Physiology 189, 54-63 [DOI] [PubMed] [Google Scholar]
- 10.Zuk P.A. et al. (2002) Human adipose tissue is a source of multipotent stem cells. Molecular Biology of the Cell 13, 4279-4295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi S. and Gronthos S. (2003) Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. Journal of Bone and Mineral Research 18, 696-704 [DOI] [PubMed] [Google Scholar]
- 12.Zannettino A.C. et al. (2008) Multipotential human adipose-derived stromal stem cells exhibit a perivascular phenotype in vitro and in vivo. Journal of Cellular Physiology 214, 413-421 [DOI] [PubMed] [Google Scholar]
- 13.Hwang J.H. et al. (2013) Combination therapy of human adipose-derived stem cells and basic fibroblast growth factor hydrogel in muscle regeneration. Biomaterials 34, 6037-6045 [DOI] [PubMed] [Google Scholar]
- 14.Lin Y.C. et al. (2013) Evaluation of a multi-layer adipose-derived stem cell sheet in a full-thickness wound healing model. Acta Biomaterialia 9, 5243-5250 [DOI] [PubMed] [Google Scholar]
- 15.Rousseau A. et al. (2013) Adipose-derived stromal cells for the reconstruction of a human vesical equivalent. Journal of Tissue Engineering and Regenerative Medicine, doi: 10.1002/term.1717 [DOI] [PubMed] [Google Scholar]
- 16.Zhang R. et al. (2012) Nuclear fusion-independent smooth muscle differentiation of human adipose-derived stem cells induced by a smooth muscle environment. Stem Cells 30, 481-490 [DOI] [PubMed] [Google Scholar]
- 17.Rao M.S. (2004) Stem sense: a proposal for the classification of stem cells. Stem Cells and Development 13, 452-455 [DOI] [PubMed] [Google Scholar]
- 18.Bianco P. et al. (2013) The meaning, the sense and the significance: translating the science of mesenchymal stem cells into medicine. Nature Medicine 19, 35-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Becker A.J., Mc C.E. and Till J.E. (1963) Cytological demonstration of the clonal nature of spleen colonies derived from transplanted mouse marrow cells. Nature 197, 452-454 [DOI] [PubMed] [Google Scholar]
- 20.Sacchetti B. et al. (2007) Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell 131, 324-336 [DOI] [PubMed] [Google Scholar]
- 21.Chan C.K. et al. (2013) Clonal precursor of bone, cartilage, and hematopoietic niche stromal cells. Proceedings of the National Academy Science of the United States of America 110, 12643-12648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crisan M. et al. (2008) A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3, 301-313 [DOI] [PubMed] [Google Scholar]
- 23.Alt E. et al. (2011) Fibroblasts share mesenchymal phenotypes with stem cells, but lack their differentiation and colony-forming potential. Biology of the Cell 103, 197-208 [DOI] [PubMed] [Google Scholar]
- 24.Mitchell J.B. et al. (2006) Immunophenotype of human adipose-derived cells: temporal changes in stromal-associated and stem cell-associated markers. Stem Cells 24, 376-385 [DOI] [PubMed] [Google Scholar]
- 25.Dominici M. et al. (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8, 315-317 [DOI] [PubMed] [Google Scholar]
- 26.Hematti P. (2012) Mesenchymal stromal cells and fibroblasts: a case of mistaken identity? Cytotherapy 14, 516-521 [DOI] [PubMed] [Google Scholar]
- 27.Gomillion C.T. and Burg K.J. (2006) Stem cells and adipose tissue engineering. Biomaterials 27, 6052-6063 [DOI] [PubMed] [Google Scholar]
- 28.Cho S.W. et al. (2005) Engineering of volume-stable adipose tissues. Biomaterials 26, 3577-3585 [DOI] [PubMed] [Google Scholar]
- 29.Halbleib M. et al. (2003) Tissue engineering of white adipose tissue using hyaluronic acid-based scaffolds. I. In vitro differentiation of human adipocyte precursor cells on scaffolds. Biomaterials 24, 3125-3132 [DOI] [PubMed] [Google Scholar]
- 30.Hemmrich K. et al. (2005) Implantation of preadipocyte-loaded hyaluronic acid-based scaffolds into nude mice to evaluate potential for soft tissue engineering. Biomaterials 26, 7025-7037 [DOI] [PubMed] [Google Scholar]
- 31.Patel P.N. et al. (2005) Poly(ethylene glycol) hydrogel system supports preadipocyte viability, adhesion, and proliferation. Tissue Engineering 11, 1498-1505 [DOI] [PubMed] [Google Scholar]
- 32.Hong L. et al. (2005) Ex vivo adipose tissue engineering by human marrow stromal cell seeded gelatin sponge. Annals of Biomedical Engineering 33, 511-517 [DOI] [PubMed] [Google Scholar]
- 33.Mauney J.R., Volloch V. and Kaplan D.L. (2005) Matrix-mediated retention of adipogenic differentiation potential by human adult bone marrow-derived mesenchymal stem cells during ex vivo expansion. Biomaterials 26, 6167-6175 [DOI] [PubMed] [Google Scholar]
- 34.Neubauer M. et al. (2005) Adipose tissue engineering based on mesenchymal stem cells and basic fibroblast growth factor in vitro. Tissue Engineering 11, 1840-1851 [DOI] [PubMed] [Google Scholar]
- 35.Vermette M. et al. (2007) Production of a new tissue-engineered adipose substitute from human adipose-derived stromal cells. Biomaterials 28, 2850-2860 [DOI] [PubMed] [Google Scholar]
- 36.Mizuno H. et al. (2008) In vivo adipose tissue regeneration by adipose-derived stromal cells isolated from GFP transgenic mice. Cells Tissues Organs 187, 177-185 [DOI] [PubMed] [Google Scholar]
- 37.Jing W. et al. (2007) Ectopic adipogenesis of preconditioned adipose-derived stromal cells in an alginate system. Cell and Tissue Research 330, 567-572 [DOI] [PubMed] [Google Scholar]
- 38.Lu F. et al. (2006) Adipose tissues differentiated by adipose-derived stem cells harvested from transgenic mice. Chinese Journal of Traumatology 9, 359-364 [PubMed] [Google Scholar]
- 39.Diekman B.O. et al. (2010) Chondrogenesis of adult stem cells from adipose tissue and bone marrow: induction by growth factors and cartilage-derived matrix. Tissue Engineering A 16, 523-533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shafiee A. et al. (2011) A comparison between osteogenic differentiation of human unrestricted somatic stem cells and mesenchymal stem cells from bone marrow and adipose tissue. Biotechnology Letters 33, 1257-1264 [DOI] [PubMed] [Google Scholar]
- 41.Zhou Z. et al. (2013) Comparison of mesenchymal stromal cells from human bone marrow and adipose tissue for the treatment of spinal cord injury. Cytotherapy 15, 434-448 [DOI] [PubMed] [Google Scholar]
- 42.Dmitrieva R.I. et al. (2012) Bone marrow- and subcutaneous adipose tissue-derived mesenchymal stem cells: differences and similarities. Cell Cycle 11, 377-383 [DOI] [PubMed] [Google Scholar]
- 43.Kern S. et al. (2006) Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 24, 1294-1301 [DOI] [PubMed] [Google Scholar]
- 44.Peng L. et al. (2008) Comparative analysis of mesenchymal stem cells from bone marrow, cartilage, and adipose tissue. Stem Cells and Development 17, 761-773 [DOI] [PubMed] [Google Scholar]
- 45.Schubert T. et al. (2011) The enhanced performance of bone allografts using osteogenic-differentiated adipose-derived mesenchymal stem cells. Biomaterials 32, 8880-8891 [DOI] [PubMed] [Google Scholar]
- 46.Danisovic L. et al. (2009) Comparison of in vitro chondrogenic potential of human mesenchymal stem cells derived from bone marrow and adipose tissue. General Physiology and Biophysics 28, 56-62 [PubMed] [Google Scholar]
- 47.Bourin P. et al. (2013) Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 15, 641-648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.De Ugarte D.A. et al. (2003) Differential expression of stem cell mobilization-associated molecules on multi-lineage cells from adipose tissue and bone marrow. Immunology Letters 89, 267-270 [DOI] [PubMed] [Google Scholar]
- 49.Niemeyer P. et al. (2007) Comparison of immunological properties of bone marrow stromal cells and adipose tissue-derived stem cells before and after osteogenic differentiation in vitro. Tissue Engineering 13, 111-121 [DOI] [PubMed] [Google Scholar]
- 50.Pachon-Pena G. et al. (2011) Stromal stem cells from adipose tissue and bone marrow of age-matched female donors display distinct immunophenotypic profiles. Journal of Cellular Physiology 226, 843-851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boeuf S. and Richter W. (2010) Chondrogenesis of mesenchymal stem cells: role of tissue source and inducing factors. Stem Cell Research & Therapy 1, 31-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Noel D. et al. (2008) Cell specific differences between human adipose-derived and mesenchymal-stromal cells despite similar differentiation potentials. Experimental Cell Research 314, 1575-1584 [DOI] [PubMed] [Google Scholar]
- 53.Rider D.A. et al. (2008) Autocrine fibroblast growth factor 2 increases the multipotentiality of human adipose-derived mesenchymal stem cells. Stem Cells 26, 1598-1608 [DOI] [PubMed] [Google Scholar]
- 54.Ikegame Y. et al. (2011) Comparison of mesenchymal stem cells from adipose tissue and bone marrow for ischemic stroke therapy. Cytotherapy 13, 675-685 [DOI] [PubMed] [Google Scholar]
- 55.Shih I.M. (1999) The role of CD146 (Mel-CAM) in biology and pathology. Journal of Pathology 189, 4-11 [DOI] [PubMed] [Google Scholar]
- 56.Ruetze M. et al. (2013) A novel niche for skin derived precursors in non-follicular skin. Journal of Dermatological Science 69, 132-139 [DOI] [PubMed] [Google Scholar]
- 57.Sorrentino A. et al. (2008) Isolation and characterization of CD146+ multipotent mesenchymal stromal cells. Experimental Hematology 36, 1035-1046 [DOI] [PubMed] [Google Scholar]
- 58.Covas D.T. et al. (2008) Multipotent mesenchymal stromal cells obtained from diverse human tissues share functional properties and gene-expression profile with CD146+ perivascular cells and fibroblasts. Experimental Hematology 36, 642-654 [DOI] [PubMed] [Google Scholar]
- 59.Brocher J. et al. (2013) Inferior ectopic bone formation of mesenchymal stromal cells from adipose tissue compared to bone-marrow: rescue by chondrogenic pre-induction. Stem Cell Res 11(3), 1393-406 [DOI] [PubMed] [Google Scholar]
- 60.Yoshimura K. et al. (2006) Characterization of freshly isolated and cultured cells derived from the fatty and fluid portions of liposuction aspirates. Journal of Cellular Physiology 208, 64-76 [DOI] [PubMed] [Google Scholar]
- 61.Maumus M. et al. (2011) Native human adipose stromal cells: localization, morphology and phenotype. International Journal of Obesity (London) 35, 1141-1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hennig T. et al. (2007) Reduced chondrogenic potential of adipose tissue derived stromal cells correlates with an altered TGFbeta receptor and BMP profile and is overcome by BMP-6. Journal of Cellular Physiology 211, 682-691 [DOI] [PubMed] [Google Scholar]
- 63.Lee R.H. et al. (2004) Characterization and expression analysis of mesenchymal stem cells from human bone marrow and adipose tissue. Cellular Physiology and Biochemistry 14, 311-324 [DOI] [PubMed] [Google Scholar]
- 64.Al-Nbaheen M. et al. (2013) Human stromal (mesenchymal) stem cells from bone marrow, adipose tissue and skin exhibit differences in molecular phenotype and differentiation potential. Stem Cell Reviews 9, 32-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Boeuf S. et al. (2009) Enhanced ITM2A expression inhibits chondrogenic differentiation of mesenchymal stem cells. Differentiation 78, 108-115 [DOI] [PubMed] [Google Scholar]
- 66.Monaco E. et al. (2012) Transcriptomics comparison between porcine adipose and bone marrow mesenchymal stem cells during in vitro osteogenic and adipogenic differentiation. PLoS ONE 7, e32481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nakanishi C. et al. (2011) Gene and protein expression analysis of mesenchymal stem cells derived from rat adipose tissue and bone marrow. Circulation Journal 75, 2260-2268 [DOI] [PubMed] [Google Scholar]
- 68.Im G.I., Shin Y.W. and Lee K.B. (2005) Do adipose tissue-derived mesenchymal stem cells have the same osteogenic and chondrogenic potential as bone marrow-derived cells? Osteoarthritis and Cartilage 13, 845-853 [DOI] [PubMed] [Google Scholar]
- 69.Liu T.M. et al. (2007) Identification of common pathways mediating differentiation of bone marrow- and adipose tissue-derived human mesenchymal stem cells into three mesenchymal lineages. Stem Cells 25, 750-760 [DOI] [PubMed] [Google Scholar]
- 70.Huang J.I. et al. (2005) Chondrogenic potential of progenitor cells derived from human bone marrow and adipose tissue: a patient-matched comparison. Journal of Orthopaedic Research 23, 1383-1389 [DOI] [PubMed] [Google Scholar]
- 71.Hildner F. et al. (2010) FGF-2 abolishes the chondrogenic effect of combined BMP-6 and TGF-beta in human adipose derived stem cells. Journal of Biomedical Materials Research A 94, 978-987 [DOI] [PubMed] [Google Scholar]
- 72.Choi Y.A. et al. (2010) Secretome analysis of human BMSCs and identification of SMOC1 as an important ECM protein in osteoblast differentiation. Journal of Proteome Research 9, 2946-2956 [DOI] [PubMed] [Google Scholar]
- 73.Polacek M. et al. (2011) The secretory profiles of cultured human articular chondrocytes and mesenchymal stem cells: implications for autologous cell transplantation strategies. Cell Transplantation 20, 1381-1393 [DOI] [PubMed] [Google Scholar]
- 74.Skalnikova H. et al. (2011) Mapping of the secretome of primary isolates of mammalian cells, stem cells and derived cell lines. Proteomics 11, 691-708 [DOI] [PubMed] [Google Scholar]
- 75.Saito Y. et al. (2013) The protective effect of adipose-derived stem cells against liver injury by trophic molecules. Journal of Surgical Research 180, 162-168 [DOI] [PubMed] [Google Scholar]
- 76.Wei X. et al. (2009) IFATS collection: the conditioned media of adipose stromal cells protect against hypoxia-ischemia-induced brain damage in neonatal rats. Stem Cells 27, 478-488 [DOI] [PubMed] [Google Scholar]
- 77.Sadat S. et al. (2007) The cardioprotective effect of mesenchymal stem cells is mediated by IGF-I and VEGF. Biochemical and Biophysical Research Communications 363, 674-679 [DOI] [PubMed] [Google Scholar]
- 78.Park Y.S. et al. (2012) Improved viability and activity of neutrophils differentiated from HL-60 cells by co-culture with adipose tissue-derived mesenchymal stem cells. Biochemical and Biophysical Research Communications 423, 19-25 [DOI] [PubMed] [Google Scholar]
- 79.Peng W. et al. (2012) Adipose-derived stem cells induced dendritic cells undergo tolerance and inhibit Th1 polarization. Cellular Immunology 278, 152-157 [DOI] [PubMed] [Google Scholar]
- 80.Puissant B. et al. (2005) Immunomodulatory effect of human adipose tissue-derived adult stem cells: comparison with bone marrow mesenchymal stem cells. British Journal of Haematology 129, 118-129 [DOI] [PubMed] [Google Scholar]
- 81.Holnthoner W. et al. (2012) Adipose-derived stem cells induce vascular tube formation of outgrowth endothelial cells in a fibrin matrix. Journal of Tissue Engineering and Regenerative Medicine, doi: 10.1002/term.1620 [DOI] [PubMed] [Google Scholar]
- 82.Rehman J. et al. (2004) Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation 109, 1292-1298 [DOI] [PubMed] [Google Scholar]
- 83.Chiellini C. et al. (2008) Characterization of human mesenchymal stem cell secretome at early steps of adipocyte and osteoblast differentiation. BMC Molecular Biology 9, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lee M.J. et al. (2010) Proteomic analysis of tumor necrosis factor-alpha-induced secretome of human adipose tissue-derived mesenchymal stem cells. Journal of Proteome Research 9, 1754-1762 [DOI] [PubMed] [Google Scholar]
- 85.Zvonic S. et al. (2007) Secretome of primary cultures of human adipose-derived stem cells: modulation of serpins by adipogenesis. Molecular & Cellular Proteomics 6, 18-28 [DOI] [PubMed] [Google Scholar]
- 86.Lee C.S. et al. (2012) Adipose stem cells can secrete angiogenic factors that inhibit hyaline cartilage regeneration. Stem Cell Research & Therapy 3, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Blaber S.P. et al. (2012) Analysis of in vitro secretion profiles from adipose-derived cell populations. Journal of Translational Medicine 10, 172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cai L. et al. (2009) IFATS collection: human adipose tissue-derived stem cells induce angiogenesis and nerve sprouting following myocardial infarction, in conjunction with potent preservation of cardiac function. Stem Cells 27, 230-237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kilroy G.E. et al. (2007) Cytokine profile of human adipose-derived stem cells: expression of angiogenic, hematopoietic, and pro-inflammatory factors. Journal of Cellular Physiology 212, 702-709 [DOI] [PubMed] [Google Scholar]
- 90.Wang M. et al. (2006) Human progenitor cells from bone marrow or adipose tissue produce VEGF, HGF, and IGF-I in response to TNF by a p38 MAPK-dependent mechanism. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology 291, R880-R884 [DOI] [PubMed] [Google Scholar]
- 91.Caplan A.I. and Dennis J.E. (2006) Mesenchymal stem cells as trophic mediators. Journal of Cellular Biochemistry 98, 1076-1084 [DOI] [PubMed] [Google Scholar]
- 92.Janicki P. et al. (2011) Prediction of in vivo bone forming potency of bone marrow-derived human mesenchymal stem cells. European Cells & Materials 21, 488-507 [DOI] [PubMed] [Google Scholar]
- 93.Kasten P. et al. (2008) Porosity and pore size of beta-tricalcium phosphate scaffold can influence protein production and osteogenic differentiation of human mesenchymal stem cells: an in vitro and in vivo study. Acta Biomaterialia 4, 1904-1915 [DOI] [PubMed] [Google Scholar]
- 94.Janicki P. et al. (2010) Chondrogenic pre-induction of human mesenchymal stem cells on beta-TCP: enhanced bone quality by endochondral heterotopic bone formation. Acta Biomaterialia 6, 3292-3301 [DOI] [PubMed] [Google Scholar]
- 95.Kasten P. et al. (2005) Ectopic bone formation associated with mesenchymal stem cells in a resorbable calcium deficient hydroxyapatite carrier. Biomaterials 26, 5879-5889 [DOI] [PubMed] [Google Scholar]
- 96.Krebsbach P.H. et al. (1997) Bone formation in vivo: comparison of osteogenesis by transplanted mouse and human marrow stromal fibroblasts. Transplantation 63, 1059-1069 [DOI] [PubMed] [Google Scholar]
- 97.Mankani M.H. et al. (2006) In vivo bone formation by human bone marrow stromal cells: reconstruction of the mouse calvarium and mandible. Stem Cells 24, 2140-2149 [DOI] [PubMed] [Google Scholar]
- 98.Hao W. et al. (2008) Collagen I gel can facilitate homogenous bone formation of adipose-derived stem cells in PLGA-beta-TCP scaffold. Cells Tissues Organs 187, 89-102 [DOI] [PubMed] [Google Scholar]
- 99.Hattori H. et al. (2006) Bone formation using human adipose tissue-derived stromal cells and a biodegradable scaffold. Journal of Biomedical Materials Research B: Applied Biomaterials 76, 230-239 [DOI] [PubMed] [Google Scholar]
- 100.Hattori H. et al. (2004) Osteogenic potential of human adipose tissue-derived stromal cells as an alternative stem cell source. Cells Tissues Organs 178, 2-12 [DOI] [PubMed] [Google Scholar]
- 101.Lin Y. et al. (2007) Ectopic and in situ bone formation of adipose tissue-derived stromal cells in biphasic calcium phosphate nanocomposite. Journal of Biomedical Materials Research A 81, 900-910 [DOI] [PubMed] [Google Scholar]
- 102.Lee S.J. et al. (2010) Enhancement of bone regeneration by gene delivery of BMP2/Runx2 bicistronic vector into adipose-derived stromal cells. Biomaterials 31, 5652-5659 [DOI] [PubMed] [Google Scholar]
- 103.Yang M. et al. (2005) In vitro and in vivo induction of bone formation based on ex vivo gene therapy using rat adipose-derived adult stem cells expressing BMP-7. Cytotherapy 7, 273-281 [DOI] [PubMed] [Google Scholar]
- 104.Steck E. et al. (2010) Discrimination between cells of murine and human origin in xenotransplants by species specific genomic in situ hybridization. Xenotransplantation 17, 153-159 [DOI] [PubMed] [Google Scholar]
- 105.Scherberich A. et al. (2007) Three-dimensional perfusion culture of human adipose tissue-derived endothelial and osteoblastic progenitors generates osteogenic constructs with intrinsic vascularization capacity. Stem Cells 25, 1823-1829 [DOI] [PubMed] [Google Scholar]
- 106.Dickhut A. et al. (2008) Chondrogenesis of mesenchymal stem cells in gel-like biomaterials in vitro and in vivo. Frontiers in Bioscience 13, 4517-4528 [DOI] [PubMed] [Google Scholar]
- 107.Jin X.B. et al. (2007) Neocartilage formation from predifferentiated human adipose derived stem cells in vivo. Acta Pharmacologica Sinica 28, 663-671 [DOI] [PubMed] [Google Scholar]
- 108.Yoon H.H. et al. (2012) Enhanced cartilage formation via three-dimensional cell engineering of human adipose-derived stem cells. Tissue Engineering A 18, 1949-1956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jin X. et al. (2007) Ectopic neocartilage formation from predifferentiated human adipose derived stem cells induced by adenoviral-mediated transfer of hTGF beta2. Biomaterials 28, 2994-3003 [DOI] [PubMed] [Google Scholar]
- 110.Jung Y. et al. (2009) In situ chondrogenic differentiation of human adipose tissue-derived stem cells in a TGF-beta1 loaded fibrin-poly(lactide-caprolactone) nanoparticulate complex. Biomaterials 30, 4657-4664 [DOI] [PubMed] [Google Scholar]
- 111.Mehlhorn A.T. et al. (2009) Chondrogenesis of adipose-derived adult stem cells in a poly-lactide-co-glycolide scaffold. Tissue Engineering A 15, 1159-1167 [DOI] [PubMed] [Google Scholar]
- 112.Pelttari K. et al. (2006) Premature induction of hypertrophy during in vitro chondrogenesis of human mesenchymal stem cells correlates with calcification and vascular invasion after ectopic transplantation in SCID mice. Arthritis & Rheumatology 54, 3254-3266 [DOI] [PubMed] [Google Scholar]
- 113.Dowthwaite G.P. et al. (2004) The surface of articular cartilage contains a progenitor cell population. Journal of Cell Science 117(Pt 6), 889-897 [DOI] [PubMed] [Google Scholar]
- 114.ter Huurne M. et al. (2012) Antiinflammatory and chondroprotective effects of intraarticular injection of adipose-derived stem cells in experimental osteoarthritis. Arthritis & Rheumatology 64, 3604-3613 [DOI] [PubMed] [Google Scholar]
- 115.Desando G. et al. (2013) Intra-articular delivery of adipose derived stromal cells attenuates osteoarthritis progression in an experimental rabbit model. Arthritis Research & Therapy 15, R22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Toghraie F. et al. (2012) Scaffold-free adipose-derived stem cells (ASCs) improve experimentally induced osteoarthritis in rabbits. Archives of Iranian Medicine 15, 495-499 [PubMed] [Google Scholar]
- 117.Dragoo J.L. et al. (2007) Healing full-thickness cartilage defects using adipose-derived stem cells. Tissue Engineering 13, 1615-1621 [DOI] [PubMed] [Google Scholar]
- 118.Im G.I., Kim H.J. and Lee J.H. (2011) Chondrogenesis of adipose stem cells in a porous PLGA scaffold impregnated with plasmid DNA containing SOX trio (SOX-5,-6 and -9) genes. Biomaterials 32, 4385-4392 [DOI] [PubMed] [Google Scholar]
- 119.Cui L. et al. (2009) Repair of articular cartilage defect in non-weight bearing areas using adipose derived stem cells loaded polyglycolic acid mesh. Biomaterials 30, 2683-2693 [DOI] [PubMed] [Google Scholar]
- 120.Matsumoto T. et al. (2010) Articular cartilage repair with autologous bone marrow mesenchymal cells. Journal of Cellular Physiology 225, 291-295 [DOI] [PubMed] [Google Scholar]
- 121.Wilke M.M., Nydam D.V. and Nixon A.J. (2007) Enhanced early chondrogenesis in articular defects following arthroscopic mesenchymal stem cell implantation in an equine model. Journal of Orthopaedic Research 25, 913-925 [DOI] [PubMed] [Google Scholar]
- 122.Lim C.T. et al. (2013) Repair of osteochondral defects with rehydrated freeze-dried oligo[poly(ethylene glycol) fumarate] hydrogels seeded with bone marrow mesenchymal stem cells in a porcine model. Tissue Engineering A 19, 1852-1861 [DOI] [PubMed] [Google Scholar]
- 123.Conejero J.A. et al. (2006) Repair of palatal bone defects using osteogenically differentiated fat-derived stem cells. Plastic and Reconstructive Surgery 117, 857-863 [DOI] [PubMed] [Google Scholar]
- 124.Cui L. et al. (2007) Repair of cranial bone defects with adipose derived stem cells and coral scaffold in a canine model. Biomaterials 28, 5477-5486 [DOI] [PubMed] [Google Scholar]
- 125.Di Bella C., Farlie P. and Penington A.J. (2008) Bone regeneration in a rabbit critical-sized skull defect using autologous adipose-derived cells. Tissue Engineering A 14, 483-490 [DOI] [PubMed] [Google Scholar]
- 126.Dudas J.R. et al. (2006) The osteogenic potential of adipose-derived stem cells for the repair of rabbit calvarial defects. Annals of Plastic Surgery 56, 543-548 [DOI] [PubMed] [Google Scholar]
- 127.Kim J.Y. et al. (2010) Evaluation of solid free-form fabrication-based scaffolds seeded with osteoblasts and human umbilical vein endothelial cells for use in vivo osteogenesis. Tissue Engineering A 16, 2229-2236 [DOI] [PubMed] [Google Scholar]
- 128.Yoon E. et al. (2007) In vivo osteogenic potential of human adipose-derived stem cells/poly lactide-co-glycolic acid constructs for bone regeneration in a rat critical-sized calvarial defect model. Tissue Engineering 13, 619-627 [DOI] [PubMed] [Google Scholar]
- 129.Chen Q. et al. (2010) Adipose-derived stem cells modified genetically in vivo promote reconstruction of bone defects. Cytotherapy 12, 831-840 [DOI] [PubMed] [Google Scholar]
- 130.Han D. and Li J. (2013) Repair of bone defect by using vascular bundle implantation combined with Runx II gene-transfected adipose-derived stem cells and a biodegradable matrix. Cell and Tissue Research 352, 561-571 [DOI] [PubMed] [Google Scholar]
- 131.Lin C.Y. et al. (2012) Immune responses during healing of massive segmental femoral bone defects mediated by hybrid baculovirus-engineered ASCs. Biomaterials 33, 7422-7434 [DOI] [PubMed] [Google Scholar]
- 132.Peterson B. et al. (2005) Healing of critically sized femoral defects, using genetically modified mesenchymal stem cells from human adipose tissue. Tissue Engineering 11, 120-129 [DOI] [PubMed] [Google Scholar]
- 133.Sheyn D. et al. (2011) Gene-modified adult stem cells regenerate vertebral bone defect in a rat model. Molecular Pharmaceutics 8, 1592-1601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.de Guzman R.C. et al. (2013) Bone regeneration with BMP-2 delivered from keratose scaffolds. Biomaterials 34, 1644-1656 [DOI] [PubMed] [Google Scholar]
- 135.Teixeira J.O. and Urist M.R. (1998) Bone morphogenetic protein induced repair of compartmentalized segmental diaphyseal defects. Archives of Orthopaedic and Trauma Surgery 117, 27-34 [DOI] [PubMed] [Google Scholar]
- 136.Wurzler K.K. et al. (1998) Radiation-induced impairment of bone healing can be overcome by recombinant human bone morphogenetic protein-2. Journal of Craniofacial Surgery 9, 131-137 [DOI] [PubMed] [Google Scholar]
- 137.Chou Y.F. et al. (2011) Adipose-derived stem cells and BMP2. Part 1. BMP2-treated adipose-derived stem cells do not improve repair of segmental femoral defects. Connective Tissue Research 52, 109-118 [DOI] [PubMed] [Google Scholar]
- 138.Keibl C. et al. (2011) Human adipose derived stem cells reduce callus volume upon BMP-2 administration in bone regeneration. Injury 42, 814-820 [DOI] [PubMed] [Google Scholar]
- 139.Mesimaki K. et al. (2009) Novel maxillary reconstruction with ectopic bone formation by GMP adipose stem cells. International Journal of Oral and Maxillofacial Surgery 38, 201-209 [DOI] [PubMed] [Google Scholar]
- 140.Sandor G.K. (2012) Tissue engineering of bone: clinical observations with adipose-derived stem cells, resorbable scaffolds, and growth factors. Annals of Maxillofacial Surgery 2, 8-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Smith D.M. et al. (2011) Regenerative surgery in cranioplasty revisited: the role of adipose-derived stem cells and BMP-2. Plastic and Reconstructive Surgery 128, 1053-1060 [DOI] [PubMed] [Google Scholar]
- 142.Levi B. et al. (2010) Human adipose derived stromal cells heal critical size mouse calvarial defects. PLoS ONE 5, e9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Niemeyer P. et al. (2010) Comparison of mesenchymal stem cells from bone marrow and adipose tissue for bone regeneration in a critical size defect of the sheep tibia and the influence of platelet-rich plasma. Biomaterials 31, 3572-3579 [DOI] [PubMed] [Google Scholar]
- 144.Cowan C.M. et al. (2004) Adipose-derived adult stromal cells heal critical-size mouse calvarial defects. Nature Biotechnology 22, 560-567 [DOI] [PubMed] [Google Scholar]
- 145.Levi B. et al. (2011) Differences in osteogenic differentiation of adipose-derived stromal cells from murine, canine, and human sources in vitro and in vivo. Plastic and Reconstructive Surgery 128, 373-386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Streckbein P. et al. (2013) Reconstruction of critical-size mandibular defects in immunoincompetent rats with human adipose-derived stromal cells. Journal of Craniomaxillofacial Surgery 41, 496-503 [DOI] [PubMed] [Google Scholar]
- 147.Wilson S.M. et al. (2012) Adipose-derived mesenchymal stem cells enhance healing of mandibular defects in the ramus of swine. Journal of Oral and Maxillofacial Surgery 70, e193-e203 [DOI] [PubMed] [Google Scholar]
- 148.Kachgal S. and Putnam A.J. (2011) Mesenchymal stem cells from adipose and bone marrow promote angiogenesis via distinct cytokine and protease expression mechanisms. Angiogenesis 14, 47-59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Niemeyer P. et al. (2008) Survival of human mesenchymal stromal cells from bone marrow and adipose tissue after xenogenic transplantation in immunocompetent mice. Cytotherapy 10(8), 784-95 [DOI] [PubMed] [Google Scholar]
- 150.Hicok K.C. et al. (2004) Human adipose-derived adult stem cells produce osteoid in vivo. Tissue Eng 10(3-4), 371-80 [DOI] [PubMed] [Google Scholar]
- 151.Supronowicz P. et al. (2011) Human adipose-derived side population stem cells cultured on demineralized bone matrix for bone tissue engineering. Tissue Eng Part A 17(5-6), 789-98 [DOI] [PubMed] [Google Scholar]
- 152.Yao J. et al. (2010) Ectopic bone formation in adipose-derived stromal cell-seeded osteoinductive calcium phosphate scaffolds. J Biomater Appl 24(7), 607-24 [DOI] [PubMed] [Google Scholar]