Abstract
Excess dietary salt intake is a major contributing factor to the pathogenesis of salt-sensitive hypertension. Strong evidence suggests that salt-sensitive hypertension is attributed to renal dysfunction, vascular abnormalities, and activation of the sympathetic nervous system. Indeed, sympathetic nerve transections or interruption of neurotransmission in various brain centers lowers arterial blood pressure (ABP) in many salt-sensitive models. The purpose of this article is to discuss recent evidence that supports a role of plasma or cerebrospinal fluid hypernatremia as a key mediator of sympathoexcitation and elevated ABP. Both experimental and clinical studies using time-controlled sampling have documented that a diet high in salt increases plasma and cerebrospinal fluid sodium concentration. To the extent it has been tested, acute and chronic elevations in sodium concentration activates the sympathetic nervous system in animals and humans. A further understanding of how the central nervous system detects changes in plasma or cerebrospinal fluid sodium concentration may lead to new therapeutic treatment strategies in salt-sensitive hypertension.
Keywords: Sympathetic nervous system, Salt-sensitive, Dahl, Deoxycorticosterone, Osmolality, Hypernatremia, Muscle sympathetic nerve activity, Humans, Rodents, Plasma, Cerebrospinal fluid, Benzamil, Transient receptor potential vanilloid-1 (TRPV1), Lamina terminalis, Blood pressure, Angiotensin II, Hypertension
SEARCH STRATEGY: NaCl, dietary salt, sodium, sympathetic, hypertension, cardiovascular disease (Years 2003–2013)
Introduction
Excess dietary salt intake is strongly correlated with cardiovascular disease and is regarded as a major contributing factor to the pathogenesis of hypertension [1–7]. Numerous pieces of evidence now suggest dietary salt (NaCl) can alter a multitude of systems including vascular, hormonal (endocrine and paracrine), renal, and neural [4]. Indeed, Guyton’s theory of impaired renal pressure-natriuresis may explain the associated alterations in blood volume and peripheral resistance, but it is now well-recognized that salt-sensitive hypertension is a multi-system disorder that involves renal dysfunction, vascular abnormalities, and neurogenic or sympathetically-mediated increases in peripheral resistance. Support for a sympathetic component arises from several lines of evidence, including: 1) salt-sensitive hypertension is associated with activation of the sympathetic nervous system [8–11], 2) sympathetic nerve transection lowers blood pressure in salt-sensitive models [12–15], and 3) interruption of neurotransmission in various brain centers lowers sympathetic nerve activity (SNA) and/or arterial blood pressure (ABP) [16–21]. The unresolved question is of the unknown origin or identity of the factor(s) linking dietary salt intake to changes in these various brain centers and SNA to, ultimately, elevated ABP. The purpose of this article is to discuss current evidence and recent insights regarding the role of plasma or cerebrospinal fluid sodium concentration as the potential link between dietary salt intake, sympathetically-mediated increases in peripheral resistance, and salt-sensitive hypertension.
Daily Sodium (or NaCl) Intake and Cardiovascular Disease
The Joint National Committee 7 (JNC7) [6] and European Society of Hypertension (ESH) / European Society of Cardiology (ESC) Guidelines [7] recommend a daily sodium intake of ~2.4 g (6 g NaCl). These recommendations are based on a number of clinical and meta-analysis studies that indicate that an elevated dietary sodium intake raises ABP and increases the risk for adverse cardiovascular events [2–5, 7, 22, 23]. It is noteworthy that researchers are less clear on whether sodium restriction below the recommended daily sodium intake is beneficial versus detrimental. For example, a recent study by O’Donnell and colleagues [22, 23] suggests a “J-Shaped” curve relating adverse cardiovascular events and urinary sodium excretion. Subjects with a low and high versus normal urinary sodium excretion had a greater prevalence of adverse cardiovascular events [23]. Severe sodium restriction may have adverse effects on the cardiovascular system, including alterations in various neural and humoral factors such as activation of the sympathetic nervous and renin-angiotensin-aldosterone systems. Therefore, discussions on the impact of sodium (or NaCl) intake should be cautious regarding the cardiovascular effects of salt restriction versus salt loading with respect to the recommended daily sodium intake.
Relationship between dietary salt intake, sodium concentration, and salt-sensitive hypertension
In experimental models, the addition of dietary sodium (NaCl) has been repeatedly demonstrated to raise ABP or further exacerbate the level of hypertension. These models include the Dahl-salt-sensitive rat [24, 25], the deoxycorticosterone model [13, 14, 26], the spontaneously hypertensive rat [24], the Grollman renal-wrap [27, 28], and chronic infusion of angiotensin II [15]. Using time-controlled sampling, several laboratories have reported that increased dietary salt intake significantly increased plasma and/or cerebrospinal fluid sodium concentrations by ~2–6 mmol/L in the majority of these salt-sensitive models [24, 25, 27, 29–32]. In many instances, the increase in plasma or cerebrospinal fluid sodium concentration occurred within the initial days preceding or parallel to the elevation in ABP [24, 25, 27]. A major issue associated with such studies to demonstrate a link between dietary salt intake, sodium concentration, and the level of blood pressure is the measurement of sodium concentration. From the above studies, the magnitude of the change in sodium concentration is relatively small (1–5 %). In some instances, this may appear equivalent to the variability between animals or even the measurement error. Therefore, the majority of studies have used a within-subjects design, a larger group size, or substantial changes in dietary salt intake.
In humans, evidence from population studies, clinical trials, and meta-analyses indicates that increased dietary salt intake also elevates ABP [2–4]. For example, sodium restriction has been repeatedly demonstrated to lower ABP to a greater extent in hypertensive versus normotensive individuals [2–4]. In addition, several studies have also provided evidence for a direct link between plasma sodium concentration and ABP in humans. He and colleagues [3] reanalyzed three different studies with varying levels of salt intake. First, a reduction in salt intake from 350 to 10–20 mmol/d over 5 days was associated with a significant decrease of ~3mmol/L plasma sodium concentration and a significant fall in ABP of hypertensive subjects. Second, a reduction of salt intake from ~170 to 100 mmol/day reduced plasma sodium concentration and systolic blood pressure. Third, an increase salt intake from 10 to 250 mmol/d increased plasma sodium concentration. In a smaller clinical study, Schmidlin and colleagues [11] reported that increased dietary sodium via NaCl or NaHCO3 from 10 to 250 mmol/d raised ABP in salt-sensitive, African-American subjects. Interestingly, the pressor response to sodium was strongly correlated with the resultant plasma sodium concentration [11]. On the other hand, evidence for increased sodium concentration in the cerebrospinal fluid is limited. To our knowledge, there is only one study that has investigated this relationship, reporting that one week of a high (16–18 g/day) versus low (1–3 g/day) NaCl level significantly increased ABP and both plasma and cerebrospinal fluid sodium concentration in 15 hypertensive subjects [33]. Collectively, these observations suggest that dietary salt may produce changes in plasma or cerebrospinal fluid sodium concentration.
We readily acknowledge that the above studies represent a fraction of the published reports regarding the relationship between dietary salt intake, plasma sodium concentration, and ABP. However, these studies were specifically selected to highlight recent evidence to support a link between these factors.
Acute increases in plasma and cerebrospinal fluid sodium concentration activate the sympathetic nervous system
Experimental investigations in animals have repeatedly demonstrated that hypernatremia or hyperosmolality activates the sympathetic nervous system [34–36]. We [35] and others [37–39] have demonstrated that acute intravenous infusions of NaCl to raise plasma sodium ~10mmol/L significantly increases lumbar SNA and ABP in rodents. This was associated with significant decreases in renal SNA [39]. Second, intracarotid infusion of hypertonic NaCl to selectively activate forebrain osmoreceptors without changes in systemic osmolality dose-dependently increases splenic, renal and/or lumbar SNA and ABP in rats [35, 40, 41] and cats [42, 43]. Third, an acute increase in cerebrospinal fluid sodium concentration by an infusion of hypertonic NaCl has been shown to increase SNA and ABP in normal rats [24, 29, 44–46]. Interestingly, the acute infusion of NaCl may produce regionally specific changes in SNA, as recent data from our laboratory [47] illustrate that an infusion of 1M NaCl (5 uL over 10 min) into the lateral ventricle to raise cerebrospinal fluid sodium concentration by 5 mmol/L significantly increased lumbar SNA and ABP but decreased renal SNA. This pressor response was abolished by ganglionic blockade [47]. It is noteworthy that others have reported that acute infusion of hypertonic NaCl into the lateral ventricle increases renal SNA [46]. The exact contribution of the various sympathetic nerves to the sodium-induced pressor response has not been investigated. Nevertheless, these observations provide convincing evidence that an acute and physiological change in plasma or cerebrospinal fluid sodium concentrations (5–10mmol/L) can activate the sympathetic nervous system and raise ABP in rodents.
Despite the above observations in animals supporting a sympathoexcitatory action of hypernatremia, a relative paucity of data currently exists in humans. Similar to studies in animals, acute intravenous infusion of hypertonic NaCl to raise serum sodium and osmolality has been reported to increase ABP by ~6–10 mmHg [48–52]. In a subset of these studies, the pressor response was associated with significant increases in plasma norepinephrine (~30–40 %) [49–51]. In addition, Farquhar and colleagues [50] reported that acute IV infusion of NaCl, which increased plasma osmolality by 8 mOsm/kg (or 4 mmol/L sodium), significantly increased muscle SNA from 14.5 to 18.1 bursts/min. However, Charkoudian and colleagues [48] reported that smaller increases in plasma osmolality (~4 mOsm/kg) do not alter muscle SNA in humans. Importantly, it has not been determined whether or not sympathetic outflow to other vascular beds (renal, cardiac, etc.) is affected by increased serum sodium or plasma osmolality in humans.
It is noteworthy that the above observations in both animals and humans were conducted in otherwise healthy, normotensive and likely salt-resistant subjects. While salt-sensitive strains of rodents are readily available, the standard laboratory rat is salt-resistant [34]. In humans, the prevalence of salt sensitivity in healthy, normotensive subjects under 40 years of age is low (~30 %) [53–55]. Data from rodent studies using intracerebroventricular infusions of NaCl have reported that significantly larger pressor and sympathoexcitatory responses are observed in Dahl-salt-sensitive versus Dahl-salt-resistant animals [45, 46]. Therefore, the sympathoexcitatory and pressor responses to changes in sodium concentration (systemic and central) may be augmented in salt-sensitive versus salt-resistant individuals. In other words, an increase in plasma or cerebrospinal fluid sodium concentration that may be considered sub-threshold in salt-resistant subjects may produce a significant elevation in SNA and ABP in salt-sensitive subjects. Whether this could reflect a greater sensitivity to sodium versus a more generalized hyperresponsiveness of a variety of stressors remains to be determined.
Chronic effects of dietary salt intake on sympathetic nervous system activity
Few studies have directly assessed how dietary salt intake chronically alters SNA, in part, due to technical limitations associated with chronic nerve recordings or concerns regarding between-animal comparisons of SNA. Regarding the latter, the majority of these studies are performed under anesthesia, which may further complicate the interpretation. In one of the few studies performed in chronically-instrumented animals, McBryde and colleagues [56] used a telemetry-based system and reported that a high-salt diet via ingestion of 0.9 % NaCl for 7 days did not alter renal SNA in normal rabbits. The data in hypertensive, salt-sensitive models is also limited. A high-salt diet combined with a chronic infusion of angiotensin II produces a sympathetically-mediated increase in ABP by 14 days [8, 15, 57]. However, this was associated with a decrease in renal SNA and no change in lumbar SNA [58]. In rabbits, Guild and colleagues [59] reported that ingestion of 0.9 % NaCl together with an infusion of angiotensin II increases renal SNA by day 21. The discrepancy could reflect the species (rabbits versus rats) or the time frame (14 versus 21 days).
Due to the limited data sets regarding changes of SNA in animals, studies have largely relied on the physiological effects of organ denervation or nerve transection to infer increased activity. In a number of salt-sensitive models, including the Dahl-salt-sensitive rat, angiotensin II, and deoxycorticosterone, renal denervation does not greatly attenuate the development of hypertension [15, 26, 60]. Interestingly, recent studies have indicated that the splanchnic sympathetic nerves may play an important role in salt-sensitive hypertension. Celiac ganglionectomy has been reported to attenuate the development of deoxycorticosterone-salt and angiotensin II-salt hypertension models [14, 15]. In addition, celiac ganglionectomy has also been reported to lower ABP after 3 weeks of high-salt diet in Dahl-salt-sensitive rats [12]. These observations suggest that chronic increases in dietary salt may elevate splanchnic SNA to increase ABP in salt-sensitive hypertension.
In humans, there are only a few studies that have investigated the relationship between chronic changes in dietary salt and SNA. Grassi and colleagues [61] reported that a low sodium intake (20 mmol/d versus 210 mmol/d for low versus regular sodium intake) increased muscle SNA and impaired baroreflex control of muscle SNA. Interestingly, this large reduction in dietary salt was not associated with changes in serum sodium concentration (141 versus 140 mmol/d for low versus regular sodium intake, respectively). Similar observations were reported with sodium restriction of 8 weeks [62]. Anderson and colleagues [63] reported that a high (~380 mmol/d) versus low (9 mmol/d) sodium diet for 5 days reduced muscle SNA and plasma norepinephrine in both normotensive and borderline hypertensive subjects. Friberg [64] reported that renal norepinephrine spillover increased during acute salt restriction (5–40mmol/d) versus normal sodium diet (160–200 mmol/d). Cardiac norepinephrine spillover remained unchanged between low versus normal sodium intake. Presently, the effects of longer duration dietary salt manipulation on measures of sympathetic outflow in humans have not been studied. Thus, it is possible that a more prolonged intervention may yield different results.
Collectively, these human studies provide evidence that acute increases in osmolality increase ABP at rest and alter neurocirculatory control. However, important gaps in our knowledge persist: (1) whether or not pressor responses to increased serum sodium/plasma osmolality in humans depend on sympathetic nervous system activation, (2) whether findings from acute sodium loading studies apply to more prolonged manipulation of serum sodium/plasma osmolality, (3) whether observed responses are altered by various factors in health and disease (i.e., hypertension, aging, etc.), (4) how increased serum sodium/plasma osmolality affects sympathetic outflow to other critical vascular beds/organs, and (5) what sites, presumably central in origin, are critical to mediating responses to increases in serum sodium/plasma osmolality?
Does plasma or cerebrospinal fluid hypernatremia contribute to elevated SNA and ABP in salt-sensitive hypertension?
A key question is whether the reported changes in plasma or cerebrospinal fluid sodium concentrations contribute to the chronic sympathoexcitation and elevated ABP in salt-sensitive hypertension. In a series of experiments, Brooks and colleagues [30] reported that plasma NaCl levels were elevated at 2–3 weeks after initiation of deoxycorticosterone-salt treatment. Interestingly, acute intravenous infusion of 5 % dextrose (in water) to lower NaCl levels significantly reduced lumbar SNA and ABP in deoxycorticosterone-salt-hypertensive animals, but had no effect in animals treated with deoxycorticosterone or salt alone. Since these infusions were performed systemically, the elevated NaCl concentrations could have activated sodium/osmoreceptors located in the gastrointestinal tract, kidney, and/or central nervous system. In a second set of experiments, hypotonic fluid was infused through the internal carotid artery to lower forebrain osmolality or NaCl concentrations by ~2 % in deoxycorticosterone-salt hypertensive animals [31]. Interestingly, the infusion of hypotonic but not isotonic fluid produced a rapid and significant reduction in ABP. The reduction in ABP occurred much sooner, when the hypotonic solution was infused through the internal carotid artery versus intravenously. While these experimental manipulations have only been performed in the deoxycorticosterone-salt model of hypertension, the findings underscore the potential importance of plasma or cerebrospinal fluid hypernatremia to the chronic sympathoexcitation and elevated ABP in salt-sensitive hypertension. Future studies are warranted in other salt-sensitive models to confirm whether this is a common mechanism.
The above studies by Brooks and colleagues [30, 31] do not distinguish whether plasma versus cerebrospinal fluid hypernatremia is the critical mediator of salt-sensitive hypertension. Indeed, several studies have reported a high-salt diet elevates cerebrospinal fluid, but not plasma and sodium concentration in Dahl-salt-sensitive rats [24, 25]. Moreover, chronic infusion of sodium-rich cerebrospinal fluid into the lateral ventricle to increase cerebrospinal fluid sodium concentrations ~5–10mmol/L produced significant and sustained increases in ABP [46]. However, it is noteworthy that studies have not directly examined whether prevention of the cerebrospinal fluid hypernatremia by intracerebroventricular infusion of hypotonic fluid attenuates salt-sensitive hypertension in Dahl rats. The relative contribution or importance of plasma versus cerebrospinal fluid sodium concentrations in different models of salt-sensitive hypertension has not been investigated.
Central signaling mechanisms in salt-sensitive hypertension—role of central sodium or osmoreceptors
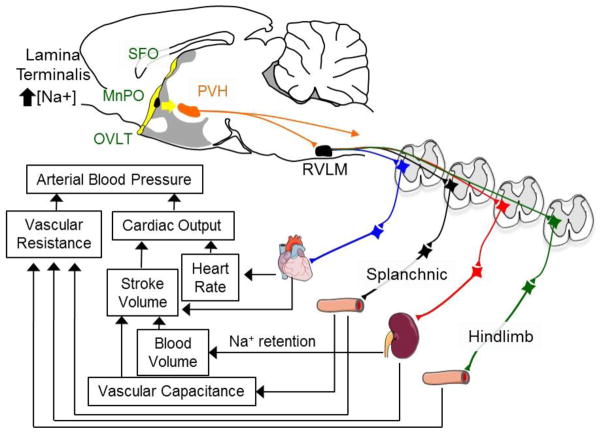
The principal set of osmoreceptors within the central nervous system is located within the forebrain lamina terminalis [35, 36, 65]. The forebrain lamina terminalis is a group of interconnected structures located along the rostral wall of the 3rd ventricle (Figure 1). It contains two circumventricular organs, the organum vasculosum of the lamina terminalis (OVLT) and the subfornical organ (SFO). These structures lack a complete blood-brain barrier, and therefore neurons in these brain regions can respond to circulating factors that neurons in other brain regions cannot. A third structure referred to as the median preoptic nucleus (MnPO) is juxtaposed between the OVLT and SFO (Figure 1). Both in vivo and in vitro electrophysiological studies have reported that the OVLT and SFO contain neurons that are intrinsically osmosensitive [65–70]. Plasma or cerebrospinal fluid hypernatremia increases Fos expression, a marker of neuronal activation, in OVLT and SFO [71–73]. Functionally, lesions of the OVLT or SFO attenuate body fluid homeostatic responses to plasma hypernatremia [74, 75].
Figure 1.
Schematic illustration of the lamina terminalis (in yellow) that consists of the organum vasculosum of the lamina termialis (OVLT), median preoptic nucleus (MnPO), and subfornical organ (SFO). The OVLT and SFO possess osmosensitive neurons. Neurons of the lamina terminalis influence sympathetic nerve activity and arterial blood pressure through polysynaptic projections to sympathetic preganglionic neurons. These actions subsequently alter vascular resistance/capacitance, blood volume, cardiac output, and arterial blood pressure.
The importance of the lamina terminalis in cardiovascular regulation and salt-sensitive hypertension was introduced by a series of reports that lesions of the anteroventral third ventricular (AV3V) region, which includes the lamina terminalis, prevented and/or attenuated hypertension in numerous experimental models of salt-sensitive hypertension [19, 20, 76]. Since these monumental observations, numerous studies have highlighted the significance of these structures in sodium-induced activation of the sympathetic nervous system. Knife-cuts placed immediately caudal to the lamina terminalis eliminate the sympathoexcitatory responses to acute hypernatremia [37]. Intracarotid injection of hypertonic NaCl increases Fos expression in OVLT neurons projecting downstream to the hypothalamic paraventricular nucleus [72]. Lesion or inhibition of the OVLT neurons attenuates the sympathoexcitatory response to intracarotid or intracerebroventricular infusion of hypertonic NaCl [40, 77]. These findings emphasize the importance of neurons in the lamina terminalis, and more specifically the OVLT, in the sympathoexcitatory effects of acute hypernatremia.
The ability of OVLT neurons to detect changes in osmolality (and perhaps sodium concentration) has been reported to depend on the transient receptor vanilloid potential 1 (TRPV1) channel [66, 67]. First, extracellular recordings from hypothalamic explants showed that increases in bath osmolality increased neuronal discharge of wild-type but not TRPV−/− mice [66]. Second, whole-cell patch-clamp recordings performed in acutely dissociated OVLT neurons indicate that bath hyperosmolality induces an inward current that is blocked by the TRPV channel blocker ruthenium red or was absent in TRPV1−/− mice [66]. Despite these elegant electrophysiological studies, there is some controversy as to whether TRPV1−/− mice show disrupted thirst responses to acute or chronic sodium loads [66, 78]. Whether the sympathetic and pressor responses to acute hypernatremia or salt-sensitive hypertension are attenuated by a blockade of TRPV1 channels in OVLT or by TRPV1−/− mice has not been investigated.
A second potential signaling mechanism postulated to directly mediate sodium-induced changes in SNA and ABP are benzamil-sensitive channels [44, 79]. Benzamil is an amiloride analog and blocks a number of non-voltage, gated sodium channels including the epithelial sodium channel, sodium-calcium exchanger, members of the acid-sensing ion channel, and the sodium/hydrogen exchanger. The potential contribution of these various ion channels in neuronal osmosensory transduction has been recently discussed [36]. A significant role for benzamil-sensitive channels in central sodium signaling has been highlighted by several key observations. First, pretreatment with benzamil eliminated the sympathoexcitatory and pressor response to acute intracerebroventricular infusion of hypertonic NaCl [29, 44, 80]. Second, a chronic intracerebroventricular infusion of benzamil prevents the hypertension induced by chronic infusion of sodium-rich cerebrospinal fluid [80]. Finally, intracerebroventricular infusion of benzamil has been reported to attenuate or prevent several forms of salt-sensitive hypertension, including deoxycorticosterone-salt, angiotensin II plus salt, Dahl-salt-sensitive, and spontaneously hypertensive rats [29, 36, 80–82]. It is noteworthy that the chronic infusion of benzamil did not affect renovascular hypertension [29]. It has been hypothesized that increased dietary salt and central hypernatremia activate the epithelial sodium channel to increase the release of endogenous oubain and subsequent sympathoexcitation, as recently reviewed [79]. Whether this benzamil-sensitive channel acts as a “sodium-sensor” versus a downstream mediator remains to be determined. Interestingly, a previous report showed that central benzamil pretreatment also attenuated the sympathoexcitatory activity and secretion of vasopressin in response to a central NaCl infusion [29]. Together, these observations highlight the importance of a central benzamil-sensitive channel, perhaps an epithelial sodium channel, in central sodium-sensing and salt-sensitive hypertension.
Neurons of the lamina terminalis do not directly innervate sympathetic preganglionic neurons. Instead, a major efferent target of these sodium- or osmotic-sensing neurons is the hypothalamic paraventricular nucleus (PVH) [35, 83]. PVH neurons influence SNA and ABP through mono- and polysynaptic pathways to several sympathetic brain regions, including the rostral ventrolateral medulla (RVLM) and spinal intermediolateral cell column (Figure 1). Evidence from several laboratories suggest that one of two pathways may mediate sodium- or osmotic-induced changes in SNA and ABP: (1) a direct vasopressinergic pathway from the PVH to the spinal cord [37], and (2) polysynaptic and glutamatergic pathways from the PVH to the spinal cord via the RVLM [35]. The relative contribution of each pathway to sodium-induced changes in SNA and ABP has been discussed elsewhere [35, 36].
Chronic dietary salt intake sensitizes sympathetic pathways
The aforementioned evidence postulates that dietary salt intake alters plasma or cerebrospinal fluid sodium concentration to activate sodium- or osmoreceptors to elevate SNA and ABP in salt-sensitive hypertension. However, the ingestion of dietary salt may have an additional action to increase the gain of central sympathetic networks. Original studies from Pawlowski-Dahm and Gordon [84] reported that excitation of sympathetic neurons in the RVLM produced greater pressor responses in animals ingesting a high- versus low-salt diet. These findings have now been replicated by two additional laboratories [85–88]. Together, the studies indicated that dietary salt intake may modulate the responsiveness of these sympathetic neurons to a number of different neurotransmitters (excitatory and inhibitory) and exacerbate numerous sympathetic reflexes [34]. Indeed, published reports suggest that chronic increases in dietary salt intake enhance sympathetic and/or pressor responses to central NaCl infusions, air-jet stress, insulin, and activation of sciatic afferents or the aortic depressor nerve [34, 46, 84, 89]. These exaggerated responses are not manifested after a short exposure to a high salt intake but require several weeks [86]. Interestingly, lesions of the AV3V region or OVLT prevent the hyperresponsiveness of RVLM neurons during high salt intake [85]. These findings suggest that dietary salt intake may have a further central effect to alter the gain of central sympathetic networks. Functionally, an increased gain of central sympathetic networks during high salt intake would result in a greater sympathetic and pressor response to a given stimulus or increase in plasma/cerebrospinal fluid sodium concentration.
Conclusions
Recent studies using time-controlled sampling have reported that increased dietary salt intake elevates plasma and/or cerebrospinal fluid sodium concentration. Numerous reports from laboratory animals suggest that an acute elevation in plasma or cerebrospinal fluid sodium concentration activates the sympathetic nervous system. In humans, evidence is limited to a few studies demonstrating that acute plasma hypernatremia evokes a similar response. The increases in plasma or cerebrospinal fluid sodium concentrations do appear to chronically activate the sympathetic nervous system and elevate ABP in experimental models of salt-sensitive hypertension. To date, this has not been directly tested in humans. This neurogenic action of dietary salt intake to raise ABP may act as a positive-feedback system as the hypernatremia activates the sympathetic nervous system to cause further sodium and fluid retention [90]. The putative cellular pathway or sodium-sensing element linking plasma or central hypernatremia to sympathoexcitation in salt-sensitive hypertension has not been identified, but these neurons likely reside in the forebrain lamina terminalis. Additional factors such as genetic background and the ability of a high-salt diet to sensitize central sympathetic networks may further enhance the sensitivity of brain networks to changes in plasma or cerebrospinal fluid sodium concentration. An increased sensitivity or responsiveness to specific populations and during chronic high salt levels functionally means that subthreshold changes in sodium concentrations may have a significant impact on SNA and ABP.
Acknowledgments
This work was supported by National Heart, Lung, and Blood Institute Grants R01 HL-090826 and R01 HL-113270 (to Sean D. Stocker), American Heart Association Established Investigator Grant (to Sean D. Stocker), and a Grant-In-Aid (to Kevin D. Monahan).
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest
Sean D. Stocker, Kevin D. Monahan, and Kirsteen N. Browning declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest have been highlighted as:
• Of importance
•• Of major importance
- 1.Appel LJ. Another major role for dietary sodium reduction: improving blood pressure control in patients with resistant hypertension. Hypertension. 2009;54(3):444–6. doi: 10.1161/HYPERTENSIONAHA.109.132944. [DOI] [PubMed] [Google Scholar]
- 2.Appel LJ, et al. The importance of population-wide sodium reduction as a means to prevent cardiovascular disease and stroke: a call to action from the American Heart Association. Circulation. 2011;123(10):1138–43. doi: 10.1161/CIR.0b013e31820d0793. [DOI] [PubMed] [Google Scholar]
- 3.He FJ, et al. Plasma sodium: ignored and underestimated. Hypertension. 2005;45(1):98–102. doi: 10.1161/01.HYP.0000149431.79450.a2. [DOI] [PubMed] [Google Scholar]
- 4.Kotchen TA, Cowley AW, Jr, Frohlich ED. Salt in health and disease—a delicate balance. N Engl J Med. 2013;368(13):1229–37. doi: 10.1056/NEJMra1212606. [DOI] [PubMed] [Google Scholar]
- 5.Strazzullo P, et al. Salt intake, stroke, and cardiovascular disease: meta-analysis of prospective studies. BMJ. 2009;339:b4567. doi: 10.1136/bmj.b4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chobanian AV, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42(6):1206–52. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 7.Mancia G, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) Eur Heart J. 2013;34(28):2159–219. doi: 10.1093/eurheartj/eht151. [DOI] [PubMed] [Google Scholar]
- 8.King AJ, et al. Whole body norepinephrine kinetics in ANG II-salt hypertension in the rat. Am J Physiol Regul Integr Comp Physiol. 2008;294(4):R1262–7. doi: 10.1152/ajpregu.00819.2007. [DOI] [PubMed] [Google Scholar]
- 9.Leenen FH, Ruzicka M, Huang BS. The brain and salt-sensitive hypertension. Curr Hypertens Rep. 2002;4(2):129–35. doi: 10.1007/s11906-002-0037-y. [DOI] [PubMed] [Google Scholar]
- 10.Osborn JW, Fink GD. Region specific changes in sympathetic nerve activity in AngII-salt hypertension. Exp Physiol. 2009 doi: 10.1113/expphysiol.2008.046326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11••.Schmidlin O, et al. Sodium-selective salt sensitivity: its occurrence in blacks. Hypertension. 2007;50(6):1085–92. doi: 10.1161/HYPERTENSIONAHA.107.091694. This smaller clinical study demonstrated that chronic sodium loading via dietary NaCl or NaHCO3 produced an increase in ABP of salt-sensitive subjects that was strongly correlated with the resultant level of plasma sodium concentration. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12•.Foss JD, Fink GD, Osborn JW. Reversal of genetic salt-sensitive hypertension by targeted sympathetic ablation. Hypertension. 2013;61(4):806–11. doi: 10.1161/HYPERTENSIONAHA.111.00474. This study reported that splanchnic denervation via celiac ganglionectomy reduced ABP in Dahl-salt-sensitive rats after 3 weeks of a high-salt diet. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacob F, et al. Role of renal nerves in development of hypertension in DOCA-salt model in rats: a telemetric approach. Am J Physiol Heart Circ Physiol. 2005;289(4):H1519–29. doi: 10.1152/ajpheart.00206.2005. [DOI] [PubMed] [Google Scholar]
- 14.Kandlikar SS, Fink GD. Splanchnic sympathetic nerves in the development of mild DOCA-salt hypertension. Am J Physiol Heart Circ Physiol. 2011;301(5):H1965–73. doi: 10.1152/ajpheart.00086.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15••.King AJ, Osborn JW, Fink GD. Splanchnic circulation is a critical neural target in angiotensin II salt hypertension in rats. Hypertension. 2007;50(3):547–56. doi: 10.1161/HYPERTENSIONAHA.107.090696. This study was the original report that demonstrated that celiac ganglionectomy reduced ABP in an experimental model of salt-sensitive hypertension. [DOI] [PubMed] [Google Scholar]
- 16.Ito S, et al. Ventrolateral medulla AT1 receptors support arterial pressure in Dahl salt-sensitive rats. Hypertension. 2003;41(3 Pt 2):744–750. doi: 10.1161/01.HYP.0000052944.54349.7B. [DOI] [PubMed] [Google Scholar]
- 17.Ito S, et al. Tonic excitatory input to the rostral ventrolateral medulla in Dahl salt-sensitive rats. Hypertension. 2001;37(2 Part 2):687–691. [PubMed] [Google Scholar]
- 18.Nakata T, et al. Paraventricular nucleus lesions attenuate the development of hypertension in DOCA/salt-treated rats. Am J Hypertens. 1989;2(8):625–30. doi: 10.1093/ajh/2.8.625. [DOI] [PubMed] [Google Scholar]
- 19.Berecek KH, et al. Vasopressin-central nervous system interactions in the development of DOCA hypertension. Hypertension. 1982;4(3 Pt 2):131–7. [PubMed] [Google Scholar]
- 20.Goto A, et al. Effect of an anteroventral third ventricle lesion on NaCl hypertension in Dahl salt-sensitive rats. Am J Physiol. 1982;243(4):H614–8. doi: 10.1152/ajpheart.1982.243.4.H614. [DOI] [PubMed] [Google Scholar]
- 21.Sanders BJ, Johnson AK. Lesions of the anteroventral third ventricle prevent salt-induced hypertension in the borderline hypertensive rat. Hypertension. 1989;14(6):619–22. doi: 10.1161/01.hyp.14.6.619. [DOI] [PubMed] [Google Scholar]
- 22.O’Donnell MJ, et al. Salt intake and cardiovascular disease: why are the data inconsistent? Eur Heart J. 2013;34(14):1034–40. doi: 10.1093/eurheartj/ehs409. [DOI] [PubMed] [Google Scholar]
- 23.O’Donnell MJ, et al. Urinary sodium and potassium excretion and risk of cardiovascular events. JAMA. 2011;306(20):2229–38. doi: 10.1001/jama.2011.1729. [DOI] [PubMed] [Google Scholar]
- 24••.Huang BS, Van Vliet BN, Leenen FH. Increases in CSF [Na+] precede the increases in blood pressure in Dahl S rats and SHR on a high-salt diet. Am J Physiol Heart Circ Physiol. 2004;287(3):H1160–6. doi: 10.1152/ajpheart.00126.2004. This paper performed simultaneous sampling and measurement of plasma and cerebrospinal fluid sodium concentration in Dahl-salt-sensitive and spontaneously hypertensive rats fed a low- or high-salt diet. The paper details that a high-salt diet selectively increases cerebrospinal fluid sodium concentration. The central hypernatremia preceded the increase in ABP. [DOI] [PubMed] [Google Scholar]
- 25••.Nakamura K, Cowley AW., Jr Sequential changes of cerebrospinal fluid sodium during the development of hypertension in Dahl rats. Hypertension. 1989;13(3):243–9. doi: 10.1161/01.hyp.13.3.243. This study performed simultaneous sampling and measurement of plasma and cerebrospinal fluid sodium concentration in Dahl-salt-sensitive rats. The authors report that a high-salt diet produced significant increases in cerebrospinal fluid sodium concentration that occurred in parallel to the rise in ABP. [DOI] [PubMed] [Google Scholar]
- 26.Kandlikar SS, Fink GD. Mild DOCA-salt hypertension: sympathetic system and role of renal nerves. Am J Physiol Heart Circ Physiol. 2011;300(5):H1781–7. doi: 10.1152/ajpheart.00972.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haywood JR, et al. Alterations in cerebrospinal fluid sodium and osmolality in rats during one-kidney, one-wrap renal hypertension. Clin Exp Pharmacol Physiol. 1984;11(5):545–9. doi: 10.1111/j.1440-1681.1984.tb00865.x. [DOI] [PubMed] [Google Scholar]
- 28.Haywood JR, Williams SF, Ball NA. Contribution of sodium to the mechanism of one-kidney, renal-wrap hypertension. Am J Physiol. 1984;247(5 Pt 2):H797–803. doi: 10.1152/ajpheart.1984.247.5.H797. [DOI] [PubMed] [Google Scholar]
- 29••.Nishimura M, et al. Benzamil blockade of brain Na+ channels averts Na(+)-induced hypertension in rats. Am J Physiol. 1998;274(3 Pt 2):R635–44. doi: 10.1152/ajpregu.1998.274.3.R635. One of the original reports that support a role of central benzamil-sensitive channels in acute and chronic sympathoexcitatory effects of dietary sodium. [DOI] [PubMed] [Google Scholar]
- 30.O’Donaughy TL, V, Brooks L. Deoxycorticosterone acetate-salt rats: hypertension and sympathoexcitation driven by increased NaCl levels. Hypertension. 2006;47(4):680–5. doi: 10.1161/01.HYP.0000214362.18612.6e. [DOI] [PubMed] [Google Scholar]
- 31•.O’Donaughy TL, Qi Y, Brooks VL. Central action of increased osmolality to support blood pressure in deoxycorticosterone acetate-salt rats. Hypertension. 2006;48(4):658–63. doi: 10.1161/01.HYP.0000238140.06251.7a. This study performed the original experiments to assess whether increases in plasma NaCl levels contribute to the sympathoexcitation and elevated ABP in deoxycorticosterone rats. [DOI] [PubMed] [Google Scholar]
- 32.Fang Z, et al. Circadian rhythm of plasma sodium is disrupted in spontaneously hypertensive rats fed a high-NaCl diet. Am J Physiol Regul Integr Comp Physiol. 2000;278(6):R1490–5. doi: 10.1152/ajpregu.2000.278.6.R1490. [DOI] [PubMed] [Google Scholar]
- 33.Kawano Y, et al. Sodium and noradrenaline in cerebrospinal fluid and blood in salt-sensitive and non-salt-sensitive essential hypertension. Clin Exp Pharmacol Physiol. 1992;19(4):235–41. doi: 10.1111/j.1440-1681.1992.tb00444.x. [DOI] [PubMed] [Google Scholar]
- 34.Stocker SD, Madden CJ, Sved AF. Excess dietary salt intake alters the excitability of central sympathetic networks. Physiol Behav. 2010;100(5):519–24. doi: 10.1016/j.physbeh.2010.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stocker SD, Osborn JL, Carmichael SP. Forebrain osmotic regulation of the sympathetic nervous system. Clin Exp Pharmacol Physiol. 2008;35(5–6):695–700. doi: 10.1111/j.1440-1681.2007.04835.x. [DOI] [PubMed] [Google Scholar]
- 36.Toney GM, Stocker SD. Hyperosmotic activation of CNS sympathetic drive: implications for cardiovascular disease. J Physiol. 2010;588(Pt 18):3375–3384. doi: 10.1113/jphysiol.2010.191940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Antunes VR, et al. A spinal vasopressinergic mechanism mediates hyperosmolality-induced sympathoexcitation. J Physiol. 2006;576(Pt 2):569–83. doi: 10.1113/jphysiol.2006.115766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brooks VL, Freeman KL, O’Donaughy TL. Acute and chronic increases in osmolality increase excitatory amino acid drive of the rostral ventrolateral medulla in rats. Am J Physiol Regul Integr Comp Physiol. 2004;287(6):R1359–68. doi: 10.1152/ajpregu.00104.2004. [DOI] [PubMed] [Google Scholar]
- 39.Weiss ML, et al. Nonuniform sympathetic nerve responses to intravenous hypertonic saline infusion. J Auton Nerv Syst. 1996;57(1–2):109–15. doi: 10.1016/0165-1838(95)00108-5. [DOI] [PubMed] [Google Scholar]
- 40.Shi P, Stocker SD, Toney GM. Organum vasculosum laminae terminalis contributes to increased sympathetic nerve activity induced by central hyperosmolality. Am J Physiol Regul Integr Comp Physiol. 2007;293(6):R2279–89. doi: 10.1152/ajpregu.00160.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen QH, Toney GM. AT(1)-receptor blockade in the hypothalamic PVN reduces central hyperosmolality-induced renal sympathoexcitation. Am J Physiol Regul Integr Comp Physiol. 2001;281(6):R1844–53. doi: 10.1152/ajpregu.2001.281.6.R1844. [DOI] [PubMed] [Google Scholar]
- 42.Schad H, Seller H. Influence of intracranial osmotic stimuli on renal nerve activity in anaesthetized cats. Pflugers Arch. 1975;353(2):107–21. doi: 10.1007/BF00599872. [DOI] [PubMed] [Google Scholar]
- 43.Tobey JC, et al. Differential sympathetic responses initiated by angiotensin and sodium chloride. Am J Physiol. 1983;245(1):R60–8. doi: 10.1152/ajpregu.1983.245.1.R60. [DOI] [PubMed] [Google Scholar]
- 44.Abrams JM, Osborn JW. A role for benzamil-sensitive proteins of the central nervous system in the pathogenesis of salt-dependent hypertension. Clin Exp Pharmacol Physiol. 2008;35(5–6):687–94. doi: 10.1111/j.1440-1681.2008.04929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang BS, et al. Neuronal responsiveness to central Na+ in 2 congenic strains of Dahl salt-sensitive rats. Hypertension. 2007;49(6):1315–20. doi: 10.1161/HYPERTENSIONAHA.106.086363. [DOI] [PubMed] [Google Scholar]
- 46.Huang BS, Wang H, Leenen FH. Enhanced sympathoexcitatory and pressor responses to central Na+ in Dahl salt-sensitive vs. -resistant rats. Am J Physiol Heart Circ Physiol. 2001;281(5):H1881–9. doi: 10.1152/ajpheart.2001.281.5.H1881. [DOI] [PubMed] [Google Scholar]
- 47.Simmonds SS, Stocker SD. Lumbar denervation attenuates the sympathetic and cardiovascular response to acute increases in cerebrospinal fluid sodium concentration. FASEB J. 2013;27:lb731. [Google Scholar]
- 48.Charkoudian N, et al. Interactions of plasma osmolality with arterial and central venous pressures in control of sympathetic activity and heart rate in humans. Am J Physiol Heart Circ Physiol. 2005;289(6):H2456–60. doi: 10.1152/ajpheart.00601.2005. [DOI] [PubMed] [Google Scholar]
- 49.Farquhar WB, et al. Blood pressure and hemodynamic responses to an acute sodium load in humans. J Appl Physiol. 2005;99(4):1545–51. doi: 10.1152/japplphysiol.00262.2005. [DOI] [PubMed] [Google Scholar]
- 50•.Farquhar WB, et al. Sympathetic neural responses to increased osmolality in humans. Am J Physiol Heart Circ Physiol. 2006;291(5):H2181–6. doi: 10.1152/ajpheart.00191.2006. One of the original reports in humans to demonstrate that acute increases in osmolality via intravenous infusion of NaCl raise muscle SNA. [DOI] [PubMed] [Google Scholar]
- 51.Peskind ER, et al. Hypertonic saline infusion increases plasma norepinephrine concentrations in normal men. Psychoneuroendocrinology. 1993;18(2):103–13. doi: 10.1016/0306-4530(93)90061-o. [DOI] [PubMed] [Google Scholar]
- 52.Wenner MM, et al. Influence of plasma osmolality on baroreflex control of sympathetic activity. Am J Physiol Heart Circ Physiol. 2007;293(4):H2313–9. doi: 10.1152/ajpheart.01383.2006. [DOI] [PubMed] [Google Scholar]
- 53.Weinberger MH. Salt sensitivity of blood pressure in humans. Hypertension. 1996;27(3 Pt 2):481–90. doi: 10.1161/01.hyp.27.3.481. [DOI] [PubMed] [Google Scholar]
- 54.Weinberger MH. Salt sensitivity is associated with an increased mortality in both normal and hypertensive humans. J Clin Hypertens (Greenwich) 2002;4(4):274–6. doi: 10.1111/j.1524-6175.2002.00924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weinberger MH. Pathogenesis of salt sensitivity of blood pressure. Curr Hypertens Rep. 2006;8(2):166–70. doi: 10.1007/s11906-006-0014-y. [DOI] [PubMed] [Google Scholar]
- 56.McBryde FD, et al. A high-salt diet does not influence renal sympathetic nerve activity: a direct telemetric investigation. Am J Physiol Regul Integr Comp Physiol. 2009;297(2):R396–402. doi: 10.1152/ajpregu.90741.2008. [DOI] [PubMed] [Google Scholar]
- 57.Kuroki MT, et al. Time-dependent changes in autonomic control of splanchnic vascular resistance and heart rate in ANG II-salt hypertension. Am J Physiol Heart Circ Physiol. 2012;302(3):H763–9. doi: 10.1152/ajpheart.00930.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yoshimoto M, et al. Chronic Angiotensin II Infusion Causes Differential Responses in Regional Sympathetic Nerve Activity in Rats. Hypertension. 2010;55(3):644–651. doi: 10.1161/HYPERTENSIONAHA.109.145110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guild SJ, et al. High dietary salt and angiotensin II chronically increase renal sympathetic nerve activity: a direct telemetric study. Hypertension. 2012;59(3):614–20. doi: 10.1161/HYPERTENSIONAHA.111.180885. [DOI] [PubMed] [Google Scholar]
- 60.Wyss JM, Sripairojthikoon W, Oparil S. Failure of renal denervation to attenuate hypertension in Dahl NaCl-sensitive rats. Can J Physiol Pharmacol. 1987;65(12):2428–32. doi: 10.1139/y87-385. [DOI] [PubMed] [Google Scholar]
- 61.Grassi G, et al. Baroreflex impairment by low sodium diet in mild or moderate essential hypertension. Hypertension. 1997;29(3):802–7. doi: 10.1161/01.hyp.29.3.802. [DOI] [PubMed] [Google Scholar]
- 62.Grassi G, et al. Short- and long-term neuroadrenergic effects of moderate dietary sodium restriction in essential hypertension. Circulation. 2002;106(15):1957–61. doi: 10.1161/01.cir.0000033519.45615.c7. [DOI] [PubMed] [Google Scholar]
- 63.Anderson EA, et al. Elevated sympathetic nerve activity in borderline hypertensive humans. Evidence from direct intraneural recordings. Hypertension. 1989;14(2):177–83. doi: 10.1161/01.hyp.14.2.177. [DOI] [PubMed] [Google Scholar]
- 64.Friberg P, et al. Evidence for increased renal norepinephrine overflow during sodium restriction in humans. Hypertension. 1990;16(2):121–30. doi: 10.1161/01.hyp.16.2.121. [DOI] [PubMed] [Google Scholar]
- 65•.Bourque CW. Central mechanisms of osmosensation and systemic osmoregulation. Nat Rev Neurosci. 2008;9(7):519–31. doi: 10.1038/nrn2400. This is a comprehensive review regarding how the central nervous system detects changes in osmolality to result in thirst and antidiuretic hormone secretion. [DOI] [PubMed] [Google Scholar]
- 66•.Ciura S, Bourque CW. Transient receptor potential vanilloid 1 is required for intrinsic osmoreception in organum vasculosum lamina terminalis neurons and for normal thirst responses to systemic hyperosmolality. J Neurosci. 2006;26(35):9069–75. doi: 10.1523/JNEUROSCI.0877-06.2006. Original report that identifies an N-variant of the TRPV1 channel as the putative osmosensory element in the central nervous system and OVLT neurons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ciura S, Liedtke W, Bourque CW. Hypertonicity sensing in organum vasculosum lamina terminalis neurons: a mechanical process involving TRPV1 but not TRPV4. J Neurosci. 2011;31(41):14669–76. doi: 10.1523/JNEUROSCI.1420-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Anderson JW, Washburn DL, Ferguson AV. Intrinsic osmosensitivity of subfornical organ neurons. Neuroscience. 2000;100(3):539–47. doi: 10.1016/s0306-4522(00)00313-4. [DOI] [PubMed] [Google Scholar]
- 69.Gutman MB, Ciriello J, Mogenson GJ. Effects of plasma angiotensin II and hypernatremia on subfornical organ neurons. Am J Physiol. 1988;254(5 Pt 2):R746–54. doi: 10.1152/ajpregu.1988.254.5.R746. [DOI] [PubMed] [Google Scholar]
- 70.Nissen R, Bourque CW, Renaud LP. Membrane properties of organum vasculosum lamina terminalis neurons recorded in vitro. Am J Physiol. 1993;264(4 Pt 2):R811–5. doi: 10.1152/ajpregu.1993.264.4.R811. [DOI] [PubMed] [Google Scholar]
- 71.Oldfield BJ, et al. Fos production in retrogradely labelled neurons of the lamina terminalis following intravenous infusion of either hypertonic saline or angiotensin II. Neuroscience. 1994;60(1):255–62. doi: 10.1016/0306-4522(94)90219-4. [DOI] [PubMed] [Google Scholar]
- 72.Shi P, et al. Intra-carotid hyperosmotic stimulation increases Fos staining in forebrain organum vasculosum laminae terminalis neurones that project to the hypothalamic paraventricular nucleus. J Physiol. 2008;586(Pt 21):5231–45. doi: 10.1113/jphysiol.2008.159665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Larsen PJ, Mikkelsen JD. Functional identification of central afferent projections conveying information of acute “stress” to the hypothalamic paraventricular nucleus. J Neurosci. 1995;15(4):2609–27. doi: 10.1523/JNEUROSCI.15-04-02609.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McKinley MJ, et al. Effect of individual or combined ablation of the nuclear groups of the lamina terminalis on water drinking in sheep. Am J Physiol. 1999;276(3 Pt 2):R673–83. doi: 10.1152/ajpregu.1999.276.3.R673. [DOI] [PubMed] [Google Scholar]
- 75.Thrasher TN, Keil LC. Regulation of drinking and vasopressin secretion: role of organum vasculosum laminae terminalis. Am J Physiol. 1987;253(1 Pt 2):R108–20. doi: 10.1152/ajpregu.1987.253.1.R108. [DOI] [PubMed] [Google Scholar]
- 76.Buggy J, et al. Prevention of the development of renal hypertension by anteroventral third ventricular tissue lesions. Circ Res. 1977;40(5 Suppl 1):I110–7. [PubMed] [Google Scholar]
- 77.Veerasingham SJ, Leenen FH. Excitotoxic lesions of the ventral anteroventral third ventricle and pressor responses to central sodium, ouabain and angiotensin II. Brain Res. 1997;749(1):157–60. doi: 10.1016/s0006-8993(96)01381-9. [DOI] [PubMed] [Google Scholar]
- 78.Taylor AC, McCarthy JJ, Stocker SD. Mice lacking the transient receptor vanilloid potential 1 channel display normal thirst responses and central Fos activation to hypernatremia. Am J Physiol Regul Integr Comp Physiol. 2008;294(4):R1285–93. doi: 10.1152/ajpregu.00003.2008. [DOI] [PubMed] [Google Scholar]
- 79.Blaustein MP, et al. How NaCl raises blood pressure: a new paradigm for the pathogenesis of salt-dependent hypertension. Am J Physiol Heart Circ Physiol. 2012;302(5):H1031–49. doi: 10.1152/ajpheart.00899.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Huang BS, Leenen FH. Brain amiloride-sensitive Phe-Met-Arg-Phe-NH(2)--gated Na(+) channels and Na(+)-induced sympathoexcitation and hypertension. Hypertension. 2002;39(2 Pt 2):557–61. doi: 10.1161/hy02t2.103004. [DOI] [PubMed] [Google Scholar]
- 81.Abrams JM, Engeland WC, Osborn JW. Effect of intracerebroventricular benzamil on cardiovascular and central autonomic responses to DOCA-salt treatment. Am J Physiol Regul Integr Comp Physiol. 2010;299(6):R1500–10. doi: 10.1152/ajpregu.00431.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang H, Leenen FH. Brain sodium channels mediate increases in brain “ouabain” and blood pressure in Dahl S rats. Hypertension. 2002;40(1):96–100. doi: 10.1161/01.hyp.0000022659.17774.e4. [DOI] [PubMed] [Google Scholar]
- 83.Toney GM, et al. Central osmotic regulation of sympathetic nerve activity. Acta Physiol Scand. 2003;177(1):43–55. doi: 10.1046/j.1365-201X.2003.01046.x. [DOI] [PubMed] [Google Scholar]
- 84.Pawloski-Dahm CM, Gordon FJ. Increased dietary salt sensitizes vasomotor neurons of the rostral ventrolateral medulla. Hypertension. 1993;22(6):929–33. doi: 10.1161/01.hyp.22.6.929. [DOI] [PubMed] [Google Scholar]
- 85.Adams JM, Bardgett ME, Stocker SD. Ventral lamina terminalis mediates enhanced cardiovascular responses of rostral ventrolateral medulla neurons during increased dietary salt. Hypertension. 2009;54(2):308–14. doi: 10.1161/HYPERTENSIONAHA.108.127803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Adams JM, et al. Increased dietary salt enhances sympathoexcitatory and sympathoinhibitory responses from the rostral ventrolateral medulla. Hypertension. 2007;50(2):354–359. doi: 10.1161/HYPERTENSIONAHA.107.091843. [DOI] [PubMed] [Google Scholar]
- 87.Adams JM, McCarthy JJ, Stocker SD. Excess dietary salt alters angiotensinergic regulation of neurons in the rostral ventrolateral medulla. Hypertension. 2008;52(5):932–937. doi: 10.1161/HYPERTENSIONAHA.108.118935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ito S, Gordon FJ, Sved AF. Dietary salt intake alters cardiovascular responses evoked from the rostral ventrolateral medulla. Am J Physiol. 1999;276(6 Pt 2):R1600–7. doi: 10.1152/ajpregu.1999.276.6.R1600. [DOI] [PubMed] [Google Scholar]
- 89.Muntzel MS, et al. Dietary salt loading exacerbates the increase in sympathetic nerve activity caused by intravenous insulin infusion in rats. Metabolism. 2007;56(3):373–379. doi: 10.1016/j.metabol.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 90.Zanchetti A. Volhard lecture: sympatho-renal interactions and blood pressure control. J Hypertens Suppl. 1986;4(6):S4–13. [PubMed] [Google Scholar]