Abstract
Background
Alcoholism is a polygenic disorder resulting from reward deficiency; polymorphisms in reward genes including serotonin transporter (5-HTT)-linked polymorphic region (5-HTTLPR), A118G in opioid receptor mu1 (OPRM1), and −141C Insertion/Deletion (Ins/Del) in dopamine receptor D2 (DRD2) as well as environmental factors (education and marital status) might affect the risk of alcoholism. Objective of the current study was to examine the main and interacting effect of these 3 polymorphisms and 2 environmental factors in contribution to alcoholism in Mexican Americans.
Methods
Genotyping of 5-HTTLPR, OPRM1 A118G, and DRD2−141C Ins/Del was performed in 365 alcoholics and 338 nonalcoholic controls of Mexican Americans who were gender-and age-matched. Alcoholics were stratified according to tertiles of MAXDRINKS, which denotes the largest number of drinks consumed in one 24-hour period. Data analysis was done in the entire data set and in each alcoholic stratum. Multinomial logistic regression was conducted to explore the main effect of 3 polymorphisms and 2 environmental factors (education and marital status); classification tree, generalized multifactor dimensionality reduction (GMDR) analysis, and polymorphism interaction analysis version 2.0 (PIA 2) program were used to study factor interaction.
Results
Main effect of education, OPRM1, and DRD2 was detected in alcoholic stratum of moderate and/or largest MAXDRINKS with education ≤12 years, OPRM1 118 A/A, and DRD2 −141C Ins/Ins being risk factors. Classification tree analysis, GMDR analysis, and PIA 2 program all supported education*OPRM1 interaction in alcoholics of largest MAXDRINKS with education ≤12 years coupled with OPRM1 A/A being a high risk factor; dendrogram showed synergistic interaction between these 2 factors; dosage-effect response was also observed for education*OPRM1 interaction. No definite effect of marital status and 5-HTTLPR in pathogenesis of alcoholism was observed.
Conclusions
Our results suggest main effect of education background, OPRM1 A118G, and DRD2 −141C Ins/Del as well as education*OPRM1 interaction in contribution to moderate and/or severe alcoholism in Mexican Americans. Functional relevance of these findings still needs to be explored.
Keywords: Alcoholism, Serotonin Transporter, Opioid Receptor mu-1, Dopamine Receptor D2, Education, Marriage, Interaction, Mexican American
Alcoholism has a significant impact on the nation’s health and welfare especially in Mexican Americans. Mexican Americans represent a prominent segment of the American mosaic and appear to be at a high risk for alcohol problems. The prevalence rate of past heavy drinking among male Mexican Americans is 3 times higher than that reported for non-Hispanic male populations (Lee et al., 1997). Furthermore, Hispanics with alcohol-induced problems, such as alcoholic liver disease (ALD), appear to fare significantly less well than those with other ethnic backgrounds. For example, the survival rate of Hispanic ALD patients after 4.5 years of follow-up is only 28%, in contrast to 66% for African Americans and 40% for Caucasians (Mendenhall et al., 1989). Studying the risk factors of alcoholism in Mexican Americans will lead to the development of diagnostic, therapeutic, and preventive strategies.
Based on family, twin pair, half sibling, and adoption studies, it has been proved that alcoholism is a polygenic complex disorder with heritability ranging between 49% and 64% (Cotton, 1979; Goodwin, 1978; Heath et al., 1997; McGue, 1999). Complex interaction of multiple genetic and environmental factors plays an important role in the development of alcoholism. Candidate gene association approach has been widely used to study the genetic risk factors for polygenic complex disorders including alcoholism. Although the pathogenic mechanism of alcoholism is not clearly understood, genes in the reward system are important and are frequently considered as candidates in association studies.
The reward system, which is a collection of brain neuro-transmission pathways, reinforces behavior by inducing pleasurable effects after stimulation of “rewards.” Primary natural rewards include those that are necessary for the survival of human beings such as food and sex. Drugs, alcohol, risky sports, etc. can also stimulate the reward system, and are referred to as “unnatural rewards” (Comings and Blum, 2000). In 1996, the reward deficiency hypothesis was proposed (Blum et al., 1996a,b) which postulates that individuals who have dysfunctions of 1 or more components of the reward system tend to be less satisfied with natural rewards and tend to abuse unnatural rewards including alcohol as a way to seek enhanced stimulation of the reward pathways. The final result of such a process is “reward deficiency syndrome” which may be comprised of negative behaviors such as alcoholism, substance abuse, and smoking.
The reward system is mainly composed of 4 neurotransmission pathways: serotonin (5-HT), opioid, dopamine, and gamma-aminobutyric acid (GABA) pathways, and these 4 pathways form a “reward cascade” (Blum et al., 1996a,b). Serotoninergic neurons release 5-HT, then 5-HT activates the opioidergic neurons and endogenous opioid is released which will inhibit the release of GABA. The primary role of GABA is to inhibit activation of dopamine neurons, so the result of inhibiting GABA activity is an increase of dopamine release. Finally, dopamine, a “pleasure molecule” (Blum et al., 2000), will produce a feeling of elation.
The common mechanism of reward deficiency is inhibition of dopaminergic neurotransmission, and utilizing natural dopaminergic repletion therapy to promote long-term dopaminergic activation will ultimately lead to a common, safe and effective modality to treat reward deficiency syndrome behaviors (Blum et al., 2008). At the same time, the final outcome of the reward cascade is decided by the function of all the 4 neurotransmission pathways. Theoretically, genetic variations leading to dysfunction of any component in these 4 pathways will likely result in the reward deficiency syndrome. Most of the reward genes are highly polymorphic, and a number of genetic polymorphisms in the functional regions of these genes have been implicated as being responsible for altering the gene functions and have been associated with alcoholism, among which are 5-HT transporter (5-HTT) linked polymorphic region (5-HTTLPR) in 5-HTT, A118G polymorphism (rs1799971) in opioid receptor mu1 (OPRM1), and −141C Insertion/Deletion (Ins/Del) polymorphism (rs1799732) in dopamine receptor D2 (DRD2).
5-HTT plays an important role in the serotoninergic transmission. It is responsible for reuptake of 5-HT in the synaptic cleft back into the presynaptic neuron, which thus terminates the synaptic actions of serotonin and recycles it into the neurotransmitter pool (Ramamoorthy et al., 1993). 5-HTTLPR is a functional polymorphism involving the insertion or deletion of a 44-bp motif in the promoter region, resulting in a long (L) allele and a short (S) allele, respectively. The short allele has a markedly reduced transcriptional efficiency than its longer counterpart (Bradley et al., 2005; Smith et al., 2004). Regarding the association between 5-HTTLPR and alcoholism, many studies in non-Mexican American populations have yielded conflicting results, while a meta-analysis based on 17 published studies (including 3,489 alcoholics and 2,325 controls mainly of European ancestry) concluded that the short allele was significantly associated with increased risk to alcoholism with an odds ratio (OR) of 1.18 (Feinn et al., 2005). For Mexican Americans, our previous study, which has a smaller sample size compared with the current study, has consistent finding with the meta-analysis (Konishi et al., 2004b).
OPRM1 is the primary site of action for opiates. The most frequently studied single nucleotide polymorphism (SNP) in this gene is A118G. OPRM1 A118G results in an amino acid change from asparagine (Asn) to aspartic acid (Asp) at position 40 of the receptor protein which is a putative N-glycosylation site. The Asp-containing receptor has 3 times higher affinity to the β-endorphin than the Asn form (Bond et al., 1998). In addition, OPRM1 118A mRNA is 1.5- to 2.5-fold more abundant than the 118G mRNA in heterozygous brain autopsy tissues, and 118G causes a 10-fold reduction in OPRM1 protein level (Zhang et al., 2005). Previous association studies regarding relationship between A118G in OPRM1 and alcoholism have produced conflicting results. In some studies, no association was found (Bergen et al., 1997; Franke et al., 2001; Zhang et al., 2006), while in some other studies 118G or 118A was found to increase alcoholism risk (Bart et al., 2005; Schinka et al., 2002). An association study of OPRM1 A118G and alcoholism in Mexican Americans has not been conducted.
Although other neurotransmitters may be important in determining the rewarding and stimulating effects of ethanol, dopamine may be critical for initiating substance use. A deficiency or absence of DRD2 receptors then predisposes individuals to a high risk for multiple addictive, impulsive, and compulsive behaviors (Bowirrat and Oscar-Berman, 2005). DRD2 is a G protein-coupled receptor located on postsynaptic dopaminergic neurons that are centrally involved in reward signal transduction (Neville et al., 2004). The DRD2 −141C Ins/Del polymorphism is located in the 5′ promoter region 141 base pairs upstream from exon1 and involves insertion or deletion of a cytosine. Compared with the insertion allele (Ins), the deletion allele (Del) is related to lower transcription activity (Arinami et al., 1997), but higher striatal DRD2 density (Jonsson et al., 1999). Ishiguro and colleagues (1998) found that the Ins allele increased the risk for alcoholism, while many other studies failed to replicate such an association (Chen et al., 2001; Parsian et al., 2000; Sander et al., 1999). In Mexican American populations, the Ins allele was found to be a risk factor for alcoholism by our previous study, in which 200 alcoholics were studied (Konishi et al., 2004b).
In the GABA pathway, GABA receptor (GABR) genes have been considered as candidates for alcoholism. The GABA receptor β3 gene (GABRβ3) encodes a major subunit of the GABAA receptor. There is a CA repeat polymorphism in GABRβ3. Noble and colleagues (1998) reported that 1-repeat allele (G1) contributed to the risk of alcoholism, but our previous study, which included 200 Mexican American alcoholics and 251 non-alcoholics, did not find an association between the G1 variant and alcoholism (Konishi et al., 2004b). Thus, GABRβ3 is not included in the current study. Besides, lack of functional information for this polymorphism, as well as sample size limitation for a multiallelic polymorphism, is the reason why GABRβ3 is excluded from further study.
Environmental factors, such as education background and marital status, also play an important role in pathogenesis of reward deficiency syndrome including alcoholism and drug abuse. In the National Longitudinal Alcohol Epidemiology Study, the risk of alcoholism among participants with <12 years of education is significantly higher (odds ratio = 1.24) than among participants with 16 years or more (Grant, 1997). With the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC, 2001–2002) participants, individuals without a college degree have higher risk of alcoholism than individuals with a college degree or more; the magnitude of risk, however, varies significantly by race-ethnicity, and there is no association between education and alcoholism among Hispanics (Gilman et al., 2008). As to marital status, never having been married was strongly associated with alcohol dependence and abuse in Swedish women (Thundal and Allebeck, 1998), and women who married or remarried decreased drinking, whereas those who became separated or divorced increased drinking (Hanna et al., 1993). For males, transition into marriage is significantly associated with greater decreases in drug use during the mid to late 20s (Flora and Chassin, 2005).
The findings regarding association of an individual polymorphism in reward genes or a single environmental factor with alcoholism have been very inconsistent partly due to the low-penetrance and minor-effect nature of every individual factor coupled with a failure to consider the complexity of gene-gene or gene-environment interactions (Goode et al., 2002; Wu et al., 2007). As what has been described above, the function of different neurotransmission pathways in reward system is closely linked with each other, so it is highly possible that the effect of a polymorphism in 1 pathway can be modified by polymorphisms in other pathways. It has been shown that after stratification of DRD2 TaqIA genotypes, a significant difference for 5-HTTLPR polymorphisms between antisocial alcoholics and antisocial non-alcoholics was found, indicating an interaction between DRD2 TaqI A and 5-HTTLPR (Wu et al., 2008). Interaction between reward genes and environmental factors has also been reported. The risk of alcoholism associated with the GABRA2 genotype varies as a function of marital status. Among individuals not currently married, the influence of GABRA2 on alcohol dependence is not detectable. However, among individuals who report a stable marriage, the influence of GABRA2 is significant (Dick et al., 2006). The interactions among 5-HTTLPR, OPRM1 A118G, DRD2 −141C Ins/Del, education background, and marital status in the development of reward deficiency syndrome have not been reported in any populations.
In the current study, for the first time, we examine the interactions among 5-HTTLPR, OPRM1 A118G, DRD2 −141C Ins/Del, education background, and marital status in contribution to alcoholism in Mexican Americans.
MATERIALS AND METHODS
Study Population
Unrelated Mexican Americans living in the Los Angeles County including 338 controls and 365 alcoholics were recruited (109 controls and 200 alcoholics were from our previous studies) (Konishi et al., 2004b; Luo et al., 2005). Controls and alcoholics were gender-and age-matched. Participants were recruited from a variety of sources including: (1) Human services, substances dependence, and mental health programs; (2) driving schools and the Alcohol Anonymous Groups organized and/or associated with these schools; (3) bars and liquor stores; (4) Hispanic churches with counseling services programs; (5) day labor centers; and (6) Hispanic newspapers, radio stations, and television stations. The alcoholic participants fulfilled the Diagnostic and Statistical Manual of Mental Disorder, Fourth Edition (DSM-IV) criteria for a current diagnosis of either alcohol dependence (303.90) or alcohol abuse (305.00). Control participants fulfilled the following criteria: (1) no current or past diagnosis of DSM-IV alcohol dependence (303.90) or alcohol abuse (305.00); and (2) no clinically unacceptable findings from physical examinations and vital signs. The inclusion criteria for both controls and alcoholics were as follows: (1) ability to give informed consent; (2) between 21 and 79 years; (3) 3 of 4 biological grandparents of Mexican heritage; (4) fluency in either Spanish or English; (5) no current use of other substances (except tobacco and caffeine), or history of such use within the past 6 months; and (6) no current or past diagnosis of mental illnesses such as schizophrenia, schizophreniform disorder, schizoaffective disorder, schizotypal disorder, major depression, or bipolar disorder. Written informed consent was obtained from each participant. The use of participants’ DNA samples was approved by the Human Subjects Committees at the University of Kansas Medical Center and Los Angeles Biomedical Research Institute at Harbor-University of California, Los Angeles Medical Center. The procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975, as revised in 1983.
Interview Instrument
Every participant was interviewed with a standard questionnaire to collect the information including gender, age, marital status, duration of education, medical history, history of smoking and alcohol consumption. Marital status was categorized into 3 types: being married, being single or living with a partner. Having been divorced, separated, and widowed was regarded as being single. Current smoking was defined as having smoked 1 or more cigarettes on 1 or more days during the past 30 days (Christophi et al., 2008). Each alcoholic was interviewed with The Semi-Structured Assessment for the Genetics of Alcoholism II (SSAGA-II) (Bucholz et al., 1994). Information of MAXDRINKS was collected according to participants’ response to the question in SSAGA “What’s the largest number of drinks you have had in a 24-hour period?”
Genotyping
Peripheral venous blood samples were collected for all participants and kept at −70°C until DNA extraction. The frozen blood was thawed and leukocyte DNA was isolated by a rapid nonenzymatic method (Lahiri and Nurnberger, 1991) or by GeneCatcher gDNA blood kits (Invitrogen, Carlsbad, CA).
OPRM1 A118G and −141C Ins /Del genotypes were determined by PCR followed by restriction fragment length polymorphism analysis according to Gelernter and colleagues (1999) and Arinami and colleagues (1997), respectively. The 5-HTTLPR genotype was determined by PCR amplification according to Collier and colleagues (1996). 5-HTTLPR and DRD2 −141C Ins /Del genotypes of 109 controls and 200 alcoholics had been determined in our previous studies (Konishi et al., 2004b).
Statistical Analysis
Hardy–Weinberg equilibrium (HWE) in controls as well as in alcoholics was tested for all the 3 polymorphisms with the HWSIM program. Pearson’s chi-square test was conducted to compare the gender, smoking status, and marital status distribution between controls and alcoholics. Age and education background difference was evaluated with both Pearson’s chi-square test and Wilcoxon rank sum test. Main effect of each factor or factor combination was explored with binary or multinomial logistic regression. Interaction among different factors was studied with classification tree analysis, generalized multifactor dimensionality reduction (GMDR) program, and polymorphism interaction analysis program version 2.0 (PIA2). The grayscale interaction dendrogram was adapted from the colorful dendrogram made by multifactor dimensionality reduction (MDR) program version 2.0-alpha (MDR 2.0-alpha). Pearson’s chi-square test, Wilcoxon rank sum test, and logistic regression were performed with SPSS 15.0 software package (SPSS Inc., Chicago, IL). p < 0.05 was taken as significance level for all statistical analyses. Bonferroni correction was applied for multiple comparisons.
RESULTS
Demographics of Participants
There were 80 (23.7%) and 69 (18.9%) females in 338 non-alcoholic controls and 365 alcoholics, respectively. There was no significant difference in gender distribution between these 2 cohorts (χ2 = 2.385, p = 0.122).
The number of young (≤30 years), middle-aged (30 to 60 years), and old (>60 years) participants in 338 controls was 93 (27.5%), 237 (70.1%), and 8 (2.4%), respectively. In the alcoholic group (365), there were 99 (27.1%) young, 257 (70.4%) middle-aged, and 9 (2.5%) old participants. The median age for controls was the same as that of alcoholics (37 years). No significant difference was found regarding age distribution with Pearson’s chi-square test (χ2 = 0.019, p = 0.991) and Wilcoxon rank sum test (Z = −1.032, p = 0.301).
Six control participants did not provide the information of smoking history. There were significantly more current smokers in 365 alcoholics than in 332 controls (45.5% vs. 17.2%, χ2 = 64.045, p < 0.001).
Seventeen controls and 8 alcoholics did not provide the information of marital status. The number of participants who were married, single and living with a partner was 120 (37.4%), 185 (57.6%), and 16 (5.0%) in 321 controls, while the number was 113 (31.7%), 215 (60.2%), and 29 (8.1%) in 357 alcoholics. No significant difference was found between these 2 cohorts regarding distribution of marital status (χ2 = 4.317, p = 0.116).
As to education background, fifteen controls and 4 alcoholics did not provide the information. The median duration of education for 323 controls and 361 alcoholics was 12 and 11 years, respectively. There were 111 (34.4%) controls and 75 (20.8%) alcoholics who had received education of more than 12 years. Significant difference was found between these 2 cohorts with Wilcoxon rank sum test (Z = −2.663, p = 0.008) and chi-square test (χ2 = 15.902, p < 0.001).
There were 2 alcoholics who did not provide the information of MAXDRINKS. For the rest 363 alcoholics, the minimum, maximum, and median MAXDRINKS was 4, 100 and 25, respectively. The skewness value was 1.203 with a standard error of 0.128, and the kurtosis value was 1.544 with a standard error of 0.255. The first and second tertile of MAXDRINKS was 22 and 35, respectively. There were 124 (34.2%) alcoholics with MAXDRINKS ≤ 22, 121 (33.3%) with MAXDRINKS 23 to 35, and 118 (32.5%) with MAXDRINKS ≥ 36.
Main Effect of Each Individual Factor on Alcoholism
Genotype distribution in controls as well as in alcoholics for each of the 3 studied polymorphisms was in HWE (p > 0.05, Table 1 and Table S1).
Table 1.
Genotype Distribution of 5-HTTLPR, OPRM1 A118G, and DRD2 −141C Ins/Del in Controls and Alcoholics
| 5-HTTLPR | n | S/S (%) | S/L (%) | L/L (%) |
| Controls | 338 | 108 (32.0) | 173 (51.2) | 57 (16.9) |
| Alcoholics | 365 | 136 (37.3) | 163 (44.6) | 66 (18.1) |
| MAXDRINKS ≤ 22 | 124 | 38 (30.6) | 61 (49.2) | 25 (20.2) |
| MAXDRINKS 23–35 | 121 | 46 (38.0) | 56 (46.3) | 19 (15.7) |
| MAXDRINKS ≥ 36 | 118 | 51 (43.2) | 45 (38.1) | 22 (18.6) |
| OPRM1 A118G | n | A/A (%) | A/G (%) | G/G (%) |
| Controls | 338 | 198 (58.6) | 120 (35.5) | 20 (5.9) |
| Alcoholics | 365 | 230 (63.0) | 123 (33.7) | 12 (3.3) |
| MAXDRINKS ≤ 22 | 124 | 70 (56.5) | 48 (38.7) | 6 (4.8) |
| MAXDRINKS 23–35 | 121 | 71 (58.7) | 47 (38.8) | 3 (2.5) |
| MAXDRINKS ≥ 36 | 118 | 88 (74.6) | 27 (22.9) | 3 (2.5) |
| DRD2 −141C Ins/Del | n | Ins/Ins (%) | Ins/Del (%) | Del/Del (%) |
| Controls | 338 | 235 (69.5) | 97 (28.7) | 6 (1.8) |
| Alcoholics | 365 | 270 (74.0) | 84 (23.0) | 11 (3.0) |
| MAXDRINKS ≤ 22 | 124 | 93 (75.0) | 29 (23.4) | 6 (1.8) |
| MAXDRINKS 23–35 | 121 | 82 (67.8) | 36 (29.8) | 2 (1.6) |
| MAXDRINKS ≥ 36 | 118 | 94 (79.7) | 18 (15.3) | 3 (2.5) |
Controlling for the confounding effect of gender, age, and smoking status, the binary logistic regression model was constructed to explore the main effect of marriage status, education background, 5-HTTLPR, OPRM1 A118G and DRD2 −141C Ins/Del on alcoholism. With all alcoholics included, the model showed that only duration of education was associated with alcoholism (p = 0.002, Table 2). Compared with education of more than 12 years, education of less than or equal to 12 years was a risk factor for alcoholism with an odds ratio (OR) of 1.792 (95%CI 1.236 to 2.604).
Table 2.
Main Effects of Environmental and Genetic Factors on Alcoholism With Multinomial Logistic Regression Controlling for the Effect of Gender, Age, and Smoking Status
| Overall | MAXDRINKS ≥ 36 | ||||||
|---|---|---|---|---|---|---|---|
| Factors | Controls/ alcoholics |
p-Value | OR (95% CI) | Control/ alcoholics |
p-Value | OR (95% CI) | |
| Marital status | Single | 185/215 | 0.254 | NA | 185/68 | 0.446 | NA |
| Married | 120/113 | 120/36 | Reference | ||||
| Living with a partner | 16/29 | 16/10 | 0.183 | ||||
| Duration of education | ≤12 years | 212/286 | 0.002 | 1.792 (1.236–2.604) | 212/96 | 0.019 | 1.966 (1.117–3.461) |
| >12 years | 111/75 | Reference | 111/21 | Reference | |||
| 5-HTTLPR | S/S | 108/136 | 0.373 | NA | 108/51 | 0.082 | NA |
| S/L & L/L | 230/229 | 230/67 | |||||
| OPRM1 | A/A | 198/230 | 0.292 | NA | 198/88 | 0.003 | 2.162 (1.300–3.597) |
| A118G A/G & G/G | 140/135 | 140/30 | Reference | ||||
| DRD2−141C | Ins/Ins | 235/270 | 0.111 | NA | 235/94 | 0.030 | 1.847 (1.062–3.211) |
| Ins/Del | Ins/Del & Del/Del | 103/95 | 103/24 | Reference | |||
| MAXDRINKS | 23–35 MAXDRINKS ≤ 22 | ||||||
|---|---|---|---|---|---|---|---|
| Factors | Controls/ alcoholics |
p-Value | OR (95% CI) | Control/ alcoholics |
p-Value | OR (95% CI) | |
| Marital status | Single | 185/75 | 0.180 | NA | 185/70 | 0.971 | NA |
| Married | 120/34 | Reference | 120/43 | Reference | |||
| Living with a partner | 16/11 | 0.051 | 16/8 | 0.677 | |||
| Duration of education | ≤12 years | 212/98 | 0.01 | 2.032 (1.186–3.481) | 212/90 | 0.155 | NA |
| >12 years | 111/22 | Reference | 111/32 | ||||
| 5-HTTLPR | S/S | 108/46 | 0.396 | NA | 108/38 | 0.552 | NA |
| S/L & L/L | 230/75 | 230/86 | |||||
| OPRM1 | A/A | 198/71 | 0.952 | NA | 198/70 | 0.605 | NA |
| A118G | A/G & G/G | 140/50 | 140/54 | ||||
| DRD2−141C | Ins/Ins | 235/82 | 0.926 | NA | 235/93 | 0.160 | NA |
| Ins/Del | Ins/Del & Del/Del | 103/39 | 103/31 | ||||
When alcoholics were divided into 3 subgroups according to tertiles of MAXDRINKS, the multinomial logistic regression analysis controlling for the effect of confounding factors indicated that alcoholics with different MAXDRINKS were related to different risk factors (Table 2). For alcoholics with the largest MAXDRINKS (≥36), education of less than or equal to 12 years, OPRM1 118 A/A, and DRD2 −141C Ins /Ins were all risk factors with the ORs being 1.966, 2.162, and 1.847, respectively. For alcoholics with medium MAXDRINKS (23 to 35), only education of less than or equal to 12 years was found to be a risk factor with an OR of 2.032. For alcoholics with the smallest MAXDRINKS (≤22), neither the environmental factors (marital status and education background), nor the genetic factors (5-HTTLPR, OPRM1 A118G and DRD2 −141C Ins/Del) were associated with alcoholism.
Interaction Among Environmental and Genetic Factors
Classification Tree Analysis
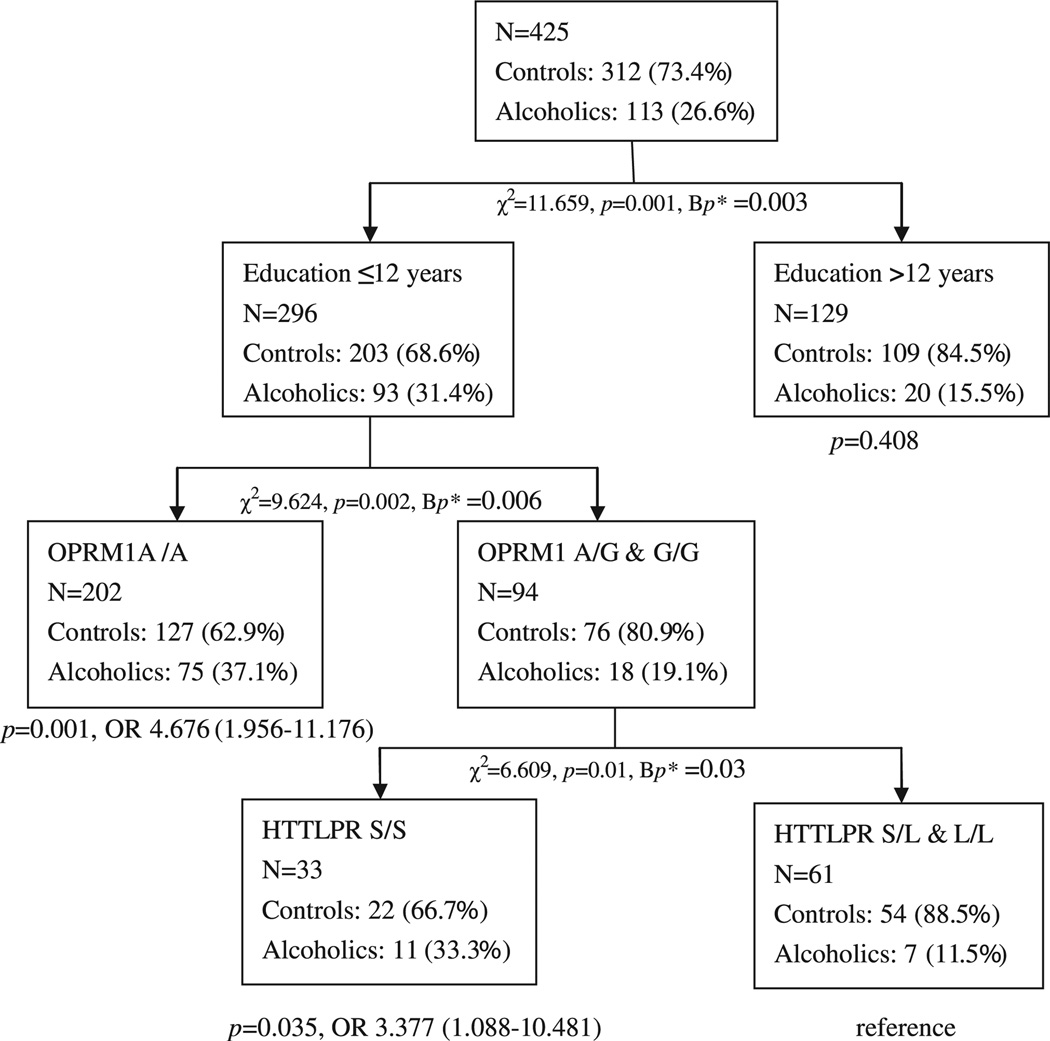
Classification tree analysis including the 2 environmental (marital status and education background) and 3 genetic factors (OPRM1, HTTLPR, and DRD2) was performed through consecutive data splitting. No factor interaction was detected when all alcoholics, alcoholic subgroup with MAXDRINKS ≤ 22 or with MAXDRINKS 23 to 35 were considered. For overall alcoholics and alcoholic subgroup with MAXDRINKS 23 to 35, only a splitting by education background can be made (p < 0.001 and p = 0.001, respectively). For alcoholic subgroup with MAXDRINKS ≤ 22, no splittings can be made. For alcoholic subgroup of MAXDRINKS ≥ 36, 3 consecutive splittings by education background, OPRM1 and HTTLPR were made, and a Bonferroni corrected p-value which was less than 0.05 was observed for each splitting (Fig. 1). Binary logistic regression controlling for effect of gender, age, smoking and DRD2 was adopted to compare the effect of each factor combination (Fig. 1). Education ≤12 years had to be combined with at least 1 genetic risk factor (OPRM1 A/A and/or HTTLPR S/S) to become a risk factor, although it was found to have main effect on pathogenesis of alcoholism, indicating interactions among education background, OPRM1 and HTTLPR.
Fig. 1.
Interaction among genetic and environmental factors with alcoholics of MAXDRINKS ≥ 36 by classification tree analysis. p-Value and OR of each factor combination were decided by binary logistic regression controlling for effect of gender, age, smoking, and DRD2.
GMDR Analysis
With the 2 environmental (marital status and education background) and 3 genetic factors (OPRM1, HTTLPR, and DRD2), GMDR analysis controlling for effect of gender, age, and smoking constructed different risk prediction models for alcoholic subgroups of different MAXDRINKS (Table 3). For overall alcoholics and alcoholics of MAXDRINKS 23 to 35, the best prediction model contained only 1 factor, the education background, whose testing balanced accuracy was 56.25 and 57.04%, respectively. For alcoholics of MAXDRINKS ≤ 22, no environmental or genetic factors were suitable for risk prediction. For alcoholics of MAXDRINKS ≥ 36, the 2-factor model consisting of education background and OPRM1 showed the best prediction efficacy with sign test p-value less than 0.05, highest cross-validation consistency (10/10) and highest testing balanced accuracy (63.16%), suggesting interaction between education background and OPRM1. Combination of education≤12 years and OPRM1 A/A served as a high risk factor for alcoholism of MAXDRINKS ≥ 36, while the other combinations served as low risk factors (Fig. 2).
Table 3.
Risk Prediction of Alcoholism by GMDR Analysis With Different Alcoholic Subgroups
| MAXDRINKS | Number of factors in the model |
Best model | Testing balanced accuracy (%) |
Cross-validation consistency |
Sign test p-value |
|---|---|---|---|---|---|
| Overall | 1 | Education | 56.25 | 10/10 | 0.0010 |
| 2 | Education*Marriage | 51.95 | 4/10 | 0.1719 | |
| 3 | Marriage*Education*OPRM1 | 52.25 | 6/10 | 0.1719 | |
| 4 | Marriage*Education*OPRM1*HTTLPR | 51.74 | 7/10 | 0.6230 | |
| 5 | Marriage*Education*OPRM1*HTTLPR*DRD2 | 50.54 | 10/10 | 0.6230 | |
| ≥36 | 1 | OPRM1 | 56.54 | 9/10 | 0.0107 |
| 2 | Education*OPRM1 | 63.16 | 10/10 | 0.0107 | |
| 3 | Marriage*Education*OPRM1 | 59.88 | 6/10 | 0.0547 | |
| 4 | Education*OPRM1*HTTLPR*DRD2 | 59.79 | 5/10 | 0.0547 | |
| 5 | Marriage*Education*OPRM1*HTTLPR*DRD2 | 62.05 | 10/10 | 0.0547 | |
| 23–35 | 1 | Education | 57.04 | 10/10 | 0.0107 |
| 2 | Education*Marriage | 50.84 | 5/10 | 0.1719 | |
| 3 | Marriage*Education*HTTLPR | 52.82 | 5/10 | 0.0547 | |
| 4 | Marriage*Education*OPRM1*HTTLPR | 48.22 | 5/10 | 0.6230 | |
| 5 | Marriage*Education*OPRM1*HTTLPR*DRD2 | 49.91 | 10/10 | 0.3770 | |
| ≤22 | 1 | Education | 51.51 | 8/10 | 0.3770 |
| 2 | HTTLPR*OPRM1 | 48.35 | 7/10 | 0.6230 | |
| 3 | Marriage*HTTLPR*DRD2 | 49.54 | 7/10 | 0.6230 | |
| 4 | Marriage*OPRM1*HTTLPR*DRD2 | 44.25 | 5/10 | 0.9893 | |
| 5 | Marriage*Education*OPRM1*HTTLPR*DRD2 | 43.58 | 10/10 | 0.9990 |
Italicized lines mean the best prediction models for the corresponding alcoholic subgroup.
Fig. 2.
Interaction between OPRM1 and education by GMDR analysis. Dark gray and light gray boxes correspond to the high- and low-risk factor combinations, respectively. The left and right bars within each box correspond to cases and controls, respectively. The top number is the sum of scores for the corresponding group of individuals (cases or controls with particular factor combination). The heights of the bars are proportional to the sum of scores in each group. For education, 0 means ≤12 years, and 1 means >12 years. For OPRM1, 0 means A/A, and 1 means A/G&G/G.
Analysis With PIA2 Program
With 7 different scoring functions including % correct, sensitivity + specificity, positive predictive value (PPV) + negative predictive value (NPV), risk ratio, odds ratio, and Gini Index, PIA2 software was used to further evaluate the efficacy of risk prediction models containing different number of factors (Table 4). For alcoholics of MAXDRINKS ≥ 36, education*OPRM1 and education*OPRM1*HTTLPR were shown to be the best 2-factor and 3-factor model with highest overall score, respectively, supporting the findings regarding factor interaction revealed by classification tree and GMDR analysis.
Table 4.
Overall Score of Factor Combinations by PIA2 Program With Alcoholics of MAXDRINKS ≥ 36
| Single-factor model (overall score) |
Two-factor model (overall score) |
Three-factor model (overall score) |
Four-factor model (overall score) |
|
|---|---|---|---|---|
| 1st | Education (296.82) | Education*OPRM1 (292.26) | Education*OPRM1*HTTLPR (297.76) | Marriage*Education*OPRM1*HTTLPR (298.73) |
| 2nd | OPRM1 (277.05) | OPRM1*HTTLPR (259.91) | Marriage*Education*OPRM1 (287.06) | Marriage*OPRM1*HTTLPR*DRD2 (286.29) |
| 3rd | HTTLPR (235.82) | Education*DRD2 (246.23) | OPRM1*HTTLPR*DRD2 (266.74) | Education*OPRM1*HTTLPR*DRD2 (277.91) |
| 4th | DRD2 (224.38) | Marriage*Education (245.81) | Education*OPRM1*DRD2 (266.11) | Marriage*Education*HTTLPR*DRD2 (250.94) |
| 5th | Marriage (186.71) | Education*HTTLPR (242.27) | Marriage*OPRM1*HTTLPR (264.18) | Marriage*Education*OPRM1*DRD2 (249.32) |
Dendrogram Regarding Education*OPRM1*HTTLPR Interaction
A dendrogram regarding the relationship among education background, OPRM1, and HTTLPR for alcoholics of MAXDRINKS ≥ 36 was made by MDR 2.0-alpha software (Fig. 3). Education background and HTTLPR were shown to be redundant with each other, while OPRM1 was synergistic with both education background and HTTLPR.
Fig. 3.
Dendrogram of interaction among education, OPRM1, and HTTLPR. The gray scale represents a continuum from Synergy to Redundancy. It ranges from black representing a high degree of synergy (positive information gain) to white representing the highest degree of redundancy (negative information gain).
Dose-Effect Response of Factor Interaction
For education*OPRM1 interaction detected with alcoholics of MAXDRINKS ≥ 36, dosage-effect response was observed by binary logistic regression controlling for effect of gender, age, smoking, and DRD2 (Table 5). Between individuals containing no and 1 risk factor, the risk for severe alcoholism with MAXDRINKS ≥ 36 was not significantly different (p = 0.694). Individuals containing both 2 risk factors, however, were more susceptible to severe alcoholism (p = 0.003, OR = 3.258).
Table 5.
Dosage–Effect Response of Education*OPRM1 Interaction With Alcoholics of MAXDRINKS ≥ 36
| Factor combination | p-Value | OR (95% CI) | |
|---|---|---|---|
| No risk factors* | Both education>12 years and OPRM1 A/G & G/G | Reference | NA |
| One risk factor* | Either education ≤12 years or OPRM1 A/A | 0.694 | NA |
| Two risk factors* | Both education ≤12 years and OPRM1 A/A | 0.003 | 3.258 (1.509–7.035) |
Risk factors include education ≤12 years and OPRM1 A/A.
DISCUSSION
In this study, we found the main effect of education background, OPRM1 A118G, and DRD2 −141C Ins/Del as well as the interaction between education background and OPRM1 A118G in modulating risk for alcoholism in Mexican Americans. Education ≤12 years, OPRM1 A/A, and DRD2 Ins/Ins were shown to be risk factors. No definite effect of marital status and 5-HTTLPR in pathogenesis of alcoholism was observed.
Individuals of lower socioeconomic status are at greater risk for alcoholism. Duration of education reflects the individual’s socioeconomic background, insofar as opportunities for schooling are influenced by parental resources (Gilman et al., 2008), so it’s not surprising to find education ≤12 years was a risk factor for alcoholism in Mexican Americans. Improving education level might serve as a preventive strategy for alcoholism in this minority group. With respect to OPRM1 A118G and DRD2 −141C Ins /Del, functional significance is not well established (Arinami et al., 1997; Bond et al., 1998; Jonsson et al., 1999; Zhang et al., 2005); the mechanism accounting for the causative effect of OPRM1 A/A and DRD2 −141C Ins/Ins still remains to be elucidated.
Regarding the main effect of DRD2 −141C Ins/Del on alcoholism in Mexican Americans, findings of our current study are similar to those revealed by our previous study with a smaller sample size (251 controls and 200 alcoholics) and chi-square test. The current results about main effect of 5-HTTLPR are conflicting with our previous ones which indicated S/S to be a risk factor, probably due to different sample sizes and statistical methods. For the main effect of the 2 environmental factors and 3 genetic factors on alcoholism considered in this study, a number of studies conducted in other ethnic groups have yielded conflicting results. This controversy might be caused by: (1) different sample size, ethnicity, and linkage disequilibrium (LD) patterns; (2) different status of population stratification and sample heterogeneity; and (3) neglect of gene-gene interactions. In our current study participants with 3 of 4 biological grandparents of Mexican heritage were recruited to ensure ethnic homogeneity and avoid population stratification. In addition, we have performed genotyping for many other polymorphisms in this population (Konishi et al., 2003, 2004a,b; Luo et al., 2005; Mendoza et al., 2001; Yang et al., 2007) finding that the allele frequencies were consistent with those reported by other studies of Mexican Americans. In addition, effect of confounding factors was controlled and factor interactions were considered, which also enhance the reliability of our findings.
Three strategies with different advantages were used simultaneously in this study to indentify interactions among the 2 environmental (education background and marital status) and 3 genetic factors (OPRM1, HTTLPR, and DRD2). The main advantage of classification tree analysis is that multiple comparisons can be corrected in a stringent way; GMDR analysis can not only control for the effect of confounders, but also verify the interactions by dividing the data into training and testing set; PIA2 program includes 7 different scoring functions for evaluation of prediction models. Combination of these 3 strategies will lead to a more reliable result. Classification tree analysis indicated education*OPRM1*HTTLPR interaction, while GMDR analysis suggested education *OPRM1 interaction. For PIA2, both the 2-factor and 3-factor interactions mentioned above were supported with both education*OPRM1 and education*OPRM1*HTTLPR being the top prediction models. In summary, all the 3 strategies converged on the education*OPRM1 interaction. Combination of education ≤12 years and OPRM1 A/A was shown to be a high risk factor for alcoholism by GMDR, which is consistent with the dendrogram illustrating synergy between education background and OPRM1. The synergistic interaction was further confirmed by dosage-effect response analysis of education*OPRM1 with logistic regression, which showed individuals with both 2 risk factors had higher risk for alcoholism than those with no or 1 risk factor. The education*OPRM1 interaction further underscores the importance of improving education level for alcoholism prevention in Mexican Americans. Polygenic disorders, such as alcoholism, are a result of the complex interplay of genetic and environmental factors. Education*OPRM1 interaction found in this current study actually represents such an environment*gene interplay. The mechanism accounting for such an interaction may be the incomplete penetrance of genotypes. OPRM1 A/A has main effect on pathogenesis of alcoholism, but the expression of genetic effect depends on environment. Only when individuals have low education level, the pathogenic genetic effect can be expressed resulting in the occurrence of alcoholism. Another possibility is that high level education can improve the brain function, including function of reward system, so the pathogenic effect of reward gene variants may be masked. These hypotheses still need to be validated with functional studies. At the same time, the reason why only OPRM1 in the reward pathway interacts with education background still remains to be elucidated.
According to previous studies (Bradley et al., 2005; Smith et al., 2004), 5-HTTLPR S allele results in decreased expression of 5-HTT and less reuptake of 5-HT, so more 5-HT will be degraded in the synaptic cleft. As a result, 5-HT in the neurotransmitter pool may not remain abundant and stable serotoninergic neurotransmission may not be maintained, breaking normal function of the reward system and increasing the risk for alcoholism. In our current study, HTTLPR was shown to be involved in the factor interaction by classification tree analysis, and the dendrogram indicated synergy between HTTLPR and OPRM1. Although the involvement of HTTLPR in the interaction was not supported by GMDR analysis, possible effect of HTTLPR on alcoholism still deserves further investigation with a larger sample size.
PIA2 program is designed to be highly sensitive at modest interactions. Even in absence of interacting factors, PIA2 will identify top scoring models, so observed interactions with PIA2 must be interpreted with caution and confirmed with alternative methods (Mechanic et al., 2008). In our current study, PIA2 indicated a top 4-factor model, which was not supported by classification tree and GMDR analysis, so the 4-factor interaction is considered as a false-positive result.
Main effects of and interactions among risk factors were detected with alcoholics of moderate or/and large MAXDRINKS. MAXDRINKS, which denotes the maximum number of drinks a person consumes in a 24-hour period, is an important phenotype used in the Collaborative Study on the Genetics of Alcoholism (COGA) study. Larger MAXDRINKS may reflect an increased tolerance for high levels of alcohol (Edenberg, 2002), so it is reasonable to take MAXDRINKS as an indicator of severity of alcoholism. For a polygenic disorder such as alcoholism, patients with severe forms of the disease always harbor more genetic and environmental risk factors, therefore, it is not surprising to detect main effect of and interaction among reward gene polymorphisms and environmental factors only in alcoholics who have the large MAXDRINKS.
The main effect and interaction of genetic factors identified in the current study might be race-specific and only exist in Mexican Americans. For other ethnic populations with high morbidity of alcoholism, the allele frequency of OPRM1 118G is very low in Africans (0.8 to 2.2%) (dbSNP), and the allele frequency of DRD2 −141C Del is as low as 2.1% in Europeans [ALlele FREquency Database (ALFRED), 1999]. Low allele frequency suggests a minor effect of these polymorphisms in pathogenesis of alcoholism in Africans and Europeans.
Although the current study might have the largest collection of Mexican Americans in the literature, given the nature of alcoholism as a polygenic disorder, the limited number of studied polymorphisms and participants might still be the limitation of the current study. Further validations from independent populations are necessary to confirm our findings. More genes in the reward pathways such as serotonin receptors, opioid receptor kappa 1, GABA receptor β1, dopamine transporter, dopamine receptor D4, etc. can be included in the interaction analysis to produce a more robust result. Alcoholics were stratified arbitrarily according to tertiles of MAXDRINKS in the current study, and the results will be more accurate if we define the severity of alcoholics with Severity of Alcohol Dependence Questionnaire (SADQ) score. None of the strategies used for interaction analysis are perfect, and development of more powerful statistical tools will be critical for future studies. More importantly, findings of current study need to be verified with functional research.
In conclusion, our results suggest a main effect of education background, OPRM1 A118G, and DRD2 −141C Ins/Del as well as education*OPRM1 interaction in contribution to moderate and/or severe alcoholism in Mexican Americans. Functional relevance of these findings still needs to be explored.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank Ms. Maria Calvillo and Glenda Mejia for participant recruitment. We thank Dr. John Tsuang for supervising the recruitment. We would like to thank Ms. Barbara Brede for critical proofreading of the manuscript. This study is supported by NIH/NIAAA grant RO1 AA12081 and the Molecular Biology Core supported by the COBRE grant P20 RR021940. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Alcohol Abuse and Alcoholism or the National Institutes of Health.
Footnotes
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article:
Table S1. Results of HWE Test
Please note: Wiley-Blackwell is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
REFERENCES
- ALlele FREquency Database. ALlele FREquency Database, ALFRED [database online] New Haven, CT: Yale University School of Medicine; 1999. Available at: http://alfred.med.yale.edu/. [Google Scholar]
- Arinami T, Gao M, Hamaguchi H, Toru M. A functional polymorphism in the promoter region of the dopamine D2 receptor gene is associated with schizophrenia. Hum Mol Genet. 1997;6:577–582. doi: 10.1093/hmg/6.4.577. [DOI] [PubMed] [Google Scholar]
- Bart G, Kreek MJ, Ott J, LaForge KS, Proudnikov D, Pollak L, Heilig M. Increased attributable risk related to a functional mu-opioid receptor gene polymorphism in association with alcohol dependence in central Sweden. Neuropsychopharmacology. 2005;30:417–422. doi: 10.1038/sj.npp.1300598. [DOI] [PubMed] [Google Scholar]
- Bergen AW, Kokoszka J, Peterson R, Long JC, Virkkunen M, Linnoila M, Goldman D. Mu opioid receptor gene variants: lack of association with alcohol dependence. Mol Psychiatry. 1997;2:490–494. doi: 10.1038/sj.mp.4000331. [DOI] [PubMed] [Google Scholar]
- Blum K, Braverman ER, Holder JM, Lubar JF, Monastra VJ, Miller D, Lubar JO, Chen TJ, Comings DE. Reward deficiency syndrome: a biogenetic model for the diagnosis and treatment of impulsive, addictive, and compulsive behaviors. J Psychoactive Drugs. 2000;32(Suppl):i–Iv. 1–112. doi: 10.1080/02791072.2000.10736099. [DOI] [PubMed] [Google Scholar]
- Blum K, Chen AL, Chen TJ, Braverman ER, Reinking J, Blum SH, Cassel K, Downs BW, Waite RL, Williams L, Prihoda TJ, Kerner MM, Palomo T, Comings DE, Tung H, Rhoades P, Oscar-Berman M. Activation instead of blocking mesolimbic dopaminergic reward circuitry is a preferred modality in the long term treatment of reward deficiency syndrome (RDS): a commentary. Theor Biol Med Model. 2008;5:24. doi: 10.1186/1742-4682-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum K, Cull JG, Braverman ER, Comings DE. Reward Deficiency Syndrome. Am Sci. 1996a;84:132–145. [Google Scholar]
- Blum K, Sheridan PJ, Wood RC, Braverman ER, Chen TJ, Cull JG, Comings DE. The D2 dopamine receptor gene as a determinant of reward deficiency syndrome. J R Soc Med. 1996b;89:396–400. doi: 10.1177/014107689608900711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond C, LaForge KS, Tian M, Melia D, Zhang S, Borg L, Gong J, Schluger J, Strong JA, Leal SM, Tischfield JA, Kreek MJ, Yu L. Single-nucleotide polymorphism in the human mu opioid receptor gene alters beta-endorphin binding and activity: possible implications for opiate addiction. Proc Natl Acad Sci USA. 1998;95:9608–9613. doi: 10.1073/pnas.95.16.9608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowirrat A, Oscar-Berman M. Relationship between dopaminergic neurotransmission, alcoholism, and Reward Deficiency syndrome. Am J Med Genet B Neuropsychiatr Genet. 2005;132B:29–37. doi: 10.1002/ajmg.b.30080. [DOI] [PubMed] [Google Scholar]
- Bradley SL, Dodelzon K, Sandhu HK, Philibert RA. Relationship of serotonin transporter gene polymorphisms and haplotypes to mRNA transcription. Am J Med Genet B Neuropsychiatr Genet. 2005;136B:58–61. doi: 10.1002/ajmg.b.30185. [DOI] [PubMed] [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, Jr, Reich T, Schmidt I, Schuckit MA. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J Stud Alcohol. 1994;55:149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Chen WJ, Chen CH, Huang J, Hsu YP, Seow SV, Chen CC, Cheng AT. Genetic polymorphisms of the promoter region of dopamine D2 receptor and dopamine transporter genes and alcoholism among four aboriginal groups and Han Chinese in Taiwan. Psychiatr Genet. 2001;11:187–195. doi: 10.1097/00041444-200112000-00002. [DOI] [PubMed] [Google Scholar]
- Christophi CA, Kolokotroni O, Alpert HR, Warren CW, Jones NR, Demokritou P, Connolly GN. Prevalence and social environment of cigarette smoking in Cyprus youth. BMC Public Health. 2008;8:190. doi: 10.1186/1471-2458-8-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier DA, Stober G, Li T, Heils A, Catalano M, Di Bella D, Arranz MJ, Murray RM, Vallada HP, Bengel D, Muller CR, Roberts GW, Smeraldi E, Kirov G, Sham P, Lesch KP. A novel functional polymorphism within the promoter of the serotonin transporter gene: possible role in susceptibility to affective disorders. Mol Psychiatry. 1996;1:453–460. [PubMed] [Google Scholar]
- Comings DE, Blum K. Reward deficiency syndrome: genetic aspects of behavioral disorders. Prog Brain Res. 2000;126:325–341. doi: 10.1016/S0079-6123(00)26022-6. [DOI] [PubMed] [Google Scholar]
- Cotton NS. The familial incidence of alcoholism: a review. J Stud Alcohol. 1979;40:89–116. doi: 10.15288/jsa.1979.40.89. [DOI] [PubMed] [Google Scholar]
- Dick DM, Agrawal A, Schuckit MA, Bierut L, Hinrichs A, Fox L, Mullaney J, Cloninger CR, Hesselbrock V, Nurnberger JI, Jr, Almasy L, Foroud T, Porjesz B, Edenberg H, Begleiter H. Marital status, alcohol dependence, and GABRA2: evidence for gene-environment correlation and interaction. J Stud Alcohol. 2006;67:185–194. doi: 10.15288/jsa.2006.67.185. [DOI] [PubMed] [Google Scholar]
- Edenberg HJ. The collaborative study on the genetics of alcoholism: an update. Alcohol Res Health. 2002;26:214–218. [PMC free article] [PubMed] [Google Scholar]
- Feinn R, Nellissery M, Kranzler HR. Meta-analysis of the association of a functional serotonin transporter promoter polymorphism with alcohol dependence. Am J Med Genet B Neuropsychiatr Genet. 2005;133B:79–84. doi: 10.1002/ajmg.b.30132. [DOI] [PubMed] [Google Scholar]
- Flora DB, Chassin L. Changes in drug use during young adulthood: the effects of parent alcoholism and transition into marriage. Psychol Addict Behav. 2005;19:352–362. doi: 10.1037/0893-164X.19.4.352. [DOI] [PubMed] [Google Scholar]
- Franke P, Wang T, Nothen MM, Knapp M, Neidt H, Albrecht S, Jahnes E, Propping P, Maier W. Nonreplication of association between mu-opioid-receptor gene (OPRM1) A118G polymorphism and substance dependence. Am J Med Genet. 2001;105:114–119. [PubMed] [Google Scholar]
- Gelernter J, Kranzler H, Cubells J. Genetics of two mu opioid receptor gene (OPRM1) exon I polymorphisms: population studies, and allele frequencies in alcohol- and drug-dependent subjects. Mol Psychiatry. 1999;4:476–483. doi: 10.1038/sj.mp.4000556. [DOI] [PubMed] [Google Scholar]
- Gilman SE, Breslau J, Conron KJ, Koenen KC, Subramanian SV, Zaslavsky AM. Education and race-ethnicity differences in the lifetime risk of alcohol dependence. J Epidemiol Community Health. 2008;62:224–230. doi: 10.1136/jech.2006.059022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goode EL, Ulrich CM, Potter JD. Polymorphisms in DNA repair genes and associations with cancer risk. Cancer Epidemiol Biomarkers Prev. 2002;11:1513–1530. [PubMed] [Google Scholar]
- Goodwin DW. Hereditary factors in alcoholism. Hosp Pract. 1978;13:121–124. 127–130. doi: 10.1080/21548331.1978.11707340. [DOI] [PubMed] [Google Scholar]
- Grant BF. Prevalence and correlates of alcohol use and DSM-IV alcohol dependence in the United States: results of the National Longitudinal Alcohol Epidemiologic Survey. J Stud Alcohol. 1997;58:464–473. doi: 10.15288/jsa.1997.58.464. [DOI] [PubMed] [Google Scholar]
- Hanna EZ, Faden VB, Harford TC. Marriage: does it protect young women from alcoholism? J Subst Abuse. 1993;5:1–14. doi: 10.1016/0899-3289(93)90119-v. [DOI] [PubMed] [Google Scholar]
- Heath AC, Bucholz KK, Madden PA, Dinwiddie SH, Slutske WS, Bierut LJ, Statham DJ, Dunne MP, Whitfield JB, Martin NG. Genetic and environmental contributions to alcohol dependence risk in a national twin sample: consistency of findings in women and men. Psychol Med. 1997;27:1381–1396. doi: 10.1017/s0033291797005643. [DOI] [PubMed] [Google Scholar]
- Ishiguro H, Arinami T, Saito T, Akazawa S, Enomoto M, Mitushio H, Fujishiro H, Tada K, Akimoto Y, Mifune H, Shioduka S, Hamaguchi H, Toru M, Shibuya H. Association study between the −141C Ins/Del and TaqI A polymorphisms of the dopamine D2 receptor gene and alcoholism. Alcohol Clin Exp Res. 1998;22:845–848. [PubMed] [Google Scholar]
- Jonsson EG, Nothen MM, Grunhage F, Farde L, Nakashima Y, Propping P, Sedvall GC. Polymorphisms in the dopamine D2 receptor gene and their relationships to striatal dopamine receptor density of healthy volunteers. Mol Psychiatry. 1999;4:290–296. doi: 10.1038/sj.mp.4000532. [DOI] [PubMed] [Google Scholar]
- Konishi T, Calvillo M, Leng AS, Lin KM, Wan YJ. Polymorphisms of the dopamine D2 receptor, serotonin transporter, and GABA(A) receptor beta(3) subunit genes and alcoholism in Mexican-Americans. Alcohol. 2004a;32:45–52. doi: 10.1016/j.alcohol.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Konishi T, Luo HR, Calvillo M, Mayo MS, Lin KM, Wan YJ. ADH1B*1, ADH1C*2, DRD2 (−141C Ins), and 5-HTTLPR are associated with alcoholism in Mexican American men living in Los Angeles. Alcohol Clin Exp Res. 2004b;28:1145–1152. doi: 10.1097/01.alc.0000134231.48395.42. [DOI] [PubMed] [Google Scholar]
- Konishi T, Smith JL, Lin KM, Wan YJ. Influence of genetic admixture on polymorphisms of alcohol-metabolizing enzymes: analyses of mutations on the CYP2E1, ADH2, ADH3 and ALDH2 genes in a Mexican-American population living in the Los Angeles area. Alcohol Alcohol. 2003;38:93–94. doi: 10.1093/alcalc/agg021. [DOI] [PubMed] [Google Scholar]
- Lahiri DK, Nurnberger JI., Jr A rapid non-enzymatic method for the preparation of HMW DNA from blood for RFLP studies. Nucleic Acids Res. 1991;19:5444. doi: 10.1093/nar/19.19.5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DJ, Markides KS, Ray LA. Epidemiology of self-reported past heavy drinking in Hispanic adults. Ethn Health. 1997;2:77–88. doi: 10.1080/13557858.1997.9961817. [DOI] [PubMed] [Google Scholar]
- Luo HR, Hou ZF, Wu J, Zhang YP, Wan YJ. Evolution of the DRD2 gene haplotype and its association with alcoholism in Mexican Americans. Alcohol. 2005;36:117–125. doi: 10.1016/j.alcohol.2005.09.003. [DOI] [PubMed] [Google Scholar]
- McGue M. Phenotyping alcoholism. Alcohol Clin Exp Res. 1999;23:757–758. doi: 10.1111/j.1530-0277.1999.tb04180.x. [DOI] [PubMed] [Google Scholar]
- Mechanic LE, Luke BT, Goodman JE, Chanock SJ, Harris CC. Polymorphism Interaction Analysis (PIA): a method for investigating complex gene-gene interactions. BMC Bioinformatics. 2008;9:146. doi: 10.1186/1471-2105-9-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendenhall CL, Gartside PS, Roselle GA, Grossman CJ, Weesner RE, Chedid A. Longevity among ethnic groups in alcoholic liver disease. Alcohol Alcohol. 1989;24:11–19. [PubMed] [Google Scholar]
- Mendoza R, Wan YJ, Poland RE, Smith M, Zheng Y, Berman N, Lin KM. CYP2D6 polymorphism in a Mexican American population. Clin Pharmacol Ther. 2001;70:552–560. doi: 10.1067/mcp.2001.120675. [DOI] [PubMed] [Google Scholar]
- Neville MJ, Johnstone EC, Walton RT. Identification and characterization of ANKK1: a novel kinase gene closely linked to DRD2 on chromosome band 11q23.1. Hum Mutat. 2004;23:540–545. doi: 10.1002/humu.20039. [DOI] [PubMed] [Google Scholar]
- Noble EP, Zhang X, Ritchie T, Lawford BR, Grosser SC, Young RM, Sparkes RS. D2 dopamine receptor and GABA(A) receptor beta3 subunit genes and alcoholism. Psychiatry Res. 1998;81:133–147. doi: 10.1016/s0165-1781(98)00084-5. [DOI] [PubMed] [Google Scholar]
- Parsian A, Cloninger CR, Zhang ZH. Functional variant in the DRD2 receptor promoter region and subtypes of alcoholism. Am J Med Genet. 2000;96:407–411. doi: 10.1002/1096-8628(20000612)96:3<407::aid-ajmg32>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Ramamoorthy S, Bauman AL, Moore KR, Han H, Yang-Feng T, Chang AS, Ganapathy V, Blakely RD. Antidepressant- and cocaine-sensitive human serotonin transporter: molecular cloning, expression, and chromosomal localization. Proc Natl Acad Sci U S A. 1993;90:2542–2546. doi: 10.1073/pnas.90.6.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander T, Ladehoff M, Samochowiec J, Finckh U, Rommelspacher H, Schmidt LG. Lack of an allelic association between polymorphisms of the dopamine D2 receptor gene and alcohol dependence in the German population. Alcohol Clin Exp Res. 1999;23:578–581. [PubMed] [Google Scholar]
- Schinka JA, Town T, Abdullah L, Crawford FC, Ordorica PI, Francis E, Hughes P, Graves AB, Mortimer JA, Mullan M. A functional polymorphism within the mu-opioid receptor gene and risk for abuse of alcohol and other substances. Mol Psychiatry. 2002;7:224–228. doi: 10.1038/sj.mp.4000951. [DOI] [PubMed] [Google Scholar]
- Smith GS, Lotrich FE, Malhotra AK, Lee AT, Ma Y, Kramer E, Gregersen PK, Eidelberg D, Pollock BG. Effects of serotonin transporter promoter polymorphisms on serotonin function. Neuropsychopharmacology. 2004;29:2226–2234. doi: 10.1038/sj.npp.1300552. [DOI] [PubMed] [Google Scholar]
- Thundal KL, Allebeck P. Abuse of and dependence on alcohol in Swedish women: role of education, occupation and family structure. Soc Psychiatry Psychiatr Epidemiol. 1998;33:445–450. doi: 10.1007/s001270050078. [DOI] [PubMed] [Google Scholar]
- Wu X, Lin X, Dinney CP, Gu J, Grossman HB. Genetic polymorphism in bladder cancer. Front Biosci. 2007;12:192–213. doi: 10.2741/2058. [DOI] [PubMed] [Google Scholar]
- Wu CY, Wu YS, Lee JF, Huang SY, Yu L, Ko HC, Lu RB. The association between DRD2/ ANKK1, 5-HTTLPR gene, and specific personality trait on antisocial alcoholism among Han Chinese in Taiwan. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:447–453. doi: 10.1002/ajmg.b.30626. [DOI] [PubMed] [Google Scholar]
- Yang M, Tsuang J, Yvonne Wan YJ. A haplotype analysis of CYP2E1 polymorphisms in relation to alcoholic phenotypes in Mexican Americans. Alcohol Clin Exp Res. 2007;31:1991–2000. doi: 10.1111/j.1530-0277.2007.00533.x. [DOI] [PubMed] [Google Scholar]
- Zhang H, Luo X, Kranzler HR, Lappalainen J, Yang BZ, Krupitsky E, Zvartau E, Gelernter J. Association between two mu-opioid receptor gene (OPRM1) haplotype blocks and drug or alcohol dependence. Hum Mol Genet. 2006;15:807–819. doi: 10.1093/hmg/ddl024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Wang D, Johnson AD, Papp AC, Sadee W. Allelic expression imbalance of human mu opioid receptor (OPRM1) caused by variant A118G. J Biol Chem. 2005;280:32618–32624. doi: 10.1074/jbc.M504942200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.