Abstract
BACKGROUND & AIMS
Technical advances have led to stool DNA (sDNA) tests that might accurately detect neoplasms on both sides of the colorectum. We assessed colorectal neoplasm detection by a next-generation sDNA test and effects of covariates on test performance.
METHODS
We performed a blinded, multicenter, case-control study using archived stool samples collected in preservative buffer from 252 patients with colorectal cancer (CRC), 133 with adenomas ≥1 cm, and 293 individuals with normal colonoscopy results (controls); two-thirds were randomly assigned to a training set and one-third to a test set. The sDNA test detects 4 methylated genes, a mutant form of KRAS, and the α-actin gene (as a reference value) using quantitative, allele-specific, real-time target and signal amplification; it also quantifies hemoglobin. We used a logistical model to analyze data.
RESULTS
The sDNA test identified 85% of patients with CRC and 54% of patients with adenomas ≥1 cm with 90% specificity. The test had a high rate of detection for all nonmetastatic stages of CRC (aggregate 87% detection rate for CRC stages I–III). Detection rates increased with adenoma size: 54% ≥1 cm, 63% >1 cm, 77% >2 cm, 86% >3 cm, and 92% >4 cm (P < .0001). Based on receiver operating characteristic analysis, the rate of CRC detection was slightly greater for the training than the test set (P = .04), whereas the rate of adenoma detection was comparable between sets. Sensitivities for detection of CRC and adenoma did not differ with lesion site.
CONCLUSIONS
Early-stage CRC and large adenomas can be detected throughout the colorectum and with high levels of accuracy by the sDNA test. Neoplasm size, but not anatomical site, affected detection rates. Further studies are needed to validate the findings in a larger population and optimize the sDNA test.
Keywords: QuARTS, Colorectal Cancer Screening, Stool Testing, Precancer Detection
Colorectal cancer (CRC) remains the number 2 cancer in terms of mortality in the United States.1 World-wide, CRC is increasingly common, now accounting for >600,000 deaths annually.2 Broadly applied preventive and early detection measures are critically needed to lessen this toll. To optimally reduce incidence and mortality by screening, an interventional tool must effectively detect both advanced precursor lesions and curable-stage cancers from throughout the colorectum and be patient-friendly, available, and affordable. No conventional screening approach fully achieves these desired attributes. For example, proximal colon neoplasms are particularly underdetected by conventional approaches, including fecal blood tests,3–5 sigmoidoscopy,6,7 and colonoscopy8–10 as currently practiced. Because of poor precursor lesion detection rates,3–5,11 fecal blood tests have had minimal impact on CRC incidence.12 It is imperative to explore alternate or complementary strategies with potential to improve CRC screening performance, especially for detection of proximal advanced neoplasms.
Stool DNA (sDNA) testing offers a biologically rational approach based on tumor exfoliation and is noninvasive, requires no diet or medication restriction, and has potential for high accuracy of neoplasm detection.13 Furthermore, test kits can be distributed widely by mail. Since fecal recovery of DNA markers from CRC14 and adenomas15 was first demonstrated, numerous corroborative studies have been reported. First-generation sDNA tests proved to be more sensitive than guaiac-type fecal blood tests for detection of CRC5 and screen-relevant neoplasms4 in multicenter studies, but their performance was compromised by various technical limitations.16 Critical advances have individually addressed such limitations with the advent of preservative buffers that prevent DNA degradation during stool shipment and storage,17,18 broadly informative marker panels,19,20 and more sensitive assay techniques that substantially improve neoplasm detection rates.19–22
We are developing a next-generation sDNA test that incorporates key innovations, including use of preservative buffer with stool collection,17 direct capture of target genes from stool supernatant, marker combinations that yield nearly 100% discrimination of CRC and adenomas from normal mucosa at the tissue level,19 and a highly sensitive assay technique, QuARTS (quantitative allele-specific real-time target and signal amplification). The QuARTS assay is suitable for high-throughput automation and achieves 100–1000-fold greater analytical sensitivity than first-generation tests.19
The present multicenter study represents the first large clinical assessment of a next-generation sDNA test that incorporates these technical advances. Our specific aims were to (1) refine marker panel and individual marker cut-offs for optimal sDNA test discrimination, (2) assess clinical performance of the sDNA test in training and test sets, and (3) identify covariates that affect neoplasm detection rates.
Methods
Study Design
This study was based on well-characterized archival stools from multiple medical centers, including referral centers (Mayo Clinic, Rochester, Minnesota; Johns Hopkins Medical Center, Baltimore, Maryland; and Toronto Cancer Care, Toronto, Ontario, Canada) and community centers in the United States and Denmark. Approval by institutional review boards was obtained. Stools had been procured from patients with CRC or at least 1 colorectal adenoma ≥1 cm. Archival stools from controls without neoplasia on colonoscopy were selected by each center to roughly match cases on age and sex. All case and control stools received were analyzed. Patients had been recruited from both clinical and screening settings. Those with known cancer syndromes or inflammatory bowel disease were excluded. A prototype multimarker stool test was performed that included 4 methylated genes (vimentin, NDRG4, BMP3, and TFPI2), mutant KRAS, a reference gene β-actin (ACTB), and hemoglobin; the assay of this full marker panel is referred to as sDNA test. To assess sDNA performance, case and control stools were distributed in balanced fashion into training and test sets (see Patient and Lesion Characteristics). A logistical regression model was constructed based on data from the training set; the model, with preselected marker cut-offs, was then independently evaluated in the test set. Training set assays were performed at Exact Sciences in Madison, Wisconsin and test set assays at Mayo Medical Laboratory in Rochester, Minnesota; all assays were run in blinded fashion.
Stool Collection and Storage
Stools were collected before bowel purgation and colonoscopy or >1 week after colonoscopy (but before neoplasm resection). Whole stools (minimum 36 g) were collected in buckets mounted to the toilet seat. A preservative buffer was added, most often immediately after defecation; and buffered stools were archived at −80°C. However, the timing of buffer addition and prestorage homogenization varied across participating centers.
Marker Selection
To select a panel of discriminant methylation markers, preliminary tissue and stool triage studies were conducted. In the tissue study,19 9 candidate genes were identified that individually or in combination yielded nearly complete separation of colorectal neoplasia from normal mucosa. These 9 candidates were then evaluated in a stool pilot study23 from which 4 methylated gene markers emerged as most discriminant: NDRG4, BMP3, vimentin, and TFPI2. Based on our previous observations,24,25 both mutant KRAS and hemoglobin complement methylation markers in stool and, accordingly, were also evaluated in the panel. Finally, β-actin was assayed as a reference gene to determine total human genome equivalents in stool; this allowed (1) assessment of sample adequacy (samples with <75 copies per reaction volume were rejected to prevent stochastic error), (2) normalization of marker levels (served as denominator for marker ratios), and (3) evaluation of human DNA level as a candidate marker itself, as we have previously found that total human DNA increases with colorectal neoplasia.15,18
Stool Processing and Target Gene Capture
Promptly after thawing, buffered stools were homogenized with a shaker device and centrifuged. A 12-mL aliquot of stool supernatant was then treated with polyvinylpolypyrroli-done (Crosby & Baker, Westport, MA) at a concentration of 50 mg/mL. Direct capture of target gene sequences by hybridization with oligonucleotide probes was performed on supernatants. Briefly, 10 mL polyvinylpolypyrrolidone-treated supernatant was denatured in 2.4 M guanidine isothiocyanate (Sigma, St Louis, MO) at 90°C for 10 min; 500 μg Sera-Mag carboxylate modified beads (ThermoFisher Scientific, Waltham, MA) functionalized with each oligonucleotide capture probe26 were subsequently added to denatured stool supernatant and incubated at room temperature for 1 hour. Sera-Mag beads were collected on a magnetic rack, and washed 3 times using 1× MOPS washing buffer (10 mM MOPS, 150 mM NaCl, pH 7.5), and then eluted out in 50 μL nuclease-free water with 20 ng/μL transfer RNA (Sigma). For the training and test sets, the 4 selected methylated markers, vimentin, NDRG4, BMP3, and TFPI2, and 1 reference gene ACTB were captured together in one hybridization reaction; the mutation marker KRAS was sequentially captured in another hybridization reaction (capture probe sequences available in Supplementary Appendix 1).
Methylation Assays
Methylation markers were quantified by the QuARTS method, as we have described.19 This method combines a polymerase-based target DNA amplification process with an invasive cleavage-based signal amplification process. The mechanism of the QuARTS assay is illustrated in Supplementary Appendix 2, and details of the assay methods with primer sequences are available in Supplement Appendix 3.
KRAS Mutations
KRAS gene was first polymerase chain reaction–amplified with primers flanking codons 12/13. QuARTS assays were then used to evaluate the 7 informative mutations on these codons. Method details including primer sequences are available in Supplementary Appendix 4.
Hemoglobin Assay
To quantify hemoglobin in stool, the HemoQuant test was performed on 2 buffered stool aliquots (each normalized to 16 mg stool) per patient, as described previously.27,28 Unlike immunochemical or guaiac-type fecal blood tests, the HemoQuant test assays heme-derived porphyrin and is not affected by stool storage.
Reference Method
Colonoscopy, in conjunction with histopathology on all colorectal lesions, served as the reference standard. In cases with multiple lesions, patients were classified based on their most advanced or largest neoplasm.
Statistical Analysis
Following previous approaches,29 a logistical regression model was developed using data from the training set to define a linear combination of age and stool marker variables that optimized the discrimination between case patients with a colorectal neoplasm (CRC or adenoma ≥1 cm) and controls with normal colonoscopy. The modeling strategy consisted of fitting age, quadratic effect of age, β-actin, fecal hemoglobin, and the sDNA methylation markers into a base model and adding quadratic and pair-wise interactions of these variables using forward variable selection with entry and removal from the model based on a P value of .05 (see model diagram in Supplementary Appendix 5). A cut-off for KRAS mutation, defined a priori, resulted in only 1 control patient being positive. Because this extremely high specificity created modeling difficulties, the effect of mutant KRAS was added after variable selection; presence of mutant KRAS was considered predictive of having a significant lesion. The 90th or 95th percentiles of the linear discriminant score within control patients (specificity) were used to define test positivity. The linear discriminant score with corresponding cut-off value was then applied to the test set. Sensitivity and specificity with corresponding 95% confidence intervals were estimated for each training and test sets separately and for combined sets.
The area under the empirical receiver operating characteristic (ROC) curve was used to compare the accuracy of nested logistic models and investigate the added value of each sDNA methylation marker to the overall linear discriminant score using a paired methods approach.30 The χ2 test was used to assess the association of test positivity with categorical clinical and lesion characteristics. The association of continuous lesion size with the linear discriminant score was evaluated using piece-wise linear regression (inflection point at 3 cm), and tests between lesion subtypes were based on the change in fit between nested models.
Inter-laboratory concordance was estimated for each sDNA methylation marker separately using the concordance correlation coefficient.31 Using this measure, both the degree of linear association between laboratories and the extent of departure from the 45-degree line of perfect agreement are considered.
Statistical significance was defined as P < .05.
Results
Patient and Lesion Characteristics
Overall, the study comprised 678 patients; median age was 60 (range, 39–92) years and 50% were women. Patients included 385 cases (252 with CRC and 133 with an adenoma ≥1 cm) and 293 controls with normal colonoscopy. Median age was 63 (range, 39–92) years for case patients and 57 (range, 41–87) years for controls; 45% of case patients and 56% of controls were women. Specimens from 456 patients were randomly assigned to the training set and 222 patients to the test set. Sets were well-balanced by age, sex, and medical centers of stool origin and by CRC and adenoma distributions and characteristic features (Table 1).
Table 1.
Summary of Patient and Lesion Characteristics
| Training set (n = 456) | Test set (n = 222) | Combined (n = 678) | |
|---|---|---|---|
| Age, y, median (range) | 61 (39–92) | 60 (40–88) | 60 (39–92) |
| Sex, % women | 50 | 51 | 50 |
| Race, % white | 82 | 80 | 81 |
| Medical center source | |||
| A/B/C/D/E, %a | 43/22/9/19/6 | 31/20/19/22/8 | 39/21/13/20/7 |
| Controls | |||
| Normal colonoscopy, n | 197 | 96 | 293 |
| Cases | |||
| Advanced adenoma | |||
| n | 89 | 44 | 133 |
| Size, cm, median (range) | 1.7 (1–8) | 1.8 (1–6) | 1.7 (1–8) |
| Site, % proximal | 44 | 46 | 44 |
| Cancer | |||
| n | 170 | 82 | 252 |
| Size, cm, median (range) | 4.0 (1–14) | 3.5 (1–8) | 4.0 (1–14) |
| Site, % proximal | 41 | 31 | 38 |
| Stage, % I/II/III/IVb | 13/37/41/9 | 12/41/33/14 | 13/38/39/11 |
Medical centers were evaluated anonymously.
Stage and site not available for some cancers.
Samples with <75 copies of β-actin (human genome equivalents) were considered inadequate for analysis; only 12 (2%) stools were excluded on this basis.
Neoplasm Detection Rates
Training set
Methylation markers and mutant KRAS were evaluated both as copy number per weight of stool and as a ratio against recovered human DNA (β-actin). Values normalized against β-actin best-discriminated case patients from control patients (data not shown) and were used to calculate all test results.
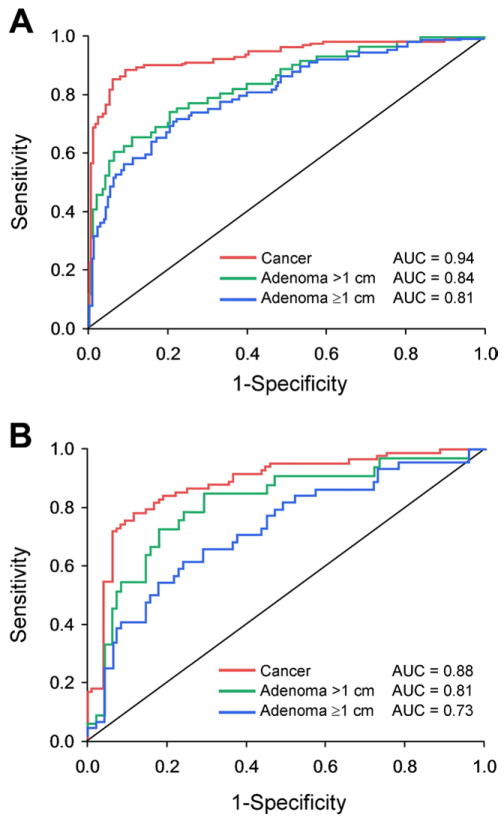
At modeled specificity cut-offs of 90%, the sDNA test detected 89% of CRCs, 62% adenomas >1 cm, and 56% of adenomas >1 cm (Table 2). Areas under the ROC curve were 0.94 for detection of CRC, 0.84 for adenomas >1 cm, and 0.82 for adenomas >1 cm (Figure 1A).
Table 2.
Neoplasm Detection Rates by a Next-Generation Stool DNA Testa
| Sensitivity, % (95% CI) | Observed specificity, % (95% CI) | |
|---|---|---|
| Training set | ||
| Cancer | 89 (83–93) | 90 (85–94) |
| Adenoma | ||
| Size >1 cm | 62 (49–74) | 90 (85–94) |
| Size ≥1 cm | 56 (45–67) | 90 (85–94) |
| Test set | ||
| Cancer | 78 (68–86) | 85 (77–92) |
| Adenoma | ||
| Size >1 cm | 64 (45–80) | 85 (77–92) |
| Size ≥1 cm | 48 (31–66) | 85 (77–92) |
| Combined | ||
| Cancer | 85 (80–89) | 89 (85–92) |
| Adenoma | ||
| Size >1 cm | 63 (52–73) | 89 (85–92) |
| Size ≥1 cm | 53 (45–62) | 89 (85–92) |
CI, confidence interval.
Stool DNA test marker panel: methylated vimentin, NDRG4, BMP3, and TFPI2; mutant KRAS; a reference gene β-actin; and hemoglobin quantity. Using a logistical regression model, a 90% specificity cut-off was determined in the training set by selecting the 90th percentile from control patients with normal colonoscopy; this cut-off was applied to the test set.
Figure 1.
Neoplasm detection by next-generation stool DNA test prototypes. ROC curves are shown for (A) training set and (B) test set. See Table 2 for definition of prototype test.
Test set
Observed specificity and sensitivity rates in the test set at modeled specificity cut-offs are shown (Table 2). At a modeled specificity cut-off of 90%, observed specificity was 85% by sDNA test. At this specificity cutoff, the detection rate for CRC was 78%, 64% for adenomas >1 cm, and 48% for adenomas ≥1 cm, areas under the ROC curve were 0.88 for CRC, 0.81 for adenomas >1 cm, and 0.73 for adenomas ≥1 cm (Figure 1B). Areas under the ROC curve were slightly higher in the training set than in the test set for detection of CRC (P = .04), but not significantly different for adenomas >1 cm (P = .31) or ≥1 cm (P = .07).
Combined sets
Data from the combined sets were used in covariate analyses, and overall performance data are summarized (Table 2). At a 90% specificity cutoff, the overall detection sensitivity for CRC was 85% (n = 252), for adenomas >1 cm 63% (n = 133), and for adenomas ≥1 cm 54% (n = 94). The model could select specificity cut-off. For example, at a 95% specificity cutoff, the sensitivity was 76% for CRC, 51% for adenomas >1 cm, and 43% for adenomas ≥1 cm. A modeled specificity cut-off of 90% was selected for covariate analyses in all following Results sections
Influence of Covariates on Neoplasm Detection
Neoplasm size
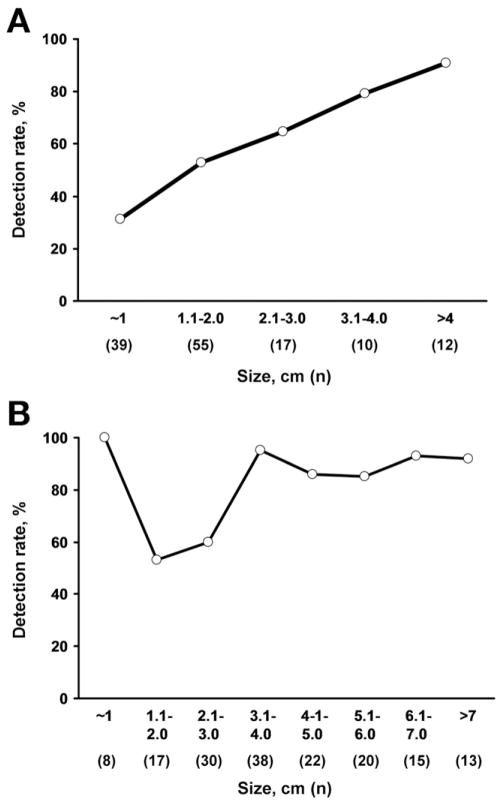
Neoplasm size had a major effect on detection rates (Figure 2). Detection increased in proportion to adenoma size (P < .0001). Adenoma detection rates according to size intervals are illustrated (Figure 2A). Adenoma detection rates according to size cut-off were 54% for adenomas ≥1 cm, 63% for those >1 cm, 77% for those >2 cm, 86% for those >3 cm, and 92% for those >4 cm. Median adenoma size of test-positives was 2.0 cm vs 1.2 cm for test-negatives (P < .0001). When adjusted for lesion size, the degree of dysplasia did not influence adenoma detection rates; detection was 69% for adenomas with high-grade dysplasia compared to 76% for comparably sized adenomas with low-grade dysplasia (P = .71).
Figure 2.
Affect of neoplasm size on stool DNA test detection rates. (A) Adenomas. Detection rates increased directly with lesion size; P < .0001. (B) Colorectal cancer. Although all 8 cancers around 1 cm in size were detected, detection rates overall increased with lesion size; P = .008.
As with adenomas, detection rates significantly increased with CRC size interval (P = .008) (Figure 2B). When neoplasm size is plotted against quantitative sDNA test scores, there was no significant difference between CRC and adenomas (P = .40) (Figure 3).
Figure 3.
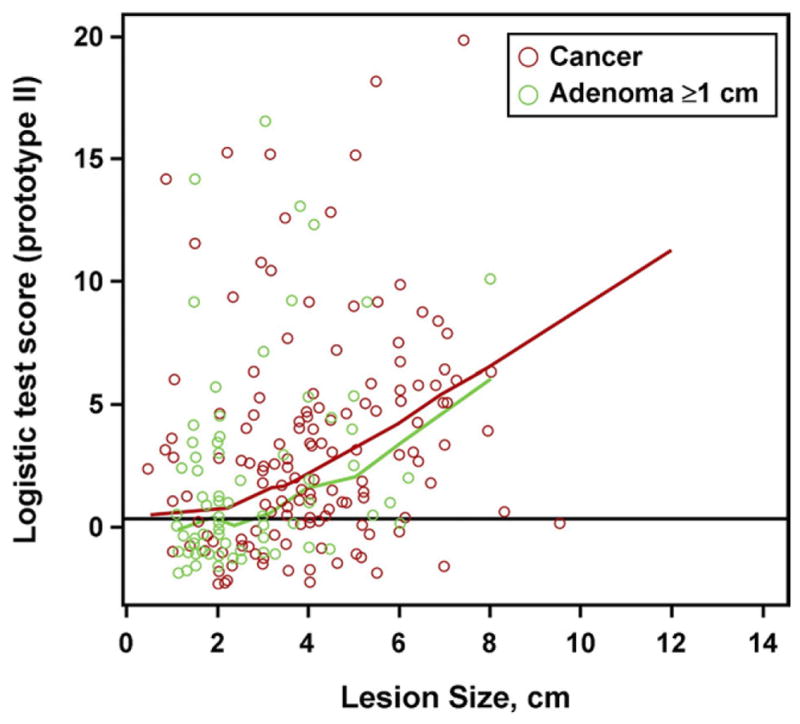
Relationship of neoplasm size to quantitative stool DNA test scores: colorectal adenoma vs cancer. When stool DNA test scores were plotted against neoplasm size by piece-wise linear regression, curves for adenomas and cancers did not differ significantly; P = .40.
Neoplasm site
Neoplasm detection rates were not affected by lesion site (Figure 4). The sDNA test detected 87% of CRC proximal to the splenic flexure vs 83% of those distal (P = .47). The test detected 55% of adenomas ≥1 cm in proximal vs 53% in the distal colorectum (P = .86).
Figure 4.
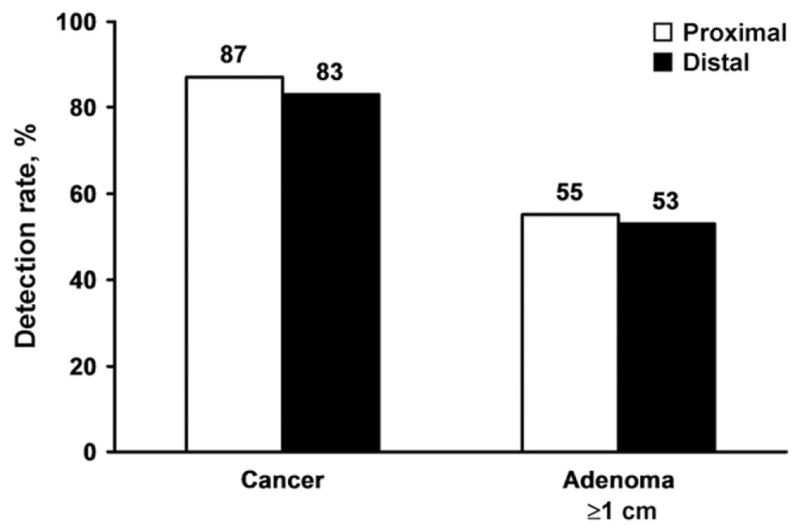
Effect of neoplasm site on detection rates by stool DNA testing. Detection rates were comparable between proximal and distal colorectal cancers (P = NS) and between proximal and distal adenomas (P = NS).
Cancer stage
Detection rates were comparably high for stages I, II, and III CRC and did not differ significantly across these stages (Figure 5). The aggregate detection rate for stages I–III CRC was 87%. In comparison, detection rates fell with stage IV CRC to 69% (P = .02 vs stages I–III).
Figure 5.
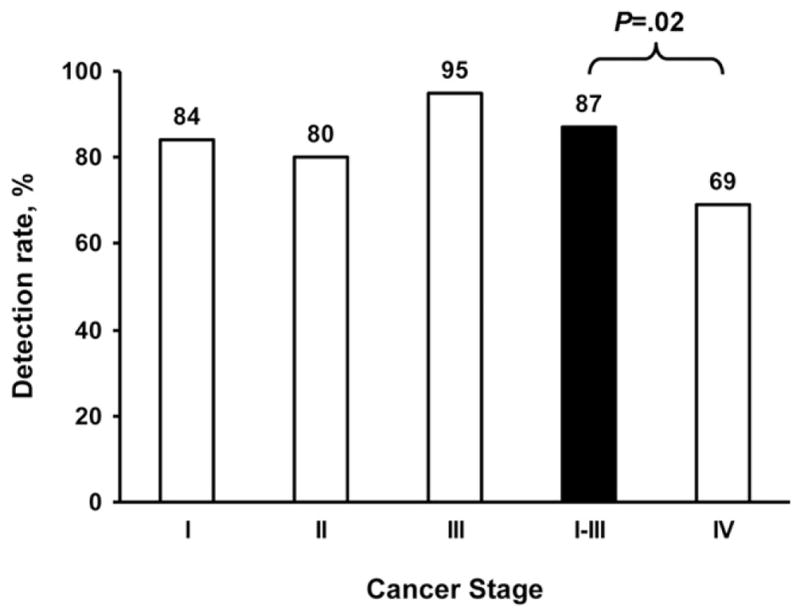
Affect of colorectal cancer stage on detection rates by stool DNA testing. Detection rates did not differ significantly between stages I, II, or III. However, aggregate sensitivity of 87% for stages I–III was significantly higher than the sensitivity of 69% for stage IV; P = .02.
Medical Center of Specimen Origin
Neoplasm detection rates varied significantly across medical centers from which stool specimens were obtained. Detection rates ranged from 70% to 94% for CRC (P = .05) and from 40% to 69% for adenomas ≥1 cm (P = .004).
Demographics
In the logistical regression model used, neither specificity nor sensitivity of the sDNA tests for neoplasm detection was affected by sex or race/ethnicity. The logistical model was adjusted for age.
Contribution of Individual Panel Markers
The discrimination of each marker component by itself was evaluated by calculating individual areas under the ROC curves for detection of combined CRC and advanced adenomas, with results as follows: NDRG4 0.75, BMP3 0.73, TFPI2 0.73, vimentin 0.69, hemoglobin (HemoQuant) 0.66, β-actin 0.64, and KRAS 0.61.
In the logistical model, hemoglobin as measured by HemoQuant contributed only modest additive discrimination to the marker panel; area under the empirical ROC curve was 0.90 (95% CI, 0.86–0.93) for the full panel vs 0.88 (95% CI, 0.84–0.91) for the panel without hemoglobin. The relative contributions of TFPI2 and vimentin to aggregate panel discrimination were also minimal. For example, at a 90% specificity cut-off, the overall sensitivity for CRC was 85% by the full marker panel and 82% by the panel without TFPI2 and vimentin; respective sensitivities for detection of adenomas >1 cm were 63% and 62%. All other panel markers were complementary.
Concordance Between Laboratories
As a check for potential technical errors, the sDNA test was blindly performed in both laboratories on a subset of 222 stools. Agreement between results was high (concordance correlation coefficient range, 0.978–0.996).
Discussion
To deliver maximum benefit, a CRC screening tool must detect both curable stage CRC and critical precursor lesions throughout the colorectum. This large multicenter study demonstrates that a next-generation sDNA test that incorporates key technical advances can achieve high detection rates of early-stage CRC and large precursor adenomas, while maintaining high specificity. Importantly, neoplasm site had no effect on detection rates by sDNA testing.
Our findings showing that next-generation sDNA testing detects curable stage CRC and large adenomas with high sensitivity substantiate and extend results from earlier small studies by our group24 and others32–36 using various multimarker panels and analytically sensitive sDNA assay methods. This study’s large size allowed us to perform both a training set and a validating test set and to critically assess the effects of lesion characteristics on results. For stage I–III CRC, the aggregate detection rate was 87%, and there were no significant differences between these individual stages. Curiously, sDNA test detection of stage IV CRC was slightly but significantly lower; we speculate that this finding might be related to hypomethylation that occurs with late progression,37 to surface necrosis and reduced colonocyte exfoliation, or to additional factors. Other noninvasive approaches, such as fecal blood testing3 and plasma-based DNA tests38,39 tend not to detect earlier-stage CRC as well as later-stage CRC. Such outcomes are not unexpected because the common biology for occult bleeding and marker entry into the blood circulation involves vascular disruption by invasion. Based on our study, detection of CRC by stool assay of exfoliated DNA markers is related to lesion size and not stage.
Prevention of CRC is the ultimate goal of screening. To accomplish this, a screening test must effectively detect those precursor lesions most likely to progress. Other noninvasive approaches to CRC screening have generally shown poor sensitivity for colorectal precursor lesions.3–5,38–40 In this study, the sDNA test detected the majority of adenomas ≥1 cm. In addition, this study is the first to demonstrate robustly that sDNA detection rates progressively increase with growth in adenoma size beyond 1 cm; for example, sDNA test detection of adenomas >3 cm exceeded 85%. This finding has important implications on precursor lesion detection by sDNA testing in a screening program, as continued growth in adenoma size >1 cm is strongly associated with progressive risk of CRC transformation.41–48 Furthermore, the high point sensitivities we observed would likely be compounded over serial screens to yield even higher program sensitivities for the highest-risk lesions. Serrated polyps, the other critical precursor lesions,49 were not evaluated in this study; however, our preliminary data suggest that serrated polyps ≥1 cm can be detected by sDNA testing at rates comparable to those observed for adenomas ≥1 cm.50
New quantitative relationships were discovered in this study based on sDNA test observations along the continuum of neoplasm size. First, detection rates of CRC and precursor adenomas were comparable at matched lesion size, suggesting that benign and malignant neoplasms exfoliate similarly. It is likely that overall detection rates are higher for CRC than for adenomas primarily because CRCs are larger rather than because of biological differences in cell shedding; median size was 4.0 cm for CRC compared to 1.7 cm for adenomas. Second, when next-generation sDNA test sensitivity is plotted against neoplasm size, an S-shape curve is generated with a lower inflexion point occurring just below a neoplasm size of 1 cm. Reflecting the effect of this lower inflexion point, detection rates for adenomas at a cut-off >1 cm were roughly 10% higher than at the conventional cut-off of ≥1 cm. Considering that neoplasm surface area is proportional to the third power of the radius, differences in surface area for lesions at these points of inflexion are appreciable; the surface area of a 3-cm neoplasm is 27 times greater than that of a 1-cm neoplasm. It is likely that proportionately few adenomas <1 cm will be detected by this method, which may be desirable clinically, as the risk of CRC progression drops precipitously below this threshold.41,46–48 For the subset of small lesions that continue to grow, they will become increasingly detectable while still at relatively low risk for malignant transformation. The test detected the large majority of neoplasms >2 or 3 cm, a size zone that includes most CRCs and the precursor lesions at highest risk of transformation.
Effective detection of proximal colon neoplasms is of paramount importance, considering that the relative incidence of proximal CRC is on the rise,51–53 that current screening modalities are less sensitive for proximal than distal neoplasms,3,4,6–10,54 and that a preponderance of interval CRCs appear to be proximal.9 This study clearly demonstrates that sDNA test detection of both CRC and large adenomas is equally high in the proximal and distal colorectum. Given this performance characteristic, sDNA testing has potential to improve the general screen detection of neoplasms throughout the colon. In addition, it would be a rational complement to colonoscopy as an interval test.
The study had important strengths. First, the large number of stools from patients with colorectal neoplasms permitted sufficient power to perform a robust covariate analysis. Second, the training and test set design involving blinded assays in separate laboratories provided objective data by which to assess test performance. And, third, samples procured from multiple centers reflected a population that was diverse with respect to geography, age, and sex.
The study also had limitations. First, while all stools were buffered, sample collection and storage were not standardized across medical centers, which may have contributed, in part, to the significant variation in test performance between centers. This points out an opportunity to further improve sDNA test performance by optimizing and standardizing stool collection and storage algorithms. Second, most patients were from referral centers, some were symptomatic, and many had undergone colonoscopy before referral. Results might not be representative of the screen setting. Third, pathology measurements of lesion size were not always available and ascertainment was based in some instances on endoscopic estimation, which is less accurate than pathologist determination and especially overestimates polyp size around 1 cm.55 Fourth, quality of colonoscopy was not controlled. It is possible that relevant neoplasms were missed in some cases, which could have had a detrimental impact on estimates of sDNA test specificity. Fifth, cases with multiple neoplasms were classified according to their largest or most advanced lesion; detailed data were not available on size and number of synchronous lesions. And, sixth, the HemoQuant assay was incorporated in the prototype tests because it is unaffected by stool storage27; however, it detects upper and lower gastrointestinal bleeding equally well and is less discriminant for colorectal bleeding.27,28 If a hemoglobin test is to be considered as an adjunct to future multimarker stool tests, it may be advantageous to use an immunochemical assay that is more specific for colorectal bleeding.56,57
Prompted by findings in this study, additional assay refinements and optimization efforts are underway that should further improve test performance. Such refinements will include simplification of the methylated marker components (reduction from 4 to 2 markers) and replacement of HemoQuant with an immunochemical hemoglobin assay.
Based on observations in this large case-control study, next-generation sDNA tests appear capable of detecting early-stage CRC and large adenomas with high sensitivity and specificity at all sites throughout the colon. Validation of optimized next-generation sDNA tests in the screening setting is now needed.
Supplementary Material
Acknowledgments
The authors thank the technical staffs at Exact Sciences Corporation and at the Clinical Molecular Genetics Laboratory within Mayo Medical Laboratories for performance of assays; Kelli Burger for statistical assistance; and Mary Devens and Julie Simonson for subject recruitment and clinical database management. Preliminary data from this study were presented at an American Association for Cancer Research meeting in October 2010 (Philadelphia, PA) and at Digestive Disease Week in May 2011 (Chicago, IL).
Funding
Exact Sciences Corporation, National Institutes of Health grant CA 62924, and grants from Charles Oswald Foundation and Virginia and D.K. Ludwig Fund for Cancer Research.
Abbreviations used in this paper
- CRC
colorectal cancer
- ROC
receiver operating characteristic
- sDNA
stool DNA
Footnotes
Conflicts of interest
These authors disclose the following: Dr Ahlquist and Mr Taylor are inventors of licensed technology. Drs Ahlquist and Vogelstein are scientific advisors to Exact Sciences. Drs Zou, Domanico, Berger, and Lidgard are employees of Exact Sciences. Mayo Clinic has licensed intellectual property to and is a minor equity investor in Exact Sciences. The remaining authors disclose no conflicts.
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at doi: 10.1053/j.gastro.2011.10.031.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Center MM, Jemal A, Ward E. International trends in colorectal cancer incidence rates. Cancer Epidemiol Biomarkers Prev. 2009;18:1688–1694. doi: 10.1158/1055-9965.EPI-09-0090. [DOI] [PubMed] [Google Scholar]
- 3.Morikawa T, Kato J, Yamaji Y, et al. A comparison of the immunochemical fecal occult blood test and total colonoscopy in the asymptomatic population. Gastroenterology. 2005;129:422–428. doi: 10.1016/j.gastro.2005.05.056. [DOI] [PubMed] [Google Scholar]
- 4.Ahlquist DA, Sargent DJ, Loprinzi CL, et al. Stool DNA and occult blood testing for screen detection of colorectal neoplasia. Ann Intern Med. 2008;149:441–450. W81. doi: 10.7326/0003-4819-149-7-200810070-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Imperiale TF, Ransohoff DF, Itzkowitz SH, et al. Fecal DNA versus fecal occult blood for colorectal-cancer screening in an average-risk population. N Engl J Med. 2004;351:2704–2714. doi: 10.1056/NEJMoa033403. [DOI] [PubMed] [Google Scholar]
- 6.Atkin WS, Edwards R, Kralj-Hans I, et al. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet. 2010;375:1624–1633. doi: 10.1016/S0140-6736(10)60551-X. [DOI] [PubMed] [Google Scholar]
- 7.Selby JV, Friedman GD, Quesenberry CP, Jr, et al. A case-control study of screening sigmoidoscopy and mortality from colorectal cancer. N Engl J Med. 1992;326:653–657. doi: 10.1056/NEJM199203053261001. [DOI] [PubMed] [Google Scholar]
- 8.Baxter NN, Goldwasser MA, Paszat LF, et al. Association of colonoscopy and death from colorectal cancer. Ann Intern Med. 2009;150:1–8. doi: 10.7326/0003-4819-150-1-200901060-00306. [DOI] [PubMed] [Google Scholar]
- 9.Brenner H, Hoffmeister M, Arndt V, et al. Protection from right- and left-sided colorectal neoplasms after colonoscopy: population-based study. J Natl Cancer Inst. 2009;102:89–95. doi: 10.1093/jnci/djp436. [DOI] [PubMed] [Google Scholar]
- 10.Brenner H, Chang-Claude J, Seiler CM, et al. Protection from colorectal cancer after colonoscopy: a population-based, case-control study. Ann Intern Med. 2011;154:22–30. doi: 10.7326/0003-4819-154-1-201101040-00004. [DOI] [PubMed] [Google Scholar]
- 11.Ahlquist DA, Wieand HS, Moertel CG, et al. Accuracy of fecal occult blood screening for colorectal neoplasia. A prospective study using Hemoccult and HemoQuant tests. JAMA. 1993;269:1262–1267. [PubMed] [Google Scholar]
- 12.Heresbach D, Manfredi S, D’Halluin PN, et al. Review in depth and meta-analysis of controlled trials on colorectal cancer screening by faecal occult blood test. Eur J Gastroenterol Hepatol. 2006;18:427–433. doi: 10.1097/00042737-200604000-00018. [DOI] [PubMed] [Google Scholar]
- 13.Ahlquist DA. Molecular detection of colorectal neoplasia. Gastroenterology. 2010;138:2127–2139. doi: 10.1053/j.gastro.2010.01.055. [DOI] [PubMed] [Google Scholar]
- 14.Sidransky D, Tokino T, Hamilton SR, et al. Identification of ras oncogene mutations in the stool of patients with curable colorectal tumors. Science. 1992;256:102–105. doi: 10.1126/science.1566048. [DOI] [PubMed] [Google Scholar]
- 15.Ahlquist DA, Skoletsky JE, Boynton KA, et al. Colorectal cancer screening by detection of altered human DNA in stool: feasibility of a multitarget assay panel. Gastroenterology. 2000;119:1219–1227. doi: 10.1053/gast.2000.19580. [DOI] [PubMed] [Google Scholar]
- 16.Ahlquist DA. Next-generation stool DNA testing: expanding the scope. Gastroenterology. 2009;136:2068–2073. doi: 10.1053/j.gastro.2009.04.025. [DOI] [PubMed] [Google Scholar]
- 17.Olson J, Whitney DH, Durkee K, et al. DNA stabilization is critical for maximizing performance of fecal DNA-based colorectal cancer tests. Diagn Mol Pathol. 2005;14:183–191. doi: 10.1097/01.pas.0000176768.18423.7e. [DOI] [PubMed] [Google Scholar]
- 18.Zou H, Harrington JJ, Klatt KK, et al. A sensitive method to quantify human long DNA in stool: relevance to colorectal cancer screening. Cancer Epidemiol Biomarkers Prev. 2006;15:1115–1119. doi: 10.1158/1055-9965.EPI-05-0992. [DOI] [PubMed] [Google Scholar]
- 19.Zou H, Cao X, Dominico M, et al. Sensitive quantification of methylated markers with a novel methylation specific technology. Clin Chem. 2010;56:A199. doi: 10.1373/clinchem.2011.171264. [DOI] [PubMed] [Google Scholar]
- 20.Zou H, Jiang X, Harrington JJ, et al. Quantitative stool DNA testing for detection of both colorectal cancer and advanced adenoma. Gastroenterology. 2009;136 (5 Suppl 1):A 625. [Google Scholar]
- 21.Zou H, Taylor WR, Harrington JJ, et al. High detection rates of colorectal neoplasia by stool DNA testing with a novel digital melt curve assay. Gastroenterology. 2009;136:459–470. doi: 10.1053/j.gastro.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 22.Diehl F, Schmidt K, Durkee KH, et al. Analysis of mutations in DNA isolated from plasma and stool of colorectal cancer patients. Gastroenterology. 2008;135:489–498. doi: 10.1053/j.gastro.2008.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahlquist D, Zou H, Domanico M, et al. Next generation stool DNA testing for detection of colorectal neoplasia: early marker evaluation. Presented at American Association for Cancer Research; Philadelphia, PA. October 2010. [Google Scholar]
- 24.Zou H, Jiang X, Harrington JJ, et al. Quantitative stool DNA testing for detection of both colorectal cancer and advanced adenoma. Gastroenterology. 2009;136(Suppl 1):A625–A626. [Google Scholar]
- 25.Zou H, Harrington JJ, Hussain FT, et al. Stool DNA and occult blood for detection of colorectal cancer: complementary markers. Gastroenterology. 2009;136(Suppl 1):A625. [Google Scholar]
- 26.Stone BB, Cohen SP, Breton GL, et al. Detection of rRNA from four respiratory pathogens using an automated Q beta replicase assay. Mol Cell Probes. 1996;10:359–370. doi: 10.1006/mcpr.1996.0049. [DOI] [PubMed] [Google Scholar]
- 27.Ahlquist DA, McGill DB, Schwartz S, et al. HemoQuant, a new quantitative assay for fecal hemoglobin. Comparison with Hemoccult. Ann Intern Med. 1984;101:297–302. doi: 10.7326/0003-4819-101-3-297. [DOI] [PubMed] [Google Scholar]
- 28.Ahlquist DA, McGill DB, Schwartz S, et al. Fecal blood levels in health and disease. A study using HemoQuant. N Engl J Med. 1985;312:1422–1428. doi: 10.1056/NEJM198505303122204. [DOI] [PubMed] [Google Scholar]
- 29.McIntosh MW, Pepe MS. Combining several screening tests: optimality of the risk score. Biometrics. 2002;58:657–664. doi: 10.1111/j.0006-341x.2002.00657.x. [DOI] [PubMed] [Google Scholar]
- 30.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 31.Lin LI. A concordance correlation coefficient to evaluate reproducibility. Biometrics. 1989;45:255–268. [PubMed] [Google Scholar]
- 32.Huang Z, Li L, Wang J. Hypermethylation of SFRP2 as a potential marker for stool-based detection of colorectal cancer and precancerous lesions. Dig Dis Sci. 2007;52:2287–2291. doi: 10.1007/s10620-007-9755-y. [DOI] [PubMed] [Google Scholar]
- 33.Itzkowitz SH, Jandorf L, Brand R, et al. Improved fecal DNA test for colorectal cancer screening. Clin Gastroenterol Hepatol. 2007;5:111–117. doi: 10.1016/j.cgh.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 34.Jiang X, Harrington JJ, Mahoney DW, et al. Detection of colorectal neoplasia by stool DNA testing: high discrimination with multi-marker quantitation. Gastroenterology. 2008;134(Suppl 1):A-484. [Google Scholar]
- 35.Nagasaka T, Tanaka N, Cullings HM, et al. Analysis of fecal DNA methylation to detect gastrointestinal neoplasia. J Natl Cancer Inst. 2009;101:1244–1258. doi: 10.1093/jnci/djp265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baek YH, Chang E, Kim YJ, et al. Stool methylation-specific polymerase chain reaction assay for the detection of colorectal neoplasia in Korean patients. Dis Colon Rectum. 2009;52:1452–1459. doi: 10.1007/DCR.0b013e3181a79533. discussion 1459–1463. [DOI] [PubMed] [Google Scholar]
- 37.Ehrlich M. DNA methylation in cancer: too much, but also too little. Oncogene. 2002;21:5400–5413. doi: 10.1038/sj.onc.1205651. [DOI] [PubMed] [Google Scholar]
- 38.Grutzmann R, Molnar B, Pilarsky C, et al. Sensitive detection of colorectal cancer in peripheral blood by septin 9 DNA methylation assay. PLoS One. 2008;3:e3759. doi: 10.1371/journal.pone.0003759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tanzer M, Balluff B, Distler J, et al. Performance of epigenetic markers SEPT9 and ALX4 in plasma for detection of colorectal precancerous lesions. PLoS One. 2010;5:e9061. doi: 10.1371/journal.pone.0009061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Diehl F, Li M, Dressman D, et al. Detection and quantification of mutations in the plasma of patients with colorectal tumors. Proc Natl Acad Sci U S A. 2005;102:16368–16373. doi: 10.1073/pnas.0507904102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muto T, Bussey HJ, Morson BC. The evolution of cancer of the colon and rectum. Cancer. 1975;36:2251–2270. doi: 10.1002/cncr.2820360944. [DOI] [PubMed] [Google Scholar]
- 42.Pickhardt PJ. The natural history of colorectal polyps and masses: rediscovered truths from the barium enema era. Am J Roentgenol. 2007;188:619–621. doi: 10.2214/AJR.06.0731. [DOI] [PubMed] [Google Scholar]
- 43.Welin S, Youker J, Spratt JS., Jr The rates and patterns of growth of 375 tumors of the large intestine and rectum observed serially by double contrast enema study (Malmoe technique) Am J Roentgenol Radium Ther Nucl Med. 1963;90:673–687. [PubMed] [Google Scholar]
- 44.Stryker SJ, Wolff BG, Culp CE, et al. Natural history of untreated colonic polyps. Gastroenterology. 1987;93:1009–1013. doi: 10.1016/0016-5085(87)90563-4. [DOI] [PubMed] [Google Scholar]
- 45.Hofstad B, Vatn M, Larsen S, et al. Growth of colorectal polyps: recovery and evaluation of unresected polyps of less than 10 mm, 1 year after detection. Scand J Gastroenterol. 1994;29:640–645. doi: 10.3109/00365529409092485. [DOI] [PubMed] [Google Scholar]
- 46.Knoernschild HE. Growth rate and malignant potential of colonic polyps: early results. Surg Forum. 1963;14:137–138. [PubMed] [Google Scholar]
- 47.Loeve F, Boer R, Zauber AG, et al. National Polyp Study data: evidence for regression of adenomas. Int J Cancer. 2004;111:633–639. doi: 10.1002/ijc.20277. [DOI] [PubMed] [Google Scholar]
- 48.Hisabe T, Tsuda S, Matsui T, et al. Natural history of small colorectal protuberant adenomas. Dig Endosc. 2010;22(Suppl 1):S43–S46. doi: 10.1111/j.1443-1661.2010.00969.x. [DOI] [PubMed] [Google Scholar]
- 49.Noffsinger AE. Serrated polyps and colorectal cancer: new pathway to malignancy. Annu Rev Pathol. 2009;4:343–364. doi: 10.1146/annurev.pathol.4.110807.092317. [DOI] [PubMed] [Google Scholar]
- 50.Hussain FT, Yab TC, Harrington JJ, et al. Noninvasive detection of serrated colorectal polyps by stool assay of methylated vimentin and mutant BRAF genes. Gastroenterology. 2010;138(Suppl 1):S102. [Google Scholar]
- 51.Gupta AK, Melton LJ, 3rd, Petersen GM, et al. Changing trends in the incidence, stage, survival, and screen-detection of colorectal cancer: a population-based study. Clin Gastroenterol Hepatol. 2005;3:150–158. doi: 10.1016/s1542-3565(04)00664-0. [DOI] [PubMed] [Google Scholar]
- 52.Cucino C, Buchner AM, Sonnenberg A. Continued rightward shift of colorectal cancer. Dis Colon Rectum. 2002;45:1035–1040. doi: 10.1007/s10350-004-6356-0. [DOI] [PubMed] [Google Scholar]
- 53.Fenoglio L, Castagna E, Comino A, et al. A shift from distal to proximal neoplasia in the colon: a decade of polyps and CRC in Italy. BMC Gastroenterol. 2010;10:139. doi: 10.1186/1471-230X-10-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kahi CJ, Hewett DG, Norton DL, et al. Prevalence and variable detection of proximal colon serrated polyps during screening colonoscopy. Clin Gastroenterol Hepatol. 2011;9:42–46. doi: 10.1016/j.cgh.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 55.Schoen RE, Gerber LD, Margulies C. The pathologic measurement of polyp size is preferable to the endoscopic estimate. Gastroin-test Endosc. 1997;46:492–496. doi: 10.1016/s0016-5107(97)70002-6. [DOI] [PubMed] [Google Scholar]
- 56.Ahlquist DAYG. Approach to the patient with occult gastrointestinal bleeding. In: Yamada T, editor. Principles of clinical gastroenterology. Chichester; Hoboken: Wiley-Blackwell; 2008. pp. 152–169. [Google Scholar]
- 57.Harewood GC, McConnell JP, Harrington JJ, et al. Detection of occult upper gastrointestinal tract bleeding: performance differences in fecal occult blood tests. Mayo Clin Proc. 2002;77:23–28. doi: 10.4065/77.1.23. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.