Non-Hodgkin lymphoma incidence is high in HIV-infected patients successfully treated with antiretroviral therapy. HIV replication, even at low levels, may be an important modifiable risk factor for non-Hodgkin lymphoma.
Keywords: non-Hodgkin lymphoma, HIV, antiretroviral therapy, incidence, viremia
Abstract
Background. The incidence of non-Hodgkin lymphoma (NHL) in human immunodeficiency virus (HIV)–infected patients remains high despite treatment with antiretroviral therapy (ART).
Methods. We evaluated NHL incidence in HIV-infected patients followed in the Centers for AIDS Research Network of Integrated Clinical Systems who started combination ART and achieved suppression of HIV. We estimated the hazard ratio for NHL by time-varying HIV viremia categories, accounting for time-varying CD4 cell count using marginal structural models.
Results. We observed 37 incident NHL diagnoses during 21 607 person-years of follow-up in 6036 patients (incidence rate, 171 per 100 000 person-years; 95% confidence interval [CI], 124–236). NHL incidence was high even among patients with nadir CD4 cell count >200 cells/µL (140 per 100 000 person-years [95% CI, 80–247]). Compared with ≤50 copies/mL, hazard ratios (HRs) for NHL were higher among those with HIV viremia of 51–500 copies/mL (HR current = 1.66 [95% CI, .70–3.94]; HR 3-month lagged = 2.10 [95% CI, .84–5.22]; and HR 6-month lagged = 1.46 [95% CI, .60–3.60]) and >500 copies/mL (HR current = 2.39 [95% CI, .92–6.21]; HR 3-month lagged = 3.56 [95% CI, 1.21–10.49]; and HR 6-month lagged = 2.50 [95% CI, .91–6.84]). Current HIV RNA as a continuous variable was also associated with NHL (HR = 1.42 per log10 copies/mL [95% CI, 1.05–1.92]).
Conclusions. Our findings demonstrate a high incidence of NHL among HIV-infected patients on ART and suggest a role of HIV viremia in the pathogenesis of NHL. Earlier initiation of potent ART and maximal continuous suppression of HIV viremia may further reduce NHL risk.
Non-Hodgkin lymphoma (NHL) is an AIDS-defining condition [1] for which incidence has declined in the modern era of antiretroviral therapy (ART) [2–7]. However, rates continue to be 4–23 times higher in human immunodeficiency virus (HIV)–infected populations compared with HIV-uninfected populations, depending on the proportion receiving ART [4–6, 8]. NHL also continues to impact survival of HIV-infected patients with little change in the proportion of NHL-associated deaths from 2000 to 2005 (11% and 10% of deaths, respectively) [9, 10].
Immune deficiency, oncogenic viruses (Epstein-Barr virus [EBV] and Kaposi sarcoma herpesvirus), HIV viremia, immune activation, and aging are intersecting factors contributing to the development of NHL in the setting of HIV [8, 11–18]. The contribution of each of these factors to NHL risk during ART is unclear. We hypothesized that any detectable HIV viremia is a driver of immune dysfunction, B-cell activation, and higher subsequent risk for NHL. We studied the incidence of NHL in a large multisite cohort of HIV-infected patients on ART and the association with HIV viremia using rigorous methods to control for level of immune deficiency and other known confounders.
METHODS
Study Population
The Centers for AIDS Research (CFAR) Network of Integrated Clinical Systems (CNICS) cohort includes >27 000 HIV-infected patients 18 years of age or older in care from 1995 to the present at 8 clinical sites across the United States: Case Western Reserve University; Fenway Community Health Center of Harvard University; Johns Hopkins University; University of Alabama at Birmingham; University of California, San Diego; University of California, San Francisco; University of North Carolina; and University of Washington [19]. The frequency of follow-up averages every 3 months; however, patients can be seen more or less often depending on clinical care. CNICS is a dynamic cohort with approximately 1800 new patients enrolling and 13% of existing patients leaving care each year. Institutional review boards at each clinical site approved the study protocols.
We examined all patients enrolled in CNICS for at least 90 days between 1998 and 2009 who started combination ART (at least 3 ARV medications) with suppression of HIV viremia and at least 3 months of follow-up. Suppression of HIV viremia was defined as achieving a single HIV RNA measurement <500 copies/mL within the first year of ART initiation. We excluded patients enrolled in CNICS before 1998 to assure HIV load testing with ultrasensitive HIV RNA polymerase chain reaction (PCR) assays (detection limit <50 copies/mL). Patients who developed NHL prior to or within 14 days of achieving initial suppression of HIV viremia were excluded.
Sources of Data
The CNICS data repository captures comprehensive clinical data for HIV-infected patients in care at each CNICS site that include standardized diagnosis, medication, laboratory, and demographic information collected through electronic health records and other institutional data systems [19]. Data quality assessment is conducted at the sites prior to data transmission and at the time of submission to the CNICS Data Management Core. After integration into the repository, data undergo extensive quality assurance procedures and data quality issues are reported to CNICS sites by the Data Management Core to investigate and correct. Data from each site are updated, fully reviewed, and integrated into the repository quarterly. The following variables were included in the analysis: demographics (ie, year of birth, sex, race/ethnicity), men who have sex with men (MSM) as a risk factor for HIV transmission, hepatitis B virus (HBV) and hepatitis C virus (HCV) coinfection, ART, CD4 cell counts, and HIV viremia determined by quantitative plasma HIV RNA PCR assays. HCV infection was defined by positive HCV antibody or HCV RNA testing within 6 months of HIV RNA suppression. HBV infection was defined by positive HBV surface antigen or HBV DNA testing within 6 months of HIV RNA suppression. Pre-ART peak HIV viremia was defined as the highest HIV RNA measurement from the start of CNICS follow-up until ART initiation. Nadir CD4 cell count was defined as the lowest CD4 cell count from the start of CNICS follow-up until ART initiation. If CD4 cell count was missing at baseline, the CD4 cell count measurement within 1 month of the date of suppression was used. Single-value imputation with the median was used for missing values of nadir CD4 cell count (<1% missing) and peak HIV viremia (2% missing).
NHL Ascertainment
At each CNICS site, incident diagnoses of NHL were reviewed using a standardized protocol to confirm the diagnosis and collect detailed information regarding NHL histopathology subtype [20]. Biopsy results confirmed 87% of systemic NHL and 68% of primary central nervous system (CNS) NHL diagnoses; the remaining NHL cases were diagnosed based on clinical, radiographic, and/or historical information. The NHL ascertainment and verification process was performed at all CNICS sites through 31 December 2009; follow-up was administratively censored after this date.
Statistical Analysis
We followed patients from date of suppression of HIV viremia (<500 copies/mL) until incident NHL, death, administrative censoring (defined above), or loss to follow-up. Loss to follow-up was defined as 1 year without a clinic visit before the administrative censoring date. Incidence rates were calculated as number of NHL diagnoses per 100 000 person-years (PY) of follow-up time.
We used marginal structural models to examine current and lagged time-varying HIV viremia as the primary predictor of NHL. HIV viremia was modeled as a continuous variable and categorized as ≤50 (reference), 51–500, and >500 copies/mL. In addition to current HIV viremia, we explored 3- and 6-month lagged values. Current HIV viremia was defined as the HIV RNA measurement at the current visit and lagged HIV viremia as HIV RNA measurements 3 and 6 months prior to visit. We adjusted for the main covariate of time-varying CD4 cell count through inverse probability (IP) weights, including current and 2-month lagged measurements. CD4 cell count was treated as a continuous variable and modeled using a restricted quadratic spline. Other covariates included in adjusted models were age (<39 vs ≥39 years), sex, race, year of HIV suppression, peak pre-ART HIV viremia, and HCV coinfection. Considering strong known associations with profound immune deficiency and EBV coinfection, we performed a sensitivity analysis where we censored the 7 cases of primary CNS lymphoma at their date of occurrence (Supplementary Table 1).
IP-weighted models were fit with and without peak pre-ART HIV viremia and HCV coinfection and the results were comparable; peak pre-ART HIV viremia and HCV coinfection were removed from the final model. A polytomous logistic regression was used to model HIV viremia categories for IP weights. Robust estimates of the variance were used in marginal structural models to account for the estimated IP weights. The distributions of the IP weights are summarized in Supplementary Table 2. Marginal structural Cox models were used to generate curves of NHL incidence over time from HIV suppression. All statistical analyses were done with SAS software, version 9.3 (SAS Institute, Cary, North Carolina).
RESULTS
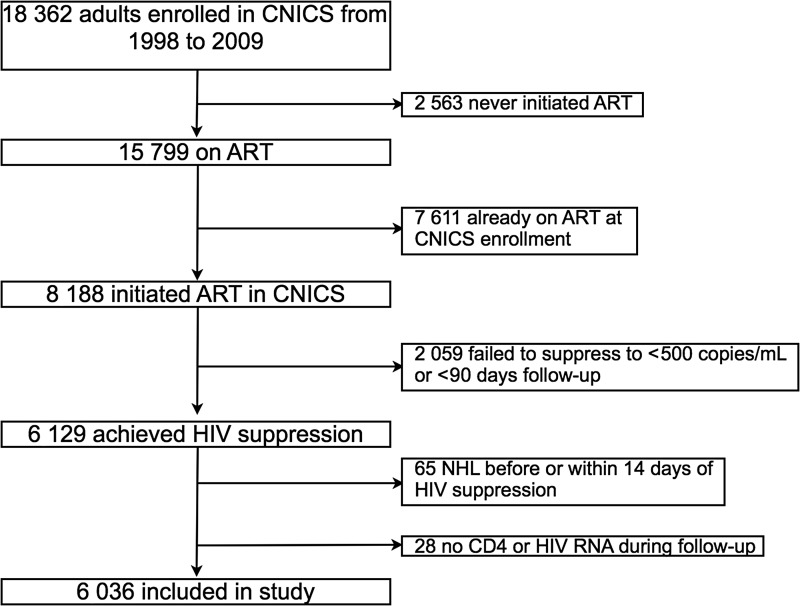
Among 18 382 HIV-infected patients enrolled in CNICS between 1998 and 2009, we studied 6036 individuals who were treatment naive at enrollment, initiated ART, and achieved suppression of HIV viremia to ≤500 copies/mL (Figure 1 and Table 1). The median age at HIV suppression was 39 years, 51% were white, 80% were male, 56% reported MSM as a risk factor for transmission of HIV infection, 7% were coinfected with HBV, and 13% were coinfected with HCV. Prior to treatment with ART, nadir CD4 cell count was 180 cells/µL, median peak pre-ART HIV viremia was 5.0 log10 copies/mL, and median peak HIV viremia after ART initiation was 2.6 log10 copies/mL. The median time from ART initiation until suppression of HIV viremia to ≤500 copies/mL was 1.9 months (interquartile range [IQR], 1.1–3.3 months). During follow-up, 2411 (40%) patients experienced a rebound in HIV RNA level >500 copies/mL at any time point, and the median time from initial suppression to rebound for these patients was 8.3 months (IQR, 3.6–18.1 months). Accounting for censoring using a Kaplan-Meier estimate, the median time to rebound was 57 months (IQR, 11 to >143). The initial ART regimen was anchored with a nonnucleoside reverse transcriptase inhibitor (NNRTI) in 2964 patients (49%), a ritonavir-boosted protease inhibitor (PI) in 1964 (33%), an unboosted PI in 561 (9%), PI (boosted or unboosted) and NNRTI in 121 (2%), and other classes of ART in 426 (7%).
Figure 1.
Patient selection. Abbreviations: ART, antiretroviral therapy; CNICS, Centers for AIDS Research Network of Integrated Clinical Systems; HIV, human immunodeficiency virus; NHL, Non-Hodgkin Lymphoma.
Table 1.
Characteristics of Analyzed Patients After Achieving HIV Suppression on Antiretroviral Therapy
| Characteristic | Overall (N = 6036) | NHL (n = 37) | No NHL (n = 5999) |
|---|---|---|---|
| Male sex | 4824 (80%) | 34 (92%) | 4790 (80%) |
| White race | 3104 (51%) | 22 (60%) | 3082 (51%) |
| Men who have sex with men | 3386 (56%) | 21 (57%) | 3365 (56%) |
| Hepatitis B virus | 397 (7%) | 3 (8%) | 394 (7%) |
| Hepatitis C virus | 801 (13%) | 9 (24%) | 792 (13%) |
| Age, y | 39 (33–46) | 42 (39–46) | 39 (33–46) |
| ART initiation year | 2005 (2002–2008) | 2003 (2001–2005) | 2005 (2002–2008) |
| Time from ART initiation to HIV suppression, mo | 1.9 (1.1–3.3) | 1.9 (1.0–3.1) | 1.9 (1.1–3.3) |
| Peak pre-ART HIV viremiaa, log10 copies/mL | 5.0 (4.6–5.5) | 5.4 (5.0–5.7) | 5.0 (4.6–5.5) |
| Peak HIV viremia after ART initiationb, log10 copies/mL | 2.6 (1.9–4.3) | 2.7 (2.3–4.8) | 2.6 (1.9–4.3) |
| Nadir CD4 cell counta, cells/µL | 180 (50–280) | 100 (30–250) | 180 (50–280) |
| CD4 cell count at HIV suppression, cells/µL | 290 (160–430) | 160 (70–350) | 290 (160–430) |
Data are presented as No. (%) or median (interquartile range).
Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; NHL, non-Hodgkin lymphoma.
a One hundred three (2%) missing peak pre-ART HIV viremia and 29 (<1%) missing nadir CD4 cell count.
b Highest HIV RNA level at any time during study follow-up.
There were 37 incident NHL diagnoses during 21 607 person-years of follow-up for a crude incidence rate of 171 per 100 000 PY (95% confidence interval [CI], 124–236), with higher incidence in patients with a nadir CD4 cell count ≤50 cells/µL (223 per 100 000 PY [95% CI, 132–376]), compared to those with higher nadir CD4 (Table 2). The median time between HIV suppression and incident NHL was 0.8 years (range, 0.1–7.5 years). Of the 37 incident NHL cases, 21 (57%) were diffuse large B cell, 7 (19%) were primary CNS, 4 (11%) Burkitt, and 5 (14%) other or unspecified lymphoma subtypes. There were 2441 patients (40%) lost to follow-up, as defined above, over the 12-year study period. The median observation time was 2.8 years with a maximum of 11.8 years.
Table 2.
Non-Hodgkin Lymphoma Incidence Rates by Nadir CD4 Cell Count
| Cell Count | No. | NHL Events | Person- Years | Rate (95% CI) per 100 000 PY |
|---|---|---|---|---|
| Overall | 6036 | 37 | 21 607 | 171 (124–236) |
| Nadir CD4 cell count, cells/µL | ||||
| ≤50 | 1566 | 14 | 6281 | 223 (132–376) |
| 51–200 | 1855 | 11 | 6783 | 162 (90–293) |
| >200 | 2615 | 12 | 8543 | 140 (80–247) |
Abbreviations: CI, confidence interval; NHL, non-Hodgkin lymphoma; PY, person-years.
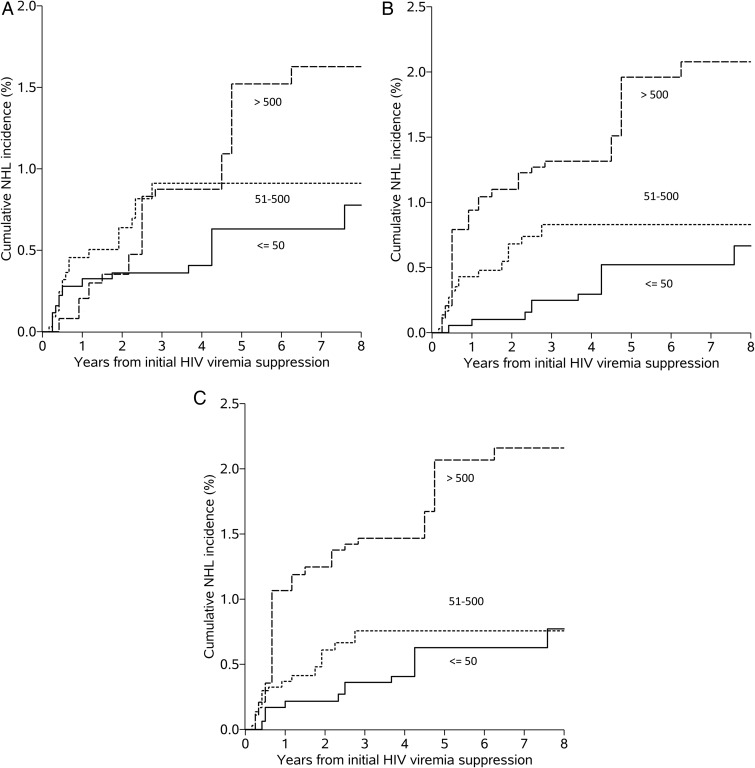
The crude and IP-weighted hazard ratios (HRs) for NHL were higher among those with current, 3-month, and 6-month lagged HIV viremia of 51–500 copies/mL and >500 copies/mL, compared with those with current HIV viremia ≤50 copies/mL (Table 3). Current HIV viremia modeled as a continuous variable showed an increased hazard (per log10 copies/mL, IP-weighted HR = 1.42 [95% CI, 1.05–1.92]). The proportional hazards assumption was not violated for the IP-weighted model (P = .745). Cumulative NHL incidence curves for current and 3- and 6-month lagged HIV viremia are displayed in Figure 2A–C.
Table 3.
Crude and Inverse Probability–Weighted Hazard Ratios for Non-Hodgkin Lymphoma
| HIV Viremia, Copies/mL | Crude HR (95% CI) | IP-Weighted HR (95% CI)a |
|---|---|---|
| Current | ||
| ≤50 (Reference) | … | … |
| 51–500 | 1.98 (.90–4.37) | 1.66 (.70–3.94) |
| >500 | 4.10 (1.77–9.51) | 2.39 (.92–6.21) |
| 3-month lagged | ||
| ≤50 (Reference) | … | … |
| 51–500 | 2.81 (1.14–6.91) | 2.10 (.84–5.22) |
| >500 | 5.70 (2.23–14.61) | 3.56 (1.21–10.49) |
| 6-month lagged | ||
| ≤50 (Reference) | … | … |
| 51–500 | 2.28 (.96–5.43) | 1.46 (.60–3.60) |
| >500 | 3.84 (1.55–9.52) | 2.50 (.91–6.84) |
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; HR, hazard ratio; IP, inverse probability.
a Marginal structural model (monthly structure) adjusted using inverse probability weighting (IPW) with combined weight for binary age, race, sex, CD4 cell count history, and year of suppression; restricted quadratic splines fit on year of human immunodeficiency virus (HIV) suppression, monthly visit variables, and CD4 cell count. IPW models included 2 lagged values from the monthly visits. P = .11, .04, and .10 for trend in non-Hodgkin lymphoma hazard across the 3 HIV viremia categories for current, 3-month lagged, and 6-month lagged, respectively.
Figure 2.
Non-Hodgkin lymphoma (NHL) incidence curves by human immunodeficiency virus (HIV) viremia category. Inverse probability weighting adjusted estimated survival curves categorized by current (A), 3-month lagged (B), and 6-month lagged (C) HIV viremia (copies/mL).
After censoring the 7 cases of primary CNS NHL (Supplementary Table 1), we observed a loss of precision (these 7 cases comprised 19% of the 37 NHL cases), and for those with 3- or 6-month lagged HIV viremia >500 copies/mL, we also observed an attenuation in the size of association (3-month lag IP-weighted HR = 2.43 [95% CI, .73–8.06]; 6-month lagged IP-weighted HR = 1.39 [95% CI, .42–4.56]). For those with a 3-month lagged HIV viremia of 51–500 cells/µL, the results were comparable to the original analysis with cases of primary CNS NHL included (IP-weighted HR = 2.11 [95% CI, .79–5.64]).
DISCUSSION
NHL continues to be an important cause of morbidity and mortality among HIV-infected individuals in the era of potent combination ART. We observed a high overall NHL incidence of 171 per 100 000 PY in a large cohort of patients receiving effective ART, far exceeding that reported in HIV-uninfected populations of approximately 10–20 per 100 000 PY [5, 8, 21, 22]. This incidence is comparable to rates of the 2 most common cancers in the general US population: prostate and breast cancer, with age-adjusted incidence rates of 152 and 124 per 100 000 PY, respectively [22]. A high incidence of NHL was noted even among patients with nadir CD4 cell count >200 cells/µL (140 per 100 000 PY), suggesting this cancer is associated with HIV infection above and beyond the level of immunodeficiency categorized as AIDS-defining by the Centers for Disease Control and Prevention in 1993 [1]. Moving forward, studies should include NHL as an important clinical endpoint along with non-AIDS-defining cancers.
In other cohort studies, high-level HIV viremia (>10 000 copies/mL), cumulative HIV viremia, and lack of ART were predictive of NHL independent of nadir and time-varying immunodeficiency [8, 11–15]. In contrast to prior studies, we focused our investigation on whether low-level detectable HIV viremia, as measured with modern ultrasensitive HIV RNA assays, was an independent predictor of NHL among patients who demonstrated HIV suppression on ART. We believe this population is most representative of the current era of HIV treatment in which the majority of patients are offered ART, resulting in an effective initial response [23]. Prior studies examined heterogeneous populations with regard to ART use and were unable to examine lower levels of HIV viremia as a predictor of clinical events such as NHL. We postulated that viral replication drives immune dysfunction and B-cell activation, which increases NHL risk. Our findings support this hypothesis. After adjusting for known confounders of older age, white race, male sex, HCV coinfection, and time-varying CD4 cell count, risk of NHL was higher when HIV viremia was above the limit of detection (50 copies/mL) in a dose-dependent relationship, although results were imprecise due to the relatively small number of NHL cases (n = 37). Replication in other cohorts, perhaps with extended follow-up, is required to further refine the association between low-level HIV viremia and NHL during ART.
Other investigations have postulated a biologic mechanism by which HIV increases NHL risk independent of its effects on T cells and resulting immune deficiency. B cells are highly activated in the setting of untreated HIV, and biomarkers of B-cell activation are associated with AIDS-associated NHL (AIDS-NHL) [18, 24]. Activation-induced cytidine deaminase (AID), a DNA-mutating enzyme upregulated by B-cell activation, is central to the development of immunoglobulin heavy-chain gene class switch recombination or somatic hypermutation in germinal center B cells [25]. Increased AID gene expression is found prior to AIDS-NHL and is induced by many viruses: EBV, HCV, human papillomavirus, and HIV [18, 26]. The HIV envelope can acquire CD40 ligand (CD40L) from host cell membrane and CD40L is a potent B-cell stimulator [27]. In vitro experiments found that CD40L-positive HIV virions induced AID gene expression and CD40L-negative HIV virions did not [27]. This induction was mediated by a direct interaction between CD40L in HIV envelope and CD40 receptor on B cells. Therefore, it is biologically plausible that HIV virions directly promote the development of B-cell NHL through stimulation of the CD40 receptor and activation of B cells.
The precision of our results was limited by the relatively few observed NHL cases. As a consequence, in our analyses adjusted using IP weighting, the HR reached statistical significance only in the 3-month lagged >500 copies/mL viremia category (IP-weighted HR = 3.56 [95% CI, 1.21–10.49]). Beyond the issue of precision, there are several possible reasons why we did not observe even stronger associations between HIV viremia and NHL risk among patients on ART. First, we were unable to assess compartmentalized HIV replication in lymphoid tissues. Throughout HIV infection and treatment with ART, lymphatic tissues, particularly gut-associated lymphoid tissue (GALT), are a major site of HIV replication, T-cell depletion, and virus persistence [28–33]. ART interruptions or poor ART penetration into GALT or other lymphatic tissue may result in preferentially high levels of HIV in these areas and localized inflammation with B-cell activation leading to lymphomagenesis. Alternatively, HIV may not be the only virus driving this process. Persistent defects in T-cell immunity could result in increased lytic EBV replication and B-cell activation or latent EBV-mediated genetic changes leading to NHL.
Potential reasons for detectable HIV viremia in this study included ART nonadherence, interruptions in drug supply, virologic failure, or intermittent release from latent reservoirs. Population-based studies report that approximately 25% of HIV-infected individuals in the United States lack health insurance; this factor has been shown to increase risk of ART discontinuation and suboptimal virologic suppression [34, 35]. Another reason for nonadherence and viremia before NHL could be symptomatic illness from an underlying undiagnosed (subclinical) lymphoma; however, we think this was unlikely in our study as associations were stronger in our 3- and 6-month lagged HIV viremia analyses. We would expect weaker associations of detectable viremia further preceding NHL, as symptoms leading to nonadherence should present or intensify in days to weeks immediately before definitive clinical diagnosis.
This study has several limitations. First, associations presented here reflect observational evidence and therefore could be influenced by unmeasured confounding. Second, 40% of included patients experienced loss to follow-up, likely due to transfer of care, and could have developed NHL outside of CNICS. Last, we were unable to provide direct comparison of NHL incidence and risk with a matched population without HIV infection. Despite these limitations, this work has several strengths. This was a multisite study from a large and diverse population with NHL diagnoses rigorously ascertained to minimize misclassification. Second, CNICS contains comprehensive and standardized information on ART, CD4 cell counts, and HIV RNA levels determined by modern ultrasensitive assays. Finally, distinct from previous studies, our study was the first to use marginal structural models to estimate the effect of time-varying HIV viremia while adjusting appropriately for confounding by time-varying immune deficiency and other known risk factors.
In conclusion, our findings highlight the importance of prompt, maximal, and sustained HIV suppression with potent combination ART. Current HIV treatment guidelines in the United States recommend initiation of ART and maximum virologic suppression for nearly all infected patients, regardless of CD4 cell count, to reduce transmission, minimize AIDS and non-AIDS clinical events, and maximize life expectancy [36]. Our study supports these recommendations and further contributes to mounting evidence that HIV replication, even at low levels, is associated with increased morbidity and mortality [14, 37–41]. In clinical practice, this will require earlier initiation of ART, maintaining insurance coverage and drug supply, switching to or intensification with modern potent ART regimens, and patient education on the importance of strict ART adherence. However, complete normalization of risk of NHL and other diseases may not be possible unless ART strategies or novel therapeutics are developed that reverse immune dysfunction and activation. Until then, providers need to be particularly vigilant for early signs and symptoms of lymphoma and prompt diagnosis in HIV-infected patients aging in the modern era of effective ART.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. These findings are presented on behalf of the Centers for AIDS Research Network of Integrated Clinical Systems (CNICS). We thank all of the CNICS investigators, data management teams, and patients who contributed to this project. We also thank Dr Otoniel Martinez-Maza for his careful reading of the manuscript and feedback.
Author contributions. C. J. A., A. L. B., and S. R. C. designed the study and acquired the data from the CNICS data management core. A. L. B. and S. R. C. selected the appropriate statistical analyses and executed them with the assistance of C. J. A. The initial draft of the manuscript was written by C. J. A., A. L. B., and S. R. C. Throughout the study, all authors participated in discussions about the design, statistical analyses, and interpretation of findings. All authors were involved in the review and editing process of the final manuscript for submission.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases at the National Institutes of Health (grant number R24 AI067039), with a supplement from the National Cancer Institute.
Potential conflicts of interest. All authors: No potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992;41:1–19. [PubMed] [Google Scholar]
- 2.Grulich AE, Li Y, McDonald AM, Correll PK, Law MG, Kaldor JM. Decreasing rates of Kaposi's sarcoma and non-Hodgkin's lymphoma in the era of potent combination anti-retroviral therapy. AIDS. 2001;15:629–33. doi: 10.1097/00002030-200103300-00013. [DOI] [PubMed] [Google Scholar]
- 3.Bedimo R, Chen RY, Accortt NA, et al. Trends in AIDS-defining and non-AIDS-defining malignancies among HIV-infected patients: 1989–2002. Clin Infect Dis. 2004;39:1380–4. doi: 10.1086/424883. [DOI] [PubMed] [Google Scholar]
- 4.Engels EA, Pfeiffer RM, Goedert JJ, et al. Trends in cancer risk among people with AIDS in the United States 1980–2002. AIDS. 2006;20:1645–54. doi: 10.1097/01.aids.0000238411.75324.59. [DOI] [PubMed] [Google Scholar]
- 5.Patel P, Hanson DL, Sullivan PS, et al. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992–2003. Ann Intern Med. 2008;148:728–36. doi: 10.7326/0003-4819-148-10-200805200-00005. [DOI] [PubMed] [Google Scholar]
- 6.Polesel J, Clifford GM, Rickenbach M, et al. Non-Hodgkin lymphoma incidence in the Swiss HIV Cohort Study before and after highly active antiretroviral therapy. AIDS. 2008;22:301–6. doi: 10.1097/QAD.0b013e3282f2705d. [DOI] [PubMed] [Google Scholar]
- 7.Silverberg MJ, Chao C, Leyden WA, et al. HIV infection and the risk of cancers with and without a known infectious cause. AIDS. 2009;23:2337–45. doi: 10.1097/QAD.0b013e3283319184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silverberg MJ, Chao C, Leyden WA, et al. HIV infection, immunodeficiency, viral replication, and the risk of cancer. Cancer Epidemiol Biomarkers Prev. 2011;20:2551–9. doi: 10.1158/1055-9965.EPI-11-0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mocroft A, Sterne JA, et al. Antiretroviral Therapy Cohort Collaboration; Variable impact on mortality of AIDS-defining events diagnosed during combination antiretroviral therapy: not all AIDS-defining conditions are created equal. Clin Infect Dis. 2009;48:1138–51. doi: 10.1086/597468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonnet F, Burty C, Lewden C, et al. Changes in cancer mortality among HIV-infected patients: the Mortalité 2005 Survey. Clin Infect Dis. 2009;48:633–9. doi: 10.1086/596766. [DOI] [PubMed] [Google Scholar]
- 11.Bohlius J, Schmidlin K, Costagliola D, et al. Collaboration of Observational HIV Epidemiological Research Europe COHERE Study Group; Incidence and risk factors of HIV-related non-Hodgkin's lymphoma in the era of combination antiretroviral therapy: a European multicohort study. Antivir Ther (Lond) 2009;14:1065–74. doi: 10.3851/IMP1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engels EA, Pfeiffer RM, Landgren O, Moore RD. Immunologic and virologic predictors of AIDS-related non-Hodgkin lymphoma in the highly active antiretroviral therapy era. J Acquir Immune Defic Syndr. 2010;54:78–84. doi: 10.1097/01.qai.0000371677.48743.8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guiguet M, Boué F, Cadranel J, et al. Effect of immunodeficiency, HIV viral load, and antiretroviral therapy on the risk of individual malignancies (FHDH-ANRS CO4): a prospective cohort study. Lancet Oncol. 2009;10:1152–9. doi: 10.1016/S1470-2045(09)70282-7. [DOI] [PubMed] [Google Scholar]
- 14.Zoufaly A, Stellbrink HJ, Heiden MA, et al. Cumulative HIV viremia during highly active antiretroviral therapy is a strong predictor of AIDS-related lymphoma. J Infect Dis. 2009;200:79–87. doi: 10.1086/599313. [DOI] [PubMed] [Google Scholar]
- 15.Bonnet F, Balestre E, Thiébaut R, et al. Factors associated with the occurrence of AIDS-related non-Hodgkin lymphoma in the era of highly active antiretroviral therapy: Aquitaine Cohort, France. Clin Infect Dis. 2006;42:411–7. doi: 10.1086/499054. [DOI] [PubMed] [Google Scholar]
- 16.Leruez-Ville M, Seng R, Morand P, et al. Blood Epstein-Barr virus DNA load and risk of progression to AIDS-related systemic B lymphoma. HIV Med. 2012;13:479–87. doi: 10.1111/j.1468-1293.2012.00998.x. [DOI] [PubMed] [Google Scholar]
- 17.van Baarle D, Wolthers KC, Hovenkamp E, et al. Absolute level of Epstein-Barr virus DNA in human immunodeficiency virus type 1 infection is not predictive of AIDS-related non-Hodgkin lymphoma. J Infect Dis. 2002;186:405–9. doi: 10.1086/341460. [DOI] [PubMed] [Google Scholar]
- 18.Epeldegui M, Vendrame E, Martínez-Maza O. HIV-associated immune dysfunction and viral infection: role in the pathogenesis of AIDS-related lymphoma. Immunol Res. 2010;48:72–83. doi: 10.1007/s12026-010-8168-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kitahata MM, Rodriguez B, Haubrich R, et al. Cohort profile: the Centers for AIDS Research Network of Integrated Clinical Systems. Int J Epidemiol. 2008;37:948–55. doi: 10.1093/ije/dym231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Achenbach CJ, Cole SR, Kitahata MM, et al. Mortality after cancer diagnosis in HIV-infected individuals treated with antiretroviral therapy. AIDS. 2011;25:691–700. doi: 10.1097/QAD.0b013e3283437f77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shiels MS, Engels EA, Linet MS, et al. The epidemic of non-Hodgkin lymphoma in the United States: disentangling the effect of HIV, 1992–2009. Cancer Epidemiol Biomarkers Prev. 2013;22:1069–78. doi: 10.1158/1055-9965.EPI-13-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, Surveillance, Epidemiology, and End Results (SEER) Program. US population data—1969–2011. Available at: www.seer.cancer.gov/popdata . Accessed 20 September 2013.
- 23.Althoff KN, Buchacz K, Hall HI, et al. U.S. trends in antiretroviral therapy use, HIV RNA plasma viral loads, and CD4 T-lymphocyte cell counts among HIV-infected persons, 2000 to 2008. Ann Intern Med. 2012;157:325–35. doi: 10.7326/0003-4819-157-5-201209040-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Breen EC, Hussain SK, Magpantay L, et al. B-cell stimulatory cytokines and markers of immune activation are elevated several years prior to the diagnosis of systemic AIDS-associated non-Hodgkin B-cell lymphoma. Cancer Epidemiol Biomarkers Prev. 2011;20:1303–14. doi: 10.1158/1055-9965.EPI-11-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Epeldegui M, Widney DP, Martínez-Maza O. Pathogenesis of AIDS lymphoma: role of oncogenic viruses and B cell activation-associated molecular lesions. Curr Opin Oncol. 2006;18:444–8. doi: 10.1097/01.cco.0000239882.23839.e5. [DOI] [PubMed] [Google Scholar]
- 26.Epeldegui M, Breen EC, Hung YP, Boscardin WJ, Detels R, Martínez-Maza O. Elevated expression of activation induced cytidine deaminase in peripheral blood mononuclear cells precedes AIDS-NHL diagnosis. AIDS. 2007;21:2265–70. doi: 10.1097/QAD.0b013e3282ef9f59. [DOI] [PubMed] [Google Scholar]
- 27.Epeldegui M, Thapa DR, De la Cruz J, Kitchen S, Zack JA, Martínez-Maza O. CD40 ligand (CD154) incorporated into HIV virions induces activation-induced cytidine deaminase (AID) expression in human B lymphocytes. PLoS One. 2010;5:e11448. doi: 10.1371/journal.pone.0011448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Veazey RS, DeMaria M, Chalifoux LV, et al. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 1998;280:427–31. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- 29.Guadalupe M, Sankaran S, George MD, et al. Viral suppression and immune restoration in the gastrointestinal mucosa of human immunodeficiency virus type 1-infected patients initiating therapy during primary or chronic infection. J Virol. 2006;80:8236–47. doi: 10.1128/JVI.00120-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schacker T, Little S, Connick E, et al. Rapid accumulation of human immunodeficiency virus (HIV) in lymphatic tissue reservoirs during acute and early HIV infection: implications for timing of antiretroviral therapy. J Infect Dis. 2000;181:354–7. doi: 10.1086/315178. [DOI] [PubMed] [Google Scholar]
- 31.Yukl SA, Gianella S, Sinclair E, et al. Differences in HIV burden and immune activation within the gut of HIV-positive patients receiving suppressive antiretroviral therapy. J Infect Dis. 2010;202:1553–61. doi: 10.1086/656722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chun T-W, Nickle DC, Justement JS, et al. Persistence of HIV in gut-associated lymphoid tissue despite long-term antiretroviral therapy. J Infect Dis. 2008;197:714–20. doi: 10.1086/527324. [DOI] [PubMed] [Google Scholar]
- 33.Zeng M, Smith AJ, Wietgrefe SW, et al. Cumulative mechanisms of lymphoid tissue fibrosis and T cell depletion in HIV-1 and SIV infections. J Clin Invest. 2011;121:998–1008. doi: 10.1172/JCI45157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muthulingam D, Chin J, Hsu L, et al. Disparities in engagement in care and viral suppression among persons with HIV. J Acquir Immune Defic Syndr. 2013;63:112–9. doi: 10.1097/QAI.0b013e3182894555. [DOI] [PubMed] [Google Scholar]
- 35.Hughes AJ, Mattson CL, Scheer S, Beer L, Skarbinski J. Discontinuation of antiretroviral therapy among adults receiving HIV care in the United States [published online ahead of print 8 December 2013] J Acquir Immune Defic Syndr. doi: 10.1097/QAI.0000000000000084. doi:10.1097/QAI.0000000000000084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thompson MA, Aberg JA, Hoy JF, et al. Antiretroviral treatment of adult HIV infection: 2012 recommendations of the International Antiviral Society–USA panel. JAMA. 2012;308:387–402. doi: 10.1001/jama.2012.7961. [DOI] [PubMed] [Google Scholar]
- 37.Mugavero MJ, Napravnik S, Cole SR, et al. Viremia copy-years predicts mortality among treatment-naive HIV-infected patients initiating antiretroviral therapy. Clin Infect Dis. 2011;53:927–35. doi: 10.1093/cid/cir526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kitahata MM, Gange SJ, Abraham AG, et al. Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med. 2009;360:1815–26. doi: 10.1056/NEJMoa0807252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thorpe J, Saeed S, Moodie EE, Klein MB; Canadian Co-infection Cohort Study CTN222. Antiretroviral treatment interruption leads to progression of liver fibrosis in HIV-hepatitis C virus co-infection. AIDS. 2011;25:967–75. doi: 10.1097/QAD.0b013e3283455e4b. [DOI] [PubMed] [Google Scholar]
- 40.Laprise C, de Pokomandy A, Baril JG, Dufresne S, Trottier H. Virologic failure following persistent low-level viremia in a cohort of HIV-positive patients: results from 12 years of observation. Clin Infect Dis. 2013;57:1489–96. doi: 10.1093/cid/cit529. [DOI] [PubMed] [Google Scholar]
- 41.Zhang S, van Sighem A, Kesselring A, et al. Episodes of HIV viremia and the risk of non-AIDS diseases in patients on suppressive antiretroviral therapy. J Acquir Immune Defic Syndr. 2012;60:265–72. doi: 10.1097/QAI.0b013e318258c651. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.