Clostridium difficile infection (CDI) causes considerable morbidity and is characterized by substantial risk of recurrence. In a decision model of strategies to treat recurrent CDI, initial treatment with fecal microbiota transplant using colonoscopic delivery is the most cost-effective approach.
Keywords: Clostridium difficile infection, vancomycin, fidaxomicin, metronidazole, fecal microbiota transplant
Abstract
Background. Clostridium difficile infection (CDI) is an important cause of morbidity and healthcare costs, and is characterized by high rates of disease recurrence. The cost-effectiveness of newer treatments for recurrent CDI has not been examined, yet would be important to inform clinical practice. The aim of this study was to analyze the cost effectiveness of competing strategies for recurrent CDI.
Methods. We constructed a decision-analytic model comparing 4 treatment strategies for first-line treatment of recurrent CDI in a population with a median age of 65 years: metronidazole, vancomycin, fidaxomicin, and fecal microbiota transplant (FMT). We modeled up to 2 additional recurrences following the initial recurrence. We assumed FMT delivery via colonoscopy as our base case, but conducted sensitivity analyses based on different modes of delivery. Willingness-to-pay threshold was set at $50 000 per quality-adjusted life-year.
Results. At our base case estimates, initial treatment of recurrent CDI using FMT colonoscopy was the most cost-effective strategy, with an incremental cost-effectiveness ratio of $17 016 relative to oral vancomycin. Fidaxomicin and metronidazole were both dominated by FMT colonoscopy. On sensitivity analysis, FMT colonoscopy remained the most cost-effective strategy at cure rates >88.4% and CDI recurrence rates <14.9%. Fidaxomicin required a cost <$1359 to meet our cost-effectiveness threshold. In clinical settings where FMT is not available or applicable, the preferred strategy appears to be initial treatment with oral vancomycin.
Conclusions. In this decision analysis examining treatment strategies for recurrent CDI, we demonstrate that FMT colonoscopy is the most cost-effective initial strategy for management of recurrent CDI.
Clostridium difficile infection (CDI) is the leading cause of nosocomial diarrhea in the United States and is associated with considerable morbidity [1]. There has also been an increase in the incidence and severity of CDI, and recognition of how community-acquired CDI contributes to the healthcare burden [2]. The economic costs of CDI exceeds $1 billion annually in the United States, with a similarly high burden in Europe [3, 4]. Contributing to this is the substantial risk of recurrent CDI. One-third of patients with CDI experience recurrent disease despite a primary cure; this proportion rises to two-thirds after the first episode of recurrence. The consequences of recurrent CDI are compounded by the fact that in patients at the highest risk of recurrent disease, risk factors for recurrence remain impossible (age, comorbidity) or difficult to modify (continued hospitalization or long-term-care facility stay, ongoing antibiotic use). Thus, identification of appropriate strategies to manage recurrent CDI is an important goal.
Our armamentarium for management of CDI and disease recurrence has increased. Both metronidazole and vancomycin are associated with substantial rates of recurrent disease [2]. Fidaxomicin promises reduced rates of recurrence, but its high cost has prohibited more widespread use [5, 6]. Other nonpharmacologic therapeutic approaches have emerged. Fecal microbiota transplant (FMT), involving luminal infusion of feces from a healthy donor to a patient, is highly effective for recurrent CDI [7–11]. FMT shows extraordinary clinical resolution rates with very low rates of recurrence, suggesting that the restoration of microflora diversity may surpass the efficacy of conventional therapy. However, the lack of comparative effectiveness studies and long-term follow-up preclude development of an optimal cost-effective treatment algorithm at a societal level.
The cost-effectiveness of a therapeutic strategy depends both on treatment-associated costs and health and cost benefits through prevention of future recurrences. A prior cost-utility analysis comparing fidaxomicin and vancomycin for the treatment of an initial episode of CDI or first recurrence suggested that fidaxomicin might be a cost-effective option under a few clinical scenarios [12]. However, there were several limitations to this analysis, including lack of a range of options for the treatment of recurrence and exclusion of promising therapies such as FMT. To date, there have been no comprehensive decision analytic models examining the optimal management of recurrent CDI that include FMT; this would be an important tool to inform clinical practice given the expanding spectrum of treatment options and increasing physician and patient interest. Thus, the aim of our study was to analyze the cost effectiveness of 4 competing strategies for the management of recurrent CDI where the first-line treatments were metronidazole, vancomycin, fidaxomicin, or FMT. We performed various sensitivity analyses to mimic relevant clinical scenarios across a range of efficacy and costs, and suggest optimal thresholds for future therapies to be cost-effective.
METHODS
Model Structure
We constructed a decision-analytic model comparing 4 strategies for the management of the recurrent CDI. The first-line therapies for the strategies were (1) metronidazole, (2) vancomycin, (3) fidaxomicin, and (4) FMT (Supplementary Figure 1A and 1B). We modeled up to 2 additional recurrences following the initial recurrence with subsequent treatments based on guidelines [6]. Strategies were compared using identical hypothetical cohorts of adult patients with a median age of 65 years [7]. Within the decision tree, patients contributed person-time in 1 of 6 health conditions: healthy, mild-moderate CDI, severe CDI, persistent recurrent disease, postcolectomy, and death. Death occurred due to severe CDI or following colectomy. The time horizon for the model was 1 year. Quality-adjusted life-years were calculated as the product of time in a particular health condition and the utility of that particular condition. All analyses were performed using TreeAge Pro 2013 (TreeAge, Williamstown, Massachusetts).
Diagnostic testing for C. difficile was done using polymerase chain reaction (PCR); all patients were initiated on treatment at diagnosis. Patients with a first recurrence of CDI were assumed to have mild-moderate disease diagnosed at an outpatient visit. Patients could be treated initially with oral metronidazole, outpatient oral vancomycin, fidaxomicin, or FMT colonoscopy (see Table 1 for drug dosing; Supplementary Figure 1A and 1B). Following treatment, patients were cured, cured but developed recurrence of mild-moderate CDI 3 weeks following cure, or were treatment failures. Patients who failed treatment were considered nonresponders who either continued to have persistent mild-moderate CDI or developed severe CDI requiring hospitalization [13]. Nonresponders with mild-moderate CDI received the same treatment as those initially achieving cure but subsequently developing recurrent CDI. The probabilities of initial cure rates (Table 1) and nonresponse sum to 1; rates of recurrence were modeled as a fraction of the population who achieved clinical cure following the initial CDI recurrence.
Table 1.
Transition Probabilities, Costs, and Utilities
| Variable | Base Case | Range | Distribution | References |
|---|---|---|---|---|
| Probabilities | ||||
| Oral metronidazole–cure | 0.710 | 0.500–0.950 | Beta (0.710; SD 0.113) | [14, 16] |
| Oral metronidazole–recurrence | 0.421 | 0.316–0.526 | Beta (0.421; SD 0.053) | [14, 16] |
| Outpatient oral vancomycin–cure | 0.916 | 0.687–1.000 | Beta (0.916; SD 0.115) | [16, 17] |
| Outpatient oral vancomycin–recurrence | 0.355 | 0.323–0.462 | Beta (0.355; SD 0.035) | [16, 17] |
| Fidaxomicin–cure | 0.937 | 0.703–1.000 | Beta (0.937; SD 0.117) | [5, 17] |
| Fidaxomicin–recurrence | 0.197 | 0.148–0.246 | Beta (0.197; SD 0.025) | [5, 17] |
| FMT colonoscopy–cure | 0.945 | 0.715–1.000 | Beta (0.945; SD 0.071) | [8–11] |
| FMT colonoscopy–recurrence | 0.091 | 0.054–0.158 | Beta (0.091; SD 0.026) | [10, 19, 20] |
| FMT duodenal infusion–cure | 0.813 | 0.610–1.000 | Beta (0.813; SD 0.098) | [38] |
| FMT duodenal infusion–recurrence | 0.063 | 0.047–0.078 | Beta (0.063; SD 0.008) | [38] |
| FMT enema–cure | 0.815 | 0.760–0.990 | Beta (0.815; SD 0.058) | [37] |
| FMT enema–recurrence | 0.091 | 0.054–0.158 | Beta (0.091; SD 0.026) | [10, 19, 20, 37] |
| Outpatient oral vancomycin pulse/taper–cure | 0.690 | 0.518–0.863 | Beta (0.690; SD 0.086) | [15, 18] |
| Outpatient oral vancomycin pulse/taper–recurrence | 0.274 | 0.206–0.343 | Beta (0.274; SD 0.034) | [5, 15, 18] |
| Severe CDI if treatment failure | 0.180 | 0.135–0.225 | Beta (0.180; SD 0.023) | [13] |
| Colectomy for severe CDI | 0.280 | 0.013–0.880 | Beta (0.280; SD 0.217) | [23–25] |
| Mortality from severe CDI, postcolectomy | 0.413 | 0.340–0.800 | Beta (0.413; SD 0.115) | [6, 25, 26] |
| Mortality from severe CDI, medical treatment | 0.580 | 0.580–0.805 | Beta (0.580; SD 0.056) | [23–26] |
| Costs, 2012 US$ | ||||
| Oral metronidazole 500 mg tid × 10 d | 22 | 17–28 | Gamma (22; SD 3) | [6], CMS |
| Intravenous metronidazole 500 mg tid × 10 d | 32 | 24–40 | Gamma (32; SD 4) | CMS |
| Outpatient oral vancomycin 125 mg qid × 10 d | 680 | 510–850 | Gamma (680; SD 85) | [6], CMS |
| Vancomycin IV compounded for oral at 125 mg qid × 10 d | 250 | 188–313 | Gamma (250; SD 31) | [6], CMS |
| Oral vancomycin 500 mg qid × 4 d prior to FMT | 1088 | 816–1360 | Gamma (1088; SD 136) | [6], CMS |
| Outpatient oral vancomycin pulse/tapera | 850 | 638–1063 | Gamma (850; SD 106) | [6], CMS |
| Fidaxomicin 200 mg bid × 10 d | 2800 | 2300–3300 | Gamma (2800; SD 250) | [6], CMS |
| Colonoscopy | 403 | 302–504 | Gamma (403; SD 50) | CPT 45378, CMS |
| Esophagogastroduodenoscopy | 305 | 229–381 | Gamma (305; SD 38) | CPT 43235, CMS |
| Cost of FMT preparation and instillationb | 112 | 84–140 | Gamma (112; SD 14) | CPT G0455, CMS |
| Cost of testing recipient prior to FMT | 119 | 89–149 | Gamma (119; SD 15) | CMS |
| Cost of testing donor prior to FMT | 528 | 396–660 | Gamma (528; SD 66) | CMS |
| Colectomy | 37 629 | 28 222–47 036 | Gamma (37629; SD 4704) | [31] |
| Median cost of hospitalization for CDI | 15 675 | 14 014–17 335 | Gamma (15675; SD 830) | [4] |
| Initial outpatient visit | 105 | 79–131 | Gamma (105; SD 13) | HCPCS 99203, CMS |
| Follow-up outpatient visits | 104 | 78–130 | Gamma (104; SD 13) | HCPCS 99214, CMS |
| Clostridium difficile nucleic acid amplification testing | 50 | 37–62 | Gamma (50; SD 6) | CMS |
| Utilities | ||||
| Healthy patient, median age 65 | 0.88 | 0.84–0.92 | Triangular (0.88, 0.84, 0.92) | [33] |
| Mild-moderate CDI | 0.82 | 0.72–0.93 | Triangular (0.82, 0.72, 0.93) | [32, 34, 35, 42] |
| Severe CDI | 0.71 | 0.50–0.72 | Triangular (0.71, 0.50, 0.72) | [32, 34, 35, 42] |
| Colectomy | 0.61 (1 mo) | 0.32–0.84 | Triangular (0.61, 0.32, 0.84) | [32, 34, 35] |
| Postcolectomy | 0.86 | 0.84–0.94 | Triangular (0.86, 0.84, 0.94) | [31, 32] |
Abbreviations: bid, twice daily; CDI, Clostridium difficile infection; CMS, Centers for Medicare and Medicaid Services; CPT, Current Procedural Terminology; FMT, fecal microbiota transplant; HCPCS, Healthcare Common Procedural Coding System; IV, intravenous; qid, 4 times daily; SD, standard deviation; tid, 3 times daily.
a Outpatient oral vancomycin pulse/taper: 125 mg qid × 10 days, followed by 125 mg every 3 days for a total of 10 additional doses.
b Does not include donor or recipient testing (see Methods).
Consistent with published guidelines, patients who received metronidazole for a first CDI recurrence were treated with oral vancomycin for a second recurrence, and outpatient oral vancomycin pulse/taper for a third recurrence [6, 14–16]. Patients who received outpatient oral vancomycin or fidaxomicin for a first CDI recurrence were given outpatient oral vancomycin pulse/taper for a second recurrence, and FMT via colonoscopy for a third recurrence [5, 17, 18]. Finally, patients who received FMT for a first CDI recurrence were given repeat FMT by the same mode of delivery for a second recurrence, and outpatient oral vancomycin pulse/taper for a third recurrence [10, 19, 20]. In the model comparing different modalities of FMT delivery, we assumed that FMT colonoscopy was performed for a third recurrence in the vancomycin or fidaxomicin strategies. In the model where FMT was not available, we assumed that fidaxomicin was used for a third recurrence. We examined 5 different treatment scenarios. The first 3 treatment scenarios compared the 3 initial pharmacologic treatment arms with each of the 3 FMT delivery methods—colonoscopy (scenario 1, base case), duodenal infusion (scenario 2), or enema (scenario 3). In the fourth scenario, we assumed that all 3 modalities of FMT delivery were available, and compared them simultaneously to the 3 pharmacologic strategies (scenario 4). Finally, recognizing that FMT may not be available as a treatment option in all patients or all settings, we compared the cost-effectiveness of the 3 pharmacologic strategies alone (scenario 5).
Hospitalized patients with severe CDI were treated with inpatient vancomycin (intravenous compounded for oral) plus intravenous metronidazole. On the basis of published data, we assumed a median hospitalization duration of 2 weeks, culminating in cure, colectomy, or death [6, 21–25]. For those with a treatment failure following a third recurrence, we also modeled a state of “persistent recurrent disease” in addition to the possibilities of cure, colectomy, and death. Persistent recurrent disease had a similar utility as mild-moderate CDI with an average cost during this time (mean 9 weeks) equivalent to 2 outpatient follow-up visits and 1 hospitalization.
Fecal Microbiota Transplant
We selected FMT delivery via colonoscopy as our base case strategy as this is the most widely adopted method. All patients undergoing FMT, regardless of mode of delivery, received 4 days of oral vancomycin 500 mg every 6 hours prior to the procedure. Because therapeutic enema is not assigned a distinct Current Procedural Terminology (CPT) code, the cost of an enema was considered to be equal to an outpatient office visit. We assumed the efficacy of one-time FMT administration based on published studies (Table 1). The FMT colonoscopy cure rate was pooled from published clinical resolution rates [8–11]. The same clinical resolution and recurrence rates were used for a second FMT after failure of the first FMT, as studies have shown that similarly high rates of cure without recurrence can be achieved with a repeat FMT.
Donor testing prior to FMT included routine laboratory screening, stool testing, and serologic testing prior to and 30 days following stool donation (Supplementary Table 1) [26]. Patients requiring a second FMT were assumed to utilize a different donor. Routine recipient testing prior to FMT primarily included serologic testing (Supplementary Table 1) [26].
Utilities
We assumed a median age of 65 years for our cohort and a utility of 0.88 for the healthy patient [7, 27]. Patients who were cured by a given treatment strategy were assumed to spend half the duration of treatment in a state of mild-to-moderate or severe disease, and the subsequent half in the healthy state. Nonresponders remained in the initial disease state through the course of treatment, and were then transitioned to mild-moderate CDI with next-line treatment, or severe CDI until they were either cured, underwent colectomy, or died.
Costs
All costs were adjusted to 2012 US dollars using the consumer price index, except for the cost of FMT [28, 29]. The total cost of FMT included the costs of donor and recipient testing, 4-day pretreatment with vancomycin, FMT preparation and instillation, and method of delivery (Table 1). The costs for donor and recipient laboratory testing prior to FMT were taken from the 2012 Clinical Diagnostic Laboratory Fee Schedule from the Centers for Medicare and Medicaid Services (Table 1; Supplementary Table 1) [26]. The cost of FMT stool preparation and instillation was based on 2013 dollars as the CPT code (G0455) for this procedure, which includes physician work for assessment of donors, preparation of fecal microbiota, and instillation, was assigned in 2013. For all other costs, utilities, and probabilities where ranges were not available, the range for sensitivity analysis varied between 25% below and above their average values [4, 6, 30–35].
Outcomes and Data Analysis
The primary outcome from the base case analysis was the incremental cost-effectiveness ratio (ICER) among the 4 competing strategies [28, 29]. A willingness-to-pay (WTP) threshold of $50 000 per quality-adjusted life-year (QALY) was used to define cost-effectiveness [36]. Model sensitivity analyses were performed using alternate methods of FMT delivery as well as scenarios where FMT may not be available or applicable. Univariate sensitivity analyses were performed on probabilities, costs, and utilities to analyze the impact of changes in model estimates on the preferred strategy. Probabilistic sensitivity analysis was performed using 100-fold distribution sampling of all variables to account for uncertainty in the model assumptions. The probability distributions and standard deviations were used to arrive at the ranges for each of our assumptions. In our one-way sensitivity analysis, each variable was tested across its entire range and thresholds were identified for influential variables. Our analysis was performed from a societal perspective.
RESULTS
Base Case Analysis
In our base case analysis, initial treatment with FMT colonoscopy was the most cost-effective strategy at our WTP threshold with an ICER of $17 016 compared with vancomycin (scenario 1, Table 2). Treatment of recurrent CDI by first-line fidaxomicin or metronidazole was both more expensive and less effective than FMT colonoscopy. FMT delivery by less effective strategies (duodenal infusion or enema) was not cost-effective (scenarios 2 and 3, Table 2), making initial oral vancomycin the preferred strategy in such settings. In a model simultaneously comparing various methods of FMT delivery, FMT colonoscopy remained the most cost-effective initial strategy (scenario 4, Table 2). Probabilistic sensitivity analysis supported findings from the base case analysis, with an ICER of $20 285 for FMT colonoscopy relative to vancomycin.
Table 2.
Base Case and Sensitivity Analyses of Competing Strategies for the Management of Recurrent Clostridium difficile Infection
| Scenario | Cost | QALY | ICER |
|---|---|---|---|
| Scenario 1: All 3 pharmacologic treatment arms, FMT via colonoscopy (base case) | |||
| Vancomycin | $2912 | 0.8580 | |
| FMT colonoscopy | $3149 | 0.8719 | $17 016 |
| Metronidazole | $3941 | 0.8292 | (Dominated)a |
| Fidaxomicin | $4261 | 0.8653 | (Dominated)a |
| Scenario 2: All 3 pharmacologic treatment arms, FMT via duodenal infusion | |||
| Vancomycin | $3531 | 0.8484 | |
| Metronidazole | $3941 | 0.8292 | (Dominated)a |
| FMT duodenal infusion | $4208 | 0.8553 | $97 352 |
| Fidaxomicin | $4628 | 0.8596 | $98 443 |
| Scenario 3: All 3 pharmacologic treatment arms, FMT via enema | |||
| Vancomycin | $3488 | 0.8485 | |
| Metronidazole | $3941 | 0.8292 | (Dominated)a |
| FMT enema | $4090 | 0.8543 | $105 003 |
| Fidaxomicin | $4602 | 0.8597 | $99 862 |
| Scenario 4 (FMT delivery via either of the 3 routes, 3 pharmacologic treatment arms) | |||
| Vancomycin | $2912 | 0.8580 | |
| FMT colonoscopy | $3149 | 0.8719 | $17 016 |
| Metronidazole | $3941 | 0.8292 | (Dominated)a |
| FMT enema | $4090 | 0.8543 | (Dominated)a |
| FMT duodenal infusion | $4208 | 0.8553 | (Dominated)a |
| Fidaxomicin | $4261 | 0.8653 | (Dominated)a |
| Scenario 5 (3 pharmacologic treatment arms alone) | |||
| Vancomycin | $2912 | 0.8580 | |
| Metronidazole | $3941 | 0.8292 | (Dominated)a |
| Fidaxomicin | $4261 | 0.8653 | $184 023b |
The willingness-to-pay threshold was $50 000 per QALY gained.
Abbreviations: FMT, fecal microbiota transplant; ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life year.
a A strategy was considered to be dominated if the preceding nondominated alternative strategy is both more effective and less expensive.
b ICER calculated for fidaxomicin relative to next nondominated strategy, which is vancomycin.
Sensitivity Analyses of Individual Variables
Initial treatment with FMT colonoscopy remained the most cost-effective strategy for recurrent CDI at cure rates >88.4% (Table 3). At cure rates lower than this threshold, vancomycin was more cost-effective because the ICER of FMT colonoscopy relative to vancomycin exceeded the WTP threshold of $50 000/QALY. If the WTP threshold was increased to $100 000/QALY, FMT colonoscopy remained the most cost-effective strategy at cure rates >84.4%. FMT colonoscopy also remained cost-effective at CDI recurrence rates <14.9% and cost up to $2724 (includes colonoscopy, donor and recipient testing, FMT preparation and instillation, and 4-day pretreatment with vancomycin).
Table 3.
One-Way Sensitivity Analyses on Fecal Microbiota Transplant Colonoscopy (Willingness to Pay $50 000)
| Variable | Base Case | Sensitivity | Threshold | Preferred Strategy | Preferred Strategy |
|---|---|---|---|---|---|
| Value | Analysis Range | Below Threshold | Above Threshold | ||
| Probabilities | |||||
| Oral metronidazole–cure | 0.710 | 0–1 | n/a | FMT colonoscopy | FMT colonoscopy |
| Oral metronidazole–recurrence | 0.421 | 0–1 | n/a | FMT colonoscopy | FMT colonoscopy |
| Outpatient oral vancomycin–cure | 0.916 | 0–1 | 0.955 | FMT colonoscopy | Vancomycin |
| Outpatient oral vancomycin–recurrence | 0.355 | 0–1 | 0.272 | Vancomycin | FMT colonoscopy |
| Fidaxomicin–cure | 0.937 | 0–1 | n/a | FMT colonoscopy | FMT colonoscopy |
| Fidaxomicin–recurrence | 0.197 | 0–1 | n/a | FMT colonoscopy | FMT colonoscopy |
| FMT-colonoscopy–curea | 0.945 | 0–1 | 0.884 | Vancomycin | FMT colonoscopy |
| FMT-colonoscopy–recurrence | 0.091 | 0–1 | 0.149 | FMT colonoscopy | Vancomycin |
| Severe CDI if treatment failure | 0.180 | 0–1 | 0.103 | Vancomycin | FMT colonoscopy |
| Costs | |||||
| Cost of colonoscopyb | $403 | $0–$1600 | $877 | FMT colonoscopy | Vancomycin |
| Cost of fidaxomicin | $2800 | $0–$4000 | $1359 | Fidaxomicin | FMT colonoscopy |
| Outpatient oral vancomycin (125 mg qid × 10 d) | $680 | $0–$1600 | $221 | Vancomycin | FMT colonoscopy |
| Utilities | |||||
| Healthy patient, median age 65 | 0.88 | 0–1 | 0.70 | Vancomycin | FMT colonoscopy |
| Mild-moderate CDI | 0.82 | 0–1 | n/a | FMT colonoscopy | FMT colonoscopy |
Abbreviations: CDI, Clostridium difficile infection; FMT, fecal microbiota transplant; n/a, not applicable; qid, 4 times daily.
a Preferred strategies at FMT colonoscopy cure rates of 0–0.648 for metronidazole, 0.648–0.659 for fidaxomicin, and 0.659–0.884 for vancomycin.
b Does not include other costs of FMT, such as donor or recipient testing, pretreatment with vancomycin, or FMT preparation and instillation (see Methods and Table 1).
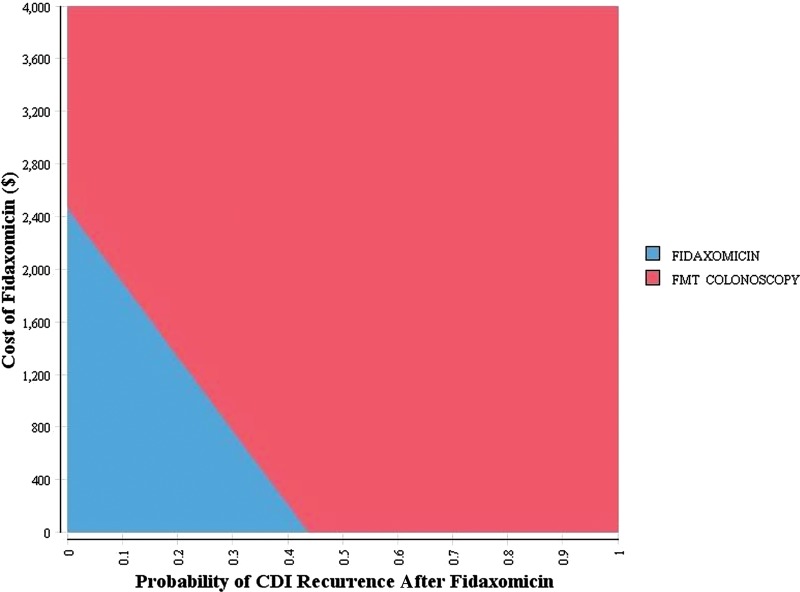
A treatment strategy with initial use of oral vancomycin for recurrent CDI required a cure rate >95.5% or CDI recurrence rate <27.2% to make it more cost-effective than FMT colonoscopy (Table 3). Our model was not sensitive to the efficacy of metronidazole or fidaxomicin. Fidaxomicin would be the most cost-effective strategy if its cost were $1359 per treatment course. Figure 1 presents the results of a 2-way sensitivity analysis between the cost and rate of recurrence of CDI with fidaxomicin. This also illustrates the efficacy and cost threshold that emerging therapies would need, assuming efficacy comparable to fidaxomicin, to be cost-effective relative to FMT colonoscopy.
Figure 1.
Two-way sensitivity analysis on cost and probability of recurrent Clostridium difficile infection with initial fidaxomicin treatment. Strategies were considered cost-effective at a willingness-to-pay threshold of $50 000 per quality-adjusted life-year. The smaller shaded area represents the most cost-effective strategy at any given cost and efficacy of fidaxomicin. Abbreviations: CDI, Clostridium difficile infection; FMT, fecal microbiota transplant.
In one-way sensitivity analyses examining other methods of FMT delivery, FMT via duodenal infusion and FMT via enema became more cost-effective than initial vancomycin if their cure rates were >85.2%.
Without FMT as a Treatment Option
In clinical settings where FMT is not available, the most cost-effective strategy was initial treatment with vancomycin; fidaxomicin had a cost-prohibitive ICER of $184 023/QALY compared with vancomycin (scenario 5, Table 2). Univariate sensitivity analysis on the cost of fidaxomicin indicated that it would be the most cost-effective strategy at a cost <$1818. Above this value, vancomycin remains the preferred strategy. At a baseline cost of fidaxomicin of $2800, vancomycin remained the preferred strategy across all cure rates of fidaxomicin.
DISCUSSION
Clostridium difficile infection is an important cause of morbidity with the effect of the initial episode often compounded by high rates of recurrence [1, 2]. Identification of cost-effective strategies for treatment of recurrent CDI is an important clinical challenge and research priority. To our knowledge, ours is the first decision analysis examining management of recurrent CDI incorporating new treatment strategies. Our decision model indicates that a strategy comprised of initial treatment with FMT colonoscopy is the most cost-effective for management of recurrent CDI. Alternative modes of FMT delivery (duodenal infusion or enema) did not meet our cost-effectiveness thresholds due to lower efficacy. In clinical situations where FMT is not available, vancomycin was the most cost-effective strategy.
Although FMT colonoscopy was the most cost-effective initial strategy, the results of our sensitivity analyses suggest that it requires a cure rate >88% to remain the preferred strategy at the traditionally accepted and more conservative WTP threshold of $50 000/QALY [36]. Considering the costs and training required to perform FMT, our analysis supports the need for continued standardization of FMT if it is to be used more routinely in the treatment of recurrent CDI. The strategy of initial treatment of the first recurrence with FMT was cost-effective at an oral vancomycin cure rate <95.5%.
We modeled FMT delivery method as a one-time administration, hence the lower cure rates for FMT via duodenal infusion or enema. In clinical trials and observational series, a second infusion by any of these methods has been shown to be highly effective with low recurrence rates [37, 38]. Our sensitivity analyses indicate that if cure rates of 85% could be consistently achieved with a single fecal preparation administration via enema or duodenal infusion, then these alternate modalities of FMT delivery would be more cost-effective than FMT colonoscopy because of their lower costs of delivery. This also suggests that less expensive FMT methods involving standardized fresh or frozen fecal preparations with comparable efficacy would likely be more cost-effective if the efficacy remains unchanged [39]. These results are also dependent on the assumption that FMT was delivered via colonoscopy for patients who developed a third recurrence in the other pharmacologic treatment arms.
Although studies have demonstrated comparable cure rates and lower recurrence rates for fidaxomicin compared with vancomycin, the use of fidaxomicin remains limited in part by cost [5, 6]. Our results suggest that fidaxomicin as a first-line treatment strategy would be cost-effective only at treatment costs <$1359 where FMT is available and or <$1818 where FMT is not available, requiring an approximately 35%–51% reduction in drug costs. Similar cost thresholds would likely need to be met for any emerging antibiotic therapies for mild-moderate CDI with efficacy comparable to fidaxomicin. Otherwise, oral vancomycin was the most cost-effective initial strategy in situations where FMT is not available or in situations where there may be other limitations to FMT, such as lack of a donor, expertise in performing FMT, insurance coverage, or patient acceptance.
Our study has several limitations. First, we did not account for potential differences in treatment efficacy or epidemiologic distribution of the more virulent North American pulsed-field gel electrophoresis type 1/restriction endonuclease analysis type B1/PCR ribotype 027 C. difficile strain [40]. Most studies examining treatment efficacy included a heterogeneous patient population with both types of strains. To date, there has only been 1 study of FMT for CDI with C. difficile strain typing, which reported a lower efficacy (89%) for the 027 strain than for the non-027 strain (100%) [9]. Second, although studies have shown increased CDI recurrence rates after second- or third-line antibiotic treatment, we did not model higher recurrence rates because of variations in risk factors for recurrence, specific antibiotic usage, and limited long-term data on recurrences following FMT [2, 6]. However, this decision was likely to have overestimated the efficacy of the antibiotic strategies in our model, biasing our results away from FMT. The utilities for mild-moderate and severe CDI had to be extrapolated from other comparable causes of diarrhea, as there are no published estimates of health utility with CDI [41, 42]. Finally, the costs attributed to FMT in our analysis included those related to donor screening and instillation of stool, but not the infrastructure and personnel costs required in establishing an FMT program.
In conclusion, using a decision analytic model examining treatments for recurrent CDI, we demonstrate that a strategy comprising initial treatment with FMT administered via colonoscopy is the most cost-effective approach. In settings where FMT is not available, initial treatment with oral vancomycin was the most cost-effective strategy. As more data become available, guidelines should consider incorporating use of FMT earlier in treatment of CDI, considering its high efficacy and low CDI recurrence rate. Future studies examining new treatments for CDI should incorporate FMT to examine comparative effectiveness if FMT becomes more widely adopted.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Author contributions. G. G. K.: study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, statistical analysis. J. S.: study concept and design, analysis and interpretation of data, critical revision of the manuscript. M. G. S.: study concept and design, analysis and interpretation of data, critical revision of the manuscript; statistical analysis. M. G.: analysis and interpretation of data, critical revision of the manuscript. A. N. A.: study concept and design, analysis and interpretation of data, critical revision of the manuscript; statistical analysis; funding, study supervision.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH).
Financial support. This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the NIH (award number T32DK007191). A. N. A. is supported by the NIH (K23 DK091742).
Potential conflicts of interest. A. N. A. has served on scientific advisory boards for Prometheus, Inc, Janssen Pharmaceuticals, and Cubist Pharmaceuticals. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Peery AF, Dellon ES, Lund J, et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology. 2012;143:1179–87. doi: 10.1053/j.gastro.2012.08.002. e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen SH, Gerding DN, Johnson S, et al. Society for Healthcare Epidemiology of America, Infectious Diseases Society of America. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA) Infect Control Hosp Epidemiol. 2010;31:431–55. doi: 10.1086/651706. [DOI] [PubMed] [Google Scholar]
- 3.Bouza E. Consequences of Clostridium difficile infection: understanding the healthcare burden. Clin Microbiol Infect. 2012;18(suppl 6):5–12. doi: 10.1111/1469-0691.12064. [DOI] [PubMed] [Google Scholar]
- 4.McGlone SM, Bailey RR, Zimmer SM, et al. The economic burden of Clostridium difficile. Clin Microbiol Infect. 2012;18:282–9. doi: 10.1111/j.1469-0691.2011.03571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Louie TJ, Miller MA, Mullane KM, et al. OPT-80-003 Clinical Study Group. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med. 2011;364:422–31. doi: 10.1056/NEJMoa0910812. [DOI] [PubMed] [Google Scholar]
- 6.Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108:478–98. doi: 10.1038/ajg.2013.4. quiz 499. [DOI] [PubMed] [Google Scholar]
- 7.Kassam Z, Lee CH, Yuan Y, Hunt RH. Fecal microbiota transplantation for Clostridium difficile infection: systematic review and meta-analysis. Am J Gastroenterol. 2013;108:500–8. doi: 10.1038/ajg.2013.59. [DOI] [PubMed] [Google Scholar]
- 8.Kelly CR, de Leon L, Jasutkar N. Fecal microbiota transplantation for relapsing Clostridium difficile infection in 26 patients: methodology and results. J Clin Gastroenterol. 2012;46:145–9. doi: 10.1097/MCG.0b013e318234570b. [DOI] [PubMed] [Google Scholar]
- 9.Mattila E, Uusitalo-Seppala R, Wuorela M, et al. Fecal transplantation, through colonoscopy, is effective therapy for recurrent Clostridium difficile infection. Gastroenterology. 2012;142:490–6. doi: 10.1053/j.gastro.2011.11.037. [DOI] [PubMed] [Google Scholar]
- 10.Rohlke F, Surawicz CM, Stollman N. Fecal flora reconstitution for recurrent Clostridium difficile infection: results and methodology. J Clin Gastroenterol. 2010;44:567–70. doi: 10.1097/MCG.0b013e3181dadb10. [DOI] [PubMed] [Google Scholar]
- 11.Yoon SS, Brandt LJ. Treatment of refractory/recurrent C. difficile-associated disease by donated stool transplanted via colonoscopy: a case series of 12 patients. J Clin Gastroenterol. 2010;44:562–6. doi: 10.1097/MCG.0b013e3181dac035. [DOI] [PubMed] [Google Scholar]
- 12.Stranges PM, Hutton DW, Collins CD. Cost-effectiveness analysis evaluating fidaxomicin versus oral vancomycin for the treatment of Clostridium difficile infection in the United States. Value Health. 2013;16:297–304. doi: 10.1016/j.jval.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 13.Wenisch JM, Schmid D, Kuo HW, et al. Hospital-acquired Clostridium difficile infection: determinants for severe disease. Eur J Clin Microbiol Infect Dis. 2012;31:1923–30. doi: 10.1007/s10096-011-1522-5. [DOI] [PubMed] [Google Scholar]
- 14.Hu MY, Maroo S, Kyne L, et al. A prospective study of risk factors and historical trends in metronidazole failure for Clostridium difficile infection. Clin Gastroenterol Hepatol. 2008;6:1354–60. doi: 10.1016/j.cgh.2008.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McFarland LV, Elmer GW, Surawicz CM. Breaking the cycle: treatment strategies for 163 cases of recurrent Clostridium difficile disease. Am J Gastroenterol. 2002;97:1769–75. doi: 10.1111/j.1572-0241.2002.05839.x. [DOI] [PubMed] [Google Scholar]
- 16.Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45:302–7. doi: 10.1086/519265. [DOI] [PubMed] [Google Scholar]
- 17.Cornely OA, Miller MA, Louie TJ, Crook DW, Gorbach SL. Treatment of first recurrence of Clostridium difficile infection: fidaxomicin versus vancomycin. Clin Infect Dis. 2012;55(suppl 2):S154–61. doi: 10.1093/cid/cis462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mullane KM, Miller MA, Weiss K, et al. Efficacy of fidaxomicin versus vancomycin as therapy for Clostridium difficile infection in individuals taking concomitant antibiotics for other concurrent infections. Clin Infect Dis. 2011;53:440–7. doi: 10.1093/cid/cir404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brandt LJ, Aroniadis OC, Mellow M, et al. Long-term follow-up of colonoscopic fecal microbiota transplant for recurrent Clostridium difficile infection. Am J Gastroenterol. 2012;107:1079–87. doi: 10.1038/ajg.2012.60. [DOI] [PubMed] [Google Scholar]
- 20.Postigo R, Kim JH. Colonoscopic versus nasogastric fecal transplantation for the treatment of Clostridium difficile infection: a review and pooled analysis. Infection. 2012;40:643–8. doi: 10.1007/s15010-012-0307-9. [DOI] [PubMed] [Google Scholar]
- 21.Byrn JC, Maun DC, Gingold DS, Baril DT, Ozao JJ, Divino CM. Predictors of mortality after colectomy for fulminant Clostridium difficile colitis. Arch Surg. 2008;143:150–4. doi: 10.1001/archsurg.2007.46. discussion 155. [DOI] [PubMed] [Google Scholar]
- 22.Dallal RM, Harbrecht BG, Boujoukas AJ, et al. Fulminant Clostridium difficile: an underappreciated and increasing cause of death and complications. Ann Surg. 2002;235:363–72. doi: 10.1097/00000658-200203000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stewart DB, Hollenbeak CS, Wilson MZ. Is colectomy for fulminant Clostridium difficile colitis life saving? A systematic review. Colorectal Dis. 2013;15:798–804. doi: 10.1111/codi.12134. [DOI] [PubMed] [Google Scholar]
- 24.Bhangu A, Nepogodiev D, Gupta A, Torrance A, Singh P West Midlands Research Committee. Systematic review and meta-analysis of outcomes following emergency surgery for Clostridium difficile colitis. Br J Surg. 2012;99:1501–13. doi: 10.1002/bjs.8868. [DOI] [PubMed] [Google Scholar]
- 25.Lamontagne F, Labbe AC, Haeck O, et al. Impact of emergency colectomy on survival of patients with fulminant Clostridium difficile colitis during an epidemic caused by a hypervirulent strain. Ann Surg. 2007;245:267–72. doi: 10.1097/01.sla.0000236628.79550.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bakken JS, Borody T, Brandt LJ, et al. Fecal Microbiota Transplantation Workgroup. Treating Clostridium difficile infection with fecal microbiota transplantation. Clin Gastroenterol Hepatol. 2011;9:1044–9. doi: 10.1016/j.cgh.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gold MR, Franks P, McCoy KI, Fryback DG. Toward consistency in cost-utility analyses: using national measures to create condition-specific values. Med Care. 1998;36:778–92. doi: 10.1097/00005650-199806000-00002. [DOI] [PubMed] [Google Scholar]
- 28.Siegel JE, Weinstein MC, Russell LB, Gold MR. Recommendations for reporting cost-effectiveness analyses. Panel on Cost-effectiveness in Health and Medicine. JAMA. 1996;276:1339–41. doi: 10.1001/jama.276.16.1339. [DOI] [PubMed] [Google Scholar]
- 29.Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB. Recommendations of the Panel on Cost-effectiveness in Health and Medicine. JAMA. 1996;276:1253–8. [PubMed] [Google Scholar]
- 30.Agency for Healthcare Research and Quality. Statistics on hospital-based care in the United States. 2009. Available at: www.ahrq.gov . Accessed 10 April 2013. [PubMed]
- 31.Muir AJ, Edwards LJ, Sanders LL, et al. A prospective evaluation of health-related quality of life after ileal pouch anal anastomosis for ulcerative colitis. Am J Gastroenterol. 2001;96:1480–5. doi: 10.1111/j.1572-0241.2001.03801.x. [DOI] [PubMed] [Google Scholar]
- 32.Prenzler A, Yen L, Mittendorf T, von der Schulenburg JM. Cost effectiveness of ulcerative colitis treatment in Germany: a comparison of two oral formulations of mesalazine. BMC Health Serv Res. 2011;11:157. doi: 10.1186/1472-6963-11-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tengs TO, Wallace A. One thousand health-related quality-of-life estimates. Med Care. 2000;38:583–637. doi: 10.1097/00005650-200006000-00004. [DOI] [PubMed] [Google Scholar]
- 34.Tsai HH, Punekar YS, Morris J, Fortun P. A model of the long-term cost effectiveness of scheduled maintenance treatment with infliximab for moderate-to-severe ulcerative colitis. Aliment Pharmacol Ther. 2008;28:1230–9. doi: 10.1111/j.1365-2036.2008.03839.x. [DOI] [PubMed] [Google Scholar]
- 35.Yen EF, Kane SV, Ladabaum U. Cost-effectiveness of 5-aminosalicylic acid therapy for maintenance of remission in ulcerative colitis. Am J Gastroenterol. 2008;103:3094–105. doi: 10.1111/j.1572-0241.2008.02130.x. [DOI] [PubMed] [Google Scholar]
- 36.Grosse SD. Assessing cost-effectiveness in healthcare: history of the $50,000 per QALY threshold. Expert Rev Pharmacoecon Outcomes Res. 2008;8:165–78. doi: 10.1586/14737167.8.2.165. [DOI] [PubMed] [Google Scholar]
- 37.Kassam Z, Hundal R, Marshall JK, Lee CH. Fecal transplant via retention enema for refractory or recurrent Clostridium difficile infection. Arch Intern Med. 2012;172:191–3. doi: 10.1001/archinte.172.2.191. [DOI] [PubMed] [Google Scholar]
- 38.van Nood E, Vrieze A, Nieuwdorp M, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368:407–15. doi: 10.1056/NEJMoa1205037. [DOI] [PubMed] [Google Scholar]
- 39.Hamilton MJ, Weingarden AR, Sadowsky MJ, Khoruts A. Standardized frozen preparation for transplantation of fecal microbiota for recurrent Clostridium difficile infection. Am J Gastroenterol. 2012;107:761–7. doi: 10.1038/ajg.2011.482. [DOI] [PubMed] [Google Scholar]
- 40.Kelly CP. Can we identify patients at high risk of recurrent Clostridium difficile infection? Clin Microbiol Infect. 2012;18(suppl 6):21–7. doi: 10.1111/1469-0691.12046. [DOI] [PubMed] [Google Scholar]
- 41.Gregor JC, McDonald JW, Klar N, et al. An evaluation of utility measurement in Crohn's disease. Inflamm Bowel Dis. 1997;3:265–76. [PubMed] [Google Scholar]
- 42.Thuresson PO, Heeg B, Lescrauwaet B, Sennfalt K, Alaeus A, Neubauer A. Cost-effectiveness of atazanavir/ritonavir compared with lopinavir/ritonavir in treatment-naive human immunodeficiency virus-1 patients in Sweden. Scand J Infect Dis. 2011;43:304–12. doi: 10.3109/00365548.2010.545835. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.