The Antibacterial Resistance Leadership Group (ARLG) is tasked with prioritizing, designing, implementing, and conducting clinical studies to address antibacterial resistance. This article outlines clinical research resources and opportunities made available by ARLG and encourages submission of proposals that address antibacterial resistance.
Keywords: antibacterial resistance, ARLG, Leadership Group, clinical trials, clinical research
Abstract
Funded by the National Institute of Allergy and Infectious Diseases, the Antibacterial Resistance Leadership Group (ARLG) is tasked with developing a clinical research agenda and conducting clinical studies to address the growing public health threat of antibacterial resistance. The ARLG has identified 4 high-priority areas of research: infections caused by gram-negative bacteria, infections caused by gram-positive bacteria, antimicrobial stewardship and infection prevention, and diagnostics. The ARLG will be accepting proposals from the scientific community for clinical research that addresses 1 or more of these high-priority areas. These studies should have the potential to transform medical practice and be unlikely to occur without ARLG support. The purpose of this article is to make interested parties aware of clinical research opportunities made available by ARLG and to encourage submission of clinical research proposals that address the problem of antibacterial resistance.
The Antibacterial Resistance Leadership Group (ARLG) (https://arlg.org) is an initiative funded by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH). Sixty-two million dollars, which will be allocated over a period of 6.5 years, is available to support clinical research in antibacterial resistance. In addition, up to $7.5 million annually of in-kind support will be available for high-resource clinical trials. The ARLG is tasked with developing and prioritizing a clinical research agenda and designing, implementing, and conducting clinical studies to address the growing public health threat of antibacterial resistance. Clinical studies may take place in selected sites, sites affiliated with Vaccine and Treatment Evaluation Units, or sites within Clinical Trials Units (CTUs) of established NIAID networks. The purpose of this article is 2-fold: (1) to make interested parties aware of clinical research resources and opportunities made available by ARLG and other NIAID initiatives; and (2) to encourage submission of clinical research proposals that address the problem of antibacterial resistance.
ARLG RESEARCH AGENDA
Antibacterial resistance is one of the world's most serious health threats. It is multifactorial, complex, and unavoidable, but potentially manageable. Reducing the overall risk of antibacterial resistance requires a sustained program that simultaneously addresses critical issues from several different perspectives.
Long-term goals of the ARLG are to facilitate development of new antibacterials or preventive or treatment strategies for multidrug-resistant gram-negative and gram-positive bacterial infections. The ARLG has identified unmet needs in antibacterial resistance and prioritized 4 areas of research (Table 1): infections caused by gram-negative bacteria, infections caused by gram-positive bacteria, antimicrobial stewardship and infection prevention, and diagnostics. To address specific unmet needs within these priority areas, the ARLG has created an organizational structure that will enable solicitation, review, and evaluation of research proposals from within the group itself and from its affiliated networks, industry, and the greater scientific community (see the section “Application and Review Process” below for more detail). The ARLG, in close collaboration with the NIAID's Division of Microbiology and Infectious Diseases (DMID), will select proposals for funding, protocol development, and study implementation. Study oversight and monitoring will be the joint responsibility of ARLG and DMID.
Table 1.
Antibacterial Resistance Leadership Group Research Priority Areas and Scientific Agenda
Infections caused by gram-negative bacteria
|
Infections caused by gram-positive bacteria
|
Antimicrobial stewardship/infection prevention
|
Diagnostics
|
To optimally address our research agenda, the ARLG will be accepting proposals from the broader scientific community for clinical research, both interventional clinical trials and observational clinical studies. These studies should have the potential to transform medical practice and be unlikely to occur without ARLG support, resources, or network access. Potential projects, for example, could include comparative effectiveness or efficacy trials; trials to optimize use of currently licensed antibacterials (dose, duration, indication) or to test clinical treatment algorithms; treatment-based prevention measures; diagnostic testing to rapidly identify resistant microbes, particularly within the context of treatment trials; observational studies; evaluation of novel infection control or antimicrobial stewardship strategies designed to prevent the development and spread of resistant organisms; and novel institutional-level approaches to prevent the development of resistance. Studies of pharmacokinetics and pharmacodynamics of antibacterials, or studies in selected populations (eg, immunocompromised hosts, patients in long-term-care settings, neonates, overweight or obese children and adults, those at risk of sexually transmitted infection) are encouraged. Project feasibility and cost are major criteria in selection of specific proposals for project development.
ARLG STRUCTURE
Executive Committee
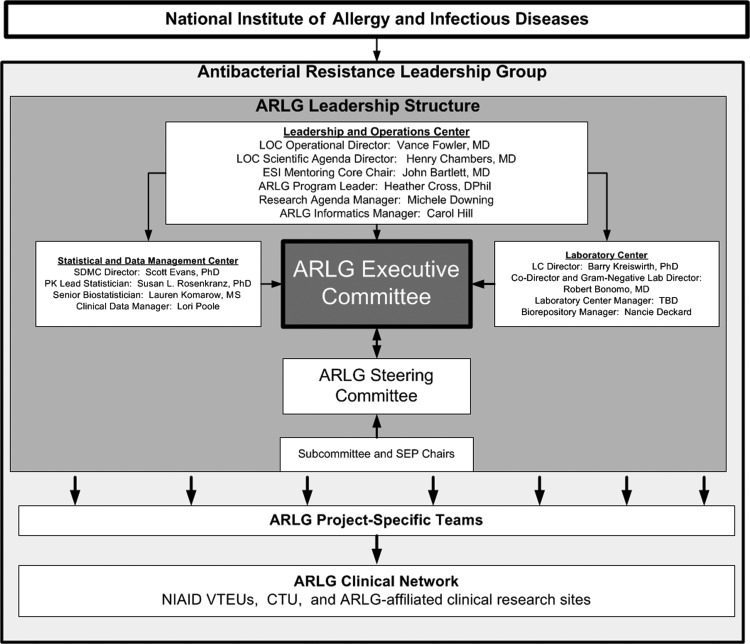
The ARLG Executive Committee (EC) (Table 2) is ultimately responsible for all ARLG activities, including high-level scientific and management decisions, finalizing the research agenda, and approving and funding studies for protocol development and implementation. The EC is charged with ensuring optimal stewardship of precious ARLG resources, including reallocation as needed. It also oversees and coordinates 3 centers: the Leadership and Operations Center (LOC), the Laboratory Center (LC), and the Statistics and Data Management Center (SDMC) (Figure 1).
Table 2.
Antibacterial Resistance Leadership Group Executive Committee Members
| Vance Fowler | PI, Leadership and Operations Center Operations Director, Executive Committee Chair |
| Henry Chambers | PI, Leadership and Operations Center Scientific Agenda Director, Steering Committee Chair |
| Scott Evans | Statistics and Data Management Center Director |
| Barry Kreiswirth | Laboratory Center Director |
| John Bartlett | Early Stage Investigator Mentoring Program Chair |
| Heather Cross | ARLG Program Leader (nonvoting member) |
| Michele Downing | Scientific Agenda Manager (nonvoting member) |
| Jane Knisely | NIAID Program Officer |
| Christine Chiou | NIAID Medical Officer |
| To be determined | Representative of participating sites |
Abbreviations: ARLG, Antibacterial Resistance Leadership Group; NIAID, National Institute of Allergy and Infectious Diseases; PI, Principle Investigator.
Figure 1.
Antibacterial Resistance Leadership Group organizational structure. Abbreviations: ARLG, Antibacterial Resistance Leadership Group; CTU, Clinical Trials Unit; ESI, early-stage investigator; LC, Laboratory Center; LOC, Leadership and Operations Center; NIAID, National Institute of Allergy and Infectious Diseases; PK, Pharmacokinetics; SDMC, Statistics and Data Management Center; SEP, Special Emphasis Panels; TBD, to be determined; VTEUs, Vaccine and Treatment Evaluation Units.
The EC coordinates its activities with DMID through its 2 representatives from NIAID, the program officer and the medical officer. These individuals ensure close communication between the EC and NIAID to advance promising projects and to facilitate access to valuable NIAID support services including Investigational New Drug (IND) sponsorship, data management and statistics support, and clinical site monitoring. The specific clinical support services to be provided are determined in collaboration with DMID staff.
Leadership and Operations Center
The LOC provides leadership and broad oversight of all network activities, including research concept prioritization, operations, protocol development and implementation, and timely publication and communication of results. This role requires coordination with the LC, the SDMC, ARLG-affiliated CTUs and other research sites, and sponsors as appropriate. The LOC is also responsible for defining and implementing network governance and resource distribution policies and procedures.
The LOC also executes the ARLG research agenda. On an individual project level, the LOC provides full support for all stages of project management. For “preaward” support, the LOC provides protocol development, study feasibility assessment, budgeting, and statistical analysis plan development. The LOC also has the capacity to oversee all aspects of study support for prioritized trials, including site monitoring. On the organizational level, the LOC leads the ongoing refinement and revision of the network's research plan, and oversees and regularly evaluates all aspects of the network's operations and performance. Finally, the LOC provides nonprotocol training and mentoring for network staff.
Laboratory Center
The LC leads development, evaluation, and implementation of the ARLG's laboratory-based research agenda and provides laboratory support for study sites and the network. The LC manages and oversees relevant laboratory services, including pharmacokinetic services and any network specimen characterization. The LC provides facilities necessary to accomplish the network's clinical research agenda, laboratory quality management programs, monitoring and evaluation of ARLG specialized laboratories, and sharing of specimens outside the network, as scientifically appropriate. The LC is responsible for oversight of bacterial isolate repositories. Finally, LC leadership fosters collaboration and harmonization of laboratory activities within the network, including clinical research site–affiliated laboratories.
Statistics and Data Management Center
The SDMC ensures leadership and expertise in biostatistics, study design, data monitoring and management, randomization, analyses, interpretation, and publication of results. The SDMC provides statistical support and expertise at virtually every phase of protocol evaluation, design, and development. Its role is to ensure complete, high-quality data by delivering innovative statistical methods in the field of antibacterial resistance and state-of-the-art clinical and laboratory data management systems. The SDMC administers data management training and education for network-affiliated clinical research sites and laboratory staff and investigators, and has a central role in standardizing and harmonizing statistics and data management activities both within the network and with other NIAID-sponsored networks.
Steering Committee
The Steering Committee (SC) facilitates communication between the ARLG leadership, Scientific Subcommittees, and Special Emphasis Panels. It reports to and advises the ARLG EC. The SC has primary responsibility for setting scientific priorities and the research agenda. This includes proposing protocols that advance the ARLG scientific agenda, reviewing research proposals, identifying new developments in the field, assessing medical needs, providing scientific oversight of protocols during design and execution, and prioritizing and reprioritizing studies as needed.
Scientific Subcommittees and Special Emphasis Panels
There are 4 Scientific Subcommittees, each corresponding to 1 of the 4 prioritized research areas: (1) the Gram-Negative Bacteria Subcommittee, (2) the Gram-Positive Bacteria Subcommittee, (3) the Antimicrobial Stewardship and Infection Prevention Subcommittee, and (4) the Diagnostics and Devices Subcommittee. There are 3 Special Emphasis Panels: (1) Special Populations, (2) Pharmacokinetics, and (3) Pediatrics. The purpose of these Special Emphasis Panels is to serve as a cross-cutting resource for the Scientific Subcommittees and the EC. The Scientific Subcommittees and Special Emphasis Panels provide topic-specific expertise and are responsible for reviewing and evaluating research proposals to ensure that high-quality protocols that advance the ARLG scientific agenda are selected.
Performance Assessment
Performance review, evaluation, and assessment are essential components of gauging ARLG success. Performance will be evaluated in 4 key areas: (1) operational performance, (2) engagement with scientific community, (3) scientific productivity, and (4) mentoring. Study progress and network performance are reported monthly to the ARLG EC, which includes DMID representatives, and annually in the Annual Progress Report to NIH. The ARLG will also undergo periodic performance assessments. Metrics include number of studies submitted, reviewed, approved, and funded; time to study implementation; number of studies implemented and completed; number of scientific presentations and publications; and number of early-stage investigators and projects supported.
APPLICATION AND REVIEW PROCESS
Research proposals can originate from any source including the general scientific community, CTUs/sites, industry, or from within ARLG infrastructure. Regardless of origin, all research proposals undergo a standardized process of thorough evaluation for scientific merit, impact, and feasibility. Further information and details about the application and review process can be found on the ARLG website (https://arlg.org). Proposals are reviewed and evaluated primarily on the basis of 4 key questions: (1) Is the proposed research responsive to the scope or mission of the ARLG? (2) Is it scientifically important? (3) Can it be completed on time and within budget? (4) Is this a unique opportunity to conduct research that could not be accomplished by other means?
Standardized forms have been developed to facilitate the submission and review process. The application consists of an online, 1-page concept sheet describing the key elements of the proposal and a 4-page form for supplemental documentation. Research proposals are reviewed by one or more Scientific Subcommittees and Special Emphasis Panels, as appropriate. For example, a study involving gram-negative infections in children would be assigned to the Gram-Negative Subcommittee for primary review and the Pediatric Special Emphasis Panel for secondary review.
There is a mechanism for fast-tracking of proposals that are time-sensitive or deemed to be of particularly high priority. Proposals will be evaluated for significance, innovation, approach, and overall impact and ranked according to the overall impact score. Reviewers’ comments and scores will be reported to and reviewed by the SC, which will make recommendations to the EC as to which projects should undergo further review for feasibility and possible implementation.
Application Types
Applications are of 3 general types: (1) research proposals, (2) early-stage investigator (ESI) seed grants, and (3) the ARLG Fellowship training program.
Research Proposals
Proposals for clinical studies (eg, randomized controlled trials including proposals from industry that may become part of regulatory submissions, case-control studies, cohort studies) to prevent, diagnose, treat, or eradicate antibiotic-resistant bacterial pathogens will be considered responsive to the scientific mission of ARLG. Applications that propose preclinical or nonclinical research, studies of nonbacterial infections, or research not directly related to antibiotic resistance will be considered nonresponsive. Research projects are designated as high resource or low resource. High-resource projects are interventional studies that pose more than minimal risk, involve vulnerable populations (eg, children), or involve administration of a drug or device requiring an IND application or Investigational Device Exemption application. In addition to funding and resources from the ARLG, high-resource studies will receive in-kind support (eg, support for study site personnel, monitoring, regulatory support, and data management services) from DMID. Examples of low-resource projects are observational studies and substudies of ongoing clinical trials. ARLG will provide the funding and resources to support low-resource projects.
ESI Seed Grants
Early-stage investigators (defined as MD, PhD, or PharmD students, graduate or postgraduate trainees, or individuals with a faculty appointment for <5 years) may apply for seed grants of $10 000 and in exceptional cases, up to $50 000. Seed grants provide a mechanism by which ESIs can obtain funds for an antibacterial resistance–related research project.
ARLG Fellowship Training Program
The ARLG Fellowship is a 2-year, fully funded, competitive opportunity to acquire expertise in antibacterial resistance–relevant clinical research. Participants gain both hands-on and formal didactic training by immersion in ARLG projects and operations and classwork leading to a Masters of Health Sciences in Clinical Research or equivalent degree. Initially, this fellowship will be at Duke due to the central location with ARLG operations at the Duke Clinical Research Institute, but later fellowships could be at other institutions, provided that appropriate infrastructure and involvement in day-to-day ARLG clinical trial operations are available. Applications for master's programs are due in spring 2014. The first ARLG Fellow will be selected in early spring 2014 and will start coursework in fall 2014.
OTHER NIAID RESOURCES AND RESEARCH OPPORTUNITIES IN ANTIBACTERIAL RESISTANCE
NIAID/DMID provides a comprehensive suite of resources in support of the ARLG and its network of investigators to facilitate development of products, including those related to antibacterial resistance. These resources are designed to fill research gaps along the product development pipeline and to help translate basic scientific findings into products. This includes diagnostics that can rapidly identify resistant microbes and drugs to treat resistant infections. DMID supports activities from basic research through clinical evaluation and development, and offers needed expertise as well as standardized, high-quality research materials and state-of-the-art technologies to eligible investigators worldwide at no cost (Table 3). For more information on all DMID resources, including staff contacts and how to access the services, please visit www.niaid.nih.gov/labsandresources/resources/dmid.
Table 3.
Division of Microbiology and Infectious Diseases Resources
Research tools and biological materials
|
Preclinical development services
|
| Clinical evaluation services supporting phase 1–4 clinical trials of vaccines, diagnostics, and therapeutics |
In conclusion, the mission of the ARLG is to prioritize, design, and execute clinical research that will reduce the public health threat of antibacterial resistance. To accomplish this will require engagement of the greater community of investigators and stakeholders. We encourage readers to visit the ARLG website (www.https://arlg.org) to learn more.
Notes
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH).
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases (NIAID), NIH (award number UM1-AI104681). R. A. B. was supported in part by the Veterans Affairs Merit Review Program, the National Institutes of Health (grant numbers AI072219-05 and AI063517-07), and the Geriatric Research Education and Clinical Center VISN 10.
Potential conflicts of interest. H. F. C. has received grant support from Cerexa, Genentech, and Cubist; has been a consultant for AstraZeneca, Theravance, and Pfizer; and has stock/stock options in Merck. R. A. B. has received grant support from AstraZeneca, Rib-X, and Merck, and has received honoraria from Cubist and AstraZeneca. S. E. C. has received grant support from Pfizer Independent Grants for Learning and Change and AdvanDx and has been a consultant for Novartis. R. S. D. has been a paid consultant for Pfizer and has received honoraria from Theravance. S. R. E. has been a paid consultant for Merck, Pfizer, Novartis, GSK, Millennium/Takeda, Roche, Cubist, MGH, Bohringer-Ingelheim, IMMPACT, Affymax, FzioMed, Alcon, Achaogen, Huntington's Study Group, Taris, Boston University, and PPRECISE; and has received honoraria or travel expenses from the American Statistical Association (ASA), Society for Clinical Trials (SCT), Food and Drug Administration (FDA), National Institutes of Health (NIH), Muscle Study Group (MSG), Drug Information Association (DIA), HIV/AIDS Network Coordination (HANC), HIV Neurobehavioral Research Center (HNRC), Harvard Medical School, Johns Hopkins University, Osaka University, and the National Cardiovascular Center of Japan. B. N. K. has 2 issued patents: System and Method for Tracking and Controlling Infections (patent number 7349808 and patent number 8046172). The patents describe the use of genotyping DNA repeat sequences to subspeciate bacterial isolates and provide discriminating data to track nosocomial infections in support of infection control measures. B. N. K. is also a consultant for Pfizer and Abbott and has grant support from Cepheid, MedImmune, and Merck. R. P. has received grant or reagent support from Pfizer, Astellas, Pocared, Tornier, Pradama, nanoMR, Curetis, BioFire, Bruker, bioMérieux, Nanosphere, and Abbott; has served or may serve as a consultant to Thermo Fisher Scientific and St Jude; has served on the IDWeek Program Planning Committee; serves on the ICAAC Program Planning Committee and NBME Committees; is an associate editor for the Journal of Clinical Microbiology; and holds patents on an antibiofilm substance, a method for biofilm removal (she has foregone her right to receive royalties in the event that this patent is licensed) and pertussis/parapertussis polymerase chain reaction. K. A. R. has received grant support for investigator-initiated research studies from Forest Laboratories and Theravance Inc; has served as a consultant to Cubist Pharmaceuticals, Durata Therapeutics Inc, Rempex Pharmaceuticals Company, Achoagen, GlaxoSmithKline, Theravance Inc, and Zavante Therapeutics; and is on the speakers’ bureaus of Forest Laboratories, Pfizer Inc, Cubist Pharmaceuticals, and Trius Inc. B. S.'s institution has received research grants on his behalf from Pfizer and Bristol-Myers Squibb, and consulting fees on his behalf from GlaxoSmithKline, Meiji, Adenium, aRigen, Cardeas, and Synthetic Biologics. V. G. F. served as Chair of the V710 Scientific Advisory Committee (Merck), has received grant support from Cerexa, Pfizer, Advanced Liquid Logic, and MedImmune; has been a paid consultant for Merck, Astellas, Affinium, Theravance, Cubist, Cerexa, Durata, Pfizer, NovaDigm, Novartis, Medicines Company, Biosynexus, MedImmune, and Inimex; and has received honoraria from Merck, Astellas, Cubist, Pfizer, Theravance, and Novartis. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.