There was no significant difference in the efficacy of daptomycin in patients with impaired renal function in methicillin-resistant Staphylococcus aureus bloodstream infection compared with vancomycin using a propensity-matched retrospective analysis.
Keywords: methicillin-resistant Staphylococcus aureus (MRSA), bloodstream infection, vancomycin, daptomycin, renal failure
Abstract
Background. Concerns regarding the efficacy of daptomycin for methicillin-resistant Staphylococcus aureus (MRSA) bloodstream infections in patients with impaired renal function are reflected in a recent package insert change by the Food and Drug Administration (FDA). However, this decision was based on a small subgroup analysis and it is unclear if this is a true association.
Methods. We conducted a retrospective cohort study of patients with MRSA bacteremia treated at a tertiary hospital from 2001 to 2011 and who received either vancomycin or daptomycin. We used propensity score and multivariable logistic regression to assess the outcome of treatment failure, via blinded adjudication, in daptomycin- vs vancomycin-treated subjects and the interaction with renal function.
Results. One hundred fifty patients were analyzed, 100 in the vancomycin arm and 50 in the daptomycin arm. The average age was 61 years, and 60% were men. Of patients treated with daptomycin or vancomycin, 29 (58%) and 51 (51%), respectively, had an estimated glomerular filtration rate (GFR) <50 mL/minute/1.73 m2. Compared with vancomycin, the usage of daptomycin in patients was not significantly associated with treatment failure in patients with a GFR >50 mL/minute/1.73 m2 (odds ratio [OR], 0.45; 95% confidence interval [CI], .11 -1.79), nor in patients with a GFR of <50 mL/minute/1.73 m2 (OR, 0.46; 95% CI, .11 -1.94). There was no significant interaction between them (P = .54).
Conclusions. In patients with MRSA bacteremia, daptomycin efficacy was not affected by GFR level and was similar to vancomycin's efficacy. Although our sample size was small, it was larger than than the one used by the FDA. However, smaller differences may be significant with a larger sample size.
For many years, vancomycin has been the mainstay of methicillin-resistant Staphylococcus aureus (MRSA) bloodstream infection (BSI) treatment. However, recent studies have suggested that daptomycin may be a preferred alternative to vancomycin, particularly in cases of vancomycin clinical failure with elevated vancomycin minimal inhibitory concentrations (MICs) [1–3]. There are some concerns with the use of daptomycin, however. In November 2010, the Food and Drug Administration (FDA) made changes to the package insert of daptomycin warning physicians of a possible decrease in efficacy of daptomycin in patients with moderate renal impairment [4]. This was based on a subgroup analysis of the original phase 3 MRSA bloodstream infection trial data showing a marked difference in clinical success 6 weeks after the last dose of antibiotics, in patients with a creatinine clearance (CrCl) of <50 mL/minute. The daptomycin-treated patients had a 14% (2/14) success rate compared with a 41% (7/17) success rate in vancomycin-treated patients [5].
However, this subgroup analysis had only a small number of total patients with a CrCl <50 mL/minute. Furthermore, this post hoc analysis excluded patients with a CrCl <30 mL/minute. This has raised concerns among physicians as to whether there is truly a difference in efficacy in these patients or if this is the result of multiple subgroup analyses and random chance, particularly as the physiologic mechanism of daptomycin's poor performance in patients with impaired renal function is unclear. As the data suggesting improved outcomes using daptomycin in patients with an elevated minimum inhibitory concentration (MIC) of S. aureus to vancomycin increase, this clinical question becomes increasingly relevant.
We performed a retrospective analysis of patients with a broad range of renal functions receiving either daptomycin or vancomycin to determine the effect of the interaction between the usage of daptomycin and impaired renal function on treatment failure (ie, whether the effect of daptomycin varies with renal function compared with vancomycin). This was done in a real-world setting, more accurately reflecting the current usage of these drugs in a broader community setting. We studied a larger number of patients and used propensity score matching to account for potential bias by indication for treatment.
METHODS
Patient Selection
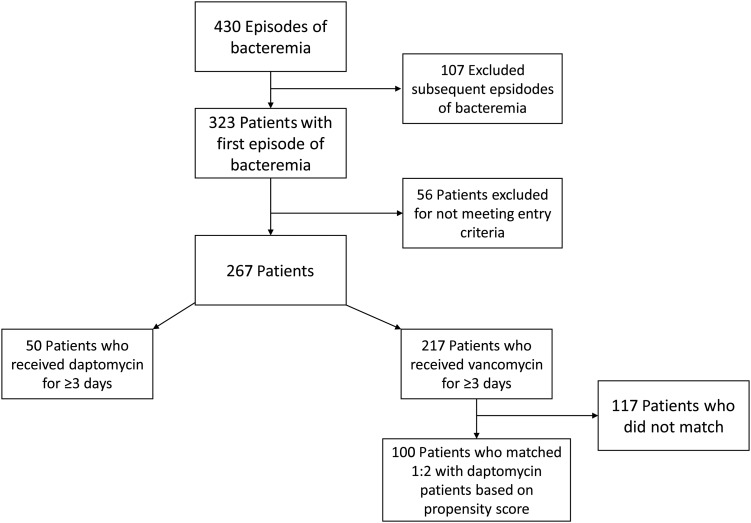
This was a retrospective cohort study using a previously established database of all patients who developed MRSA bacteremia at a single tertiary care hospital between January 2001 and August 2011. To be included in the study, patients must have been aged >18 years and have had at least 1 positive blood culture for MRSA. Only first episodes of MRSA BSI were evaluated. Patients must have received either vancomycin or daptomycin for at least 3 consecutive days to be included in the current analysis. Most patients in our clinical setting were switched to daptomycin from vancomycin for a variety of clinical reasons. To be included in our study, patients in the daptomycin group may have received vancomycin for up to 10 days prior to this switch. Alternatively, patients could have received >10 days of vancomycin prior to the switch as long as the ratio of total daptomycin-treated days to days of prior antibiotics remained ≥2, indicating that the majority of their treatment was done with daptomycin. Patients were excluded if there was any intravascular foreign material not removed within 4 days of the first positive blood culture, if the patient had a polymicrobial bloodstream infection, or if pneumonia was likely the source of the BSI (Figure 1). Renal function was assessed on the first day of bacteremia, using the Modification of Diet in Renal Disease (MDRD) method for calculating the glomerular filtration rate (GFR). Daptomycin was always dosed at a goal of ≥6 mg/kg based on actual body weight. The decision to use doses >6 mg/kg was up to the treating physician. Vancomycin was dosed for a goal trough level of 10–20 µg/mL until 2009 when this was changed to 15–20 µg/mL and subsequently monitored by clinical pharmacists. All patients at our institution since 2007 with S. aureus BSI receive a mandatory infectious disease consultation done by a rotation of approximately 25 physicians.
Figure 1.
Patient selection flow diagram illustrating the patient selection process.
Statistical Analysis
Daptomycin-treated patients were matched with vancomycin-treated patients in a 1:2 ratio using a propensity score and an optimal matching algorithm. After excluding subsequent episodes of BSI, and patients who did not meet our inclusion criteria, a propensity score was built on a data set of 267 patients to predict the use of daptomycin using baseline variables, obtained via an initial brief medical record review, present prior to the initiation of any antibiotics on the first day of positive blood cultures (Figure 1). Variables included in the propensity score were age, sex, race, APACHE II score, Charlson comorbidity index, functional status of the patient, and the presence of a vancomycin MIC of 2 mg/L by broth microdilution. In addition, as the total days of bacteremia was a very strong predictor of receiving daptomycin, the days of positive blood cultures prior to a possible switch to daptomycin, in those patients who were switched, was also included. Within the vancomycin group, this was defined as the total days of positive blood cultures as there was no switch in this group. This resulted in 50 patients treated with daptomycin matched to 100 patients treated with vancomycin. We determined that we would have a power of 83% to detect an interaction difference (ie, a variation in the effect of daptomycin compared with vancomycin in patients with a GFR both greater than and less than 50 mL/minute/1.73 m2) as large as that seen in original trial data [3, 4]. Renal impairment was evaluated using a categorical variable dividing the GFR into those less or greater than 50 mL/minute/1.73 m2. This was done after evaluating the nonlinear relationship of GFR to treatment failure with a natural inflection point at 50 mL/minute/1.73 m2.
After matching, additional chart review was performed to gather more detailed comorbidity information. Using this additional information, further adjustment was performed using multivariable conditional logistic regression to evaluate for the main effects of daptomycin compared with vancomycin in those patients with a GFR greater than or less than 50 mL/minute/1.73 m2 as well as the interaction between them.
The multivariable model was constructed using significant (P < .05) results from a univariable analysis of multiple factors and their association with treatment failure. These variables included the presence of endocarditis, liver disease (defined as a Child-Pugh class B or greater), the risk level of the source of infection, and year of treatment to account for variation in clinical treatment over time. In addition, the total number of days of MRSA-active antibiotics (including vancomycin) prior to a switch to daptomycin was forced into the model. This variable was defined as 0 in the vancomycin group. The source of BSI was classified into 3 categories, as previously described: low risk (associated mortality rate <10%), which included urinary tract, ear-nose-larynx, gynecologic, and several manipulation-related sources; intermediate risk (associated mortality rate 10%–20%), which included central line–associated, osteoarticular, soft tissue, and unknown sources; and high risk (associated mortality rate >20%), which included endovascular, abdominal, and central nervous system sources [1]. Sensitivity analyses of those patients in whom recurrence data were not available were performed assuming both a similar rate of recurrence in this group as the remaining patients in that group, and assuming that every nonevaluable patient was a recurrence did not significantly change our results. All statistical analyses were performed with the R statistical software package (version 2.13.1)
Outcome Assessment
Treatment failure was a composite endpoint consisting of in-hospital mortality, recurrence of MRSA BSI within 30 days of cessation of antibiotic therapy, or persistent bacteremia ≥5 days after the start of drug of interest (vancomycin or daptomycin, respectively). This outcome was independently assessed by 2 blinded adjudicators with disagreements settled by consensus. Each component of the composite endpoint was assessed individually as well. Tufts University institutional review board approval was obtained for this study.
RESULTS
Study Population Characteristics
The average age of study patients was 61 years, and 60% of the subjects were men. There were no statistically significant differences between comparison groups on variables that were included in the propensity score (Table 1). There was a significantly greater proportion of patients with a hematologic malignancy and recent prior surgery in the vancomycin group, whereas patients in the daptomycin group had a larger proportion of low- and high-risk sources of infection. There was no significant difference in renal function between the 2 groups, with 29 patients (58%) in the daptomycin group having a GFR of <50 mL/minute/1.73 m2 vs 51 patients (51%) in the vancomycin group. There was also little variation in renal function throughout admission with an average change in creatinine from the first day of bacteremia to discharge of −0.37 mg/dL.
Table 1.
Clinical Characteristics of the Comparison Groups After Matching
| Characteristic | Daptomycin (n = 50) | Vancomycin (n = 100) | P Value |
|---|---|---|---|
| Variables used in the propensity score | |||
| Mean age, y | 58.34 | 61.64 | .27 |
| Male sex | 28 (56) | 62 (62) | .48 |
| Race | |||
| White | 36 (72) | 78 (78) | .42 |
| Black | 8 (16) | 10 (10) | .29 |
| Asian | 4 (8) | 10 (10) | .69 |
| Hispanic | 2 (4) | 2 (2) | .47 |
| Mean APACHE II score | 13.22 | 13.98 | .55 |
| Mean Charlson comorbidity index | 5.76 | 6.13 | .48 |
| Vancomycin MIC ≥2 mg/L (by broth microdilution) | 10 (20) | 22 (22) | .78 |
| Functional status | |||
| Independent | 17 (34) | 30 (30) | .62 |
| Partly dependent | 20 (40) | 40 (40) | 1 |
| Fully dependent | 13 (26) | 30 (30) | .61 |
| Total days of positive blood cultures prior to switch | 5 (±5.23) | 3.68 (±4.95) | .14 |
| Variables collected via chart review after matching | |||
| Liver disease (Child-Pugh B or greater) | 10 (20) | 20 (20) | 1 |
| Diabetes (type 1 or type 2) | 23 (46) | 36 (36) | .24 |
| Congestive heart failure (any stage) | 16 (32) | 25 (25) | .37 |
| Coronary artery disease | 16 (32) | 25 (39) | .4 |
| Solid malignancy | 2 (4) | 13 (13) | .083 |
| Hematologic malignancy | 2 (4) | 15 (15) | .045 |
| Chornic obstructive pulmonary disease | 8 (16) | 17 (17) | .88 |
| Prior surgery within 30 d | 1 (2) | 21 (21) | .002 |
| History of stroke | 6 (12) | 9 (9) | .56 |
| Endocarditis | 13 (26) | 11 (11) | .018 |
| Risk level of source | |||
| Low | 13 (26) | 6 (6) | <.001 |
| Intermediate | 17 (34) | 72 (72) | <.001 |
| High | 20 (40) | 22 (22) | .021 |
| Immunosuppression | 10 (20) | 20 (20) | 1 |
| HIV infection | 3 (6) | 3 (3) | .38 |
| Hemodialysis | 11 (22) | 19 (19) | .67 |
| Prior history of MRSA infection (not bloodstream) | 26 (52) | 42 (42) | .25 |
| Average GFR | 53.8 (±37.4) | 60.5 (±44.6) | .31 |
| GFR <50 mL/min/1.73 m2 | 27 (58) | 51 (51) | .42 |
| Creatinine clearance <30 mL/min | 12 (24) | 25 (25) | .99 |
| Outcomes | |||
| Failure composite outcome | 17 (34) | 51 (51) | .048 |
| In-hospital mortality | 8 (16) | 35 (35) | .015 |
| Persistent bacteremia | 7 (14) | 21 (21) | .3 |
| Recurrence | 6 (12) | 5 (5) | .12 |
Data are presented as No. (%) unless otherwise specified. The upper variables represent those baseline variables available and used in the construction of the propensity score acquired after an initial brief records review. The lower variables were collected after matching via additional records review. For the variable of “total days of positive blood cultures prior to a switch,” the total days of positive blood cultures was used for those patients in the vancomycin group.
Abbreviations: GFR, glomerular filtration rate; HIV, human immunodeficiency virus; MIC, minimum inhibitory concentration; MRSA, methicillin-resistant Staphylococcus aureus.
Antibiotic Usage and Safety
Patients in the vancomycin group received vancomycin for an average of 21 days with a median first vancomycin level of 15.3 µg/mL (range, 8.2–25.6) drawn after a mean of 2 days. Patients in the daptomycin group received an average of 28 days of daptomycin therapy after a mean of 7 days of prior therapy with a range of 0–21 days. The average dose of daptomycin was 6.8 mg/kg (range, 5.1–10.8 mg/kg). All daptomycin patients with a CrCl of <30 mL/minute (n = 37) received the drug at least every 48 hours as recommended by the manufacturer, including some patients on hemodialysis. Some patients (n = 12) on hemodialysis received it at every dialysis session. Only 2 (4%) of the patients received daptomycin as primary initial therapy. Most of the patients previously received vancomycin (82%), whereas 12% received linezolid and 4% received clindamycin. The most common reasons for switching to daptomycin were persistently positive blood cultures (13/50 [26%]), a decision made by the infectious disease consultants (11/50 [22%]), clinical failure in the opinion of the treating physician (7/50 [14%]), and unknown (6/50 [12%]). In the daptomycin group, only 2 patients (4%) had creatinine phosphokinase elevations >1000 IU/L while on daptomycin, necessitating cessation of the drug. These 2 patients were receiving doses of 5.7 mg/kg and 8 mg/kg.
Outcome Descriptions
For the outcome of recurrence, 19% of the patients in vancomycin group and 14% of the patients in the daptomycin group were lost to follow-up at discharge and could not be evaluated for recurrence of MRSA BSI. The unadjusted data show a significantly higher rate of failure and mortality in the vancomycin group and a suggestion of increased recurrence in the daptomycin group (Table 1).
Associations With Failure
Univariable analysis showed several factors associated with treatment failure that were then used in the multivariable model (Table 2). These included endocarditis, liver disease, the risk level of the source of infection, total days of MRSA-active antibiotics given prior to a switch to daptomycin (forced into the model), and year of treatment (accounting for change in clinical care and antibiotic usage over time). In our multivariable model, compared with vancomycin, neither the usage of daptomycin in patients with a GFR >50 mL/minute/1.73 m2 (odds ratio [OR], 0.45; 95% confidence interval [CI], .11–1.79) nor in patients with a GFR of <50 mL/minute/1.73 m2 (OR, 0.46; 95%CI, .11–1.94) was significantly associated with treatment failure (Table 3). Additionally, there was no significant interaction between them (P = .54), indicating that any differences in treatment effect were not significant. Similar nonsignificant results were seen for the individual outcomes of mortality, persistent bacteremia, and recurrence (Table 3). Within this same multivariable model, significant liver disease (OR, 4.14; P = .005) and the highest risk source of infection (OR 5.11; P = .028) remained significant predictors of failure, and the presence of endocarditis was nearly a significant predictor of failure (OR, 3.45; P = .08).
Table 2.
Univariate Analysis of Factors Associated With Treatment Failure
| Factor | OR | P Value |
|---|---|---|
| Daptomycin usage | 0.49 | .05 |
| GFR <50 mL/min/1.73 m2 | 1.34 | .37 |
| Coronary artery disease | 1.35 | .38 |
| Central line or devices present | 1.73 | .1 |
| Congestive heart failure | 1.06 | .88 |
| Chronic obstructive pulmonary disease | 1.67 | .24 |
| Days of MRSA-active antibiotics given prior to daptomycin | 1.04 | .37 |
| Days prior to any MRSA active antibiotic given | 0.93 | .64 |
| Diabetes | 0.78 | .46 |
| Endocarditis | 4.56 | .003 |
| Hemodialysis | 0.84 | .67 |
| Hematologic malignancy | 1.85 | .24 |
| HIV | 0.59 | .55 |
| History of stroke | 1.43 | .51 |
| History of any MRSA infection | 1.34 | .37 |
| History of MRSA bacteremia | 1.12 | .77 |
| Immunosuppression | 1.37 | .44 |
| Liver disease | 3.00 | .011 |
| Prior MRSA active antibiotic (overall) | .34 | |
| Vancomycin | 0.41 | .54 |
| Clindamycin | 1.57 | .99 |
| Linezolid | 0.20 | .37 |
| Prior surgery | 0.81 | .65 |
| Reason for switch to daptomycin (overall) | .065 | |
| Risk level of source (overall) | <.001 | |
| Solid malignancy | 0.30 | .07 |
| Time to vancomycin level | 0.99 | .94 |
| Vancomycin level | 1.01 | .77 |
| Year treated | 1.27 | .002 |
Abbreviations: GFR, glomerular filtration rate; HIV, human immunodeficiency virus; MRSA, methicillin-resistant Staphylococcus aureus; OR, odds ratio.
Table 3.
Results of the Multivariable Analysis
| Outcome | Daptomycin and GFR >50 mL/min/1.73 m2 (95% CI) | Daptomycin and GFR <50 mL/min/1.73 m2 (95% CI) | Test of Interaction |
|---|---|---|---|
| Failure | 0.45 (.11–1.79) | 0.46 (.11–1.94) | P = .54 |
| Mortality | 0.57 (.11–3.1) | 0.54 (.09–3.44) | P = .21 |
| Persistent bacteremia | 0.29 (.03–2.24) | 0.29 (.03–2.6) | P = .99 |
| Recurrence | 3.93 (.43–37.26) | 3.87 (.41–38.19) | P = .4 |
Analysis shows, compared to vancomycin, the association with treatment failure, mortality, persistent bacteremia, and recurrence with daptomycin usage in patients with a GFR >50 mL/min/1.73 m2 and <50 mL/min/1.73 m2 and the interaction between them. The nonsignificant test of interaction (P > .05) indicates that the effect of daptomycin on a particular outcome did not significantly differ between patients with a GFR greater than or less than 50 mL/min/1.73 m2. Additional variables adjusted for as described in results.
Abbreviations: CI, confidence interval; GFR, glomerular filtration rate.
Additional Analyses
Further evaluation of the effect of renal function was undertaken by stratifying renal function by the Kidney Disease Outcome Quality Initiative (KDOQI) stages of chronic kidney disease. In this model, neither daptomycin usage (P = .84) nor any stage of chronic kidney disease (P > .33 for all) was significantly associated with failure. Furthermore, there was no significant interaction between any stage of kidney disease and daptomycin usage (P > .28 for all).
In examining the rate of recurrence, 19% of the patients in the vancomycin group and 14% of the patients in the daptomycin group were lost to follow-up at discharge and could not be evaluated for recurrence of MRSA BSI. Sensitivity analyses assuming both a similar rate of failure in this group as the remaining patients in that group and assuming that every nonevaluable patient was a recurrence did not significantly change our results. Additionally, an analysis was performed defining persistent bacteremia as ≥5 days of positive blood cultures from the start of any therapy. This analysis was also not significantly different from our primary results. To account for a possible bias in the outcome of persistent bacteremia, as a component of the number of days of positive blood cultures was used in the propensity score, we performed an additional analysis using an alternative definition of failure that did not include the outcome of persistent bacteremia. This analysis was also not significantly different from our primary results.
DISCUSSION
In this study, we evaluated if there was a significant variation in the effect of daptomycin with renal function. We were unable to detect a significant interaction. We feel that our data should somewhat reassure clinicians if they choose to use daptomycin to treat MRSA BSI in patients with renal impairment.
Our results may differ from the trial data for a number of reasons. We had a greater number of patients in our evaluation, including more than double the number of patients with a GFR of <50 mL/minute/1.73 m2, granting increased power. The original data were from a post hoc subgroup analysis within the clinical trial, with a small number of patients, and possible significant clinical or comorbidity differences between the subgroups. We accounted for differences in our population in multiple ways, including propensity score matching and further multivariable regression. Our patient population also more likely reflects the current usage patterns of daptomycin and vancomycin in a real-world setting. Additional advantages of our study include a blinded assessment of outcome and the evaluation across a wide array of renal functions.
We used the MDRD equation to calculate renal function as opposed to the Cockcroft-Gault CrCl more commonly used for drug dosage calculations. We chose to use GFR, as this may be a more accurate representation of a patient's renal function compared with the Cockcroft-Gault creatinine clearance [6]. This method is also likely more readily available to clinicians, often being automatically calculated in many electronic medical records. Furthermore, there was no association or interaction with the usage of daptomycin across a range of renal function, suggesting a lack of a relationship with renal impairment regardless of how it was calculated.
However, there are some limitations to this study. Due to the retrospective nature of our study, we may not have been able to control for all measured and unmeasured confounders that may affect this association. Residual confounding that differentially affects strata defined by renal function could mask true effect modification. Arguing against this is that our model identifies liver disease, a high-risk source of infection, and endocarditis as predictors of failure, similar to prior literature [7, 8]. This indicates that our model at least accounts for several factors associated with failure, as others have seen. We also attempted to account for a possible bias in favor of daptomycin using an alternative definition of persistent bacteremia that “counted” days of positive blood cultures prior to a switch to daptomycin as well.
There may be bias in the outcome of persistent bacteremia because a component of the number of positive blood cultures was used in the propensity score. This was done as the number of positive blood cultures was the strongest indicator for the usage of daptomycin in our analysis and we also felt clinically that it was a strong predictor of use. However, this may have minimized the differences seen in the outcome of persistent bacteremia, making the odds ratio for failure closer to 1 for this outcome. To account for this, we performed an additional analysis removing persistent bacteremia from our composite failure outcome.
We also performed a limited evaluation of the safety aspects of each drug. Given the complexity of assessing for renal failure attributable to vancomycin and the fact that this outcome was not central to our clinical question of efficacy, we elected to not evaluate vancomycin-related nephrotoxicity. In addition, we did not control for the concurrent use of non-MRSA active β-lactam drugs, as some data have suggested a potential benefit to this practice. However, only 3 patients in our study received significant (>3 days) concurrent β-lactam therapy with a MRSA-active antibiotic and all those patients received <10 days of concurrent therapy.
Finally, despite having a greater number of patients than in the original study, the power of this study to detect difference smaller than the original phase 3 subgroup data in the interaction between daptomycin and renal impairment is limited. We were unable to find significant effect modification of renal function on the impact of daptomycin. However, this study includes every patient treated with daptomycin for a substantial period of at least 8 years at a large tertiary hospital. If a treatment difference exists between daptomycin and vancomycin in patients with impaired renal function, it is likely small and certainly smaller than the large difference seen in the original data that caused the FDA's change in the package insert.
As the usage of daptomycin for MRSA BSI and the prevalence of kidney disease continue to increase, physicians may be reassured by this data. Whereas data from a randomized, controlled comparative efficacy trial would be ideal to definitively answer this question, a proposed trial by Cubist (ClinicalTrials.gov, NCT01104662) was terminated, possibly due to difficulties with recruitment, early in June 2012 after recruiting only 92 subjects. With our data, we were unable to detect that renal impairment of any kind has a significant impact on the efficacy of daptomycin in the treatment of MRSA bacteremia. Although this is the first study to evaluate this question, further nonrandomized prospective or larger retrospective studies may be needed to provide additional reassurance to clinicians.
Notes
Acknowledgments. The authors thank Dr Jennifer Chow and Dr Cheleste Thorpe for their assistance in outcome adjudication and Dr John Griffith for additional statistical assistance.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH).
Financial support. This work was supported by the National Center for Research Resources (grant number UL1 RR025752) and the National Center for Advancing Translational Sciences, NIH (grant number UL1 TR000073). A. W. is supported by the NIH (2T32AI055412-06).
Potential conflicts of interest. Y. G. has received payment for consulting and speaking from Cubist. D. R. S. consults for CSL Behring, Genetech, Millenium, Genzyme, Boeringer Ingelheim, Massachusetts Biologic Public Health Laboratories, Merck, Microbiotix, and Seres Health. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Moore CL, Osaki-Kiyan P, Haque NZ, Perri MB, Donabedian S, Zervos MJ. Daptomycin versus vancomycin for bloodstream infections due to methicillin-resistant Staphylococcus aureus with a high vancomycin minimum inhibitory concentration: a case-control study. Clin Infect Dis. 2012;54:51. doi: 10.1093/cid/cir764. [DOI] [PubMed] [Google Scholar]
- 2.Murray KP, Zhao JJ, Davis SL, et al. Early use of daptomycin versus vancomycin for methicillin-resistant Staphylococcus aureus bacteremia with vancomycin minimum inhibitory concentration >1 mg/L: a matched cohort study. Clin Infect Dis. 2013;56:1562–9. doi: 10.1093/cid/cit112. [DOI] [PubMed] [Google Scholar]
- 3.Sakoulas G, Brown J, Lamp KC, et al. Clinical outcomes of patients receiving daptomycin for the treatment of Staphylococcus aureus infections and assessment of clinical factors for daptomycin failure: a retrospective cohort study utilizing the Cubicin Outcomes Registry and Experience. Clin Ther. 2009;31:1936–45. doi: 10.1016/j.clinthera.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 4.Cubicin (daptomycin) package insert, 30 November 2010. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/021572s022s023s024s027s030s032lbl.pdf. Accessed 15 August 2011.
- 5.FDA label and approval history, 30 November 2010. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/appletter/2010/021572s022,s023,s024,s027,s030,s032ltr.pdf. Accessed 15 August 2011.
- 6.Teruel Briones JL, Gomis Couto A, Sabater J, et al. Validation of the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation in advanced chronic renal failure. Nefrologia. 2011;31:677. doi: 10.3265/Nefrologia.pre2011.Sep.11014. [DOI] [PubMed] [Google Scholar]
- 7.Walraven CJ, North MS, Marr-Lyon L, Deming P, Sakoulas G, Mercier RC. Site of infection rather than vancomycin MIC predicts vancomycin treatment failure in methicillin-resistant Staphylococcus aureus bacteraemia. J Antimicrob Chemother. 2011;66:2386. doi: 10.1093/jac/dkr301. [DOI] [PubMed] [Google Scholar]
- 8.Pastagia M, Kleinman LC, Lacerda de la Cruz EG, Jenkins SG. Predicting risk for death from MRSA bacteremia. Emerg Infect Dis. 2012;18:1072. doi: 10.3201/eid1807.101371. [DOI] [PMC free article] [PubMed] [Google Scholar]