Among all cases of Enterobacter bacteremia at 2 academic hospitals over 6 years, we found 100% efficacy of single-agent cefepime at clearing bacteremia ≤1 day and similar rates of in-hospital mortality compared with carbapenems.
Keywords: Enterobacter, ampC, bacteremia, cefepime, comparative effectiveness
Abstract
Background. Carbapenems are recommended for treatment of Enterobacter infections with AmpC phenotypes. Although isolates are typically susceptible to cefepime in vitro, there are few data supporting its clinical efficacy.
Methods. We reviewed all cases of Enterobacter species bacteremia at 2 academic hospitals from 2005 to 2011. Outcomes of interest were (1) persistent bacteremia ≥1 calendar day and (2) in-hospital mortality. We fit logistic regression models, adjusting for clinical risk factors and Pitt bacteremia score and performed propensity score analyses to compare the efficacy of cefepime and carbapenems.
Results. Three hundred sixty-eight patients experienced Enterobacter species bacteremia and received at least 1 antimicrobial agent, of whom 52 (14%) died during hospitalization. Median age was 59 years; 19% were neutropenic, and 22% were in an intensive care unit on the day of bacteremia. Twenty-nine (11%) patients had persistent bacteremia for ≥1 day after antibacterial initiation. None of the 36 patients who received single-agent cefepime (0%) had persistent bacteremia, as opposed to 4 of 16 (25%) of those who received single-agent carbapenem (P < .01). In multivariable models, there was no association between carbapenem use and persistent bacteremia (adjusted odds ratio [aOR], 1.52; 95% CI, .58–3.98; P = .39), and a nonsignificant lower odds ratio with cefepime use (aOR, 0.52; 95% CI, .19–1.40; P = .19). In-hospital mortality was similar for use of cefepime and carbapenems in adjusted regression models and propensity-score matched analyses.
Conclusions. Cefepime has a similar efficacy as carbapenems for the treatment of Enterobacter species bacteremia. Its use should be further explored as a carbapenem-sparing agent in this clinical scenario.
Antimicrobial resistance among Gram-negative bacilli (GNR) is a growing worldwide public health threat [1–4]. Enterobacter spp. infections are particularly problematic because these pathogens can harbor chromosomally encoded AmpC-type β-lactamases, which confer resistance to most β-lactamase inhibitors and can develop resistance during therapy through enzyme induction and stable derepression [5–8]. Not unexpectedly, the resultant delays in initiation of active antimicrobial therapy in these infections are associated with substantially increased mortality [9]. Because of these concerns, many experts have recommended carbapenems as the preferred treatment for resistant GNR bloodstream infections [4, 10, 11]. On the other hand, there has been an increased awareness that these bacteria may also exhibit resistance to carbapenems, either directly from carbapenemase production or through combinations of other β-lactamases with outer membrane porin changes [12–15], making empirical and isolate-specific choices difficult in daily practice.
Cefepime is a poor inducer of and relatively more stable to the AmpC β-lactamase [6]. Moreover, because of its zwitteronic structure, cefepime rapidly passes through bacterial cell membranes, thereby enhancing access to its enzymatic target [16]. In vitro data suggest that cefepime, unlike other cephalosporins, maintains activity against AmpC-producing isolates [17]. A recent study using phenotypic testing to identify AmpC β-lactamases found 96% of AmpC-producing isolates were susceptible to cefepime in vitro [18]. Moreover, surveillance data suggest that 80%–90% of Enterobacter spp. bloodstream infections are susceptible to cefepime (based on European Committee on Antimicrobial Susceptibility Testing threshold of a cefepime minimum inhibitory concentration [MIC] of ≤1 µg/mL for susceptible isolates), a rate similar to or higher than that of many Enterobacteriaceae [19], and that cefepime maintains activity for ceftazidime-resistant Enterobacter spp. isolates [20].
Yet, human studies of the comparative efficacy of cefepime vs carbapenems for resistant GNR bacteremia have shown contrasting results [21–24]. Notably, a recent study of patients with extended-spectrum β-lactamase (ESBL)–producing bacteria found higher failure and mortality for patients receiving cefepime vs carbapenems, especially among those who had isolates with MICs at the higher end of the susceptible range (eg, 4 or 8 µg/mL), although the number of patients who received cefepime was small (n = 17) [22]. In contrast, a recent study found no differences in mortality or length of hospital stay among patients receiving cefepime or carbapenems specifically for AmpC-producing β-lactamase enteric GNR blood stream infections [18]. There is a relative paucity of data on the efficacy of cefepime specifically for Enterobacter spp. infections, which usually harbor AmpC and variably harbor plasmid-encoded ESBL-type β-lactamases. If effective, cefepime might serve an important role as a carbapenem-sparing agent in these cases [25].
We sought to assess the comparative efficacy of empiric cefepime vs other antimicrobial agents for the treatment of Enterobacter spp. bacteremia during a 6-year period at 2 major academic hospitals. We hypothesized that cefepime would have similar efficacy as carbapenems for this indication.
METHODS
Patients and Data Collection
We reviewed microbiology databases at Brigham and Women's Hospital and Massachusetts General Hospital, both in Boston, Massachusetts, between January 2005 and March 2011 for cases of Enterobacter spp. bacteremia. We included all cases of Enterobacter bacteremia that received antibacterial treatment during hospitalization. We also collected the following data: sex, age, hospital (Brigham and Women's Hospital vs Massachusetts General Hospital), antimicrobial use, dosing and duration, neutropenia (absolute neutrophil count <500 cells/μL) at the time of bacteremia, comorbidities (extracted by International Classification of Diseases, Ninth Revision code groups from the corresponding hospital admission), documented source of a second positive Enterobacter spp. culture, intensive care unit (ICU) location at time of bacteremia, all data required for Pitt bacteremia score calculation (blood pressure, temperature, mental status, use of mechanical ventilation, use of vasopressors, and prior cardiac arrest on the day of bacteremia [26–28]), Enterobacter species, results of antibiotic susceptibility testing, days to bacteremia clearance, and in-hospital mortality. Because antibiotics were initiated before isolate identification and susceptibility results, antibiotic selection and dosing was empirical and determined by ordering providers.
Species Identification and Susceptibility Testing
Species were identified using the Vitek 2 ID-GNB Panel, and susceptibilities were determined by use of the Vitek system [29]. We used the Clinical and Laboratory Standards Institute (CLSI) M100-S22 criteria [30] to determine antibacterial susceptibility of each isolate, with the following breakpoints: ceftriaxone MIC ≤ 1 μg/mL or Kirby-Bauer disc diameter ≥22 mm; cefepime MIC ≤ 8 μg/mL or Kirby-Bauer disc diameter ≥18 mm; imipenem or meropenem (to represent carbapenem susceptibility) MIC ≤ 1 μg/mL or Kirby-Bauer disc diameter ≥23 mm; and ciprofloxacin (to represent quinolone susceptibility) MIC ≤ 1 μg/mL or Kirby-Bauer disc diameter ≥21 mm. We did not analyze a subset of carbapenem and ceftriaxone susceptibility data collected before July 2006 because susceptibility results were recorded with breakpoints that could not be interpreted with current CLSI criteria (ie, meropenem MIC ≤ 2 or ceftriaxone MIC ≤ 8).
Statistical Analyses
For exposures of interest, we categorized patients by the empiric antimicrobial agent(s) they received after blood cultures that grew Enterobacter were obtained. Antimicrobial agents of interest included cefepime, ceftriaxone, ceftazidime, meropenem or imipenem (hereafter described as carbapenems), ciprofloxacin or levofloxacin (hereafter described as quinolones), gentamicin, or other antibacterial agents (used in <5% of the study population; included amikacin, amoxicillin/clavulanic acid, ampicillin/sulbactam, cefoxitin, piperacillin/tazobactam, and tigecycline). We also created categories for patients who received 2 or 3 antibiotics if the additional agents were initiated within 24 hours of initiation of the first antibiotic. We selected 2 primary outcomes of interest: (1) persistent bacteremia ≥1 calendar day after antibiotic initiation and 2) in-hospital mortality. For clearance of bacteremia analyses, we excluded patients who did not have confirmatory blood cultures on the day after initiation of antibiotics, unless they died within 48 hours of antibiotic initiation, in which case they were allocated as having persistent bacteremia. We restricted analyses to the first episode of Enterobacter spp. bacteremia for each patient. For the mortality outcome, all patients were included independent of subsequent culture results.
We summarized patient characteristics and tested for differences between those with or without persistent bacteremia and those with and without in-hospital mortality, using χ2 tests for categorical variables and rank-sum tests for non-normally distributed continuous variables. To assess for efficacy by antimicrobial agent used, we fit univariable and multivariable logistic regression models, adjusted for demographic (age, sex, and hospital) and clinical characteristics (neutropenia, days between positive culture and antibiotic initiation, location in the ICU, and Pitt bacteremia score), separately for both bacterial clearance and mortality outcomes. We fit additional models including International Classification of Diseases, Ninth Revision–derived comorbidities that were associated with outcomes of interest in univariable modeling (ie, prior solid organ transplant in the persistent bacteremia model and each of hematologic malignancy, cardiac disease, chronic obstructive pulmonary disease, and chronic renal insufficiency in the mortality models). Because none of these models appreciably changed the magnitude or significance of effect sizes for antibiotic use after addition of Pitt bacteremia score [27, 28], we removed comorbidities from the final models. We performed all analyses independently for (1) patients who received only a single antimicrobial agent and (2) patients who received antimicrobial agents alone or in combination with other agents.
To compare efficacy between cefepime and carbapenems directly, we performed propensity-matched analyses for both the persistent bacteremia and mortality outcomes. For each analysis, we estimated the propensity to receive either a carbapenem or cefepime using the following predictors: age, sex, hospital, neutropenia, duration between positive blood culture and antibiotic initiation, location in the ICU at the time of bacteremia, and Pitt bacteremia score. We used nearest-neighbor matching without replacement, with a caliper length of 0.20 [31]. We estimated the absolute risk difference of persistent bacteremia and mortality by receipt of cefepime vs carbapenem in unmatched analysis (total cohort) and in subgroups that were restricted to subjects within a matched propensity pair. We performed these analyses both for patients who received a single antimicrobial agent and for those who received single or combination antimicrobial therapy.
For patients receiving cefepime or carbapenems, we also examined clearance rates for isolates with ceftriaxone resistance to serve as a surrogate marker of either ESBL or derepressed AmpC β-lactamases [6]. For patients at Brigham and Women's Hospital, where susceptibility testing for the cephamycins, cefoxitin, or cefotetan is routinely performed, we assessed for rates of bacterial clearance among a subset of isolates with cephamycin resistance (as a surrogate marker of AmpC β-lactamase production) [6]. Lastly, we performed a subanalysis among those who received cefepime to determine the impact of cefepime dosing and MIC distribution on bacterial clearance.
Ethics Statement
This study was approved by the Partners Human Research Committee, which oversees research at both participating hospitals.
RESULTS
Cohort Characteristics
A total of 368 patients experienced Enterobacter spp. bacteremia at the 2 hospitals during the study period, received at least 1 antimicrobial agent before discharge, and were included in our mortality analyses (Table 1). Of these, 271 had subsequent blood cultures within 1 calendar day of initiating antimicrobials and were included in our analyses of clearance of bacteremia. These patients were 56% male and had a median age of 59 years (interquartile range [IQR], 48–69). At the time of bacteremia, 19% were neutropenic and 22% were in an ICU (Table 2). The cohort included subjects with multiple comorbidities, including 39% with a solid organ malignancy, 21% with a hematologic malignancy, and 6% each with a prior solid organ or hematopoietic stem cell transplant. The median Pitt bacteremia score was 2 (IQR, 1–3; range, 0–8).
Table 1.
Patients With Enterobacter spp. Bacteremia During the Period 2005–2011 at 2 Major Hospitals in Boston, Massachusetts
| Characteristic | Total Cohort (n = 368) | Survived Hospitalization (n = 315) | Died During Hospitalization (n = 52) | P Valuea |
|---|---|---|---|---|
| Clinical characteristics | ||||
| Male sex, No. (%) | 204 (55) | 181 (57) | 23 (44) | .07 |
| Median age, y (IQR) | 59 (48–69) | 58 (47–69) | 63 (54–74) | .04 |
| Neutropenic, No. (%) | 66 (18) | 50 (16) | 16 (31) | .01 |
| ICU on day of bacteremia, No. (%) | 90 (24) | 62 (20) | 28 (54) | <.001 |
| Comorbid medical conditions, No. (%) | ||||
| Solid organ malignancy | 139 (38) | 118 (38) | 21 (41) | .62 |
| Hematologic malignancy | 66 (18) | 51 (16) | 15 (29) | .02 |
| Diabetes mellitus | 91 (25) | 81 (26) | 10 (20) | .34 |
| Cardiac disease | 147 (40) | 119 (38) | 28 (55) | .02 |
| COPD | 41 (11) | 31 (10) | 10 (20) | .04 |
| Chronic renal insufficiency | 88 (24) | 64 (20) | 24 (47) | <.001 |
| Liver disease | 71 (19) | 59 (19) | 12 (24) | .43 |
| Solid organ transplant | 18 (5) | 15 (5) | 3 (6) | .74 |
| Hematopoietic stem cell transplant | 19 (5) | 16 (5) | 3 (6) | .82 |
| Secondary culture source, No. (%) | ||||
| Cardiovascular catheter tip | 18 (5) | 18 (6) | 0 (0) | .08 |
| Abdominal | 15 (4) | 11 (3) | 4 (8) | .16 |
| Respiratory | 24 (7) | 16 (5) | 8 (15) | .01 |
| Urinary | 27 (7) | 24 (8) | 3 (6) | .64 |
| Blood culture positive only | 276 (75) | 238 (75) | 38 (73) | .74 |
| Pitt bacteremia score, No. (%) | <.001 | |||
| 0 | 67 (18) | 63 (20) | 4 (8) | |
| 1–4 | 262 (71) | 232 (73) | 30 (58) | |
| 5–8 | 39 (11) | 21 (7) | 18 (35) | |
| Enterobacter cloacae, No. (%) | 288 (28) | 242 (77) | 46 (88) | .06 |
| Massachusetts General Hospital, No. (%) | 223 (61) | 193 (61) | 30 (58) | .62 |
| Median days from positive culture to antibiotic initiation (IQR) | 0 (0–1) | 0 (0–1) | 0 (0–0.5) | .01 |
| Persistent bacteremia >1 d, No. (%) | 29 (11) | 22 (9) | 7 (18) | .10 |
| Antimicrobial susceptibilityb | ||||
| Ceftriaxone susceptible (n = 335), No. (%) | 227 (68) | 207 (72) | 20 (41) | <.001 |
| Cefepime susceptible (n = 363), No. (%) | 351 (97) | 303 (97) | 48 (92) | .04 |
| Carbapenem susceptible (n = 278), No. (%) | 263 (95) | 223 (95) | 40 (93) | .62 |
| Quinolone susceptible (n = 364), No. (%) | 307 (84) | 268 (86) | 39 (75) | .05 |
| Antimicrobial(s) used, No. (%) | ||||
| Ceftriaxone | 39 (11) | 37 (12) | 2 (4) | .44 |
| Ceftazidime | 77 (21) | 64 (20) | 13 (25) | .09 |
| Cefepime | 137 (37) | 116 (37) | 21 (40) | .59 |
| Cefepime dose per 24 h | .46 | |||
| <2 g | 27 (20) | 21 (18) | 6 (30) | |
| 2–4 g | 88 (66) | 77 (68) | 11 (55) | |
| 6 g | 19 (14) | 16 (14) | 3 (15) | |
| Carbapenem | 74 (20) | 56 (18) | 18 (35) | .01 |
| Quinolone | 168 (46) | 147 (47) | 21 (40) | .40 |
| Gentamicin | 53 (14) | 48 (15) | 5 (10) | .29 |
| Any other antibiotic | 33 (9) | 27 (9) | 6 (12) | .49 |
| Any 2 antibiotics | 201 (55) | 172 (55) | 29 (56) | .88 |
| Any 3 antibiotics | 38 (10) | 28 (9) | 10 (19) | .02 |
Abbreviations: COPD, chronic obstructive pulmonary disease; ICU, intensive care unit; IQR, interquartile range.
a P values are for differences between those who did and did not clear bacteremia within 1 day, calculated with χ2 tests for categorical variables and rank-sum tests for continuous, non-normally distributed variables.
b Susceptibility testing determined by the Vitek system and classified using Clinical and Laboratory Standards Institute M100-S22 [30].
Table 2.
Patients With Enterobacter spp. Bacteremia and Subsequent Blood Cultures Within 1 Day of Antimicrobial Initiation to Assess Clearance of Bacteremia
| Characteristic | Total Cohort (n = 271) | Cleared Bacteremia <1 d (n = 242) | Persistent Bacteremia ≥1 d (n = 29) | P Valuea |
|---|---|---|---|---|
| Clinical characteristics | ||||
| Male sex, No. (%) | 152 (58) | 140 (58) | 12 (41) | .10 |
| Median age, y (IQR) | 59 (48–69) | 59 (50–69) | 58 (44–64) | .25 |
| Neutropenic, No. (%) | 51 (19) | 42 (17) | 9 (31) | .09 |
| ICU on day of bacteremia, No. (%) | 59 (22) | 47 (19) | 12 (41) | .01 |
| Comorbid medical conditions, No. (%) | ||||
| Solid organ malignancy | 105 (39) | 95 (40) | 10 (36) | .59 |
| Hematologic malignancy | 56 (21) | 52 (22) | 4 (14) | .36 |
| Diabetes mellitus | 67 (25) | 63 (26) | 4 (14) | .17 |
| Cardiac disease | 107 (40) | 92 (38) | 15 (54) | .12 |
| COPD | 27 (10) | 24 (10) | 3 (11) | .91 |
| Chronic renal insufficiency | 64 (24) | 56 (23) | 8 (29) | .54 |
| Liver disease | 59 (22) | 52 (22) | 7 (25) | .69 |
| Solid organ transplant | 17 (6) | 12 (5) | 5 (18) | .01 |
| Hematopoietic stem cell transplant | 16 (6) | 16 (7) | 0 (0) | .16 |
| Secondary culture source, No. (%) | ||||
| Cardiovascular catheter tip | 12 (4) | 9 (4) | 3 (10) | .10 |
| Abdominal | 12 (4) | 12 (5) | 0 (0) | .22 |
| Respiratory | 15 (6) | 13 (5) | 2 (7) | .73 |
| Urinary | 20 (7) | 16 (7) | 4 (14) | .16 |
| Blood culture positive only | 205 (76) | 185 (76) | 20 (69) | .38 |
| Pitt bacteremia score, No. (%) | .62 | |||
| 0 | 53 (20) | 49 (20) | 4 (14) | |
| 1–4 | 198 (73) | 176 (73) | 22 (76) | |
| 5–8 | 20 (7) | 17 (7) | 3 (10) | |
| Enterobacter cloacae, No. (%) | 212 (78) | 188 (78) | 24 (83) | .53 |
| Massachusetts General Hospital, No. (%) | 160 (59) | 144 (60) | 16 (56) | .65 |
| Median days from positive culture to antibiotic initiation (IQR) | 0 (0–1) | 0 (0–1) | 0 (0–1) | .02 |
| In-hospital death, No. (%) | 38 (14) | 30 (13) | 7 (25) | .08 |
| Antimicrobial susceptibilityb | ||||
| Ceftriaxone susceptible (n = 249), No. (%) | 172 (69) | 159 (71) | 13 (52) | .051 |
| Cefepime susceptible (n = 267), No. (%) | 260 (97) | 237 (97) | 29 (100) | .35 |
| Carbapenem susceptible (n = 203), No. (%) | 191 (94) | 171 (94) | 20 (91) | .50 |
| Quinolone susceptible, No. (%) | 229 (85) | 211 (88) | 18 (62) | <.001 |
| Antimicrobial(s) used, No. (%) | ||||
| Ceftriaxone | 28 (10) | 26 (11) | 2 (7) | .52 |
| Ceftazidime | 61 (23) | 54 (22) | 7 (24) | .82 |
| Cefepime | 107 (39) | 99 (41) | 8 (28) | .17 |
| Cefepime dose per 24 h | .74 | |||
| <2 g | 22 (21) | 21 (22) | 1 (13) | |
| 2–4 g | 66 (63) | 60 (62) | 6 (75) | |
| 6 g | 17 (16) | 16 (17) | 1 (13) | |
| Carbapenem | 57 (21) | 48 (19) | 9 (31) | .16 |
| Quinolone | 121 (45) | 107 (44) | 14 (48) | .68 |
| Gentamicin | 39 (14) | 35 (15) | 4 (14) | .92 |
| Other antibiotic | 24 (9) | 21 (9) | 3 (10) | .77 |
| Any 2 antibiotics | 149 (55) | 132 (55) | 17 (59) | .68 |
| Any 3 antibiotics | 27 (10) | 26 (11) | 1 (3) | .21 |
Abbreviations: COPD, chronic obstructive pulmonary disease; ICU, intensive care unit; IQR, interquartile range.
a P values are for differences between those who did and did not clear bacteremia within 1 day, calculated with χ2 tests for categorical variables and rank-sum tests for continuous, non-normally distributed variables.
b Susceptibility testing determined by the Vitek system and classified using Clinical and Laboratory Standards Institute M100-S22 [30].
Persistent Bacteremia
Twenty-nine (11%) patients had persistent bacteremia ≥1 calendar day after initiation of antimicrobial agents. Those with persistent bacteremia were more likely to be in the ICU at the time of bacteremia (41% vs 19%; P = .01) and to have received a solid organ transplant (5% vs 18%; P = .001) and were less likely to have organisms susceptible to ceftriaxone (52% vs 71%; P = .051) or quinolones (62% vs 88%; P < .001). Twenty-five percent of patients had a documented additional positive culture source for Enterobacter spp. (Table 1).
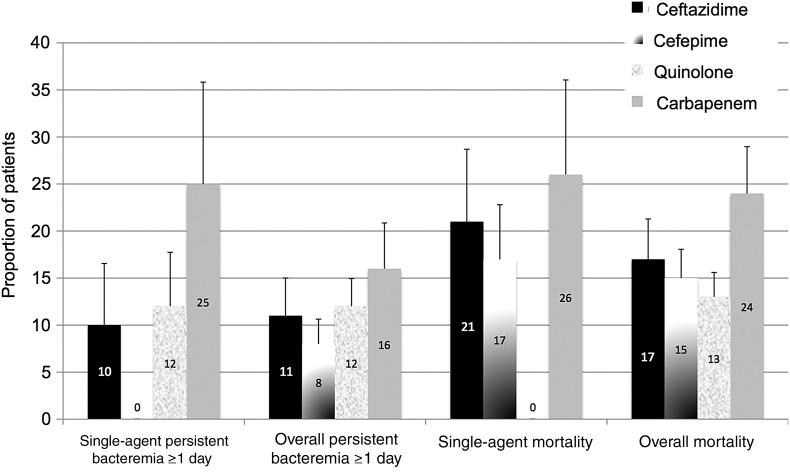
Crude rates of persistent bacteremia ≥1 day are reflected in Table 3 and Figure 1. None of the 36 (0%) subjects who received cefepime monotherapy had persistent bacteremia beyond 1 day, compared with 4 of 16 (25%) who received single-agent carbapenem (P < .01). Among patients who received a single antibacterial agent (n = 122), the adjusted odds of persistent bacteremia (adjusted for sex, age, neutropenia, Pitt bactermia score, comorbidities, location in the ICU, days from positive blood culture to antibiotic initiation, and hospital) were lowest with cefepime (adjusted odds ratio [aOR], 0) and highest for carbapenems (aOR, 8.80; 95% confidence interval [CI], 1.00–77.8; P = .05). When all patients were analyzed (single or combination therapy, n = 271), no antimicrobial was associated with statistically significant odds of bacteremia clearance within 1 day of initiation. Those receiving cefepime had the lowest adjusted odds of persistent bacteremia (aOR, 0.50; 95% CI, .18–1.35; P = .17), whereas those receiving carbapenems had the highest odds of persistent bacteremia (aOR, 1.51; 95% CI, .58–3.96; P = .40).
Table 3.
Odds of Persistent Enterobacter Bacteremia ≥1 Day After Antimicrobial Initiation by Regimen
| Antimicrobial Agent Received | Proportion Persistent Bacteremia >24 h | Crude Estimate |
Adjusted Estimate |
||||
|---|---|---|---|---|---|---|---|
| Crude Odds Ratio | 95% CI | P Value | Adjusted Odds Ratioa | 95% CI | P Value | ||
| Received a single agent (n = 122) | 12/122 (10) | ||||||
| Ceftriaxone | 0/3 (0%) | 0 | N/A | N/A | 0 | N/A | N/A |
| Ceftazidime | 2/21 (10%) | 0.96 | .19–4.73 | .96 | 0.70 | .08–5.95 | .74 |
| Quinolone | 4/32 (12%) | 1.46 | .41–5.24 | .56 | 3.81 | .54–26.80 | .18 |
| Gentamicin | 1/6 (17%) | 1.91 | .20–17.84 | .57 | 0.95 | .07–13.01 | .97 |
| Carbapenem | 4/16 (25%) | 4.08 | 1.07–15.62 | .04 | 8.80 | 1.00–77.79 | .05 |
| Cefepime | 0/36 (0%) | 0 | NA | NA | 0 | NA | NA |
| Received alone or in combination with other agents (n = 271) | 29/271 (11) | ||||||
| Ceftriaxone | 2/28 (7%) | 0.62 | .14–2.74 | .52 | 0.76 | .15–3.80 | .74 |
| Ceftazidime | 7/61 (11%) | 1.11 | .45–2.73 | .82 | 0.75 | .22–2.53 | .64 |
| Quinolone | 14/121 (12%) | 1.18 | .54–2.55 | .68 | 1.38 | .59–3.24 | .46 |
| Gentamicin | 4/39 (10%) | 0.95 | .31–2.88 | .92 | 0.87 | .26–2.92 | .82 |
| Carbapenem | 9/57 (16%) | 1.82 | .78–4.25 | .17 | 1.51 | .58–3.96 | .40 |
| Cefepime | 8/106 (8%) | 0.56 | .24–1.31 | .18 | 0.50 | .18–1.35 | .17 |
| Any 2 agents | 17/149 (11%) | 1.18 | .54–2.58 | .68 | 1.10 | .48–2.52 | .83 |
| Any 3 agents | 1/27 (4%) | 0.30 | .04–2.27 | .24 | 0.23 | .04–1.09 | .06 |
Abbreviations: CI, confidence interval; NA, not applicable.
a Analyses adjusted for age, sex, neutropenia at time of bacteremia, intensive care unit location at time of bacteremia, Pitt bacteremia score, days from positive culture to antibiotic initiation, and hospital. Reference category for each estimate is all other regimens.
Figure 1.
Crude rates of persistent bacteremia ≥1 day and mortality by empiric antibacterial agent among patients with Enterobacter spp. bacteremia. Data are divided into those who received a single agent and those who received any number of antibacterial agents (overall bacteremia and overall mortality). Numbers within the bars refer to the proportion estimate, and error bars mark the standard error of each estimate.
In-Hospital Mortality
Crude rates of in-hospital mortality are presented in Table 4. Among patients receiving a single agent, the lowest rates of mortality were seen with ceftriaxone (n = 0/5, 0%) and quinolones (n = 0/44, 0%), followed by cefepime (n = 7/42, 17%), ceftazidime (n = 6/28, 21%), carbapenems (n = 5/19, 26%), and gentamicin (n = 2/6, 33%). A similar pattern was seen among patients who received these agents in any combination (alone or with other agents). Although carbapenem use was associated with increased mortality in univariable models (aOR, 2.45; 95% CI, 1.29–4.64; P = .01), in multivariable analyses adjusted for demographic and clinical characteristics, no agents were statistically associated with increased mortality. Odds of mortality were similar among those receiving cefepime (aOR, 1.50; 95% CI, .73–3.47; P = .25) and those receiving a carbapenem (aOR, 1.82; 95% CI, .82–3.80; P = .11).
Table 4.
Odds of In-Hospital Mortality by Regimen
| Antimicrobial Agent Received | Proportion With in-Hospital Death | Crude Estimate |
Adjusted Estimate |
||||
|---|---|---|---|---|---|---|---|
| Crude Odds Ratio | 95% CI | P Value | Adjusted Odds Ratioa | 95% CI | P Value | ||
| Received a single agent (n = 166) | 23/166 (14%) | ||||||
| Ceftriaxone | 0/5 (0%) | 0 | NA | NA | 0 | NA | NA |
| Ceftazidime | 6/28 (21%) | 1.94 | .68–5.47 | .21 | 8.14 | 1.09–60.93 | .04 |
| Quinolone | 0/44 (0%) | 0 | NA | NA | 0 | NA | NA |
| Gentamicin | 2/6 (33%) | 3.31 | .57–19.20 | .18 | 1.28 | .10–16.26 | .85 |
| Carbapenem | 5/19 (26%) | 2.56 | .82–7.95 | .10 | 1.78 | .42–7.53 | .44 |
| Cefepime | 7/42 (17%) | 1.35 | .51–3.55 | .54 | 1.71 | .42–6.94 | .46 |
| Received alone or in combination with other agents (n = 368) | 52/368 (14%) | ||||||
| Ceftriaxone | 2/39 (5%) | 0.30 | .07–1.28 | .11 | 0.56 | .12–2.57 | .45 |
| Ceftazidime | 13/77 (17%) | 1.31 | .66–2.59 | .44 | 0.87 | .31–2.38 | .78 |
| Quinolone | 21/168 (13%) | 0.77 | .43–1.41 | .40 | 0.95 | .48–1.86 | .87 |
| Gentamicin | 5/53 (9%) | 0.59 | .22–1.56 | .29 | 0.70 | .24–2.04 | .52 |
| Carbapenem | 18/74 (24%) | 2.45 | 1.29–4.64 | .01 | 1.82 | .82–3.80 | .11 |
| Cefepime | 21/136 (15%) | 1.18 | .65–2.15 | .59 | 1.59 | .73–3.47 | .25 |
| Any 2 agents | 29/201 (14%) | 1.05 | .58–1.89 | .88 | 1.05 | .54–2.03 | .89 |
| Any 3 agents | 10/38 (26%) | 2.44 | 1.11–5.38 | .03 | 2.37 | .97–5.79 | .06 |
Abbreviations: CI, confidence interval; NA, not applicable.
a Analyses adjusted for age, sex, neutropenia at time of bacteremia, Pitt bacteremia score, days from positive culture to antibiotic initiation, and hospital. Reference category for each estimate is all other regimens.
Propensity Score Analyses
Results of propensity score–matched analyses are displayed in Table 5. In constructing propensity scores, we found that, in the total cohort, patients were more likely to receive a cefepime vs a carbapenem if they were at Massachusetts General Hospital (aOR, 12.4; 95% CI, 4.1–37.7) and less likely to receive cefepime if they were in the ICU at the time of bacteremia (aOR, 0.3; 95% CI, .1–.9) or had a delay in time to antibiotic initiation (aOR, 0.5; 95% CI, .3–1.0). Whereas in the total cohort subjects were more likely to clear bacteremia within 24 hours with receipt of cefepime vs a carbapenem, we found no differences in efficacy in terms of either persistent bacteremia or in-hospital mortality in analyses restricted to subjects with a propensity score–matched pair. There were no differences in bacteremia clearance among patients receiving cefepime or carbapenems for those with isolates with cephalosporin resistance. Among isolates that were resistant to ceftriaxone, 5 of 28 (18%) of those receiving cefepime and 6 of 32 (19%) of those receiving a carbapenem had persistent bacteremia. In an analysis restricted to those with cephamycin resistance, persistent bacteremia was found in 3 of 20 (15%) of those who received cefepime vs 1 of 9 (11%) of those who received a carbapenem.
Table 5.
Comparative Efficacy for Cefepime vs Carbapenems for the Treatment of Enterobacter spp. Bacteremia With Both Crude and Propensity Score–Matched Subgroups
| Outcome and Analysis Specification | Proportion With Outcome Among Those Receiving Carbapenems | Proportion With Outcome Among Those Receiving Cefepime | Absolute Risk Difference | P Value |
|---|---|---|---|---|
| Outcome: persistent bacteremia ≥1 d | ||||
| Single agent | ||||
| Crude analysis | 4/16 (25%) | 0/36 (0%) | 0.25 | .002 |
| Propensity score analysis | 2/8 (25%) | 0/9 (0%) | 0.25 | .11 |
| Combination therapy | ||||
| Crude analysis | 8/42 (19%) | 7/91 (8%) | 0.11 | .054 |
| Propensity score analysis | 4/26 (15%) | 3/28 (11%) | 0.04 | .61 |
| Outcome: in-hospital mortality | ||||
| Single agent | ||||
| Crude analysis | 5/19 (26%) | 7/42 (17%) | 0.10 | .38 |
| Propensity score analysis | 3/10 (30%) | 2/12 (17%) | 0.13 | .46 |
| Combination therapy | ||||
| Crude analysis | 15/55 (27%) | 18/117 (15%) | 0.12 | .07 |
| Propensity score analysis | 8/34 (24%) | 5/40 (13%) | 0.11 | .21 |
MIC and Dosing Analyses
In patients who received cefepime with evaluable MIC results, only 2 of 74 (3%) patients with an isolate with a cefepime MIC of ≤2 μg/mL had persistent bacteremia within 24 hours vs 6 of 23 (26%) patients with an MIC ≥ 4 μg/mL (P < .001). Specifically, the proportion of subjects who received cefepime alone or in combination with any other antibacterial agents and had clearance of bacteremia within 1 day by cefepime MIC were 66 of 68 (97%) for ≤1 μg/mL, 7 of 7 (100%) for 2 μg/mL, 16 of 21 (76%) for 4 μg/mL, 0 of 1 (0%) for 8 μg/mL, and 1 of 1 (100%) for >8 μg/mL. Among the 30 participants who received single-agent cefepime with evaluable MIC results, all 30 (100%) cleared bacteremia within 1 calendar day with the following MICs (n = 26 for ≤1 μg/mL; n = 1 for 2 μg/mL; n = 2 for 4 μg/mL; n = 1 for ≥64 μg/mL). Because all patients who received single-agent cefepime cleared bacteremia within 24 hours, we were not able to detect an effect of cefepime dosing on persistence of bacteremia (10 [29%] received <2 grams/24 hours, 20 [57%] received 2–4 grams/24 hours, 5 [14%] received 6 grams/24 hours). Of those receiving low dose cefepime (<2 grams/24 hours), the median age was 73 years (IQR, 60–80), and median creatinine was 2.9 mg/dL (IQR, 1.5–4.1). This is compared with a median creatinine of 1.0 mg/dL (IQR, 0.8–1.5) for those receiving a dose >2 grams/24 hours, suggesting dosing was adjusted for creatinine clearance. Among those receiving cefepime in combination with other agents, we did not detect a difference in persistent bacteremia by cefepime dose received: <2 grams/24 hours (n = 5/26, 5%), 2–4 grams/24 hours (n = 6/65, 9%), 6 grams/24 hours (n = 1/17, 6%; P = .74).
DISCUSSION
During 6 years of observation at 2 major academic hospitals, we found similar rates of efficacy for use of cefepime vs carbapenems for the treatment of Enterobacter spp. bacteremia. Although no antimicrobial agent or class demonstrated statistically significantly improved clearance of bacteremia, among those who received a single antimicrobial, we found lower crude rates of persistent bacteremia with cefepime (n = 0/36, 0%) than with a carbapenem (n = 4/16, 25%). In multivariable analyses adjusted for clinical characteristics, we found that those who received cefepime were least likely to have persistent bacteremia (aOR, 0.50; P = .19). In both multivariable regression and propensity score–matched analyses, there were no differences in risk of in-hospital mortality between patients receiving carbapenems or cefepime. Importantly, we found similar rates of bacterial clearance in subsets of patients with Enterobacter spp. isolates that were resistant to either ceftriaxone or a second-generation cephamycin, for which cefepime cleared bacteremia at rates of >85%. These data suggest that cefepime could be considered as effective as carbapenems for the treatment of Enterobacter spp. bacteremia. In particular, we would suggest cefepime as an agent of choice for isolates with an MIC of ≤2 μg/mL because approximately 97% of patients with such isolates cleared bacteremia within 1 day of cefepime initiation.
Our results support prior data that cefepime is effective for the treatment of Enterobacter spp. infections, based on the knowledge that these species often have a chromosomally encoded AmpC-type β-lactamase to which cefepime is relatively more stable than other cephalosporins [6, 19]. A recent study, which used phenotypic testing to identify the presence of AmpC β-lactamases among GNR bacteremia isolates, found similar outcomes with use of cefepime or carbapenems for mortality and length of hospital stay (n = 64 in the comparative analysis) [18]. A second study of critically ill patients with Enterobacter aerogenes blood stream infections (n = 43) found equivalent rates of bacterial clearance and mortality and reduced antibiotic exposure with cefepime vs carbapenem use [21]. Prior in vitro studies have also supported the activity of cefepime against these isolates [16, 17].
Our data differ from prior studies that more broadly consider treatment of bloodstream infections caused by all species of Enterobacteriaceae, or those with ESBL-type enzymes. For example, a small retrospective review demonstrated increased mortality and treatment failure with use of cefepime vs carbapenems for treatment of bacteremia with phenotypic ESBL-type Enterobacteriaceae [22]. Other studies have also demonstrated inferiority of cefepime for ESBL producers [23, 24]. In contrast with these studies, we found equivalent rates of bacteremia clearance in those receiving cefepime and carbapenems among isolates resistant to later-generation cephalosporins. Our study included more cases treated with cefepime (n = 136) than prior studies and was restricted to cases of Enterobacter spp. infections. The difference between our studies and prior evaluations of cefepime for Enterobacteriaceae in general largely suggests key differences due to bacterial species or β-lactamase type. This hypothesis is supported by the fact that, although ceftriaxone resistance in Escherichia or Klebsiella spp. are usually due to plasmid-encoded ESBLs, the same pattern of resistance in Enterobacter spp. (and other AmpC-containing species) is more often associated with AmpC overexpression [6, 19, 32]. Because cefepime is relatively stable to AmpC, Enterobacter strains are therefore more likely to be susceptible to cefepime than would be the case for Escherichia coli or Klebsiella spp. While guidelines and practitioners sometimes group treatment of multidrug resistant GNR infections [33], the distinction between resistance patterns for AmpC vs other ESBL β-lactamases might allow more targeted therapeutic recommendations by species type.
A strength of our study was its relatively large sample size from 2 major academic centers with different empiric prescribing practices. Our use of both microbiologic outcomes (clearance of bacteremia within 1 day) and clinical outcomes (mortality) augments the validity of our findings. However, relatively few subjects received a single antibiotic, mitigating our ability to make definitive pair-wise comparative efficacy conclusions. The data are also limited by the observational nature of data collection, which precludes our ability to discount residual confounding in our analyses. This weakness was demonstrated by our finding of a nonsignificant increase in mortality among those who received 3 antimicrobials, despite adjustment for age, ICU stay, Pitt bacteremia score, and neutropenia. Because both hospitals in our study were in the same geographic location, our results might not reflect expected outcomes in areas with different antimicrobial susceptibility patterns. Finally, although we relied on phenotypic resistance testing and were unable to verify AmpC β-lactamase production, phenotypic testing, and particular cefoxitin resistance, has been demonstrated to be a reliable predictor of genotypic AmpC β-lactamase production [34, 35].
In summary, in an observational cohort of >300 patients, we found that cefepime had similar efficacy as carbapenems for the treatment of Enterobacter spp. bacteremia. Future guidelines should consider cefepime for treatment of blood-stream infections with this organism, particularly those with an MIC ≤ 2 μg/mL. Future studies with use of randomization or confirmatory genotypic testing for AmpC β-lactamases will be useful to corroborate our findings.
Notes
Financial support. M. J. S. receives support from the National Institute of Mental Health (NIH K23 MH099916) and T. F. O. receives support from the National Institute of General Medical Sciences (NIH R01 GM103525).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Canton R, Akova M, Carmeli Y, et al. Rapid evolution and spread of carbapenemases among Enterobacteriaceae in Europe. Clin Micro and Infect. 2012;18:413–31. doi: 10.1111/j.1469-0691.2012.03821.x. [DOI] [PubMed] [Google Scholar]
- 2.Ho J, Tambyah PA, Paterson DL. Multiresistant Gram-negative infections: a global perspective. Curr Opin Infect Dis. 2010;23:546–53. doi: 10.1097/QCO.0b013e32833f0d3e. [DOI] [PubMed] [Google Scholar]
- 3.Moellering RC., Jr NDM-1—a cause for worldwide concern. New Eng J Med. 2010;363:2377–9. doi: 10.1056/NEJMp1011715. [DOI] [PubMed] [Google Scholar]
- 4.Pitout JD, Laupland KB. Extended-spectrum beta-lactamase-producing Enterobacteriaceae: an emerging public-health concern. Lancet Infect Dis. 2008;8:159–66. doi: 10.1016/S1473-3099(08)70041-0. [DOI] [PubMed] [Google Scholar]
- 5.Choi SH, Lee JE, Park SJ, et al. Emergence of antibiotic resistance during therapy for infections caused by Enterobacteriaceae producing AmpC β-lactamase: implications for antibiotic use. Antimicrob Agents Chemother. 2008;52:995–1000. doi: 10.1128/AAC.01083-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacoby GA. AmpC beta-lactamases. Clin Microbiol Rev. 2009;22:161–82. doi: 10.1128/CMR.00036-08. Table of Contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chow JW, Fine MJ, Shlaes DM, et al. Enterobacter bacteremia: clinical features and emergence of antibiotic resistance during therapy. Ann Intern Med. 1991;115:585–90. doi: 10.7326/0003-4819-115-8-585. [DOI] [PubMed] [Google Scholar]
- 8.Kaye KS, Cosgrove S, Harris A, Eliopoulos GM, Carmeli Y. Risk factors for emergence of resistance to broad-spectrum cephalosporins among Enterobacter spp. Antimicrob Agents Chemother. 2001;45:2628–30. doi: 10.1128/AAC.45.9.2628-2630.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwaber MJ, Carmeli Y. Mortality and delay in effective therapy associated with extended-spectrum beta-lactamase production in Enterobacteriaceae bacteraemia: a systematic review and meta-analysis. J Antimicrob Chemother. 2007;60:913–20. doi: 10.1093/jac/dkm318. [DOI] [PubMed] [Google Scholar]
- 10.Paterson DL, Bonomo RA. Extended-spectrum β-lactamases: a clinical update. Clin Micro Review. 2005;18:657–86. doi: 10.1128/CMR.18.4.657-686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vardakas KZ, Tansarli GS, Rafailidis PI, Falagas ME. Carbapenems versus alternative antibiotics for the treatment of bacteraemia due to Enterobacteriaceae producing extended-spectrum beta-lactamases: a systematic review and meta-analysis. J Antimicrob Chemother. 2012;67:2793–803. doi: 10.1093/jac/dks301. [DOI] [PubMed] [Google Scholar]
- 12.Nordmann P, Naas T, Poirel L. Global spread of carbapenemase-producing Enterobacteriaceae. Emerg Infect Dis. 2011;17:1791–8. doi: 10.3201/eid1710.110655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nordmann P, Cuzon G, Naas T. The real threat of Klebsiella pneumoniae carbapenemase-producing bacteria. Lancet Infect Dis. 2009;9:228–36. doi: 10.1016/S1473-3099(09)70054-4. [DOI] [PubMed] [Google Scholar]
- 14.Jacoby GA, Mills DM, Chow N. Role of β-lactamases and porins in resistance to ertapenem and other beta-lactams in Klebsiella pneumoniae. Antimicrob Agents and Chemother. 2004;48:3203–6. doi: 10.1128/AAC.48.8.3203-3206.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martinez-Martinez L, Pascual A, Hernandez-Alles S, et al. Roles of beta-lactamases and porins in activities of carbapenems and cephalosporins against Klebsiella pneumoniae. Antimicrob Agents Chemother. 1999;43:1669–73. doi: 10.1128/aac.43.7.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nikaido H, Liu W, Rosenberg EY. Outer membrane permeability and beta-lactamase stability of dipolar ionic cephalosporins containing methoxyimino substituents. Antimicrob Agents Chemother. 1990;34:337–42. doi: 10.1128/aac.34.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martinez-Martinez L, Conejo MC, Pascual A, et al. Activities of imipenem and cephalosporins against clonally related strains of Escherichia coli hyperproducing chromosomal beta-lactamase and showing altered porin profiles. Antimicrob Agents Chemother. 2000;44:2534–6. doi: 10.1128/aac.44.9.2534-2536.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tamma PD, Girdwood SC, Gopaul R, et al. The use of cefepime for treating AmpC beta-lactamase-producing Enterobacteriaceae. Clin Infect Dis. 2013;57:781–8. doi: 10.1093/cid/cit395. [DOI] [PubMed] [Google Scholar]
- 19.Gales AC, Castanheira M, Jones RN, Sader HS. Antimicrobial resistance among Gram-negative bacilli isolated from Latin America: results from SENTRY Antimicrobial Surveillance Program (Latin America, 2008–2010) Diagn Microbiol Infect Dis. 2012;73:354–60. doi: 10.1016/j.diagmicrobio.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 20.Pfaller MA, Sader HS, Fritsche TR, Jones RN. Antimicrobial activity of cefepime tested against ceftazidime-resistant Gram-negative clinical strains from North American Hospitals: report from the SENTRY Antimicrobial Surveillance Program (1998–2004) Diagn Microbiol Infect Dis. 2006;56:63–8. doi: 10.1016/j.diagmicrobio.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 21.Goethaert K, Van Looveren M, Lammens C, et al. High-dose cefepime as an alternative treatment for infections caused by TEM-24 ESBL-producing Enterobacter aerogenes in severely-ill patients. Clin Microbiol Infect. 2006;12:56–62. doi: 10.1111/j.1469-0691.2005.01290.x. [DOI] [PubMed] [Google Scholar]
- 22.Lee NY, Lee CC, Huang WH, Tsui KC, Hsueh PR, Ko WC. Cefepime therapy for monomicrobial bacteremia caused by cefepime-susceptible extended-spectrum β-lactamase-producing Enterobacteriaceae: MIC matters. Clin Infect Dis. 2013;56:488–95. doi: 10.1093/cid/cis916. [DOI] [PubMed] [Google Scholar]
- 23.Chopra T, Marchaim D, Veltman J, et al. Impact of cefepime therapy on mortality among patients with bloodstream infections caused by extended-spectrum-beta-lactamase-producing Klebsiella pneumoniae and Escherichia coli. Antimicrob Agents Chemother. 2012;56:3936–42. doi: 10.1128/AAC.05419-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Labombardi VJ, Rojtman A, Tran K. Use of cefepime for the treatment of infections caused by extended spectrum β-lactamase-producing Klebsiella pneumoniae and Escherichia coli. Diagn Microbiol infect Dis. 2006;56:313–5. doi: 10.1016/j.diagmicrobio.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 25.Ramphal R, Ambrose PG. Extended-spectrum beta-lactamases and clinical outcomes: current data. Clin Infect Dis. 2006;42(Suppl 4):S164–72. doi: 10.1086/500663. [DOI] [PubMed] [Google Scholar]
- 26.Paterson DL, Ko WC, Von Gottberg A, et al. International prospective study of Klebsiella pneumoniae bacteremia: implications of extended-spectrum beta-lactamase production in nosocomial Infections. Ann Intern Med. 2004;140:26–32. doi: 10.7326/0003-4819-140-1-200401060-00008. [DOI] [PubMed] [Google Scholar]
- 27.Feldman C, Alanee S, Yu VL, et al. Severity of illness scoring systems in patients with bacteraemic pneumococcal pneumonia: implications for the intensive care unit care. Clin Microbiol Infect. 2009;15:850–7. doi: 10.1111/j.1469-0691.2009.02901.x. [DOI] [PubMed] [Google Scholar]
- 28.Rhee JY, Kwon KT, Ki HK, et al. Scoring systems for prediction of mortality in patients with intensive care unit-acquired sepsis: a comparison of the Pitt bacteremia score and the Acute Physiology and Chronic Health Evaluation II scoring systems. Shock. 2009;31:146–50. doi: 10.1097/SHK.0b013e318182f98f. [DOI] [PubMed] [Google Scholar]
- 29.Livermore DM, Struelens M, Amorim J, et al. Multicentre evaluation of the VITEK 2 Advanced Expert System for interpretive reading of antimicrobial resistance tests. J Antimicrob Chemother. 2002;49:289–300. doi: 10.1093/jac/49.2.289. [DOI] [PubMed] [Google Scholar]
- 30.Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; twenty-second informational supplement. 2012 CLSI document M100-S22. Wayne, PA: Clinical and Laboratory Standards Institute. [Google Scholar]
- 31.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46:399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alvarez M, Tran JH, Chow N, Jacoby GA. Epidemiology of conjugative plasmid-mediated AmpC beta-lactamases in the United States. Antimicrob Agents Chemother. 2004;48:533–7. doi: 10.1128/AAC.48.2.533-537.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009. Update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49:1–45. doi: 10.1086/599376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peter-Getzlaff S, Polsfuss S, Poledica M, et al. Detection of AmpC beta-lactamase in Escherichia coli: comparison of three phenotypic confirmation assays and genetic analysis. J Clin Microbiol. 2011;49:2924–32. doi: 10.1128/JCM.00091-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Polsfuss S, Bloemberg GV, Giger J, Meyer V, Bottger EC, Hombach M. Practical approach for reliable detection of AmpC beta-lactamase-producing Enterobacteriaceae. J Clin Microbiol. 2011;49:2798–803. doi: 10.1128/JCM.00404-11. [DOI] [PMC free article] [PubMed] [Google Scholar]