Abstract
Background
Clinical staging of esophageal cancer has improved with PET/CT and endoscopic ultrasound. Despite such progress, small single center studies have questioned the reliability of clinical staging of T2N0 esophageal cancer. This study broadly examines the adequacy of clinical staging of T2N0 disease using the Society of Thoracic Surgeons database.
Methods
We retrospectively studied 810 clinical stage T2N0 patients from 2002-2011. There were 58 excluded because of incomplete pathologic staging data. Clinical stage, pathologic stage, and preoperative characteristics were recorded. Logistic regression analysis was utilized to identify factors associated with upstaging at the time of surgery.
Results
Among 752 clinical stage T2N0 patients, 35.9 %(270) received induction therapy prior to surgery. Of 482 patients that went directly to surgery, 27.4%(132) were confirmed as pathologic T2N0, 25.9%(125) were downstaged(i.e. T0-1N0), while 46.7%(225) were upstaged at surgery (T3-4N0 or TanyN1-3). Exclusive tumor upstaging(i.e. pathological T3-4N0) accounted for 18.2%(41), while exclusive nodal upstaging(i.e. pathological T1-2N1-3) accounted for 44.5%(100). Combined tumor and nodal upstaging(i.e. pathological T3-4N1-3) accounted for 37.3%(84). Among patients who received induction therapy, 38.1%(103) were upstaged vs. 46.7%(225) without induction therapy(p=0.026). Comparing the induction therapy group and the primary surgical group, postoperative 30-day mortality(3.7% vs. 3.7%, p=1.0) and morbidity(46.3% vs. 45%, p=0.76) were similar.
Conclusion
Despite advances in staging techniques clinical staging of T2N0 esophageal cancer remains unreliable. Recognizing T2N0 as a threshold for induction therapy in esophageal cancer, many surgeons have opted to treat T2N0 disease with induction therapy, despite the fact that one quarter of these patients will be pathological T1N0. While this study demonstrated similar perioperative morbidity and mortality with and without induction therapy, further study is needed to examine the impact of upstaging on long-term survival.
Introduction
While there is debate regarding the appropriate treatment of T1 carcinomas limited to the esophageal mucosa, submucosal tumors (i.e. T1b) are generally treated with primary surgery without induction therapy.[1] In contrast, locally advanced but non-metastatic esophageal carcinomas (T3N1-3) are generally approached with induction chemoradiotherapy followed by resection. The premise of this approach is based on the increased likelihood of occult systemic disease with increasing tumor size and nodal involvement. While controversy may still exist regarding the role of induction therapy in locally advanced disease, there are increasing data to suggest a survival advantage with a multimodality approach.[2-5] Historically, clinical T2N0 disease has been approached with primary surgery as the standard treatment. However, concern over the potential presence of occult nodal disease has brought into question the role of induction therapy vs. primary surgery for this small subset of patients.
Recent revisions in the staging system have highlighted the prognostic significance of accurate pathologic staging for esophageal carcinoma.[6, 7] The two most important factors in this staging are depth of tumor invasion and nodal disease. While these factors are more easily determined in pathologic specimens after surgery, these factors are also important in guiding treatment decisions prior to surgery. The addition of Positron Emission Tomography (PET)/CT scans and endoscopic ultrasound (EUS) have improved our ability to clinically stage esophageal cancer and both are standard recommended staging tools per the National Comprehensive Cancer Network (NCCN) clinical practice guidelines.[8] While the addition of EUS has improved clinical staging, the accuracy is lower for early stage lesions (i.e. T1-2N0) vs. advanced stage lesions (i.e. T3N1-3).[9-12] The issues outlined above have brought into question the reliability of current clinical staging techniques in T2N0 esophageal cancer and the relative role of multi-modality therapy in this subset of patients. Important work by Rice et al initially highlighted the inaccuracy of clinically staging of T2N0 esophageal carcinoma in a small series of patients.[9, 13] This and other single center studies questioning the accuracy of clinical T2N0 esophageal cancer have been limited by small sample size.[9, 14-16]
In this study, we utilized the Society of Thoracic Surgeons (STS) General Thoracic Surgery Database (GTSDB) to overcome the limitation of small sample size that has plagued other studies. Using the database, we investigated the accuracy of clinical staging of T2N0 esophageal cancer, examined current practice patterns in terms of treatment regimens, and examined the impact of induction therapy on peri-operative morbidity and mortality in this subset of clinically staged T2N0 patients.
Patients and Methods
Patient population
All patients with clinical stage T2N0 who underwent esophageal resection between January 2002 and December 2011 were retrospectively identified using the STS GTSDB database. There were 6886 patients undergoing esophagectomy for esophageal cancer with 810 patients classified as clinical stage T2N0. Fifty eight patients were excluded because of incomplete pathologic staging. Clinical stage (c), pathologic stage (p), preoperative demographics and co-morbidity, perioperative characteristics, and 30-day morbidity and mortality were recorded. There were 2 versions of STS GTSD. Version 2.07 spanned the time period of 2002-2007 while Version 2.081 spanned the period of 2008-2011. Tumor histology, histologic grade, and tumor location were not defined in version 2.07 of the database but were included in version 2.081.
All study subjects had biopsy-proven esophageal cancer. Patients with either adenocarcinoma or squamous cell carcinoma were included. Clinical and pathologic staging definitions were defined by the 6th Edition of the American Joint Committee on Cancer (AJCC) for staging esophageal cancer for Version 2.07 and the 7th Edition for Version 2.081 once this became available.[7] The study patients either underwent primary surgical resection or received induction therapy followed by surgery.
Clinical stage and pathologic stage were required fields in the STS GTSDB and were reviewed for the purposes of this study. Neither a PET/CT nor an EUS were required fields within the database, though it is assumed that the majority of subjects underwent these staging tests.
Statistical Methods
Descriptive statistics for continuous variables were expressed as mean ± standard deviation. Categorical data were expressed as counts and proportions. Fisher's exact test was used to analyze differences among the categorical data. Logistic regression analysis was used to identify covariates (among baseline patient variables) associated with upstaging. Sensitivity was defined as TP/(TP+FN). Specificity was defined as TN/(TN+FP). Positive predictive value was defined as TP/(TP+FP). Negative predictive value was defined as TN/(TN+FN). Accuracy was defined as (TP+TN)/(TP+FP+FN+TN). P-values less than 0.05 were considered statistically significant. The statistical software, Statistical Analysis Software (SAS) and R were used for the analyses.
Results
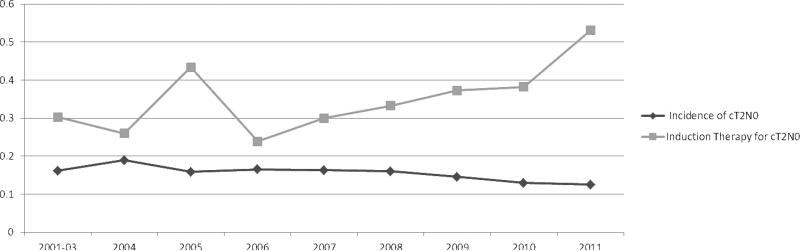
From the STS GTSD, we identified 810 patients clinically staged as T2N0 (cT2N0) who underwent resection between January 2002 to December 2011 with 58 excluded because of incomplete pathologic staging. Table 1 outlines perioperative characteristics of all cT2N0 evaluable patients (N=752). Among 752 cT2N0 patients, 35.9% (270) received induction therapy prior to surgery while 64.1% (482) went directly to surgery. Among the 270 patients receiving induction therapy, 83.3% (225) underwent chemoradiotherapy, 11.9% (32) underwent chemotherapy only, and 4.8% (13) underwent radiation therapy only. Among 359 patients where histologic type was recorded, 18.7% (67) were squamous cell carcinomas while 81.3% (292) were adenocarcinomas. Among 361 with reported histologic grade of the tumor, 15% (54) were GX (undetermined), 10.8% (39) were G1 (well differentiated), 38.0% (137) were G2 (moderately differentiated), 35.7% (129) were G3 (poorly differentiated), and 0.6% (2) were G4 or (undifferentiated). Table 2 outlines the distribution of pathologic stages for cT2N0 patients. To exclude the confounding issue of induction therapy's impact upon pathologic staging, we specifically analyzed the subset of cT2N0 patients that did not undergo induction therapy (N=482). Of 482 patients that went directly to surgery, 27.4% (132) were confirmed as pT2N0, 25.9% (125) were downstaged (i.e. T0-1N0), while 46.7% (225) were upstaged at surgery (T3-4N0 or TanyN1-3) (Table 2). Exclusive tumor upstaging (i.e. pT3-4N0) accounted for 18.2% (41), while exclusive nodal upstaging (i.e. pT1-2N1-3) accounted for 44.5% (100). Combined tumor and nodal upstaging (i.e. pT3-4N1-3) accounted for 37.3% (84), thus nodal descriptor upstaging accounted for 81.8% (184). Figure 1 outlines the annual incidence of cT2N0 esophageal cancer and the proportion of cT2N0 patients receiving induction therapy from 2001-2012. While the incidence of cT2N0 disease remained relatively constant over the study period as a proportion of all clinically staged esophageal cancers, the proportion of cT2N0 patients receiving induction appears to be on an upward trend over the past few years.
Table 1.
Characteristics of 752 Evaluable Patients with cT2N0 Esophageal Cancer.
| 752 cT2N0 Patients | |
|---|---|
| N (%) | |
| Age (Mean ± SD) | 63.8±11.1 |
| Gender | |
| Male | 626(82.2%) |
| Female | 126(16.8%) |
| Race | N=732 |
| Caucasian | 683(93.3%) |
| Black | 31(4.2%) |
| Hispanic | 11(1.5%) |
| Asian | 7(1.0%) |
| Zubrod score | N=736 |
| 0=Normal Activity, no symptoms | 177(24.0%) |
| 1=Symptoms but fully ambulatory | 507(68.9%) |
| 2=Symptoms but in bed <50% time | 34(4.6%) |
| 3=Symptoms but in bed 50-100% time | 15(2.0%) |
| 4=Bedridden | 2(0.3%) |
| 5=Moribund | 1(0.1%) |
| ASA Risk Class | N=743 |
| I-II | 180(24.2%) |
| III | 521(70.1%) |
| IV-V | 42(5.7%) |
| BMI (kg/m2) | N=709 |
| Mean±/SD | 28.1±5.6 |
| CHF | N=670 |
| No | 659(98.4%) |
| Yes | 11(1.6%) |
| CAD | N=673 |
| No | 515(76.5%) |
| Yes | 158(23.5%) |
| PVD | N=670 |
| No | 619(92.4%) |
| Yes | 51(7.6%) |
| Hypertension | N=679 |
| No | 279(41.1%) |
| Yes | 400(58.9%) |
| Prior Thoracic Surgery | N=670 |
| No | 596(89.0%) |
| Yes | 74(11.0%) |
| Preoperative Induction therapy | |
| None | 482(64.1%) |
| Chemotherapy/XRT | 225(29.9%) |
| Chemotherapy only | 32(4.3%) |
| XRT only | 13(1.7%) |
Table 2.
Pathologic stage for 752 Evaluable Patients with clinical T2N0 Esophageal Cancer.
| Induction therapy (n=270) | Surgery (n=482) | Total (n=752) | |
|---|---|---|---|
| Pathologic TN pattern | N (%) | N (%) | N (%) |
| TisN0 | 4(1.4) | 7(1.4) | 11(1.5) |
| T0N0 | 39(14.4) | 1(0.2) | 40(5.3) |
| T1N0 | 41(15.2) | 117(24.3) | 158(21.0) |
| T2N0 | 83(30.7) | 132(27.4) | 215(28.6) |
| T0N1 | 10(3.7) | 1(0.2) | 11(1.5) |
| T1N1 | 7(2.6) | 35(7.3) | 42(5.6) |
| T1N2 | 0(0) | 3(0.6) | 3(0.4) |
| T2N1 | 20(7.4) | 56(11.6) | 76(10.1) |
| T2N2 | 1(0.4) | 3(0.6) | 4(0.5) |
| T2N3 | 0(0) | 2(0.4) | 2(0.3) |
| T3N0 | 27(10.0) | 40(8.3) | 67(8.9) |
| T3N1 | 30(11.1) | 68(14.1) | 98(13.0) |
| T3N2 | 2(0.7) | 9(1.9) | 11(1.5) |
| T3N3 | 4(1.5) | 5(1.0) | 9(1.2) |
| T4N0 | 1(0.4) | 1(0.2) | 2(0.3) |
| T4N1 | 1(0.4) | 1(0.2) | 2(0.3) |
| T4N2 | 0(0) | 1(0.2) | 1(0.1) |
| cT2N0 to pathologic TN status | |||
| Down-Stage (pT0-1N0) | 84(31.1)* | 125(25.9) | 209(27.8) |
| No change (pT2N0) | 83(30.7)# | 132(27.4) | 215(28.6) |
| Upstage (pT3-4N0 or pTanyN1-3) | 103(38.1)** | 225(46.7) | 328(43.6) |
p=0.15 induction therapy group vs. primary surgery group
p=0.37 induction therapy group vs. primary surgery group
p=0.026 induction therapy group vs. primary surgery group
Figure 1.
Incidence of Clinical T2N0 Diagnosis and Rate of Induction Therapy Given to Patients with cT2N0 Esophageal Cancer
Table 3 outlines the sensitivity, specificity, Positive Predictive Value (PPV), Negative Predictive Value (NPV), and accuracy for cT2N0 diagnosis among patients undergoing surgery without induction therapy. The overall accuracy for the cT2 primary tumor diagnosis was 76.6% while the accuracy of the cN0 nodal diagnosis was 74.4%. This is in comparison to the accuracy of cT2N0 diagnosis of 79.6% outlined in Table 3.
Table 3.
Sensitivity, Specificity, Positive Predictive Value (PPV), Negative Predictive Value (NPV), and Accuracy for Clinical Staging of T2N0 Esophageal Cancer*
| Pathologic Stage | Clinical stage | ||||||
|---|---|---|---|---|---|---|---|
| cT2N0 | NOT cT2N0 | Accuracy | Sensitivity | Specificity | PPV | NPV | |
| pT2N0 | 132 | 98 | 79.6% | 57.4% | 82.2% | 27.4% | 94.3% |
| NOT pT2N0 | 350 | 1614 | |||||
Analysis includes all patients undergoing primary esophagectomy without induction therapy for esophageal cancer in the STS Thoracic Database
Patients who received induction therapy had a slightly different pattern of discordance between clinical and pathological staging compared to the primary surgery group (Table 2). In the induction group 31.1% (84) of patients were downstaged to pT0-1N0 compared to 25.9% (125) in the surgery group (p=0.15). Fewer patients had pathological upstaging in the induction therapy group vs. the primary surgery group (38.1% versus 46.7%, p=0.026,). When comparing the induction therapy and surgery group, upstaging occurred due to tumor (T) alone (Induction 27.2%, Surgery 18.2%), nodal disease (N) alone (36.9%, 44.4%) and T and N (35.9%, 37.3%). Among the group of patients that were upstaged at surgery from cT2N0, nodal upstaging accounted for 81.7% of upstaging in the primary surgery group vs. 72.7% in the induction therapy group (p=0.09). Interestingly, 39 of the 40 patients that had T0N0 tumors in the final pathology were in the induction therapy group and were likely a result of complete pathological response. The incidence of complete pathologic response in the induction group was 14.4% (39/270).
Thirty-day mortality was 3.7% in both the induction therapy group (10/270) as well as the primary surgical group (18/482, p=1.0). Furthermore, there was no detectable difference in the overall complication rate between the induction therapy group and the primary surgical group (46.3% vs. 45%, p=0.76) (Table 4). Anastomotic complications requiring surgical intervention were more common in the primary surgery group vs. the induction therapy group (7.3% vs. 3.0%, p=0.014). Furthermore, there was no difference in the incidence of any complication between patients that were clinically understaged vs. those that were appropriately staged or overstaged.
Table 4.
Post-operative Complications after Esophagectomy in cT2N0 patients Undergoing Induction Therapy (N=270) vs. Primary Surgery (N=482)
| Induction Therapy (n=270) | Primary Surgery (n=482) | Total (n=752) | ||
|---|---|---|---|---|
| Complication | N(%) | N(%) | N(%) | P |
| Air leak > 5 days | 3(1.1) | 3(0.6) | 6(0.8) | 0.67 |
| Atelectasis requiring bronchoscopy | 13(4.8) | 32(6.6) | 45(6.0) | 0.34 |
| Pneumonia | 19(7.0) | 51(10.6) | 70(9.3) | 0.12 |
| Adult Respiratory Distress Syndrome | 9(3.3) | 11(2.3) | 20(2.7) | 0.48 |
| Pulmonary Embolus | 4(1.5) | 9(1.9) | 13(1.7) | 0.78 |
| Ventilator support > 48 hours | 11(4.1) | 15(3.1) | 26(3.5) | 0.53 |
| Reintubation | 28(10.4) | 72(14.9) | 100(13.3) | 0.093 |
| Tracheostomy | 17(6.3) | 27(5.6) | 44(5.9) | 0.75 |
| Other pulmonary event | 19(7.0) | 37(7.7) | 56(7.4) | 0.88 |
| Atrial arrhythmia requiring treatment | 56(20.7) | 86(17.8) | 142(18.9) | 0.33 |
| Anastomotic complication requiring medical treatment only | 17(6.3) | 33(6.8) | 50(6.6) | 0.88 |
| Anastomotic complication requiring surgical treatment | 8(3.0) | 35(7.3) | 43(5.7) | 0.014 |
| Chylothorax requiring drainage/medical treatment only | 11(4.1) | 6(1.2) | 17(2.3) | 0.019 |
| Chylothorax requiring surgical treatment | 4(1.5) | 11(2.3) | 15(2.0) | 0.59 |
| Complications (at least one) | 125(46.3) | 217(45) | 342(45.5) | 0.76 |
Examining the group that underwent surgery without induction therapy, we attempted to identify factors associated with discrepancies between clinical and pathological staging. In this group, 86.3% (182) of the patients had adenocarcinoma and 13.7% (29) had squamous cell carcinoma. The grade distribution was Gx (13, 6.1%), G1 (24, 11.2%), G2 (90, 42.1%), G3 (85, 39.7%), and G4 (2, 0.9%). Pathological type of tumor was not associated with pathologic upstaging among cT2N0 patients. Using univariate regression analysis: age, histologic tumor grade, gender, Zubrod score, and the absence of prior thoracic procedures were associated with tumor upstaging. However, on multivariate analysis, only male gender, higher Zubrod score, and absence of prior thoracic surgical procedures were associated with tumor upstaging at final pathology (Table 5). Histologic tumor grade was not included in the multivariate analysis because it was only reported in Version 2.081 of the database. Other variables: including co-morbidities, race, age, smoking, American Society of Anesthesiologist (ASA) risk score, BMI, tumor histology, and tumor size did not correlate with tumor upstaging. A separate analysis specifically examining tumor histology demonstrated a similar proportion of patients clinically understaged among patients with adenocarcinoma vs. squamous cell carcinoma (data not shown).
Table 5.
Predictors of Pathologic Upstaging in cT2N0 Patients Undergoing Primary Surgical Resection without Induction Therapy. Univariate and Multivariate Analysis of 482 Patients.
| Characteristic | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |
| Gender | 0.01 | 0.02 | ||||
| Female | 1.00 | 1.00 | ||||
| Male | 1.78 | 1.10-2.88 | 1.85 | 1.07-3.18 | ||
| Zubrod Score | 0.03 | 0.04 | ||||
| 0 | 1.00 | 1.00 | ||||
| 1 | 1.75 | 1.15-2.66 | 1.69 | 1.06-2.69 | ||
| 2 | 2.71 | 0.98-7.47 | 3.71 | 1.23-11.18 | ||
| 3 | 1.72 | 0.47-6.27 | 1.42 | 0.35-5.71 | ||
| Prior Thoracic Surgery | 0.03 | 0.01 | ||||
| No | 1.00 | 1.00 | ||||
| Yes | 0.51 | 0.27-0.96 | 0.45 | 0.23-0.86 | ||
Comment
While the techniques of clinical staging cannot be confirmed within the current versions of the GTSD, this study confirms the findings presented in previous single center studies regarding the inaccuracy of clinical staging of T2N0 esophageal cancer.[9, 14-16] From these data, the reliability of the cT2N0 diagnosis was poor with only 27.4% confirmed pT2N0. While 46.7% were apparently understaged and ultimately upstaged at surgery, 25.9% were clinically overstaged and ultimately downstaged at surgery to pT0-1N0. In a similar study by Rice et al, among 53 cT2N0 patients, only 13% were found to be pT2N0 while 55% were downstaged at surgery (pT0-1N0) and 32% were upstaged.[9] Similar studies of cT2N0 disease have also been limited by small sample size given the infrequency of this clinical stage of esophageal cancer in any single institutional experience. The STS GTSD provided the opportunity to evaluate this subset of patients using a multi-institutional contemporary cohort. Unfortunately, based on the distribution of pathologic stage, the clinical staging of T2N0 disease remains unpredictable.
As with other small studies, the majority of patients that were clinically understaged were mis-staged due to occult nodal disease.[9, 14] Specifically, occult nodal disease played a role in 81.8% of the clinically understaged patients. Unfortunately, details on the clinical staging techniques were not a required field within the database. Thus, we do not know the specifics of how EUS was performed or if EUS-fine needle aspiration (FNA) was utilized consistently. Performance of EUS-FNA improves both the specificity and the sensitivity for identification of nodal metastases vs. the utilization of EUS clinical criteria alone.[20, 21] Performance of FNA of a subclinical lymph node that may contain metastatic disease will improve the sensitivity of EUS with no additional risk added to the procedure. Thus, more consistent utilization of EUS-FNA in these presumed clinical T2N0 patients is a potential area of improvement in the assessment of nodal disease. Regarding the 18.2% of patients that were understaged based upon inaccurate assessment of the depth of tumor invasion alone, a more meticulous approach to ultrasound evaluation of the entire lesion may help to limit this problem. Careful evaluation of the entire lesion with use of higher frequency probes that provide greater detail of the layers of the esophagus(12 or 20 MHz) may be an appropriate approach to patients initially assessed as clinical T1-2N0.[22] It would seem reasonable to add a more detailed assessment with higher frequency probes if the decision to offer induction therapy is impacted directly by the clinical stage.
In this study, there were proportionately fewer patients upstaged at surgery after induction therapy compared to those who underwent primary resection. This is presumably multifactorial, although induction therapy likely has a beneficial effect on tumor eradication and clearing of nodal metastases. Alternatively, given the limitation of the database capturing only surgical patients, there may be a subset that had disease progression during the administration of induction therapy or failed to come to resection because of treatment-related morbidity. The proportion of patients who are intended to undergo resection after induction therapy, but who do not make it to surgery is generally small and often related to disease progression.[23, 24] Disease progression with induction therapy presumably would be less likely in cT2N0 disease compared to more locally advanced disease.
Despite the concept that limited disease (i.e. T1-2N0) is appropriately treated with primary surgical therapy, it appears that surgeons’ perception of the unreliable staging of T2N0 has prompted a more prolific use of induction therapy in these patients. Overall, 36% received some sort of induction regimen, although in the final year 53% received induction therapy. In earlier publications of cT2N0 disease, the percentage that received induction therapy was small ranging from 5-13%.[13, 14] Given the high incidence of occult nodal disease and the unreliability of clinical staging of T2N0 disease, some authors have advocated for routine administration of induction therapy in this population.[15, 16] While our data confirms the inaccuracy of clinical staging, this broad approach of offering induction therapy to all of these patients would mean that over 50% of those treated in this manner would have limited disease, 26% with T1N0, and 27.4% with T2N0 disease. The ultimate question regarding the management of this subset of patients is whether the survival benefit of offering induction therapy to cT2N0 patients outweighs the risk/cost associated with induction treatment. Unfortunately this is a significant limitation of this study in that long-term survival is not currently a component of the STS database. Currently From the current STS data presented here, there was no difference in 30-day mortality or morbidity after resection with or without induction therapy. Some studies have demonstrated higher perioperative morbidity among patients receiving induction therapy prior to esophageal resection.[25] While there is some controversy regarding the impact of induction therapy on perioperative morbidity, it may be that such an impact is more pronounced in patients with more advanced disease vs. more localized disease. This is one piece of the puzzle that would favor induction therapy in this group.
The more obvious answer to what treatment we offer these patients would be to simply improve upon the accuracy of clinical staging in this population. By univariate analysis, increasing histologic tumor grade (i.e. more poorly differentiated tumors) was associated with pathologic upstaging although this was not analyzed in the multivariate model because of incomplete data as described. This is congruent with previous findings from our single center study on cTT1-2N0 disease.[14] From the multivariate analysis we found that male gender and a higher Zubrod functional score (worse functional status) was associated with upstaging at surgery. Another limitation of the STS database for the purposes of this study is that there are factors that could potentially impact upstaging that were not consistently recorded. Such factors may include tumor length and PET uptake in the primary tumor which has previously been shown to be predictive of upstaging in cT1-2N0 patients.[14]
Utilizing the STS general thoracic surgery database, this is the first large scale study of clinical staging inaccuracies among patients with presumably limited disease. Despite the current limitations of the database and the lack of granularity regarding clinical staging techniques, it is clear that improvements in clinical staging are necessary to guide therapy. Technical improvements in clinical staging techniques as well as the potential use of surrogate markers to supplement clinical staging may improve the accuracy of staging of early esophageal cancers. It would seem that additional efforts to improve upon clinical staging would be more cost efficient, and perhaps safer, than broadly treating all cT2N0 patients with induction therapy. Alternatively, however, the equivalent rate of perioperative morbidity and mortality would favor the routine use of induction therapy in light of the high rate of occult nodal disease. Until we can confirm improvements in clinical staging, however, further multi-institutional studies are necessary to assess the relative survival benefit of induction therapy in this subset of patients.
Discussion
2. Richard E. Clark Paper for General Thoracic Surgery: Evaluation of the Reliability of Clinical Staging of T2 N0 Esophageal Cancer: A Review of the STS General Thoracic Surgery Database. Paper presented by Traves Crabtree, MD, St. Louis, MO. crabtreet@wustl.edu
Discussion by Cameron D. Wright, MD, Massachusetts cdwright@partners.org Dr. C. Wright (Boston, MA): I would like to congratulate Dr. Crabtree on an excellent presentation; very thoughtful and clear. I would also like to thank him and his colleagues for providing me a manuscript in a timely fashion. The manuscript is elegantly written with a very thorough analysis of both the data and a discussion of the findings.
There are two obvious limitations within the general thoracic database that pertain to this paper. One is prior to the current version of the database, we did not collect the method of staging of esophageal cancer. So it is unknown how many patients had EUS versus CT versus PET-CT in determining this T2 N0 clinical staging status. Furthermore, as he mentioned at the conclusion, we don't have a survival analysis to know, did it make any difference. The message of this paper is clear, though. Like Dr. Rice's seminal report in 2007 where only approximately 10% of patients were correctly staged, only one quarter of the patients that we clinically stage as T2N0 have the correct stage assigned, furthermore, most of them are seriously understaged. I have two questions.
Do you have a suggested strategy to refine the stage estimate when a T2 N0 lesion is diagnosed, perhaps by using a higher megahertz ultrasound probe, in order to better delineate the T1 T2 interface, which, in the end, is the most important aspect of this staging where we want to get it right? We want to make sure that we don't give induction therapy to T1 N0 patients.
2. Richard E. Clark Paper for General Thoracic Surgery: Evaluation of the Reliability of Clinical Staging of T2 N0 Esophageal Cancer: A Review of the STS General Thoracic Surgery Database. Response by Traves Crabtree, MD, St. Louis, MO.
DR. CRABTREE: Dr. Wright, thank you. That is a excellent question. I think certainly to delineate the different tumor stages between T1 and T2 or T2 and T3 that a higher frequency probe would intuitively allow us to see that subtle interface better. There have been some studies, however, to suggest that it is not the panacea. The probe is a little bit more difficult to utilize; it is a smaller probe. So sometimes if it's in a dilated esophagus there is a lot of air around it making visualization more difficult. So while you may have better resolution, it wouldn't solve all of the problems.
There were some things that we couldn't look at in the database that may be clinical predictors. One that wasn't in the multivariate analysis because it wasn't included throughout the database was tumor differentiation; poorly differentiated tumors were more likely to be clinically understaged. Another thing would be tumor length. As you can imagine, the longer the tumor the more room for error. Also, SUV uptake by PET is another factor that we identified in a previous small study that could potentially predict clinical understaging, although we were not able to evaluate that here. Certainly in addition to using a higher frequency probe because of the missed advanced tumor stage, more liberal use of FNA at the time of EUS I think would be a very important factor as well.
DR. WRIGHT: Thank you. And my final question relates to, does this really matter? We have to interpret this study in the context of the CROSS trial that was just reported this year, which included T2 N0 patients in their induction strategy. In a large series of 366 patients with a relatively nontoxic induction program, there was a dramatic doubling in survival with induction therapy for locally advanced esophageal cancer, which in their study included T2 N0 patients. In fact, one of your last slides indicated that the surgeons of America are voting with their feet, and now more than 50% of the people with T2 N0 lesions are having induction therapy. I must say in my center, our oncologists strongly believe all T2 N0 patients should have induction therapy because of the very high likelihood of missed N1 disease, as you have shown elegantly in this study. So, again, does this really matter?
DR. CRABTREE: Another excellent question. It was interesting, in the CROSS trial, if you look at their hazard ratio model, induction therapy actually mostly favored the patients that were clinical N0 and there was no survival advantage in the patients that were clinically N1. So it could be that this subset of patients with I guess a lower burden of nodal disease in this setting, occult nodal disease, may be the patients that would potentially benefit from this induction therapy.
I agree, I think potentially now I will likely change my practice to offer these patients induction therapy, although ultimately it would be nice to see long-term survival data in this subset to really confirm that. But I think this is another piece of the puzzle that supports the CROSS trial data as well. I will say, like the database, only 15% of the patients in the CROSS trial were T2 N0. So it was a small subset of patients.
2. Richard E. Clark Paper for General Thoracic Surgery: Evaluation of the Reliability of Clinical Staging of T2 N0 Esophageal Cancer: A Review of the STS General Thoracic Surgery Database. Paper presented by Traves Crabtree, MD, St. Louis, MO. crabtreet@wustl.edu
Discussion by Douglas E. Wood, MD, Washington dewood@u.washington.edu Dr. D. Wood (Seattle, WA): That is a great presentation and delivered some new insights, and Dr. Wright correctly pointed out the limitation of the staging modalities over this time period, which is significantly limiting. My biggest question has to do with institutional variation or experience and its impact on reliability. I know that wasn't part of your study and I don't even know whether maybe there is enough numbers to be valid, but we certainly know with EUS that operator experience is a very important contributor to accuracy. The biggest argument I would say from your paper for induction therapy for clinical T2 is that most of the clinical T2 patients are not pathologic T2, just as Dr. Wright pointed out. But if in an institution you had a high degree of clinical accuracy and actually mostly did have T2 N0, would that potentially be a reason to continue your strategy of primary surgery rather than induction therapy, which is largely driven by the unexpected presence of N1 disease?
2. Richard E. Clark Paper for General Thoracic Surgery: Evaluation of the Reliability of Clinical Staging of T2 N0 Esophageal Cancer: A Review of the STS General Thoracic Surgery Database. Response by Traves Crabtree, MD, St. Louis, MO.
DR. CRABTREE: Dr. Wood, thank you. Actually if you look at the data, it's a coin toss: about 50% are actually going to be pathologic T1 or T2 N0 and about 50% are going to have a higher pathologic stage.
With regards to the experience of centers, it is still a little hard to tease out in the database. At the completion of this study there were 108 centers participating in the thoracic database. Only about 20 centers contributed more than 10 patients to this actual study. So 100 or so studies had less than 10 patients. I can't tell you about the details of how they performed EUS, the experience with EUS, but it's intuitive. The data in the endoscopy literature clearly shows that a greater experience improves the accuracy of EUS.
2. Richard E. Clark Paper for General Thoracic Surgery: Evaluation of the Reliability of Clinical Staging of T2 N0 Esophageal Cancer: A Review of the STS General Thoracic Surgery Database. Paper presented by Traves Crabtree, MD, St. Louis, MO. crabtreet@wustl.edu Discussion by Steven R. DeMeester, MD, California sdemeester@surgery.usc.edu Dr. S. DeMeester (Los Angeles, CA): I enjoyed your paper very much. First a comment.
The most important risk factor for dying from esophageal cancer is lymph node disease. So if you look at your group that were overstaged, those less than T2 N0, add to those the true T2 N0, and then add any T3 N0 patients, who also have a very good survival rate because they are node negative, the subgroup that really might benefit from neoadjuvant therapy, the group at risk for systemic disease, ie those with nodal disease, becomes very small.
My question is, in the STS database, do we know what happens to the patients that get induction therapy? If you look at the CROSS trial and every published trial on neoadjuvant therapy, a number drop out because of disease progression during therapy or because they get too sick to subsequently have their surgery. Does the STS database know how many patients never made it to surgery with relatively early stage disease for which they were getting toxic chemotherapy?
2. Richard E. Clark Paper for General Thoracic Surgery: Evaluation of the Reliability of Clinical Staging of T2 N0 Esophageal Cancer: A Review of the STS General Thoracic Surgery Database. Response by Traves Crabtree, MD, St. Louis, MO.
DR. CRABTREE: Dr. DeMeester, thank you. That is an excellent point. I saw you are also doing the pro-con talk on the treatment of clinical T2 N0 disease.
No, the only patients that we have in the database are the patients that actually underwent esophagectomy. It was interesting in the CROSS trial that the rate of fallout in that trial was only about 6%, and most of those patients were from disease progression.
2. Richard E. Clark Paper for General Thoracic Surgery: Evaluation of the Reliability of Clinical Staging of T2 N0 Esophageal Cancer: A Review of the STS General Thoracic Surgery Database. Paper presented by Traves Crabtree, MD, St. Louis, MO. crabtreet@wustl.edu
Discussion by Wayne L. Hofstetter, MD, Texas whofstetter@mdanderson.org Dr. W. Hofstetter (Houston, TX): Talking about better staging, we have to enter into a discussion about EMR and using EMR more liberally to try and delineate those T0 and T1 patients. So I think the key to us getting rid of that 25% over-diagnosis is the liberal use of diagnostic EMR.
2. Richard E. Clark Paper for General Thoracic Surgery: Evaluation of the Reliability of Clinical Staging of T2 N0 Esophageal Cancer: A Review of the STS General Thoracic Surgery Database. Response by Traves Crabtree, MD, St. Louis, MO.
DR. CRABTREE: Thank you, Dr. Hofstetter. I think we do have to be careful, I agree with the ones that are specifically T1, attempting to determine whether they are T1a or T1b. You potentially increase the risk of perforation if you do EMR on a T2 lesion.
2. Richard E. Clark Paper for General Thoracic Surgery: Evaluation of the Reliability of Clinical Staging of T2 N0 Esophageal Cancer: A Review of the STS General Thoracic Surgery Database. Paper presented by Traves Crabtree, MD, St. Louis, MO. crabtreet@wustl.edu
Discussion by Hiran Fernando, MD, Massachusetts hiran.fernando@bmc.org Dr. H. Fernando (Boston, MA): I would echo Dr. DeMeester's comment that we have to focus on the patients who have nodal disease. There is about a 10% difference between your two groups of patients who were found to have nodal disease. The other factor to take into account is that after induction therapy, your ability to get a good lymph node dissection and your total lymph node counts are going to be less. So it may just be that you are not removing as many lymph nodes, and you may be understaging patients as well. So the question is, does it really matter whether we give induction therapy or not for these patients?
2. Richard E. Clark Paper for General Thoracic Surgery: Evaluation of the Reliability of Clinical Staging of T2 N0 Esophageal Cancer: A Review of the STS General Thoracic Surgery Database. Response by Traves Crabtree, MD, St. Louis, MO.
DR. CRABTREE: No doubt we need to look at long-term survival to look at the relative benefit of induction therapy, but I don't have the data on the adequacy of nodal dissection in the induction therapy group or primary surgical group. I think it does matter that we are potentially offering surgery to a quarter of patients with T1 N0 disease. That might be a group that we would like to avoid induction therapy in.
DR. FERNANDO: But the actual difference is only about 10% in patients who had nodal disease between the two groups, and that may have been a factor related to the adequacy of that nodal dissection.
DR. CRABTREE: Perhaps. I don't have the data to confirm or deny that. Ultimately, the only way to answer this question is to determine the impact of induction therapy on longterm survival in clinical T2N0 patients. These data don't afford us that opportunity.
2. Richard E. Clark Paper for General Thoracic Surgery: Evaluation of the Reliability of Clinical Staging of T2 N0 Esophageal Cancer: A Review of the STS General Thoracic Surgery Database. Paper presented by Traves Crabtree, MD, St. Louis, MO. crabtreet@wustl.edu
Discussion by John R. Benfield, MD, California j.benfield@ucla.edu
Dr. J. Benfield (Los Angeles, CA): As we are approaching the 50th anniversary of the Society of Thoracic Surgeons, these excellent papers cause me to reflect about the history of the database that is now about 25 years old. The New York Times had reported misleading cardiac surgery outcomes that were not risk-adjusted. The late Paul Ebert, a Past President of the AATS, and other database visionaries, saw the need for risk-adjusted outcomes to inform our patients, and to guide us in therapy. This database is widely acknowledged for its groundbreaking originality and importance. It is the kind of initiative that should be brought forward to the press that so often portrays physicians as greedy and self-serving. The public would appreciate knowing that our primary and over-riding interested is in the welfare of our patients.
DR. CRABTREE: Thank you very much, sir.
Abbreviations and Acronyms
- AJCC
American Joint Committee on Cancer
- ASA
American Society of Anesthesiologists
- BMI
Body Mass Index
- c
Clinical Staging
- CT
Computed Tomography
- CHF
Congestive Heart Failure
- CAD
Coronary Artery Disease
- EMR
Esophageal Mucosal Resection
- EUS
Esophageal Ultrasound
- FNA
Fine Needle Aspiration
- GTSDB
General Thoracic Surgery Database
- Gx
Grade undetermined
- G1
Grade well differentiated
- G2
Grade moderately differentiated
- G3
Grade poorly differentiated
- G4
Grade undifferentiated
- N
Lymph Nodes
- MHz
Megahertz
- NCCN
National Comprehensive Cancer Network
- NPV
Negative Predictive Value
- p
Pathologic Staging
- PVD
Peripheral Vascular Disease
- PPV
Positive Predictive Value
- PET
Positron Emission Tomography
- XRT
Radiation Therapy
- SAS
Statistical Analysis Software
- STS
Society of Thoracic Surgeons
- T
Tumor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the 49th Annual Meeting of the Society of Thoracic Surgeons, Los Angeles, CA, Jan 27-30, 2013. Winner of the Thoracic Richard E. Clark Award.
References
- 1.Urschel JD, Vasan H, Blewett CJ. A meta-analysis of randomized controlled trials that compared neoadjuvant chemotherapy and surgery to surgery alone for resectable esophageal cancer. Am J Surg. 2002;183(3):274–9. doi: 10.1016/s0002-9610(02)00795-x. [DOI] [PubMed] [Google Scholar]
- 2.Graham AJ, et al. Defining the optimal treatment of locally advanced esophageal cancer: a systematic review and decision analysis. Ann Thorac Surg. 2007;83(4):1257–64. doi: 10.1016/j.athoracsur.2006.11.061. [DOI] [PubMed] [Google Scholar]
- 3.van Hagen P, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366(22):2074–84. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- 4.Tepper J, et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol. 2008;26(7):1086–92. doi: 10.1200/JCO.2007.12.9593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malthaner R, Wong RK, Spithoff K. Preoperative or postoperative therapy for resectable oesophageal cancer: an updated practice guideline. Clin Oncol (R Coll Radiol) 2010;22(4):250–6. doi: 10.1016/j.clon.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 6.Li Z, Rice TW. Diagnosis and staging of cancer of the esophagus and esophagogastric junction. Surg Clin North Am. 2012;92(5):1105–26. doi: 10.1016/j.suc.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 7.Rice TW, Blackstone EH, Rusch VW. 7th edition of the AJCC Cancer Staging Manual: esophagus and esophagogastric junction. Ann Surg Oncol. 2010;17(7):1721–4. doi: 10.1245/s10434-010-1024-1. [DOI] [PubMed] [Google Scholar]
- 8.Network NCC. NCCN Clinical Practice Guidelines in Oncology. Esophageal and esophagogastric junction cancers. 2011 www.nccn.org, Editor.
- 9.Rice TW, et al. T2N0M0 esophageal cancer. J Thorac Cardiovasc Surg. 2007;133(2):317–24. doi: 10.1016/j.jtcvs.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 10.DeWitt J, et al. Endoscopic ultrasound for esophageal and gastroesophageal junction cancer: Impact of increased use of primary neoadjuvant therapy on preoperative locoregional staging accuracy. Dis Esophagus. 2005;18(1):21–7. doi: 10.1111/j.1442-2050.2005.00444.x. [DOI] [PubMed] [Google Scholar]
- 11.Kutup A, et al. Quality control of endoscopic ultrasound in preoperative staging of esophageal cancer. Endoscopy. 2007;39(8):715–9. doi: 10.1055/s-2007-966655. [DOI] [PubMed] [Google Scholar]
- 12.Pech O, et al. Accuracy of endoscopic ultrasound in preoperative staging of esophageal cancer: results from a referral center for early esophageal cancer. Endoscopy. 2010;42(6):456–61. doi: 10.1055/s-0029-1244022. [DOI] [PubMed] [Google Scholar]
- 13.Rice TW, et al. Role of clinically determined depth of tumor invasion in the treatment of esophageal carcinoma. J Thorac Cardiovasc Surg. 2003;125(5):1091–102. doi: 10.1067/mtc.2003.404. [DOI] [PubMed] [Google Scholar]
- 14.Crabtree TD, et al. Endoscopic ultrasound for early stage esophageal adenocarcinoma: implications for staging and survival. Ann Thorac Surg. 2011;91(5):1509–15. doi: 10.1016/j.athoracsur.2011.01.063. discussion 1515-6. [DOI] [PubMed] [Google Scholar]
- 15.Zhang JQ, et al. Neoadjuvant chemoradiation therapy is beneficial for clinical stage T2 N0 esophageal cancer patients due to inaccurate preoperative staging. Ann Thorac Surg. 2012;93(2):429–35. doi: 10.1016/j.athoracsur.2011.10.061. discussion 436-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stiles BM, et al. Clinical T2-T3N0M0 esophageal cancer: the risk of node positive disease. Ann Thorac Surg. 2011;92(2):491–6. doi: 10.1016/j.athoracsur.2011.04.004. discussion 496-8. [DOI] [PubMed] [Google Scholar]
- 17.Barbour AP, et al. Endoscopic ultrasound predicts outcomes for patients with adenocarcinoma of the gastroesophageal junction. J Am Coll Surg. 2007;205(4):593–601. doi: 10.1016/j.jamcollsurg.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 18.Heeren PA, et al. Influence of tumor characteristics on the accuracy of endoscopic ultrasonography in staging cancer of the esophagus and esophagogastric junction. Endoscopy. 2004;36(11):966–71. doi: 10.1055/s-2004-825956. [DOI] [PubMed] [Google Scholar]
- 19.Kelly S, et al. A systematic review of the staging performance of endoscopic ultrasound in gastro-oesophageal carcinoma. Gut. 2001;49(4):534–9. doi: 10.1136/gut.49.4.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vazquez-Sequeiros E. Nodal staging: number or site of nodes? How to improve accuracy? Is FNA always necessary? Junctional tumors--what's N and what's M? Endoscopy. 2006;38(Suppl 1):S4–8. doi: 10.1055/s-2006-946642. [DOI] [PubMed] [Google Scholar]
- 21.Vazquez-Sequeiros E, et al. Routine vs. selective EUS-guided FNA approach for preoperative nodal staging of esophageal carcinoma. Gastrointest Endosc. 2006;63(2):204–11. doi: 10.1016/j.gie.2005.08.053. [DOI] [PubMed] [Google Scholar]
- 22.Yoshinaga S, et al. Endoscopic ultrasound using ultrasound probes for the diagnosis of early esophageal and gastric cancers. World J Gastrointest Endosc. 2012;4(6):218–26. doi: 10.4253/wjge.v4.i6.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gilbert S, et al. Impact of patient selection, disease progression, and adverse events on esophageal cancer outcomes after trimodality therapy. Ann Thorac Surg. 2012;94(5):1659–66. doi: 10.1016/j.athoracsur.2012.05.044. [DOI] [PubMed] [Google Scholar]
- 24.Walsh TN, et al. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med. 1996;335(7):462–7. doi: 10.1056/NEJM199608153350702. [DOI] [PubMed] [Google Scholar]
- 25.Murthy SC, et al. Induction chemoradiotherapy increases pleural and pericardial complications after esophagectomy for cancer. J Thorac Oncol. 2009;4(3):395–403. doi: 10.1097/JTO.0b013e318195a625. [DOI] [PubMed] [Google Scholar]