Abstract
Infantile Pompe disease is a fatal genetic muscle disorder caused by a deficiency of acid alpha-glucosidase, a glycogen-degrading lysosomal enzyme. We constructed a plasmid containing a 5'-shortened human acid alpha-glucosidase cDNA driven by the cytomegalovirus promoter, as well as the aminoglycoside phosphotransferase and dihydrofolate reductase genes. Following transfection in dihydrofolate reductase-deficient Chinese hamster ovary cells, selection with Geneticin, and amplification with methotrexate, a cell line producing high levels of the alpha-glucosidase was established. In 48 hr, the cells cultured in Iscove's medium with 5 mM butyrate secreted 110-kDa precursor enzyme that accumulated to 91 micrograms.ml-1 in the medium (activity, > 22.6 mumol.hr-1.ml-1). This enzyme has a pH optimum similar to that of the mature form, but a lower Vmax and Km for 4-methylumbelliferyl-alpha-D-glucoside. It is efficiently taken up by fibroblasts from Pompe patients, restoring normal levels of acid alpha-glucosidase and glycogen. The uptake is blocked by mannose 6-phosphate. Following intravenous injection, high enzyme levels are seen in heart and liver. An efficient production system now exists for recombinant human acid alpha-glucosidase targeted to heart and capable of correcting fibroblasts from patients with Pompe disease.
Full text
PDF

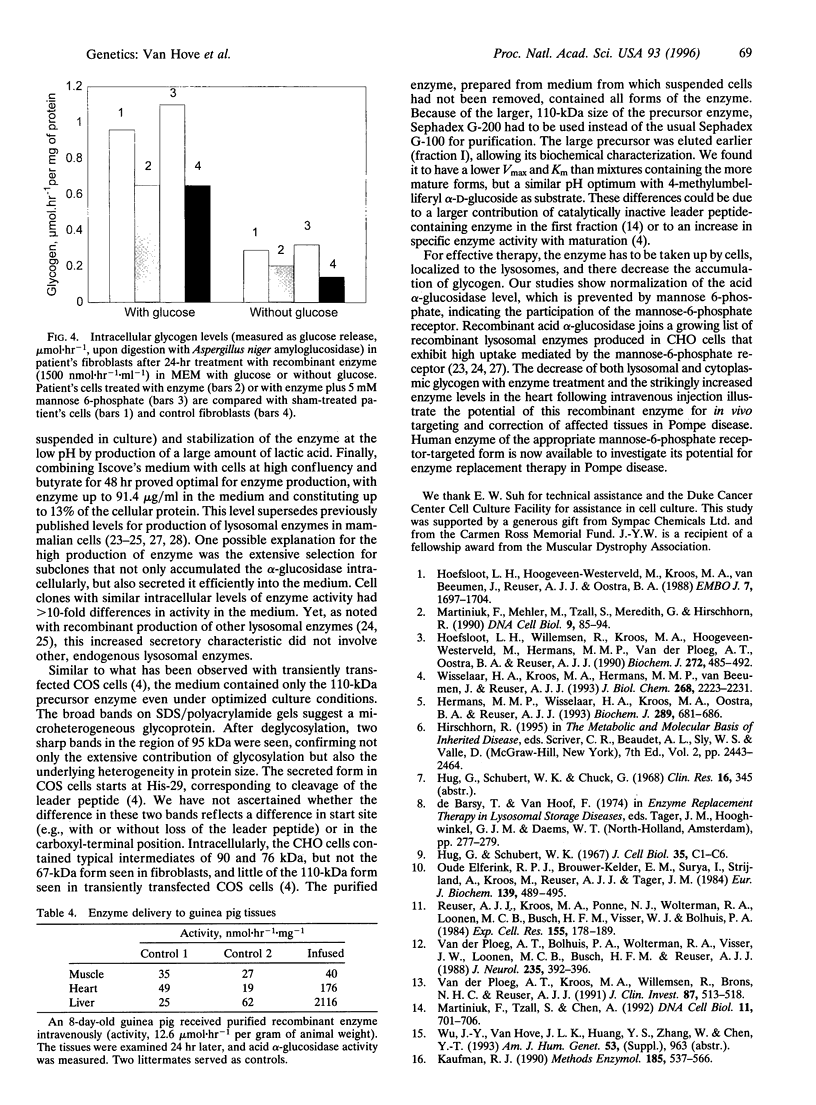

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anson D. S., Taylor J. A., Bielicki J., Harper G. S., Peters C., Gibson G. J., Hopwood J. J. Correction of human mucopolysaccharidosis type-VI fibroblasts with recombinant N-acetylgalactosamine-4-sulphatase. Biochem J. 1992 Jun 15;284(Pt 3):789–794. doi: 10.1042/bj2840789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsoum J. Introduction of stable high-copy-number DNA into Chinese hamster ovary cells by electroporation. DNA Cell Biol. 1990 May;9(4):293–300. doi: 10.1089/dna.1990.9.293. [DOI] [PubMed] [Google Scholar]
- Bielicki J., Hopwood J. J., Wilson P. J., Anson D. S. Recombinant human iduronate-2-sulphatase: correction of mucopolysaccharidosis-type II fibroblasts and characterization of the purified enzyme. Biochem J. 1993 Jan 1;289(Pt 1):241–246. doi: 10.1042/bj2890241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Dorner A. J., Wasley L. C., Kaufman R. J. Increased synthesis of secreted proteins induces expression of glucose-regulated proteins in butyrate-treated Chinese hamster ovary cells. J Biol Chem. 1989 Dec 5;264(34):20602–20607. [PubMed] [Google Scholar]
- Hermans M. M., Wisselaar H. A., Kroos M. A., Oostra B. A., Reuser A. J. Human lysosomal alpha-glucosidase: functional characterization of the glycosylation sites. Biochem J. 1993 Feb 1;289(Pt 3):681–686. doi: 10.1042/bj2890681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoefsloot L. H., Hoogeveen-Westerveld M., Kroos M. A., van Beeumen J., Reuser A. J., Oostra B. A. Primary structure and processing of lysosomal alpha-glucosidase; homology with the intestinal sucrase-isomaltase complex. EMBO J. 1988 Jun;7(6):1697–1704. doi: 10.1002/j.1460-2075.1988.tb02998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoefsloot L. H., Willemsen R., Kroos M. A., Hoogeveen-Westerveld M., Hermans M. M., Van der Ploeg A. T., Oostra B. A., Reuser A. J. Expression and routeing of human lysosomal alpha-glucosidase in transiently transfected mammalian cells. Biochem J. 1990 Dec 1;272(2):485–492. doi: 10.1042/bj2720485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hug G., Schubert W. K. Lysosomes in type II glycogenosis. Changes during administration of extract from Aspergillus niger. J Cell Biol. 1967 Oct;35(1):C1–C6. doi: 10.1083/jcb.35.1.c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannou Y. A., Bishop D. F., Desnick R. J. Overexpression of human alpha-galactosidase A results in its intracellular aggregation, crystallization in lysosomes, and selective secretion. J Cell Biol. 1992 Dec;119(5):1137–1150. doi: 10.1083/jcb.119.5.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakkis E. D., Matynia A., Jonas A. J., Neufeld E. F. Overexpression of the human lysosomal enzyme alpha-L-iduronidase in Chinese hamster ovary cells. Protein Expr Purif. 1994 Jun;5(3):225–232. doi: 10.1006/prep.1994.1035. [DOI] [PubMed] [Google Scholar]
- Kaufman R. J. Selection and coamplification of heterologous genes in mammalian cells. Methods Enzymol. 1990;185:537–566. doi: 10.1016/0076-6879(90)85044-o. [DOI] [PubMed] [Google Scholar]
- Martiniuk F., Mehler M., Tzall S., Meredith G., Hirschhorn R. Sequence of the cDNA and 5'-flanking region for human acid alpha-glucosidase, detection of an intron in the 5' untranslated leader sequence, definition of 18-bp polymorphisms, and differences with previous cDNA and amino acid sequences. DNA Cell Biol. 1990 Mar;9(2):85–94. doi: 10.1089/dna.1990.9.85. [DOI] [PubMed] [Google Scholar]
- Martiniuk F., Tzall S., Chen A. Recombinant human acid alpha-glucosidase generated in bacteria: antigenic, but enzymatically inactive. DNA Cell Biol. 1992 Nov;11(9):701–706. doi: 10.1089/dna.1992.11.701. [DOI] [PubMed] [Google Scholar]
- Oude Elferink R. P., Brouwer-Kelder E. M., Surya I., Strijland A., Kroos M., Reuser A. J., Tager J. M. Isolation and characterization of a precursor form of lysosomal alpha-glucosidase from human urine. Eur J Biochem. 1984 Mar 15;139(3):489–495. doi: 10.1111/j.1432-1033.1984.tb08032.x. [DOI] [PubMed] [Google Scholar]
- Park H. K., Kay H. H., McConkie-Rosell A., Lanman J., Chen Y. T. Prenatal diagnosis of Pompe's disease (type II glycogenosis) in chorionic villus biopsy using maltose as a substrate. Prenat Diagn. 1992 Mar;12(3):169–173. doi: 10.1002/pd.1970120305. [DOI] [PubMed] [Google Scholar]
- Reuser A. J., Koster J. F., Hoogeveen A., Galjaard H. Biochemical, immunological, and cell genetic studies in glycogenosis type II. Am J Hum Genet. 1978 Mar;30(2):132–143. [PMC free article] [PubMed] [Google Scholar]
- Reuser A. J., Kroos M. A., Ponne N. J., Wolterman R. A., Loonen M. C., Busch H. F., Visser W. J., Bolhuis P. A. Uptake and stability of human and bovine acid alpha-glucosidase in cultured fibroblasts and skeletal muscle cells from glycogenosis type II patients. Exp Cell Res. 1984 Nov;155(1):178–189. doi: 10.1016/0014-4827(84)90779-1. [DOI] [PubMed] [Google Scholar]
- Reuser A. J., Kroos M., Oude Elferink R. P., Tager J. M. Defects in synthesis, phosphorylation, and maturation of acid alpha-glucosidase in glycogenosis type II. J Biol Chem. 1985 Jul 15;260(14):8336–8341. [PubMed] [Google Scholar]
- Van der Ploeg A. T., Kroos M. A., Willemsen R., Brons N. H., Reuser A. J. Intravenous administration of phosphorylated acid alpha-glucosidase leads to uptake of enzyme in heart and skeletal muscle of mice. J Clin Invest. 1991 Feb;87(2):513–518. doi: 10.1172/JCI115025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisselaar H. A., Kroos M. A., Hermans M. M., van Beeumen J., Reuser A. J. Structural and functional changes of lysosomal acid alpha-glucosidase during intracellular transport and maturation. J Biol Chem. 1993 Jan 25;268(3):2223–2231. [PubMed] [Google Scholar]
- van der Ploeg A. T., Bolhuis P. A., Wolterman R. A., Visser J. W., Loonen M. C., Busch H. F., Reuser A. J. Prospect for enzyme therapy in glycogenosis II variants: a study on cultured muscle cells. J Neurol. 1988 Sep;235(7):392–396. doi: 10.1007/BF00314479. [DOI] [PubMed] [Google Scholar]