Summary
Background
The cascade of HIV care has become a focal point for implementation efforts to maximise the individual and public health benefits of antiretroviral therapy. We aimed to characterise longitudinal changes in engagement with the cascade of HIV care in British Columbia, Canada, from 1996 to 2011.
Methods
We used estimates of provincial HIV prevalence from the Public Health Agency of Canada and linked provincial population-level data to define, longitudinally, the numbers of individuals in each of the eight stages of the cascade of HIV care (HIV infected, diagnosed, linked to HIV care, retained in HIV care, highly active antiretroviral therapy (HAART) indicated, on HAART, adherent to HAART, and virologically suppressed) in British Columbia from 1996 to 2011. We used sensitivity analyses to determine the sensitivity of cascade-stage counts to variations in their definitions.
Findings
13 140 people were classified as diagnosed with HIV/AIDS in British Columbia during the study period. We noted substantial improvements over time in the proportions of individuals at each stage of the cascade of care. Based on prevalence estimates, the proportion of unidentified HIV-positive individuals decreased from 49·0% (estimated range 36·2–57·5%) in 1996 to 29·0% (11·6–40·7%) in 2011, and the proportion of HIV-positive people with viral suppression reached 34·6% (29·0–43·1%) in 2011.
Interpretation
Careful mapping of the cascade of care is crucial to understanding what further efforts are needed to maximise the beneficial effects of available interventions and so inform efforts to contain the spread of HIV/AIDS.
Funding
British Columbia Ministry of Health, US National Institute on Drug Abuse (National Institutes of Health).
Introduction
The introduction of highly active antiretroviral therapy (HAART) in 1996 was a substantial advance in HIV care.1 HAART can stop HIV replication on a sustained basis and, as a result, plasma HIV-1 RNA concentration (or plasma viral load) becomes undetectable.2 International guidelines have uniformly recognised that sustained, full suppression of plasma viral load is needed to optimise the therapeutic effect of HAART.2–4 This viral suppression allows immune reconstitution to take place, leading to long-term disease remission and prolonged survival.5,6 During the past decade, HAART has become more potent, better tolerated, and less complex,7 thus improving health outcomes for patients at all stages of disease progression.
In the past few years, results of several studies have shown the secondary benefit of HAART in preventing HIV transmission,8–10 and expansion of treatment is increasingly recognised as a key strategy in prevention, with efforts to expand access to HAART increasing worldwide.11 The notion of treatment as prevention has been embraced as a crucial component of combination prevention strategies,12,13 and discourse has shifted towards the best implementation strategy for such programmes.14
To achieve a reduction in HIV transmission, HAART programmes have to ensure the effectiveness and quality of a cascade of services, from testing and referral to care, to ensuring continued adherence to treatment.15,16 Indeed, the cascade of HIV care has become a focal point for implementation efforts to maximise the beneficial effects of HIV treatment for individuals and populations, emphasised by WHO as the central assessment and monitoring metric for treatment as prevention in global AIDS response and progress reporting.17
Deficits in individual engagement in HIV care, including late diagnosis, suboptimum linkage to and retention in HIV care, low HAART coverage, and poor adherence to treatment, pose serious barriers to the achievement of the best possible individual and population health outcomes.16,18 A thorough understanding of the points within the cascade of care continuum at which individuals are lost—referred to as leakage—will inform efforts to optimise HAART roll-out strategies in the interest of reducing HIV incidence in populations. We aimed to characterise longitudinal changes in engagement with the cascade of HIV care in British Columbia from the beginning of the HAART era in 1996 to the end of 2011.
Methods
Data sources and analysis
We used data from several sources to determine the cascade of HIV care. First, we used annual estimates of HIV prevalence for British Columbia from the Public Health Agency of Canada, derived from a multiple-method approach based on back-calculation from HIV/AIDS surveillance data and other sources.19 Estimates from the Public Health Agency of Canada preceded the construction of the linked database used in this study.
We used a series of linked provincial datasets (comprising the linked database of the STOP HIV/AIDS initiative) to estimate the number of identified HIV-positive individuals in the various stages of the cascade of HIV care (HIV infected, diagnosed, linked to HIV care, retained in HIV care, HAART indicated, on HAART, adherent to HAART, and virologically suppressed). The BC Centre for Disease Control is the provincial agency that centralises all HIV testing data and receives reports of new HIV diagnoses from the British Columbia Public Health Microbiology and Reference Laboratory, which does all confirmatory testing in the province. Furthermore, mandatory HIV reporting legislation has been in place in British Columbia since 2003; individuals can choose to have their identifiable information suppressed in HIV case reports to the Public Health Microbiology and Reference Laboratory (non-nominal vs nominal reporting). For individuals aged 18 months or older, the BC Centre for Disease Control uses a screening test (ELISA) to detect HIV antibodies, with HIV diagnosis confirmed on the basis of a reactive western blot or nucleic acid amplification test.
We used data for plasma viral load, CD4 cell count testing, and HAART use from the BC Centre for Excellence in HIV/AIDS population-based registries. The BC Centre for Excellence is the agency that centrally distributes all antiretroviral drugs in the province. It maintains comprehensive clinical guidelines for the management of HIV/AIDS, which have remained consistent with those published by the International AIDS Society (IAS)-USA every 2 years since 1996.1–7 All measurements of plasma viral load in British Columbia are done under the auspices of the BC Centre for Excellence at the virology laboratory of St Paul’s Hospital (Vancouver, BC), thus 100% of data for plasma viral load are captured. Additionally, an estimated 80% of all CD4 cell count measurements done in the province were captured in the BC Centre for Excellence data.20
We supplemented these data with the medical services plan physician billing database, which captures all fee-for-service care in the province, including HIV-related physician visits and other services; the provincial discharge abstract database, which records inpatient care; the British Columbia PharmaNet database, which captures all non-antiretroviral drug dispensations (used to assess administrative loss to follow-up); and the British Columbia Vital Statistics database.
Linkage and preparation of the de-identified individual-level database was facilitated by the British Columbia Ministry of Health, and fully described in a previous report.21 We excluded individuals from the cascade of care after death or administrative loss to follow-up, defined as having no record of death and no health administrative records from any of the linked databases for a period of at least 18 months before the end of study follow-up (March 31, 2012). Intermittent losses to follow-up were therefore not excluded by this definition; any return to care within 18 months of the conclusion of follow-up would entail continued inclusion in the cohort.
The BC Centre for Excellence received ethical approval to do this study from the University of British Columbia ethics review committee at St Paul’s Hospital, Providence Health Care site (P05-123). The programme also conforms to British Columbia’s Freedom of Information and Protection of Privacy Act.
Definitions
Whenever possible, our definitions for the eight stages of the cascade of HIV care (panel 1) followed or were adapted from evidence-based standards.22–24
Panel 1. Operational definitions for the eight stages of the cascade of HIV care.
HIV infected
Based on HIV prevalence estimates reported by the Public Health Agency of Canada*
HIV diagnosed
Defined as the first instance of any one of:
a confirmed HIV-positive test
detectable plasma viral load†
an HIV-related MSP billing or hospital admission
a reported AIDS-defining illness
dispensation of antiretroviral therapy
Linked to HIV care
Among HIV-diagnosed individuals, defined as:
Retained in HIV care
Among individuals linked to HIV care, defined as:
HIV-related physician visits or diagnostic tests (CD4 cell count or plasma viral load test) ≥3 months apart within the calendar year
or at least two antiretroviral drug dispensations ≥3 months apart, within the calendar year
HAART indicated
Among individuals retained in HIV care but not currently on HAART; defined as meeting the primary or secondary IAS-USA initiation criteria within the calendar year:
1996: CD4 count <500 cells per μL, plasma viral load ≥30 000 copies per mL, or AIDS-defining illness
1997–99: plasma viral load >5000 copies per mL or AIDS-defining illness
2000–01: CD4 count <500 cells per μL, plasma viral load ≥30 000 copies per mL, or AIDS-defining illness
2002–07: CD4 count ≤200 cells per μL or AIDS-defining illness
2008–09: CD4 count ≤350 cells per μL or AIDS-defining illness
2010–11: CD4 count ≤500 cells per μL or AIDS-defining illness
On HAART
Among individuals with HAART indicated, defined as receiving at least two antiretroviral drug dispensations ≥3 months apart, within the calendar year
Adherent to HAART
Among individuals on HAART, defined as having at least 80% adherence§ in the calendar year, or from the point of antiretroviral initiation for those who began treatment within the calendar year
Virologically suppressed
Among individuals adherent to HAART, defined as having at least one episode (≥3 months) with an undetectable plasma viral load† within the calendar year
MSP=medical services plan. HAART=highly active antiretroviral therapy.
IAS=International AIDS Society.
HIV infection, diagnosis, and linkage to care were all fixed classifications. Once infected, diagnosed, or linked to care, an individual is counted as such for each subsequent calendar year until death or administrative loss to follow-up. Individual classifications in each of the subsequent stages of care varied over time: from one calendar year to another, individuals can be lost to care after a period of retention, become ineligible for HAART, drop out of HAART, or become non-adherent and not virologically suppressed after periods of stable, suppressive treatment. The denominator in each step of the cascade is the sum of the preceding stage—ie, the number of individuals with suppressed plasma viral load is calculated as a proportion of the number who are adherent to HAART, the number of individuals adherent to HAART is calculated as a proportion of the number on HAART, and so on.
Because cascade-stage definitions are bound to differ across settings on the basis of data availability and differences in data-generating processes, we did sensitivity analyses on selected stage classifications to assess the effect of our definitions on the results. We did sensitivity analyses on the specific definitions of linkage to HIV care, retention in HIV care, on HAART, and viral suppression. The sensitivity analysis for definitions of viral suppression also assessed an alternative definition of the denominator for this stage that included all individuals with at least one measurement of plasma viral load within the calendar year.
Finally, although viral suppression is the most relevant endpoint for individual health benefits derived from treatment, a dose-response relation between plasma viral load and the risk of HIV transmission suggests that public health benefits extend beyond full viral suppression.9,25 We therefore present data for aggregate plasma viral load for the population of individuals who received at least one plasma viral load test in each calendar year throughout the study period. We used the highest annual measurement of plasma viral load for each individual in this analysis to provide a conservative estimate of aggregate viral load for those with a recorded measurement, thus probably overestimating the aggregate amount of virus at any point in time during the calendar year.
Role of the funding source
The sponsor of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. BN had full access to all the data in the study and JSGM had final responsibility for the decision to submit for publication. The British Columbia Ministry of Health facilitated access to components of the linked database.
Results
Estimates of annual HIV prevalence, along with empirically derived counts of diagnosed cases are presented in figure 1. We estimated the remaining stages of the cascade of HIV care by use of linked individual data for the population in British Columbia (figure 2). 13 140 people were classified as diagnosed with HIV/AIDS in British Columbia during the study period. The proportion of HIV-positive individuals diagnosed with either a positive HIV test or otherwise identified as HIV-positive increased from 51·0% in 1996 to 71·0% in 2011. Although the proportion of HIV-positive individuals linked to HIV care was 4·1–9·6% less than the proportion diagnosed throughout the study period, retention in HIV care lagged far behind linkage, reaching 80·5% of those diagnosed in 2011. The numbers of individuals indicated for and accessing HAART were close to those for retention in HIV care, apart from during 2000–06, when guidelines for initiation of HAART were changed such that treatment was only indicated for patients with CD4 counts of less than 200 cells per μL. Finally, the proportion of HIV-positive individuals with viral suppression increased from 0·7% in 1996 to 34·6% (estimated range 29·0–43·1%) in 2011, with steep increases between 1996 and 2000 (from 0·7% to 13·7%) and between 2003 and 2011 (from 16·5% to 34·6%). The appendix provides absolute numbers for each stage of the cascade.
Figure 1. Estimated annual HIV prevalence and number of individuals diagnosed.
Prevalence estimates are based on unpublished data from the Public Health Agency of Canada (Archibald C, Public Health Agency of Canada, personal communication).
Figure 2. The cascade of HIV care.
HAART=highly active antiretroviral therapy.
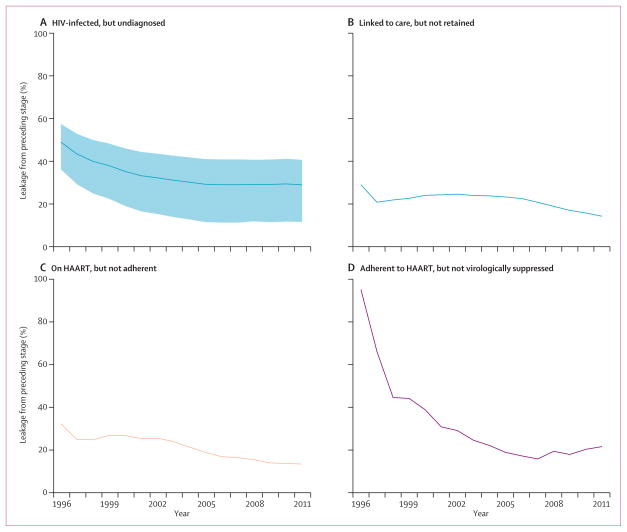
The proportion of people infected but undiagnosed fell from 49·0% (estimated range 36·2–57·5%) to 29·0% (11·6–40·7) during the study period (figure 3); during the same period the proportion of individuals linked to but not retained in care remained fairly constant at 20·0%. Among people on HAART, the proportion not adherent decreased from 24·0% in 2003 to 13·4% in 2011. The greatest gains were realised in viral suppression—the proportion of people adherent but not virologically suppressed decreased from 95·2% in 1996 to 21·6% in 2011.
Figure 3. Changes in leakage from the cascade of HIV care.
Shaded region in (A) represents HIV prevalence range estimates from the Public Health Agency of Canada (Archibald C, Public Health Agency of Canada, personal communication). HAART=highly active antiretroviral therapy.
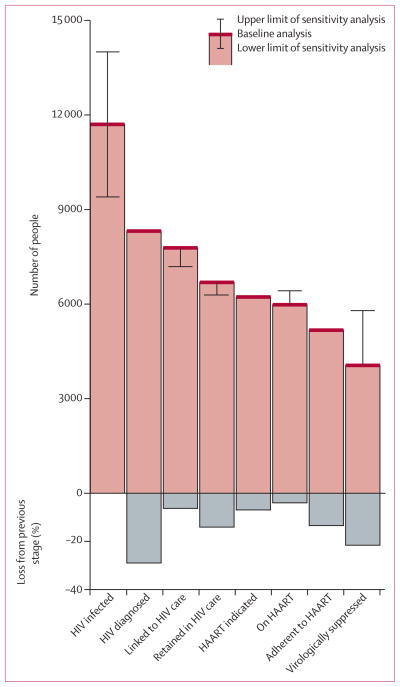
Sensitivity analyses revealed some differences dependent on cascade definitions (figures 4, 5). Excluding CD4 cell counts and plasma viral load testing from definitions of linkage and retention in HIV care resulted in figures up to 18 percentage points lower than the baseline definitions. Separating HIV-related physician visits from other fee-for-service billings captured in the medical services plan dataset made little difference in proportions for linkage and retention. Our more conservative on-HAART classification, which required at least two dispensations at least 3 months apart, resulted in a difference of nearly 20 percentage points in positive classification compared with the most liberal definition of any antiretroviral dispensation within the calendar year; however, this difference decreased to less than five percentage points in 2011.
Figure 4. Sensitivity analyses for cascade-stage definitions.
Viral suppression thresholds: <500 copies per mL for 1996, <400 copies per mL for 1997–98, and <50 copies per mL for 1999–2011. HAART=highly active antiretroviral therapy. MSP=medical services plan. *Takes into account all diagnosed individuals in the numerator of the estimated proportion, including those who did not meet the baseline thresholds for adherence, being on HAART, HAART indicated, or retained in or linked to care.
Figure 5. Cascade of HIV care, including estimates of HIV prevalence and ranges from sensitivity analyses.
Prevalence estimates are based on unpublished data from the Public Health Agency of Canada (Archibald C, Public Health Agency of Canada, personal communication). Error bars represent plausible intervals from sensitivity analyses of cascade-stage definitions. HAART=highly active antiretroviral therapy.
Classifications of viral suppression were sensitive to the definitions used. Our most conservative definition, which required suppressed plasma viral load measurements at least 3 months apart, positively classified up to 49% of diagnosed individuals in 2011. An alternative definition that required two consecutive findings of suppressed plasma viral load resulted in an additional four percentage points of diagnosed individuals being classified as suppressed; use of a constant threshold of plasma viral load of less than 500 copies per mL increased the proportion by an additional nine percentage points. Finally, the most liberal definition, which required only one measurement in which plasma viral load was undetectable in a calendar year, classified roughly five percentage points of additional cases as suppressed from 1998 onwards. Use of the same threshold of a single measurement of less than 500 copies per mL, but with the inclusion of individuals not adherent to treatment (and thus not included in other classifications), resulted in 15–25 percentage points of additional viral load suppression from 1998 onward.
Aggregate plasma viral load measurements, based on the highest available measurement for each individual who received a test in each calendar year, showed decreasing proportions of people in high viral load strata and gains in viral suppression (figure 6).
Figure 6. Aggregate HIV-1-RNA concentrations in HIV-positive individuals.
Data represent the highest plasma viral load measurement for each individual who received a plasma viral load test in each calendar year. *Viral suppression thresholds: <500 copies per mL for 1996, <400 copies per mL for 1997–98, and <50 copies per mL for 1999–2011.
Discussion
In British Columbia, we noted substantial improvements in the proportions of people diagnosed, on HAART, and virologically suppressed, largely as a result of increased testing intensity,8 changes in IAS-USA treatment initiation guidelines,1,2,7 improvements in compliance with HIV care guidelines,26 and clinical response to treatment.27 With the linked administrative data system established as part of the STOP HIV/AIDS initiative, the cascade of care provides an easily interpretable framework to analyse data for the numbers and demographic characteristics of people lost to care at various points on the HIV care continuum; to track HIV-related disparities and health inequities; to provide a basis to inform potential redistribution of resources to improve the efficiency and quality of care and reduce health disparities; to provide a basis for continued assessment of the effect of the various provincial governing bodies responsible for HIV/AIDS care on the coverage, use, and quality of health care for people with HIV, allowing identification of any difficulties encountered and informing future planning; and to provide a window into provincial health policy that can be used as a template for national efforts (panel 2).22
Panel 2. Research in context.
Systematic review
We searched PubMed for articles published in English up to April 31, 2013, that included the terms “HIV” and “cascade”, “continuum”, or “retention” in the abstract. One peer-reviewed study16 estimated the cascade of HIV care for the US population, and this estimate was later updated by the US Centers for Disease Control,28 which suggested that 40% or fewer HIV-positive individuals were retained in care, and 19–25% were virologically suppressed.
Interpretation
Although each stage in the cascade of HIV care in British Columbia has improved with time, gaps in diagnosis and retention in care remain. Our longitudinal characterisation of the cascade allows for explicit consideration of changes in cascade leakage between stages over time. The results of our sensitivity analyses showed that cascade-stage counts are sensitive to the definitions of cascade stages used, particularly with respect to how viral suppression is measured. In view of the absence of evidence-based standards for cascade-stage definitions, caution should be used when comparing our results with those from other jurisdictions. Our methods could be useful in the design of HIV surveillance systems internationally.
Future efforts in the province should focus on the engagement of individuals linked to or retained in HIV care, but not accessing HAART. Further expansion of HIV testing is also a priority; however, uncertainty exists with respect to the number, distribution, and characteristics of undiagnosed HIV-positive individuals in British Columbia. Well designed epidemiological studies to better define this population are needed to inform future HIV testing campaigns. Furthermore, the definition of cascades of care stratified by key demographic characteristics (mode of transmission, age, ethnic origin, etc), characteristics of those lost to care at various stages, and within specific geographical regions would allow for better targeted surveillance systems that can be tracked over time to monitor progress. Such improvements are the key focal points for HIV surveillance in British Columbia in the future, and can inform similar efforts nationally and internationally.
Each stage of the cascade can be affected by several individual and systemic barriers; however, financial constraints consistently play a prominent part. Even within a universal health-care system, most jurisdictions in Canada charge some form of copayment or deductible against prescription drugs, including antiretroviral therapy. For example, as of 2006, Ontario’s annual prescription drug plan for non-elderly people with a net annual household income of less than CAN$100 000 entailed a deductible of $150–4089, and a user copayment of $2 per prescription, with no maximum annual contribution.29 The effect of prescription drug cost-sharing on access to antiretroviral therapy in Canada is unclear; higher copayments have been associated with reduced adherence and increased treatment interruption in US settings.30,31 British Columbia is unique in Canada, because HAART and laboratory and medical monitoring of HIV-infected individuals is universally covered and fully subsidised. Our results are thus likely to represent a best-case scenario, in which individuals are not subject to financial disincentives and state-of-the-art antiretroviral management is consistently recommended and available.
Nonetheless, we noted substantial leakage in the cascade of HIV care, particularly at the stages of retention in care, which is an independent predictor of survival.32 Late initiation of antiretroviral therapy (CD4 count <200 cells per μL or an AIDS-defining illness) was favoured by guidelines between 2002 and 2007.33–35 As of 2010, therapeutic guidelines have increasingly recommended that antiretroviral therapy be offered to most infected individuals immediately on diagnosis and, increasingly, irrespective of CD4 cell count.2 These new guidelines negate the necessity of a HAART-indicated classification in future cascades of care, and should decrease loss from the retention stage in the future. Further efforts, such as the refinement of HIV primary care guidelines, intensive case management, outreach, and quality improvement initiatives are urgently needed to ensure sustained engagement in appropriate care and to allow re-engagement by individuals lost to HIV care.
Our sensitivity analyses support the use of the selected cascade stage definitions and provide a basis of comparison for similar efforts in other jurisdictions. Some reports3,22,36 have defined retention in HIV care as having an HIV-related physician visit on two or more occasions at least 3 months apart in a 12 month period. We extended this definition to include any HIV care (physician visit, on HAART, or having a routine CD4 cell count or plasma viral load test). Data for plasma viral load testing and CD4 cell counts in the absence of HIV- related physician visits comprised a large proportion of those defined as linked and retained in care, and are thus a useful measure of entry into care after HIV diagnosis1 and an important component of HIV surveillance.
The sensitivity analyses for definitions of viral suppression showed the greatest variation. National estimates in the USA suggest that between 19% and 28% of the HIV-infected population were virologically suppressed in 2010.19,37 Our threshold for viral suppression required at least two plasma viral load readings below the threshold for suppression (<50 copies per mL from 1999 onwards) at least 3 months apart, consistent with definitions of retention in HIV care and being on HAART. Furthermore, our denominator included only patients adherent to HAART, consistent with the cascade-of-care model. These definitions were chosen solely for their value as indicators of individual and public health benefit.
Although a comparison with previous US estimates is indirect, with a threshold of one plasma viral load measurement of less than 50 copies per mL in a calendar year, among any HIV-positive individuals who received a plasma viral load test (previous thresholds of plasma viral load were <200 or <500 copies per mL), the proportion of individuals in British Columbia classified as virologically suppressed was 70% (5792/8308) of those diagnosed, and 50% (5792/11 700) of the estimated infected population in 2011. Using a plasma viral load threshold of less than 200 copies per mL, the North American AIDS cohort Collaboration on Research and Design investigators38 reported that 72% of US participants were virologically suppressed; however, the investigators used as a denominator the number of individuals linked to HIV care. Despite being widely used, our sensitivity analysis suggests that definitions based on single measurements of plasma viral load suppression probably misclassify as suppressed a substantial proportion of individuals who are not stably engaged in treatment in a given calendar year.
Despite the comprehensive scope of the data systems used, our study has several limitations related to measurements at each stage of the cascade of care. First, the number of prevalent cases is not known and was instead derived from a national modelling effort on the basis of common assumptions about key model parameters across provinces.39 In view of the significantly decreasing rates of new cases of HIV in British Columbia compared with stable or increasing rates elsewhere in Canada,20 these estimates might be positively biased. Second, although administrative loss to follow-up was accounted for, emigration from the province, particularly in 2010 and 2011 in view of the study cutoff point, might have resulted in us overestimating the numbers of people diagnosed and linked to care, thereby underestimating proportions of individuals receiving HAART and those virologically suppressed. Earlier annual administrative losses to follow-up (between 1996 and 2009) were in the range of 1·50% (70 of 4656 diagnosed) in 1997 to 1·98% (102 of 5160 diagnosed) in 1998; therefore, this misclassification was probably small. Third, the study cohort was defined partly on the basis of health administrative data; therefore, some cases could have been misclassified, potentially missing some undiagnosed cases. We have described the procedures used to construct and validate the cohort elsewhere21—specifically, we applied case-finding algorithms to identify HIV-positive individuals identified as such only from health administrative databases.
Outpatient care delivered in some inner-city health clinics had billing by session rather than fee-for-service, and was therefore not captured in the medical services plan database. Our definition of retention in HIV care thus incorporated antiretroviral dispensations, for which we have complete capture. Also, the number of individuals with nominal and therefore linkable HIV diagnoses was underestimated, particularly before 2003, when HIV became reportable and systematic follow-up of all new HIV diagnoses commenced, improving data quality for identifiers. As a result, our previous analysis estimated that only 52% of individuals accessing HIV care in British Columbia had a linked HIV test available,21 which would result in our overestimating the proportion of undiagnosed infections before 2003.
With respect to the subsequent cascade stages, incomplete capture of CD4 cell count measurements could have resulted in underestimates for retention in HIV care over time. Measurement of adherence to HAART was based on refill compliance, which might overestimate true adherence. Furthermore, an informal audit done in 2010 (Barrios R, unpublished) showed that 125 (2·4%) of 5264 individuals eligible for antiretroviral therapy in the area under the jurisdiction of the Vancouver Coastal Health Authority were on treatment and being monitored in the context of industry-sponsored clinical trials, and thereby not captured by the BC Centre for Excellence databases; our estimates for treatment uptake, adherence, and viral suppression are thus slightly lower than actual numbers, resulting in a small conservative bias. Finally, assessments of need for antiretroviral therapy were simplified from actual recommendations, focusing on standard CD4 cell counts, plasma viral load tests, and AIDS-defining illness thresholds for eligibility, and not taking into account secondary or individual-specific considerations.
Our results show a steady improvement in the engagement of people with HIV within the cascade of care in British Columbia during the HAART era. Careful mapping of the cascade of care is crucial to improve our understanding of how to maximise the beneficial effects of available interventions and to inform efforts to contain the spread of HIV/AIDS. A high-quality HIV surveillance system actively linked to relevant administrative health records is essential for such an endeavour.
Supplementary Material
Acknowledgments
This study was funded by the British Columbia Ministry of Health-funded Seek and Treat for Optimal Prevention of HIV & AIDS pilot project. We acknowledge the assistance of David Milan and Suzanne Humphreys in early efforts towards this report, and all the British Columbia Ministry of Health and Vancouver Coastal Health decision support staff involved in data access and procurement, including Monika Lindegger (Clinical Prevention Services, BC Centre for Disease Control), Elsie Wong (Public Health Agency of Canada [PHAC]), Al Cassidy (British Columbia Ministry of Health Registries), Joleen Wright (Vancouver Coastal Health decision support), and Karen Luers (Vancouver Coastal Health decision support). We also thank Chris Archibald for providing PHAC estimates of annual HIV prevalence for British Columbia, Canada. BN is a Canadian Institutes of Health Research Bisby Fellow. BN and VDL are funded by the Michael Smith Foundation for Health Research.
JSGM has received grants from Abbott Laboratories, bioLytical Laboratories, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Janssen Pharmaceuticals, Merck, and ViiV Healthcare. He has also received support from the British Columbia Ministry of Health; a Knowledge Translation Award from the Canadian Institutes of Health Research; and an Avant-Garde Award (1DP1DA026182) from the US National Institute on Drug Abuse (National Institutes of Health); the International AIDS Society; UNAIDS; WHO; the US National Institutes of Health Office of AIDS Research; the US National Institute of Allergy and Infectious Diseases; the US President’s Emergency Plan For AIDS Relief; the Bill & Melinda Gates Foundation; the French National Agency for Research on AIDS and Viral Hepatitis; and the Public Health Agency of Canada.
The STOP HIV/AIDS Study Group
Patty Daly (Vancouver Coastal Health), Perry R W Kendall (British Columbia Ministry of Health and Faculty of Medicine, University of British Columbia), Ciro Panessa (British Columbia Ministry of Health), and Nancy South (British Columbia Ministry of Health).
Footnotes
Unpublished data (Archibald C, Public Health Agency of Canada, personal communication).
Based on plasma viral load testing technology available at the time of measurement; virological suppression thresholds: <500 copies per mL for 1996, <400 copies per mL for 1997–98, and <50 copies per mL for 1999–2011.
Plasma viral load test, CD4 cell count, HIV-related physician visit, or antiretroviral drugs dispensed.
Refers to the number of days of drugs dispensed, divided by the total number of days in care.
Contributors
BN and JSGM had the initial idea for the research. BN led in the preparation of the report and contributed to the analysis. GC led the analysis. BY, KC, and VDL also contributed to the analysis. KH, MG, HS, and RSH contributed to the preparation of the report. JSGM, RB, RG, and RSH contributed to the procurement of the data on which the study is based. All authors contributed to data interpretation and approved the submitted version of the report.
Conflicts of interest
All other authors declare that they have no conflicts of interest.
References
- 1.Carpenter CC, Fischl MA, Hammer SM, et al. Antiretroviral therapy for HIV infection in 1996. Recommendations of an international panel. International AIDS Society-USA. JAMA. 1996;276:146–54. [PubMed] [Google Scholar]
- 2.Thompson MA, Aberg JA, Cahn P, et al. Antiretroviral treatment of adult HIV infection: 2010 recommendations of the International AIDS Society-USA Panel. JAMA. 2010;304:321–33. doi: 10.1001/jama.2010.1004. [DOI] [PubMed] [Google Scholar]
- 3.WHO. [accessed Nov 2, 2012];ART guidelines for adults and adolescents—evidence map. 2010 http://www.who.int/hiv/topics/treatment/evidence/en/index.html.
- 4.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Washington, DC: US Department of Health & Human Services; 2011. [accessed March 12, 2012]. http://aidsinfo.nih.gov/contentfiles/AdultandAdolescentGL.pdf. [Google Scholar]
- 5.Hogg RS, O’Shaughnessy MV, Gataric N, et al. Decline in deaths from AIDS due to new antiretrovirals. Lancet. 1997;349:1294. doi: 10.1016/S0140-6736(05)62505-6. [DOI] [PubMed] [Google Scholar]
- 6.The Antiretroviral Therapy in Lower Income Countries (ART-LINC) Collaboration and ART Cohort Collaboration (ART-CC) groups. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet. 2006;367:817–24. doi: 10.1016/S0140-6736(06)68337-2. [DOI] [PubMed] [Google Scholar]
- 7.Thompson MA, Aberg JA, Hoy JF, et al. Antiretroviral treatment of adult HIV infection: 2012 recommendations of the International Antiviral Society-USA Panel. JAMA. 2012;308:387–402. doi: 10.1001/jama.2012.7961. [DOI] [PubMed] [Google Scholar]
- 8.Montaner JSG, Lima VD, Barrios R, et al. Association of highly active antiretroviral therapy coverage, population viral load, and yearly new HIV diagnoses in British Columbia, Canada: a population-based study. Lancet. 2010;376:532–39. doi: 10.1016/S0140-6736(10)60936-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wood E, Kerr T, Marshall BDL, et al. Longitudinal community plasma HIV-1-RNA concentrations and incidence of HIV-1 among injecting drug users: a prospective cohort study. BMJ. 2009;338:b1649. doi: 10.1136/bmj.b1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen MS, Chen YQ, McCauley M, et al. for the HPTN 052 study team. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Granich RM, Gilks CF, Dye C, De Cock KM, Williams BG. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet. 2009;373:48–57. doi: 10.1016/S0140-6736(08)61697-9. [DOI] [PubMed] [Google Scholar]
- 12.Dieffenbach CW, Fauci AS. Universal voluntary testing and treatment for prevention of HIV transmission. JAMA. 2009;301:2380–82. doi: 10.1001/jama.2009.828. [DOI] [PubMed] [Google Scholar]
- 13.Coates TJ, Richter L, Caceres C. Behavioural strategies to reduce HIV transmission: how to make them work better. Lancet. 2008;372:669–84. doi: 10.1016/S0140-6736(08)60886-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Granich R, Gupta S, Suthar A, et al. for the ART in Prevention of HIV and TB Research Writing Group. Antiretroviral therapy in prevention of HIV and TB: update on current research efforts. Curr HIV Res. 2011;9:446–69. doi: 10.2174/157016211798038597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burns DN, Dieffenbach CW, Vermund SH. Rethinking prevention of HIV type-1 infection. Clin Infect Dis. 2010;51:725–31. doi: 10.1086/655889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gardner EM, McLees MP, Steiner JF, del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis. 2011;52:793–800. doi: 10.1093/cid/ciq243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.WHO. Meeting report on framework for metrics to support effective treatment as prevention; 2–3 April 2012; Geneva, Switzerland. Geneva: World Health Organization; 2012. [accessed Nov 12, 2012]. http://www.who.int/iris/handle/10665/75387. [Google Scholar]
- 18.Eldred L, Malitz F. Introduction to the supplemental issue on the HRSA SPNS outreach initiative. AIDS Patient Care STDS. 2007;21 (suppl 1):S1–2. [Google Scholar]
- 19.British Columbia Centre for Disease Control. [accessed Oct 15, 2012];HIV and sexually transmitted infections. 2010 http://www.bccdc.ca/NR/rdonlyres/2035512C-DBEC-495B-A332-C410EE9520C7/0/CPS_Report_STI_HIV_2010_annual_report_FINAL_20111122.pdf.
- 20.Hogg RS, Heath K, Lima VD, et al. Disparities in the burden of HIV/AIDS in Canada. PLoS One. 2012;7:e47260. doi: 10.1371/journal.pone.0047260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nosyk B, Colley G, Chan K, et al. for the STOP HIV/AIDS Study Team. Application of case-finding algorithms for identifying individuals with human immunodeficiency virus from administrative data in British Columbia, Canada. PLoS One. 2013;8:e54416. doi: 10.1371/journal.pone.0054416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Institute of Medicine. Monitoring HIV care in the United States: a strategy for generating national estimates of HIV care and coverage. Washington, DC: The National Academies Press; 2012. [PubMed] [Google Scholar]
- 23.Thompson MA, Mugavero MJ, Amico KR, et al. Guidelines for improving entry into and retention in care and antiretroviral adherence for persons with HIV: evidence-based recommendations from an International Association of Physicians in AIDS Care Panel. Ann Intern Med. 2012;156:817–33. doi: 10.7326/0003-4819-156-11-201206050-00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.US Department of Health & Human Services, Health Resources and Services Administration. [accessed Feb 12, 2013];HAB HIV core clinical performance measures for adult/adolescent clients: group 1. http://hab.hrsa.gov/deliverhivaidscare/files/habgrp1pms08.pdf.
- 25.Quinn TC, Wawer MJ, Sewankambo N, et al. for the RAKAI project study group. Viral load and heterosexual transmission of human immunodeficiency virus type 1. N Engl J Med. 2000;342:921–29. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 26.Lima VD, Le A, Nosyk B, et al. Development and assessment of a composite programme assessment score for initial HIV therapy. PLoS One. 2012;7:e47859. doi: 10.1371/journal.pone.0047859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nosyk B, Min JE, Lima VD, Yip B, Hogg RS, Montaner JSG on behalf of the STOP HIV/AIDS Study Group. HIV-1 disease progression during highly active antiretroviral therapy: an application using population-level data in British Columbia: 1996–2011. J Acquir Immune Defic Syndr. 2013;63:653–59. doi: 10.1097/QAI.0b013e3182976891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.US Centers for Disease Control and Prevention. [accessed Feb 3, 2013];HIV in the United States: the stages of care. http://www.cdc.gov/nchhstp/newsroom/docs/2012/Stages-of-CareFactSheet-508.pdf.
- 29.Demers V, Melo M, Jackevicius C, et al. Comparison of provincial prescription drug plans and the impact on patients’ annual drug expenditures. CMAJ. 2008;178:405–09. doi: 10.1503/cmaj.070587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnston SS, Juday T, Seekins D, Espindle D, Chu BC. Association between prescription cost sharing and adherence to initial combination antiretroviral therapy in commercially insured antiretroviral-naïve patients with HIV. J Manag Care Pharm. 2012;18:129–45. doi: 10.18553/jmcp.2012.18.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Das-Douglas M, Riley ED, Ragland K, et al. Implementation of the Medicare Part D prescription drug benefit is associated with antiretroviral therapy interruptions. AIDS Behav. 2009;13:1–9. doi: 10.1007/s10461-008-9401-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tripathi A, Youmans E, Gibson JJ, Duffus WA. The impact of retention in early HIV medical care on viro-immunological parameters and survival: a statewide study. AIDS Res Hum Retroviruses. 2011;27:751–58. doi: 10.1089/AID.2010.0268. [DOI] [PubMed] [Google Scholar]
- 33.Carpenter CC, Cooper DA, Fischl MA, et al. Antiretroviral therapy in adults: updated recommendations of the International AIDS Society-USA Panel. JAMA. 2000;283:381–90. doi: 10.1001/jama.283.3.381. [DOI] [PubMed] [Google Scholar]
- 34.Yeni PG, Hammer SM, Carpenter CC, et al. Antiretroviral treatment for adult HIV infection in 2002: updated recommendations of the International AIDS Society-USA Panel. JAMA. 2002;288:222–35. doi: 10.1001/jama.288.2.222. [DOI] [PubMed] [Google Scholar]
- 35.Yeni PG, Hammer SM, Hirsch MS, et al. Treatment for adult HIV infection: 2004 recommendations of the International AIDS Society-USA Panel. JAMA. 2004;292:251–65. doi: 10.1001/jama.292.2.251. [DOI] [PubMed] [Google Scholar]
- 36.Mugavero MJ, Davila JA, Nevin CR, Giordano TP. From access to engagement: measuring retention in outpatient HIV clinical care. AIDS Patient Care STDS. 2010;24:607–13. doi: 10.1089/apc.2010.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen M, Rhodes PH, Hall HI, Kilmarx PH, Branson BM, Valleroy LA. Prevalence of undiagnosed HIV infection among persons aged ≥13 years—National HIV surveillance system, United States, 2005–2008. MMWR Morb Mortal Wkly Rep. 2012;61 (suppl):57–64. [PubMed] [Google Scholar]
- 38.Althoff KN, Buchacz K, Hall HI, et al. for the North American AIDS Cohort Collaboration on Research and Design. US trends in antiretroviral therapy use, HIV RNA plasma viral loads, and CD4 T-lymphocyte cell counts among HIV-infected persons, 2000 to 2008. Ann Intern Med. 2012;157:325–35. doi: 10.7326/0003-4819-157-5-201209040-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang Q, Boulos D, Yan P, et al. Estimates of the number of prevalent and incident human immunodeficiency virus (HIV) infections in Canada, 2008. Can J Public Health. 2010;101:486–90. doi: 10.1007/BF03403969. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.