Summary
Tumor endothelial cells (ECs) promote cancer progression in ways beyond their role as conduits supporting metabolism. However, it is not understood how vascular niche-derived paracrine factors, known as angiocrine factors, provoke tumor aggressiveness. Here, we show that FGF4 produced by B-Cell lymphoma cells (LCs) through activating FGFR1 upregulates the Notch-ligand Jagged1 (Jag1) on neighboring tumor ECs. In turn, upregulation of Jag1 on ECs reciprocally induces Notch2-Hey1 in LCs. This crosstalk enforces aggressive CD44+IGF1R+CSF1R+ LC phenotypes, including extra-nodal invasion and chemoresistance. Inducible EC-selective deletion of Fgfr1 or Jag1 in the Eμ-Myc lymphoma model or impairing Notch2 signaling in mouse and human LCs diminished lymphoma aggressiveness and prolonged mouse survival. Thus, targeting the angiocrine FGF4-FGFR1/Jag1-Notch2 loop could inhibit LC aggressiveness and enhance chemosensitivity.
INTRODUCTION
Vascular endothelial cells (ECs) are a specialized component of the tumor microenvironment that can orchestrate tumor growth and invasion (Beck et al., 2011; Bergers and Hanahan, 2008; Butler et al., 2010a; Calabrese et al., 2007; Carmeliet and Jain, 2011; Charles et al., 2010; Ghajar et al., 2013; Lu et al., 2013; Rakhra et al., 2010; Trimboli et al., 2009; Weis and Cheresh, 2011). During regeneration, tissue-specific ECs provide instructive paracrine cues, known as angiocrine growth factors, that trigger proliferation of repopulating progenitor cells (Brantley-Sieders et al., 2011; Butler et al., 2012; Butler et al., 2010a; Butler et al., 2010b; Ding et al., 2014; Ding et al., 2010; Ding et al., 2011; Ding et al., 2012; Potente et al., 2011; Red-Horse et al., 2007). However, the mechanism by which EC-derived angiocrine factors influence tumor behaviors is unknown (Gilbert and Hemann, 2010; Leite de Oliveira et al., 2012; Nakasone et al., 2012; Schmitt et al., 2000).
Notch signaling is a pivotal modulator of lymphomagenesis (Aster et al., 2008; Espinosa et al., 2010; Liu et al., 2010; Lobry et al., 2013), enhancing Myc activity and upregulating receptors such as IGF1R (Medyouf et al., 2011; Weng et al., 2006). The Jagged (Jag) and Delta-like (Dll) families of Notch ligands induce Notch signaling (Gridley, 2010; Siekmann and Lawson, 2007). Both Jag1 and Dll4 are preferentially expressed by ECs during tumor progression but have distinct roles in neoplastic tissue (Rehman and Wang, 2006; Sethi et al., 2011; Vilimas et al., 2007). Dll4 is expressed by sprouting ECs and appears to regulate EC expansion (proliferative angiogenesis), whereas juxtacrine activation of Notch receptors on tumor cells appears to be mediated by EC-derived Jag1 (inductive angiogenesis) (Lu et al., 2013; Sonoshita et al., 2011). However, mechanisms controlling expression of these Notch-ligands in tumor ECs are undefined (Benedito et al., 2009; Corada et al., 2010; High et al., 2008; Hoey et al., 2009; Hofmann et al., 2010; Noguera-Troise et al., 2006; Ridgway et al., 2006; Tung et al., 2012). Moreover, the paucity of EC-specific mouse genetic models has handicapped elucidation of the EC-derived angiocrine signals regulating the fate and behavior of tumors (Lu et al., 2013).
Malignant lymphoma cells (LCs) are composed of heterogeneous cell subpopulations, with a subset of LCs possessing more aggressive features (Dierks et al., 2007; Hoey et al., 2009; Kelly et al., 2007). Although chemotherapy eliminates the majority of proliferating LCs, a subpopulation of aggressive LCs manifests resistance, ultimately leading to lymphoma relapse. Because the surrounding microenvironment can support tumor cells (Hanahan and Coussens, 2012; Lane et al., 2009; Memarzadeh et al., 2007; Rakhra et al., 2010; Reimann et al., 2010; Scadden, 2012; Zhang et al., 2012), we reasoned that elucidating the microenvironmental signals (i.e. tumor vascular niche) influencing aggressive LCs, such as lymphoma initiating cells (LICs), could provide effective lymphoma treatment strategies.
RESULTS
ECs support expansion of LCs with aggressive features
To identify the crosstalk between ECs and LCs without the confounding influence of supplementation with exogenous serum and angiogenic growth factors, we devised a serum and growth factor-free platform to propagate LCs in co-culture with ECs. To this end, we transduced ECs, such as human umbilical vein ECs, with the adenoviral E4ORF1 gene. E4ORF1 transduced ECs (VeraVec ECs) -referred for simplicity here as ECs- are non-transformed but have low level Akt signaling that permits their serum-free survival while retaining their tissue-specific vascular attributes as well as the capacity to form functional contact-inhibited monolayers in vitro and perfused, patent blood vessels in vivo (Butler et al., 2012; Butler et al., 2010b; Nolan et al., 2013; Seandel et al., 2008). Indeed, because maintenance of VeraVec ECs do not require recombinant angiogenic factors (e.g. VEGF-A and FGF-2), serum, or other xenobiotic factors, these ECs can be used in co-culture models to screen and to identify the instructive vascular niche-like functions and angiocrine factors supporting the expansion of organ-specific stem and progenitor cells (Butler et al., 2010b; Ding et al., 2014; Ding et al., 2010; Ding et al., 2011) and possibly tumor cells. To reveal the angiocrine influence of ECs on LCs, we compared expansion of B220+CD19+ LCs isolated from Eμ-Myc mice in three conditions: serum containing medium (LCSerum), in serum and growth factor-free medium (LC), or in serum and growth factor-free medium with co-cultured ECs (LCEC). We found that serum-free co-culture of LCs with ECs supported greater LC proliferation than serum alone (Figures 1A–B, and S1A–B). Subcutaneous co-injection of LCs with ECs into immunodeficient NOD-SCID-IL2Rγ−/− (NSG) mice significantly enhanced tumor growth, compared to LCs injected alone (Figure S1C). The growth rate of LCEC in wild-type (WT) C57/B6 mice was significantly higher than LCSerum early after subcutaneous injection (Figure S1D–E). We then assessed the serial methylcellulose colony formation (MCF) capacity of expanded LCs. LCEC had 5-fold greater MCF potential than did LCSerum (Figure 1C–D). Limiting dilution transplantation into NSG mice showed that LCEC cells contained more lethal LCs than did LCSerum cells (Figure 1E). Thus, ECs establish an inductive vascular niche that enforces outgrowth of aggressive LCs.
Figure 1. Expansion of Myc+ LCs with aggressive LIC features after co-culture with ECs in serum and cytokine-free conditions.
(A) Representative images of Eμ-Myc mouse LCs cultured with EC. Scale bar= 200 μm.
(B) Quantification of LC number of Eμ-Myc mouse LCs cultured in the absence of ECs (LC), with EC (LCEC), or with serum supplementation (LCSerum). *, p<0.02; n= 5. All data are presented as mean±SEM throughout.
(C, D) Representative image (C) and the colony number (D) of colony forming capacity of LCs after serial passage. Five clones were passed every step. *p<0.02; Scale bar= 500 μm.
(E) Survival curve of NSG mice intraperitoneally (i.p.) transplanted with the indicated numbers of LCs; n= 6–8.
(F, G) Quantification (F) and representative image (G) of proliferation of LCs after treatment of indicated concentrations of doxorubicin. n= 5. Scale bar= 1000 μm.
(H) Survival of NSG mice inoculated with 1x105 indicated LCs and treated or not treated with doxorubicin (chemo) as indicated; n= 6–8.
(I) Heat-map presenting the expression level of indicated transcripts in LCEC and LCSerum.
(J) Representative flow cytometry graph of CD44 and IGF1R expression in LCs.
(K) Representative time-lapse microscopy image of single colony expansion of LCEC (top) and flow cytometry analysis of the clonally derived CD44+IGF1R+CSF1R+ LCs (bottom). Scale bar= 25 μm.
(L, M) Serial colony forming ability (L) and growth inhibition after doxorubicin treatment (M) comparing CD44+IGF1R+CSF1R+ and CD44−IGF1R−CSF1R− LCs. *, p<0.025; n= 4.
(N) Tumorigenicity of indicated LCs was compared by limiting dilution transplantation into NSG mice; n= 6 and 10 in CD44+IGF1R+CSF1R+ and CD44−IGF1R−CSF1R− LC-injected groups, respectively.
(O) Survival of NSG mice implanted with 105 indicated LCs then treated with or without 50 mg/kg doxorubicin; n= 6–10.See also Figure S1 and Table S1.
We then evaluated whether ECs could confer chemotherapy resistance to LCs by treating LCEC and LCSerum with doxorubicin. Indeed, LCEC were less sensitive to doxorubicin than LCSerum in vitro (Figure 1F, G). Doxorubicin prolonged survival of NSG mice transplanted with LCSerum but not mice transplanted with LCEC (Figure 1H). Thus, co-culture with ECs promoted a chemoresistant phenotype in the B220+CD19+Myc+ B-Cell LCs.
To investigate the mechanism underlying the aggressiveness of LCEC, we profiled the transcriptome of LCEC. Co-culture with ECs induced transcripts characteristic of LICs, including CD44, IGF1R and CSF1R (Hanahan and Weinberg, 2011; Medyouf et al., 2011) (Figure 1I, Table S1). Flow cytometry analysis and quantitative PCR (qPCR) confirmed that EC coculture-mediated upregulation of corresponding proteins was due to generation of a CD44+IGF1R+CSF1R+ LC subset (Figures 1J, S1F–G). To assess the functional activity of the CD44+IGF1R+CSF1R+ subpopulation, we obtained clonally-derived LCs by serial dilution in co-culture with ECs (Figure 1K) and compared their activity to CD44−IGF1R−CSF1R− LCs. The CD44+IGF1R+CSF1R+ LCs yielded more serial methylcellulose colonies, caused higher lethality in limiting dilution transplantation, and were less sensitive to doxorubicin than CD44−IGF1R−CSF1R− LCs (Figures 1L–O, S1H–J). Thus, EC co-culture enabled outgrowth of more aggressive and chemoresistant CD44+IGF1R+CSF1R+ LCs.
ECs support LCs via Jag1-dependent juxtacrine activation of Notch2 pathway
We next investigated the mechanism by which ECs stimulate aggressiveness in LCs. Transcription profiling showed upregulation of the Notch downstream transcriptional effector Hey1 in LCEC (Figure 1I). qPCR confirmed specific upregulation of Hey1 in LCEC (Figure 2A), and in the aggressive CD44+IGF1R+CSF1R+ LC subpopulation (Figure S2A–C). To test whether Hey1 upregulation confers aggressive LC features, we studied how loss- and gain-of-function of Notch1, Notch2 and Hey1 in LCs altered their expansion (Figures 2B, S2D–F). Genetic silencing using shRNA to Notch2 (shNotch2) or Hey1 (shHey1), but not Notch1 (shNotch1), abrogated EC-driven expansion of LCs. In contrast, Hey1 overexpression (Hey1 OE) phenocopied the effect of EC co-culture (Figures 2C–D, S2G–H). Notch pathway inhibition using the γ-secretase inhibitor compound-E similarly abrogated LC growth after co-culture with ECs. Immunoblot and immunostaining for Notch intracellular domains (NICDs) and chromatin immunoprecipitation (ChIP) of RBPJ showed selective activation of Notch2, but not Notch1, in LCEC (Figures 2E–G, S2I–J). Therefore, co-culture with ECs selectively activates Notch2 in LCs, resulting in Hey1-dependent expansion of LCs.
Figure 2. Angiocrine effects of endothelial Jag1 on Notch2 activation and propagation of Myc+ mouse LCs.
(A) Expression level of Notch pathway effecter Hey1, Hes1, and Hey2 in LCs.
(B) Approaches to define Notch pathway activation in mouse LCs. Notch1, Notch2 and Hey1 in LCs was silenced by shRNA (shNotch1, shNotch2, shHey1), and Notch pathway was blocked by compound E. Cell expansion, Notch activation, colony formation, and hepatic invasiveness were then compared.
(C, D) Quantification (C) and representative image (D) of expansion of LCs co-cultured with ECs (LC+EC) or cultured in serum-free medium (LC). Srb, scrambled shRNA; OE Hey1, overexpression of Hey1; n= 4; Scale bar= 25 μm.
(E, F) Notch1 and Notch2 intracellular domains (ICD) were detected in LCs by immunoblot (E) and Notch2 ICD in LCs was examined by immunostaining (F). White arrowheads indicate nuclear Notch2 ICD. Scale bar= 10 μm.
(G) ChIP analysis of RBPJ activity in LCs after Notch inhibition. shN1 and shN2 denote shRNA against Notch1 and Notch2, respectively. n= 4.
(H–K) Aggressive features of LCs were tested after disruption of Notch2-Hey1 pathway. Extra-nodal invasiveness of LCs was examined by intrasplenic injection into NSG mice (H). Hepatic tumor load was examined by H&E (I) and fluorescent microscopy (K). Quantification of hepatic tumor load is shown in (J); n= 4; Scale bar= 100 μm in (I) and 1 mm in (K).
(L) Expression of Notch ligands in feeder ECs after co-culture with LCs (EC + LC); n= 4.
(M, N) LCs were co-cultured with ECs transduced with Scrambled shRNA (ECSrb) and Jag1 shRNA (ECshJag1). Notch activation in LCs was tested by Hey1 upregulation (M) and fluorescent intensity of RBPJ-driven GFP reporter in LCs (N); n= 5.
(O, P) Representative image (O) and expansion (P) of LCs cultured alone or co-cultured with ECShJag1 or ECSrb. *, p<0.025; n= 5. Scale bar= 50 μm.
See also Figure S2.
Next, we tested how disruption of Notch signaling affected in vivo hepatic tumor seeding following intrasplenic injection (Ding et al., 2010) (Figure 2H). Knockdown of Notch2 or Hey1, or administration of Compound E, significantly reduced hepatic tumor load but Notch1 knockdown had little effect (Figures 2I–K, S2K–L). Thus, juxtacrine activation of Notch2-Hey1 by ECs promotes extra-nodal invasion, a feature of aggressive lymphomas.
While ECs express several Notch ligands, Jag1 was primarily upregulated by ECs after co-culture with LCs (Figure 2L). This finding implicates EC Jag1 as the ligand activating Notch2 in LCs because LCs express negligible Jag1 (Figure S2M). To test this, we knocked down Jag1 in ECs (ECshJag1) and co-cultured these feeder cells with Myc+ LCs expressing a Notch reporter (RBPJ-driven GFP). ECshJag1 were less effective than control ECs transduced with scrambled shRNA (ECSrb) in supporting serum and growth factor-free expansion of LCs and in inducing Hey1 upregulation and Notch activation (Figure 2M–P). Notably, inhibition of the Notch2-Hey1 pathway in CD44+IGF1R+ LCs after co-culture with ECs had little effect on the aggressive feature (Figure S2N–O). Thus, propagation of aggressive LICs in vitro is driven by EC Jag1 expression.
EC-specific upregulation of Jag1 in human lymphoma tissue accompanies propagation of perivascularly localized aggressive LCs
To investigate whether human LCs upregulate Jag1 in ECs, we stained primary human Burkitt’s lymphoma sections for Jag1 and Hey1. Jag1 was specifically expressed by tumor ECs but not by LCs in all tested lymphomas (Figures 3A–B, S3A, Table S2). Notably, Hey1 was preferentially expressed in LCs adjacent to Jag1+ tumor ECs (Figure 3A, C). We next investigated whether ECs foster propagation of human Burkitt’s LCs that harbor a translocated MYC gene under the control of immunoglobulin heavy chain gene regulatory elements, as modeled by the Eμ-Myc+ mouse. Co-culture of CD19+ human Burkitt’s LCs with ECs triggered Notch2-mediated signaling in LCs and utgrowth of the CD44+IGF1R+ subpopulation (Figures 3D–F, S3B–F). Expansion of human lymphoma colony forming cells and hepatic lymphoma load were also promoted by co-culture with ECs, compared with serum culture (Figure 3G–J). Disruption of the Notch2-Hey1 pathway during co-culture by knockdown of Jag1 in ECs (ECshJag1) or Notch2 in LCs (LCshNotch2), or use of compound E abrogated EC-dependent expansion, colony formation, and extra-nodal invasiveness of human LCs (Figures 3G–N, S3G–J). Notably, after intrasplenic injection into NSG mice, human LCEC induced Jag1 expression in the ECs surrounding the lymphoma nodules but not tumor-free hepatic regions (Figure 3O). Thus, expansion of invasive human Burkitt’s LCs is also driven by instructive angiocrine factors supplied by the vascular niche.
Figure 3. Influence of angiocrine Jag1 on expansion and aggressive features of human B-Cell LCs.
(A–C) Expression of Hey1 and Jag1 in patient Burkitt’s lymphoma with MYC translocation were examined. VE-cadherin was stained to identify ECs (A). Quantification of Jag1 expression in lymphoma ECs (B) and Hey1 in perivascular LCs (C) is shown. Scale bar = 100 μm and 20 μm in inset.
(D) CD44 and IGF1R expression on human LCs cultured with ECs (LCEC) or with serum (LCSerum).
(E, F) Immunoblot analysis (E) and immunostaining (F) analysis of Notch ICD in LC cultured with serum or with indicated ECs. Scale bar= 25 μm.
(G, H) Representative image (G) and quantification (H) of colony formation capacity of human LCSerum, and LCs co-cultured with ECs transduced with Scrambled (LCEC-srb) or Jag1 shRNA (LCEC-shJag1). n= 4; Scale bar= 100 μm.
(I, J) Representative image of hepatic lymphoma (I) and quantification of tumor colony number burden (J) of human LCEC-srb or LCEC-shJag1 intrasplenically injected into NSG mice. LCSerum were also injected for comparison. Scale bar= 50 μm.
(K–N) Inhibition of Notch1, Notch2, Hey1 was performed in human LCs before EC co-culture, and EC-dependent expansion (K, L) and hepatic tumor load of (M, N) of LCs were determined; n= 4; Scale bar= 1000 μm in (K) and 50 μm in (M).
(O) Jag1 expression in host ECs within the hepatic lymphoma nodule was assessed 14 days after intrasplenic injection of human LCs into NSG mice. Lymphoma mass in the liver is denoted by dotted line. Scale bar= 50 μm (20 μm in inset).
LCs induce FGFR1 signaling in ECs to prime a Jag1+ vascular niche that reciprocally reinforces lymphoma propagation and chemoresistance
We have found that during organ regeneration activation of VEGF-A/VEGFR2 and FGF/FGFR1 signaling in ECs induces expression of angiocrine factors (Ding et al., 2014; Ding et al., 2010; Ding et al., 2011). Hence, we tested if LCs might co-opt these mechanisms to upregulate Jag1 in tumor ECs and form a malignant vascular niche. Both microarray expression and qPCR analysis showed that co-culture with ECs upregulated FGF4 in mouse and human LCs (Figure 4A). Whereas normal human lymph nodes have negligible FGF4, we found significant expression of FGF4 protein in human Burkitt’s lymphoma tissue and preferential activation of FGFR1 in the lymphoma-associated ECs (Figure S4A). To examine whether FGFR1-mediated signaling in ECs was necessary for Jag1 induction, we exposed control ECs and FGFR1-deficient ECs to serum-free or conditioned media (CM) derived from LCs. CM derived from mouse LCEC activated Jag1 expression in ECs in a FGFR1-dependent manner (Figure 4B–C). Notably, during EC-LC co-culture shRNA knockdown of FGFR1 in ECs or of FGF4 in LCs blocked Jag1 induction and FGFR1 signaling in ECs (Figures 4D, S4B–D). Importantly, after intrasplenic injection, FGF4-deficient human LCs failed to upregulate Jag1 in the ECs of liver lymphoma nodules of recipient mice (Figure 4E). Therefore, LCs supply FGF4 to activate FGFR1 on ECs to reinforce Jag1-mediated vascular niche function, driving Notch2-dependent expansion of aggressive LCs.
Figure 4. Reciprocal instigatory interactions between LCs and co-cultured ECs in vitro and host ECs in vivo.
(A) Expression of angiogenic factors VEGF-A, SDF1, FGF2, and FGF4 in mouse and human LCs co-cultured with ECs (LC + EC) or in serum-containing medium (LC); n=4.
(B–D) ECs transduced with FGFR1 shRNA were stimulation with LC conditioned medium (CM). LCs were transduced with Scrambled (LCSrb) and Fgf4 shRNA (LCshFGF4). Jag1 and FGFR1 protein levels (B) and FGFR1 activation (as determined by phosphorylation of FRS-2) (C) in ECs were examined and quantification (D); n= 4.
(E) After human LCs were transplanted into NSG mice via intrasplenic injection, Jag1 induction in ECs associated with hepatic lymphoma nodule was determined. Lymphoma mass is delineated from normal tissue by dotted line. Jag1 expression in lymphoma ECs is indicated in inset. Scale bar= 100 μm and 20 μm in inset.
(F, G) Schematic representation of generating Fgfr1iΔEC/iΔEC and control Fgfr1iΔEC/+ mice (F) and quantification of growth of subcutaneously injected B6RV2 mouse LCs in these mice (G).
(H–K) B6RV2 lymphoma in the liver was examined in Fgfr1iΔEC/iΔEC and control mice (H). Histological studies of hepatic tumor load was determined by H&E staining (I) and fluorescent microscopy scan (J) of liver lobe 14 days after intrasplenic injection of LCs. Proliferation of LCs was determined by Ki67 staining (K). Scale bar= 50 μm in (I), (K) and 1mm in (J).
See also Figure S4.
To determine whether this “feed-forward” loop drives lymphoma tumorigenesis in vivo, we conditionally deleted Fgfr1 specifically in adult ECs by crossing VE-cadherin-CreERT2 mice with Fgfr1loxP/loxP mice (Figure 4F) and using tamoxifen to delete Fgfr1 specifically in ECs (Fgfr1iΔEC/iΔEC) (Figure S4E, F). To control for Cre-mediated toxicity, we used EC-specific haplodeficient Fgfr1iΔEC/+ as control mice. We used a murine B6RV2 lymphoma transplantation model to examine the lymphoma growth. Fgfr1iΔEC/iΔEC, but not Fgfr1iΔEC/+ mice, were inhospitable to subcutaneously and intrasplenically injected B6RV2 LCs, resulting in reduced tumor growth and hepatic colonization (Figure 4G–K). Therefore, activation of FGFR1 in ECs is required for LCs to establish a pro-tumorigenic vascular niche.
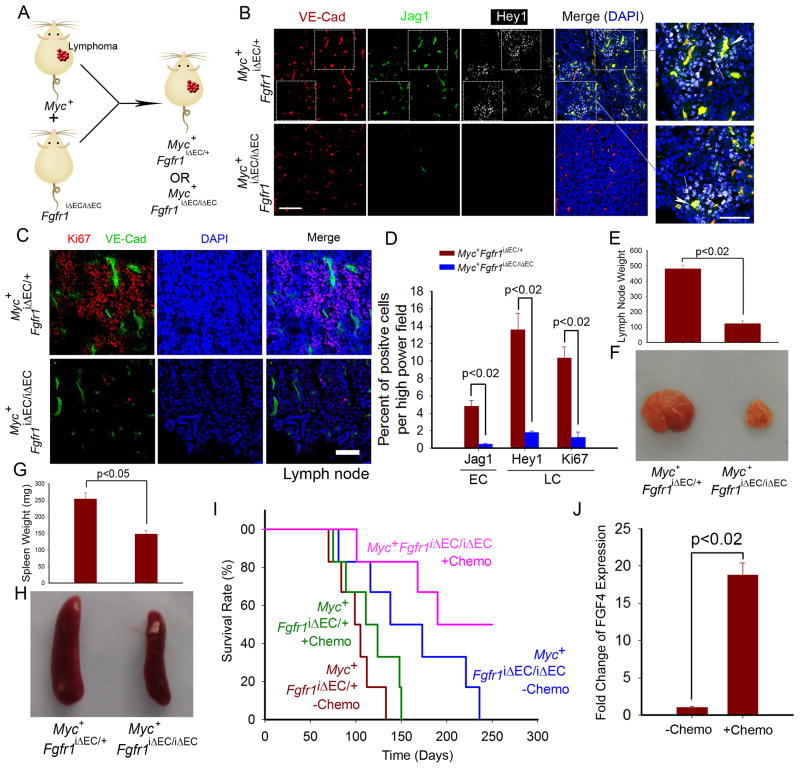
We next crossed Eμ-Myc mice with Fgfr1iΔEC/iΔEC and control mice (Figure 5A) to assess the role of FGFR1 in establishing a malignant vascular niche. Jag1 was preferentially expressed by VE-cadherin+ ECs within the lymphoma of control (Myc+Fgfr1iΔEC/+) mice, but not by ECs of Myc+Fgfr1iΔEC/iΔEC lymphomas (Figure 5B). Complementary expression of Hey1 was seen in the perivascular LCs of control but not Myc+Fgfr1iΔEC/iΔEC mice. As a result, Myc+Fgfr1iΔEC/iΔEC mice survived longer than Myc+Fgfr1iΔEC/+ controls, owing to reduced tumor cell proliferation (Figure 5C–I).
Figure 5. Lymphoma propagation and chemoresistance in Eμ-Myc mice with EC-specific deletion of Fgfr1 (Myc+Fgfr1iΔEC/iΔEC).
(A) Generation of Myc+Fgfr1iΔEC/iΔEC and control Myc+Fgfr1iΔEC/+ mice.
(B–D) Expression of Jag1 and Hey1 in lymphoma tissue was determined in Myc+Fgfr1iΔEC/iΔEC and control mice (B). White arrow indicates expression of Jag1 on VE-cadherin+ ECs. LC proliferation was tested by Ki67 staining (C). Quantification of Jag1 upregulation in ECs, Hey1 and proliferation marker Ki67 in LCs is presented in (D); n =4. Scale bar= 50 μm in B (20 μm in inset).
(E–H) Weight (E, G) and representative images (F, H) of lymph node (E, F) and spleen (G, H) in Myc+Fgfr1iΔEC/iΔEC and control mice. n= 4.
(I) Survival of Myc+Fgfr1iΔEC/iΔEC and control mice with or without treatment of 100 mg/kg of doxorubicin every week; n= 5–6. (J) FGF4 mRNA expression in LCs after treatment of doxorubicin; n= 4.
Because ECs promoted the expansion of chemoresistant LCs, we investigated whether EC-specific deletion of Fgfr1 sensitized Eμ-Myc lymphomas to doxorubicin treatment. Survival of Myc+Fgfr1iΔEC/iΔEC, but not Fgfr1iΔEC/+ mice, was prolonged by chemotherapy (Figure 5I). LCs treated with doxorubicin upregulated FGF4 expression, enabling a vascular niche that confers chemoresistance to LCs (Figure 5J). Hence, FGFR1-mediated signaling deploys Jag1 in tumor ECs, establishing a pro-tumorigenic vascular niche that shelters resident LCs from chemotherapy-induced cytotoxicity.
Endothelial Jag1 in tumor capillaries subverts indolent LCs to manifest aggressive features
To investigate whether enhanced chemoresistance and invasiveness of LCs upon co-cultured with Jag1+ ECs was due to selective enrichment of an aggressive LC subclone or bestowing aggressive attributes to indolent LCs by tumorigenic vascular niche, we co-cultured clonally derived CD44−IGF1R− CSF1R− LCs (triple-negative LCs) with ECs (Figure 6A). After co-culture with ECs, the triple-negative LC progeny acquired ~14-fold greater MCF capacity (Figure 6B–C). Immunophenotypic analysis showed that 9.5% of LCs from the single triple-negative clone are CD44+IGF1R+ after co-cultured with EC (Figure 6D). Notably, after EC co-culture, triple-negative LCs were more lethal to NSG mice following i.p. injection (Figure 6E). All of these effects were attenuated by co-culture with Jag1-deficient ECs (Figure 6B–E). Therefore, the aggressive lymphoma tumor phenotype is conferred upon LCs by angiocrine signals emanating from a tumorigenic vascular microenvironment.
Figure 6. Acquisition of aggressive LIC-like features in LCs by Jag1-expressing vascular niche.
(A–E) Aggressive traits of CD44−IGF1R−CSF1R− indolent LC colonies after co-culture with ECs was investigated (A). CD44−IGF1R−CSF1R− LC colonies were co-cultured with ECSrb and ECShJag1 and tested for colony forming capacity (B, C), CD44 and IGF1R expression (D), and lethality in NSG mice after injection of 5 x103 indicated LCs (E).
(F) Different LC colonies were transplanted into mice with EC-specific deletion of Jag1 (Jag1iΔEC/iΔEC). Jag1iΔEC/+ mice were used as control.
(G, H) Propagation of CD44+IGF1R+CSF1R+ LCs in Jag1iΔEC/iΔEC and control mice was determined after subcutaneous (G) and intrasplenic transplantation. Representative H&E staining of liver is shown in (H); n= 4. Scale bar = 50 μm.
(I, J) Acquisition of aggressive LIC features in CD44−IGF1R−CSF1R− LCs after transplantation to control and Jag1iΔEC/iΔEC mice. LCs were isolated at day 28 after subcutaneous injection from enlarging tumor mass and analyzed for CD44, IGF1R, and CSF1R (I) and serial colony formation capacity (J). Each derived clone was injected into 3 mice.
See also Figure S5.
We then assessed tumorigenicity and extra-nodal invasiveness of LCs in Jag1iΔEC/+ control and Jag1iΔEC/iΔEC mice (Figure 6F). LCs transplanted into Jag1iΔEC/iΔEC mice yielded smaller tumors than did the same LCs inoculated into Jag1iΔEC/+ mice (Figure 6G–H, S5A). To test whether host EC Jag1 altered CD44−IGF1R−CSF1R− LCs in vivo, individual triple-negative LC clones were injected subcutaneously into Jag1iΔEC/iΔEC, Jag1iΔEC/+ (Figure 6I), or WT mice (Figure S5B). Following expansion in the recipient mice, transplanted triple-negative LCs acquired over 6-fold greater triple-positive immunophenotype and ~5-fold higher MCF in Jag1iΔEC/+ mice than in Jag1iΔEC/iΔEC mice (Figure 6J). Therefore, host EC-derived Jag1 subverts indolent LCs to manifest aggressive phenotypes.
Deletion of Jag1 in ECs of Eμ-Myc mice (Myc+Jag1iΔEC/iΔEC) abolishes de novo generation of aggressive LCs
To assess the lymphoma-promoting role of EC Jag1, we selectively deleted Jag1 in ECs of Eμ-Myc mice (Myc+Jag1iΔEC/iΔEC), using Myc+Jag1iΔEC/+ as controls. We then isolated nodal B-cell LCs and assayed for their aggressive attributes (Figure 7A). During serial methylcellulose culture, LCs from Myc+Jag1iΔEC/iΔEC mice had fewer CD44+IGF1R+ LCs and yielded substantially fewer colonies than LCs from control mice (Figure 7B–D). LCs derived from Myc+Jag1iΔEC/iΔEC mice were less capable in killing the recipient mice and were more sensitive to doxorubicin compared to LCs isolated from Myc+Jag1iΔEC/+ mice (Figure 7E–F). LCs from Myc+Jag1iΔEC/iΔEC mice also gave rise to fewer and smaller hepatic tumors after intrasplenic seeding (Figure 7G–H). Therefore, the pro-tumorigenic Jag1+ vascular niche endows aggressive features to LCs, driving lymphomagenesis, extra-nodal invasiveness, and chemoresistance.
Figure 7. Generation of invasive and chemoresistant triple-positive LC subpopulation in Myc+ mice with conditional deletion of Jag1 in ECs (Myc+Jag1iΔEC/iΔEC).
(A) Analysis of aggressive attributes of LCs from Myc+Jag1iΔEC/iΔEC and control Myc+Jag1iΔEC/+ mice.
(B) Percentage of CD44+IGF1R+ LC subset in Myc+Jag1iΔEC/iΔEC and control mice.
(C, D) Colony forming capacity of LCs. Five clones isolated from Myc+ mice were picked for each passage. Representative images (C) and quantification of colony (D) are shown. Scale bar = 500 μm.
(E) Lethality of LCs from control (left) and Myc+Jag1iΔEC/iΔEC (right) mice after limiting dilution transplantation into NSG mice (E).
(F) Survival of NSG mice injected with 105 indicated LCs with or without treatment of 50 mg/kg doxorubicin; n = 5–8.
(G, H) Representative image (G) and quantification of tumor colony number (H) of hepatic tumor in NSG mice after injection of LCs. Scale bar= 1mm.
Vascular niche-derived Jag1 confers Notch-dependent chemoresistance to Myc+ LCs
To unravel the role of EC-derived Jag1 in lymphoma pathogenesis and development of chemoresistance, we administered doxorubicin to Myc+Jag1iΔEC/iΔEC and control mice (Figure 8A, B). Notch activity was tracked in LCs by crossing Myc+Jag1iΔEC/iΔEC mice with transgenic Notch reporter (TNR) mice expressing GFP upon Notch activation (Butler et al., 2010b). Genetic ablation of Jag1 in ECs reduced tumor load and improved survival of Myc+Jag1iΔEC/iΔEC mice, compared to control Myc+Jag1iΔEC/+ mice (Figures 8B, S6A). Moreover, LCs from Myc+Jag1iΔEC/iΔEC mice were more sensitive to chemotherapy, manifesting as enhanced survival (64% after 8 months) compared to a median survival of ~150 days in controls. Notch was activated in LCs positioned adjacent to VE-cadherin+ ECs in control mice, but not in Myc+Jag1iΔEC/iΔEC mice (Figure 8C, D), indicating that Jag1 supplied by ECs activate Notch signaling in LCs. The enhanced survival of Myc+Jag1iΔEC/iΔEC mice was associated with greater LC death (Figure 8E, F). Notably, when EC Jag1 is intact, a subset of perivascularly localized LCs with activated Notch signaling (reported by TNR-GFP cells) were protected from doxorubicin-induced cell death. Therefore, Jag1-expressing ECs establish a chemoresistant microenvironment for LCs via juxtacrine Notch activation (Figure 8G).
Figure 8. Chemoresistance of lymphoma in Myc+Jag1iΔEC/iΔEC and control Myc+Jag1iΔEC/+ mice.
(A) To test the role of angiocrine Jag1 in stimulating chemoresistance, control and Myc+Jag1iΔEC/iΔEC mice were treated with doxorubicin at 100 mg/kg once a week for 4 consecutive weeks.
(B) Survival rate of Myc+Jag1iΔEC/iΔEC and control mice. Chemo indicates mouse group treated with 100 mg/kg doxorubicin.
(C, D) Jag1 expression in VE-cadherin+ ECs, Notch activation (GFP expression) in LCs was measured with or without chemotherapy (C). Quantification of GFP intensity is shown (D). Compound E was injected into control mice to compare the degree of Notch inhibition; n = 4; Scale bar= 50 μm.
(E, F) TUNEL staining image (E) and quantification of TUNEL+ cells (F) in the lymphoma of Myc+ mice after chemotherapy; n = 4; Scale bar= 50 μm.
(G) Angiocrine Jag1 activates Hey1 to stimulate the emergence of LCs exhibiting aggressive LIC features. Expanding LCs reciprocally activate FGFR1 on ECs and induce Jag1 upregulation, further reinforcing Jag1-mediated angiocrine support of aggressive LCs with LIC attributes.
See also Figure S6.
To assess whether aberrant EC Jag1 expression could cause vascular abnormalities that might compromise tumor blood supply, we examined vascular perfusion in Myc+Jag1iΔEC/iΔEC mice by intravenously injecting B4-isolectin to label patent ECs. The majority of VE-cadherin+ ECs within the lymphoma were recognized by B4-isolectin. Furthermore, tissue staining using the hypoxia marker, pimonidazole, showed little hypoxia in the lymphoma mass of Myc+Jag1iΔEC/iΔEC mice (Figure S6B–D). Therefore, vascular blood supply and oxygen delivery were not compromised in Myc+Jag1iΔEC/iΔEC mice. Taken together, we employed a variety of mouse/human lymphoma models to demonstrate that angiocrine expression of Jag1 activates lymphoma Notch to promote tumorigenicity without affecting the passive perfusion function of tumor vasculature.
DISCUSSION
Tumor initiating cells are believed to acquire aggressive phenotypes via cell-autonomous mechanisms. Here, we challenge this paradigm and demonstrate that in certain lymphomas, the pro-tumorigenic vascular niche dictates tumor aggressiveness. We show that tumor ECs convert indolent CD44−IGF1R−CSF1R− LCs to more aggressive CD44+IGF1R+CSF1R+ LIC-like cells manifesting greater tumorigenicity, extranodal invasion and chemoresistance. The majority of these aggressive tumor features were dependent upon FGF4-driven Jag1 expression by tumor ECs. Disruption of this juxtacrine/angiocrine loop at any level—FGF4, FGFR1, Jag1, Notch2—severely diminished the aggressiveness of both human and murine lymphomas. Thus, Eμ-Myc driven oncogenesis is not sufficient to provoke aggressive lymphoma behaviors. The LC phenotype is plastic and is determined by cues emanating from the malignant vascular niche rather than by cell-autonomous signaling pathways alone.
The properties of the malignant vascular niche are co-opted via activation of FGF4/FGFR1 signaling in tumor ECs. This signaling induces host ECs to express Jag1 in proximity to neighboring LCs. Importantly, the FGFR1-Jag1 feed-forward signaling loop promotes LC chemoresistance and is further reinforced by chemotherapy administration. In this way, angiocrine Jag1 functionalizes a chemoresistance niche that activates Notch signaling in perivascular LCs and spares them from chemotherapeutics such as doxorubicin. These results suggest a paradigm of tumor propagation whereby a dynamic neoplasm-primed tumor microenvironment shelters tumor cells from chemotherapy and instructively directs them to grow locally and invade distal organs. Thus, targeting the malignant vascular niche should sensitize LCs to chemotherapy and improve outcomes.
Indeed, EC-specific deletion of Fgfr1 or Jag1 in the Fgfr1iΔEC/iΔEC and Jag1iΔEC/iΔEC mice enhanced chemosensitivity and improved mouse survival by abolishing Notch activation in Myc+ LCs after doxorubicin treatment. Similarly, in human lymphoma specimens we found Jag1 upregulated in tumor ECs and the Notch pathway activated in perivascular human LCs, as originally observed in the murine lymphoma models. These findings highlight the functional interplay between ECs and tumor cells that depends on the FGF-Notch paracrine/juxtacrine loop. Upregulation of endothelial Jag1 is central to this loop as it endows both mouse and human LCs with aggressive LC features. Similarly, as yet unrecognized and distinct angiocrine pathways are likely activated within the microenvironment of other tumor types.
Blockade of Jag1 expressed by tumor ECs reduced lymphoma progression without compromising perfusion of the tumor vasculature. Jag1 appeared dispensable for most homeostatic vascular functions because deletion of endothelial Jag1 in adult mice caused no excess mortality or morbidity. Thus, inhibiting the instructive angiocrine signals from a tumor-primed vascular niche can effectively target aggressive LC features. Anti-angiocrine therapeutics need not interfere with tumor perfusion and therefore should not be compromised by tumor hypoxia and rebound angiogenesis that can lead to paradoxical tumor growth (Ebos et al., 2009; Paez-Ribes et al., 2009).
Comparative analyses of human and mouse lymphoma tissues suggest that our findings may have clinical relevance. Jag1 is upregulated in ECs in human lymphoma specimens. Jag1 upregulation in ECs endows both mouse and human LCs with aggressive LC features. Whether induced expression of Jag1 and activation of Notch pathway in human tumor ECs may also portend poor prognosis is unknown and can only be determined in double blind multi-center clinical studies. Based on our data, we speculate that patients harboring tumors with the capacity of inducing functional Notch ligands in tumor ECs may be at higher risk for tumor relapse and chemoresistance and be treated with more aggressive therapeutic protocols.
Taken together, our findings demonstrate that tumor cells primes a maladapted vascular niche that reciprocally confers tumors with aggressive, lethal properties: augmenting tumor growth, fostering chemoresistance and promoting extra-nodal invasion. Indeed, function of lymphoma propagating or initiating cells depends on pro-tumorigenic state of the vascular niche and can not be entirely attributed to cell-autonomous malignant properties of tumor cells. For example, an authentic tumor initiating cell may fail to engraft host tissues with an inhospitable vascular niche and assays used to identify tumor-initiating cells need to be modified to account for the activation state of vascular niche. Similarly, differences in host EC functions may underlie the tumor tropisms that select common metastatic sites. This study introduces promising therapeutic approaches to improve clinical outcomes for patients with aggressive lymphomas by ejecting LCs from the pro-tumorigenic vascular niche to limit local tumor growth and extra-nodal invasion while sensitizing LCs to chemotherapy.
EXPERIMENTAL PROCEDURES
Transgenic Reporter and Gene-Targeted Animals
Jag1loxP/loxP mice were provided by Dr. Thomas Gridley (Jackson laboratories), and Fgfr1loxp/loxp mice were obtained from Dr. Michael Simons (Yale University School of Medicine). Generation of inducible EC-specific Fgfr1 knockout mice was carried out as described (Ding et al., 2010; Ding et al., 2011; Wang et al., 2010). In brief, Fgfr1loxp/loxp or Jag1loxp/loxp mice were bred with VE-cadherin-CreERT2 (cdh5-PAC-CreERT2) transgenic mice and treated with tamoxifen to induce EC-specific gene deletion (Fgfr1iΔEC/iΔEC or Jag1iΔEC/iΔEC), as described in “supplemental Experimental Procedures”. Lymphomagenesis was induced by transgenic Eμ-driven Myc. Myc+Jag1iΔEC/iΔEC mice were crossed with Transgenic Notch Reporter (TNR) mice in which the activation of Notch pathway results in GFP expression. All animal experiments were carried out under the guidelines of National Institute of Health and approved by Institutional Animal Care and Use Committee (IACUC) at Weill Cornell Medical College, using age/weight/genetic background-matched animals.
Human Burkitt’s Lymphoma Tissues/Samples
Burkitt’s lymphoma patient specimens were obtained from Weill Cornell Medical College. The procedure was approved by Institutional Review Board (IRB) at Weill Cornell Medical College. Patient-related information was identified in Table S2. Human Burkitt’s LCs without Epstein-Barr-Virus infection were purchased from American Type Culture Collection (ATCC).
In vitro modeling of vascular niche for LC co-culture
To maintain EC survival in serum/growth factor-free conditions without confounding effects of supplementation with serum, bovine brain extracts and recombinant angiogenic factors (i.e. VEGF-A, FGF-2, EGFs, PDGFs, angiopoietins), primary freshly purified ECs, such as human umbilical vein ECs (HUVECs) or adult tissue-specific mouse ECs were transduced with E4ORF1 gene (VeraVec ECs, Angiocrine Bioscience, New York). VeraVec ECs maintain their native vascular and microvascular attributes and produce physiological levels of tissue-specific angiocrine factors (Butler et al., 2012; Butler et al., 2010b; Nolan et al., 2013; Seandel et al., 2008). As such, VeraVec ECs establish a responsive unbiased vascular niche model to unequivocally interrogate the role of angiocrine factors in fostering the homeostasis of tumor cells and LICs. Both Eμ-Myc mouse LCs and Human Burkitt’s LCs (ATCC) harboring the c-MYC translocation were utilized for co-culture with VeraVec ECs as described in “supplemental Experimental Procedures”. For simplicity, we referred to VeraVec ECs as ECs.
To investigate the angiocrine contribution of vascular niche to aggressiveness of LCs/LICs, Jag1 shRNA or scrambled shRNA (Open biosystems) was used to knock down Jag1 in ECs. Experimental procedures of shRNA knockdown of Notch pathway (Notch1, 2 and Hey1) in LCs and Notch ligand Jag1 in ECs is described in “supplemental Experimental Procedures” section.
Subcutaneous Inoculation and Hepatic Tumor Seeding Model
To monitor tumor propagation in vivo, 5x105 human LCs, 1x105 murine Eμ-Myc LCs were injected i.p. or 2x106 LCs were injected subcutaneously into immunodeficient NSG and mice with indicated genetic backgrounds. A liver seeding model via intrasplenic transplantation of LCs was performed as described (Ding et al., 2010). Briefly, mice were anesthetized, and 5x105 mCherry labeled human LCs or 1x105 mouse LCs were injected into the parenchyma of the spleen. Splenectomy was carried out after the injection. Mice were sacrificed 14 days after intrasplenic transplantation and hepatic tumor load was analyzed by H&E staining and immunofluorescence whole scan of liver lobe.
γ-secretase inhibitor compound E was utilized to abolish Notch pathway activation in LCs. LCs were incubated with 1 μM compound E. For in vivo Notch inhibition, compound E was i.p. injected to mice at 2 mg/kg. Notch2 activation was also determined as described in “supplemental Experimental Procedures” section.
Flow cytometric analysis of LCs
For flow cytometry analysis, LCs were filtered through a 30-μm strainer, preblocked with Fc block (CD16/CD32; BD Biosciences), and then incubated with the primary antibodies: CD44, CD19, B220 (eBiosciences); IGF1R (Abcam); Notch 1, 2 (Biolegend). Primary antibodies were conjugated to Alexa Fluor dyes using antibody labeling kits (Invitrogen) following the instructions of manufacturer. GFP-expressing cells were detected by their own fluorescence, as described in “supplemental Experimental Procedures”.
Immunofluorescent Staining
For immunofluorescence study, cryopreserved sections were incubated with antibodies to mouse VE-Cadherin (R&D), Jag1 (Abcam), and Ki67 (Dako) supplemented with 10% normal donkey serum/1% BSA/0.1% Tween-20, followed by incubation with fluorophore-conjugated second antibodies (Jackson Immuno Research). Images were captured on AxioVert LSM710 microscope (Zeiss).
Methylcellulose Colony Assay
Mouse and human LCs formed colonies upon serum culture (LCSerum) or EC co-culture (LCEC). Single colonies of LCSerum and LCEC were dispersed in methylcellulose respectively. Colonies formed were randomly picked and serially passaged. Colony number was quantified upon each serial passage.
Statistical Analysis of Data
All data were presented as mean ± SEM. Comparison between different groups were made using Student’s t test and analysis of variance (ANOVA). Statistical significance was considered as p < 0.05.
Supplementary Material
Highlights.
Lymphoma cells (LCs) produce FGF4 to co-opt host endothelial cell (EC) function
Activated FGFR1 triggers ECs to deploy Jag1 functionalizing a malignant vascular niche
Angiocrine Jag1 on ECs stimulates adjacent LCs via Notch2 to activate LC Hey1
Subverted vascular niche provokes aggressive LC phenotypes and chemoresistance
Significance.
Blood vessels within lymphomas are not just passive conduits delivering nutrients but contain specialized ECs that constitute a maladapted niche actively instigating aggressiveness during tumor progression. Here, we show that tumor ECs supply angiocrine factors to promote chemotherapy resistance and extra-nodal invasiveness of CD44+IGF1R+CSF1R+ LCs. LCs produce FGF4 to induce expression of Notch-ligand Jag1 in ECs. In turn, EC-derived angiocrine Jag1 activates Notch2 in LCs to promote tumor invasiveness and chemoresistance. Interfering with the FGF4/Jag1 cross-talk between LCs and ECs decreases tumor progression and promotes sensitivity to chemotherapy, thereby increasing survival of tumor-bearing mice. Targeting pro-tumorigenic angiocrine factors supplied by lymphoma blood vessels promises efficacious approaches to block tumorigenesis and restore chemotherapy sensitivity.
Acknowledgments
B.-S.D. is supported by a National Scientist Development Grant from the American Heart Association (number 12SDG1213004) and Druckenmiller Fellowship from the New York Stem Cell Foundation. S.R. is supported by the Ansary Stem Cell Institute, the Howard Hughes Medical Institute, Empire State Stem Cell Board and New York State Department of Health grants (C024180, C026438, C026878, C028117), National Heart, Lung, and Blood Institute grants R01HL097797, R01HL119872 and RC2HL101846, National Institute of Diabetes and Digestive and Kidney Diseases grant R01DK095039, National Cancer Institute grant U54CA163167, Qatar National Priorities Research Foundation grant NPRP08-663-3-140 and the Qatar Foundation BioMedical Research Program. S.R. is the founder and consultant to Angiocrine Bioscience, New York, NY. J.M.B. receives research support from Angiocrine Bioscience, New York, NY. J.S. is suppored by National Cancer Institute grants CA159175 and CA163167, by National Heart, Lung, and Blood Institute grants HL119872 and HL055748 and by a Leukemia & Lymphoma Society Scholar award.
Footnotes
Accession Number:
The microarray data are deposited at Gene Expression Omnibus under accession number GSE46368.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aster JC, Pear WS, Blacklow SC. Notch signaling in leukemia. Annu Rev Pathol. 2008;3:587–613. doi: 10.1146/annurev.pathmechdis.3.121806.154300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck B, Driessens G, Goossens S, Youssef KK, Kuchnio A, Caauwe A, Sotiropoulou PA, Loges S, Lapouge G, Candi A, et al. A vascular niche and a VEGF-Nrp1 loop regulate the initiation and stemness of skin tumours. Nature. 2011;478:399–403. doi: 10.1038/nature10525. [DOI] [PubMed] [Google Scholar]
- Benedito R, Roca C, Sorensen I, Adams S, Gossler A, Fruttiger M, Adams RH. The notch ligands Dll4 and Jagged1 have opposing effects on angiogenesis. Cell. 2009;137:1124–1135. doi: 10.1016/j.cell.2009.03.025. [DOI] [PubMed] [Google Scholar]
- Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8:592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brantley-Sieders DM, Dunaway CM, Rao M, Short S, Hwang Y, Gao Y, Li D, Jiang A, Shyr Y, Wu JY, Chen J. Angiocrine factors modulate tumor proliferation and motility through EphA2 repression of Slit2 tumor suppressor function in endothelium. Cancer Res. 2011;71:976–987. doi: 10.1158/0008-5472.CAN-10-3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler JM, Gars EJ, James DJ, Nolan DJ, Scandura JM, Rafii S. Development of a vascular niche platform for expansion of repopulating human cord blood stem and progenitor cells. Blood. 2012;120:1344–1347. doi: 10.1182/blood-2011-12-398115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler JM, Kobayashi H, Rafii S. Instructive role of the vascular niche in promoting tumour growth and tissue repair by angiocrine factors. Nat Rev Cancer. 2010a;10:138–146. doi: 10.1038/nrc2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler JM, Nolan DJ, Vertes EL, Varnum-Finney B, Kobayashi H, Hooper AT, Seandel M, Shido K, White IA, Kobayashi M, et al. Endothelial cells are essential for the self-renewal and repopulation of Notch-dependent hematopoietic stem cells. Cell Stem Cell. 2010b;6:251–264. doi: 10.1016/j.stem.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, Oh EY, Gaber MW, Finklestein D, Allen M, et al. A perivascular niche for brain tumor stem cells. Cancer cell. 2007;11:69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles N, Ozawa T, Squatrito M, Bleau AM, Brennan CW, Hambardzumyan D, Holland EC. Perivascular nitric oxide activates notch signaling and promotes stem-like character in PDGF-induced glioma cells. Cell Stem Cell. 2010;6:141–152. doi: 10.1016/j.stem.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corada M, Nyqvist D, Orsenigo F, Caprini A, Giampietro C, Taketo MM, Iruela-Arispe ML, Adams RH, Dejana E. The Wnt/beta-catenin pathway modulates vascular remodeling and specification by upregulating Dll4/Notch signaling. Dev Cell. 2010;18:938–949. doi: 10.1016/j.devcel.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierks C, Grbic J, Zirlik K, Beigi R, Englund NP, Guo GR, Veelken H, Engelhardt M, Mertelsmann R, Kelleher JF, et al. Essential role of stromally induced hedgehog signaling in B-cell malignancies. Nature medicine. 2007;13:944–951. doi: 10.1038/nm1614. [DOI] [PubMed] [Google Scholar]
- Ding BS, Cao Z, Lis R, Nolan DJ, Guo P, Simons M, Penfold ME, Shido K, Rabbany SY, Rafii S. Divergent angiocrine signals from vascular niche balance liver regeneration and fibrosis. Nature. 2014;505:97–102. doi: 10.1038/nature12681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding BS, Nolan DJ, Butler JM, James D, Babazadeh AO, Rosenwaks Z, Mittal V, Kobayashi H, Shido K, Lyden D, et al. Inductive angiocrine signals from sinusoidal endothelium are required for liver regeneration. Nature. 2010;468:310–315. doi: 10.1038/nature09493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding BS, Nolan DJ, Guo P, Babazadeh AO, Cao Z, Rosenwaks Z, Crystal RG, Simons M, Sato TN, Worgall S, et al. Endothelial-derived angiocrine signals induce and sustain regenerative lung alveolarization. Cell. 2011;147:539–553. doi: 10.1016/j.cell.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, Saunders TL, Enikolopov G, Morrison SJ. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481:457–462. doi: 10.1038/nature10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebos JM, Lee CR, Cruz-Munoz W, Bjarnason GA, Christensen JG, Kerbel RS. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer cell. 2009;15:232–239. doi: 10.1016/j.ccr.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa L, Cathelin S, D’Altri T, Trimarchi T, Statnikov A, Guiu J, Rodilla V, Ingles-Esteve J, Nomdedeu J, Bellosillo B, et al. The Notch/Hes1 pathway sustains NF-kappaB activation through CYLD repression in T cell leukemia. Cancer cell. 2010;18:268–281. doi: 10.1016/j.ccr.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghajar CM, Peinado H, Mori H, Matei IR, Evason KJ, Brazier H, Almeida D, Koller A, Hajjar KA, Stainier DY, et al. The perivascular niche regulates breast tumour dormancy. Nature cell biology. 2013;15:807–817. doi: 10.1038/ncb2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert LA, Hemann MT. DNA damage-mediated induction of a chemoresistant niche. Cell. 2010;143:355–366. doi: 10.1016/j.cell.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gridley T. Notch signaling in the vasculature. Curr Top Dev Biol. 2010;92:277–309. doi: 10.1016/S0070-2153(10)92009-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer cell. 2012;21:309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- High FA, Lu MM, Pear WS, Loomes KM, Kaestner KH, Epstein JA. Endothelial expression of the Notch ligand Jagged1 is required for vascular smooth muscle development. Proc Natl Acad Sci U S A. 2008;105:1955–1959. doi: 10.1073/pnas.0709663105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoey T, Yen WC, Axelrod F, Basi J, Donigian L, Dylla S, Fitch-Bruhns M, Lazetic S, Park IK, Sato A, et al. DLL4 blockade inhibits tumor growth and reduces tumor-initiating cell frequency. Cell Stem Cell. 2009;5:168–177. doi: 10.1016/j.stem.2009.05.019. [DOI] [PubMed] [Google Scholar]
- Hofmann JJ, Zovein AC, Koh H, Radtke F, Weinmaster G, Iruela-Arispe ML. Jagged1 in the portal vein mesenchyme regulates intrahepatic bile duct development: insights into Alagille syndrome. Development. 2010;137:4061–4072. doi: 10.1242/dev.052118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly PN, Dakic A, Adams JM, Nutt SL, Strasser A. Tumor growth need not be driven by rare cancer stem cells. Science. 2007;317:337. doi: 10.1126/science.1142596. [DOI] [PubMed] [Google Scholar]
- Lane SW, Scadden DT, Gilliland DG. The leukemic stem cell niche: current concepts and therapeutic opportunities. Blood. 2009;114:1150–1157. doi: 10.1182/blood-2009-01-202606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leite de Oliveira R, Deschoemaeker S, Henze AT, Debackere K, Finisguerra V, Takeda Y, Roncal C, Dettori D, Tack E, Jonsson Y, et al. Gene-targeting of Phd2 improves tumor response to chemotherapy and prevents side-toxicity. Cancer cell. 2012;22:263–277. doi: 10.1016/j.ccr.2012.06.028. [DOI] [PubMed] [Google Scholar]
- Liu H, Chi AW, Arnett KL, Chiang MY, Xu L, Shestova O, Wang H, Li YM, Bhandoola A, Aster JC, et al. Notch dimerization is required for leukemogenesis and T-cell development. Genes Dev. 2010;24:2395–2407. doi: 10.1101/gad.1975210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobry C, Ntziachristos P, Ndiaye-Lobry D, Oh P, Cimmino L, Zhu N, Araldi E, Hu W, Freund J, Abdel-Wahab O, et al. Notch pathway activation targets AML-initiating cell homeostasis and differentiation. J Exp Med. 2013;210:301–319. doi: 10.1084/jem.20121484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Ye X, Fan F, Xia L, Bhattacharya R, Bellister S, Tozzi F, Sceusi E, Zhou Y, Tachibana I, et al. Endothelial Cells Promote the Colorectal Cancer Stem Cell Phenotype through a Soluble Form of Jagged-1. Cancer cell. 2013;23:171–185. doi: 10.1016/j.ccr.2012.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medyouf H, Gusscott S, Wang H, Tseng JC, Wai C, Nemirovsky O, Trumpp A, Pflumio F, Carboni J, Gottardis M, et al. High-level IGF1R expression is required for leukemia-initiating cell activity in T-ALL and is supported by Notch signaling. J Exp Med. 2011;208:1809–1822. doi: 10.1084/jem.20110121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memarzadeh S, Xin L, Mulholland DJ, Mansukhani A, Wu H, Teitell MA, Witte ON. Enhanced paracrine FGF10 expression promotes formation of multifocal prostate adenocarcinoma and an increase in epithelial androgen receptor. Cancer cell. 2007;12:572–585. doi: 10.1016/j.ccr.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakasone ES, Askautrud HA, Kees T, Park JH, Plaks V, Ewald AJ, Fein M, Rasch MG, Tan YX, Qiu J, et al. Imaging tumor-stroma interactions during chemotherapy reveals contributions of the microenvironment to resistance. Cancer cell. 2012;21:488–503. doi: 10.1016/j.ccr.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguera-Troise I, Daly C, Papadopoulos NJ, Coetzee S, Boland P, Gale NW, Lin HC, Yancopoulos GD, Thurston G. Blockade of Dll4 inhibits tumour growth by promoting non-productive angiogenesis. Nature. 2006;444:1032–1037. doi: 10.1038/nature05355. [DOI] [PubMed] [Google Scholar]
- Nolan DJ, Ginsberg M, Israely E, Palikuqi B, Poulos MG, James D, Ding BS, Schachterle W, Liu Y, Rosenwaks Z, et al. Molecular signatures of tissue-specific microvascular endothelial cell heterogeneity in organ maintenance and regeneration. Developmental Cell. 2013;26:204–219. doi: 10.1016/j.devcel.2013.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Vinals F, Inoue M, Bergers G, Hanahan D, Casanovas O. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer cell. 2009;15:220–231. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146:873–887. doi: 10.1016/j.cell.2011.08.039. [DOI] [PubMed] [Google Scholar]
- Rakhra K, Bachireddy P, Zabuawala T, Zeiser R, Xu L, Kopelman A, Fan AC, Yang Q, Braunstein L, Crosby E, et al. CD4(+) T cells contribute to the remodeling of the microenvironment required for sustained tumor regression upon oncogene inactivation. Cancer cell. 2010;18:485–498. doi: 10.1016/j.ccr.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Red-Horse K, Crawford Y, Shojaei F, Ferrara N. Endothelium-microenvironment interactions in the developing embryo and in the adult. Dev Cell. 2007;12:181–194. doi: 10.1016/j.devcel.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Rehman AO, Wang CY. Notch signaling in the regulation of tumor angiogenesis. Trends Cell Biol. 2006;16:293–300. doi: 10.1016/j.tcb.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Reimann M, Lee S, Loddenkemper C, Dorr JR, Tabor V, Aichele P, Stein H, Dorken B, Jenuwein T, Schmitt CA. Tumor stroma-derived TGF-beta limits myc-driven lymphomagenesis via Suv39h1-dependent senescence. Cancer cell. 2010;17:262–272. doi: 10.1016/j.ccr.2009.12.043. [DOI] [PubMed] [Google Scholar]
- Ridgway J, Zhang G, Wu Y, Stawicki S, Liang WC, Chanthery Y, Kowalski J, Watts RJ, Callahan C, Kasman I, et al. Inhibition of Dll4 signalling inhibits tumour growth by deregulating angiogenesis. Nature. 2006;444:1083–1087. doi: 10.1038/nature05313. [DOI] [PubMed] [Google Scholar]
- Scadden DT. Rethinking stroma: lessons from the blood. Cell Stem Cell. 2012;10:648–649. doi: 10.1016/j.stem.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt CA, Rosenthal CT, Lowe SW. Genetic analysis of chemoresistance in primary murine lymphomas. Nature medicine. 2000;6:1029–1035. doi: 10.1038/79542. [DOI] [PubMed] [Google Scholar]
- Seandel M, Butler JM, Kobayashi H, Hooper AT, White IA, Zhang F, Vertes EL, Kobayashi M, Zhang Y, Shmelkov SV, et al. Generation of a functional and durable vascular niche by the adenoviral E4ORF1 gene. Proc Natl Acad Sci U S A. 2008;105:19288–19293. doi: 10.1073/pnas.0805980105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi N, Dai X, Winter CG, Kang Y. Tumor-derived JAGGED1 promotes osteolytic bone metastasis of breast cancer by engaging notch signaling in bone cells. Cancer cell. 2011;19:192–205. doi: 10.1016/j.ccr.2010.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siekmann AF, Lawson ND. Notch signalling limits angiogenic cell behaviour in developing zebrafish arteries. Nature. 2007;445:781–784. doi: 10.1038/nature05577. [DOI] [PubMed] [Google Scholar]
- Sonoshita M, Aoki M, Fuwa H, Aoki K, Hosogi H, Sakai Y, Hashida H, Takabayashi A, Sasaki M, Robine S, et al. Suppression of colon cancer metastasis by Aes through inhibition of Notch signaling. Cancer cell. 2011;19:125–137. doi: 10.1016/j.ccr.2010.11.008. [DOI] [PubMed] [Google Scholar]
- Trimboli AJ, Cantemir-Stone CZ, Li F, Wallace JA, Merchant A, Creasap N, Thompson JC, Caserta E, Wang H, Chong JL, et al. Pten in stromal fibroblasts suppresses mammary epithelial tumours. Nature. 2009;461:1084–1091. doi: 10.1038/nature08486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung JJ, Tattersall IW, Kitajewski J. Tips, Stalks, Tubes: Notch-Mediated Cell Fate Determination and Mechanisms of Tubulogenesis during Angiogenesis. Cold Spring Harb Perspect Med. 2012;2:a006601. doi: 10.1101/cshperspect.a006601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilimas T, Mascarenhas J, Palomero T, Mandal M, Buonamici S, Meng F, Thompson B, Spaulding C, Macaroun S, Alegre ML, et al. Targeting the NF-kappaB signaling pathway in Notch1-induced T-cell leukemia. Nature medicine. 2007;13:70–77. doi: 10.1038/nm1524. [DOI] [PubMed] [Google Scholar]
- Wang Y, Nakayama M, Pitulescu ME, Schmidt TS, Bochenek ML, Sakakibara A, Adams S, Davy A, Deutsch U, Luthi U, et al. Ephrin-B2 controls VEGF-induced angiogenesis and lymphangiogenesis. Nature. 2010;465:483–486. doi: 10.1038/nature09002. [DOI] [PubMed] [Google Scholar]
- Weis SM, Cheresh DA. Tumor angiogenesis: molecular pathways and therapeutic targets. Nature medicine. 2011;17:1359–1370. doi: 10.1038/nm.2537. [DOI] [PubMed] [Google Scholar]
- Weng AP, Millholland JM, Yashiro-Ohtani Y, Arcangeli ML, Lau A, Wai C, Del Bianco C, Rodriguez CG, Sai H, Tobias J, et al. c-Myc is an important direct target of Notch1 in T-cell acute lymphoblastic leukemia/lymphoma. Genes & development. 2006;20:2096–2109. doi: 10.1101/gad.1450406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Trachootham D, Liu J, Chen G, Pelicano H, Garcia-Prieto C, Lu W, Burger JA, Croce CM, Plunkett W, et al. Stromal control of cystine metabolism promotes cancer cell survival in chronic lymphocytic leukaemia. Nature cell biology. 2012;14:276–286. doi: 10.1038/ncb2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.