Abstract
Background
Cultured brain tumors can form neurospheres harboring tumorigenic cells with self renewal and differentiation capacities. Renewable neurosphere formation has clinical predictive value in adult malignant gliomas, yet its prognostic role for pediatric brain tumors is unknown.
Methods
Established neurosphere conditions were used for culturing samples from glial, embryonal and mixed glioneuronal tumors from 56 pediatric patients. Potential associations between neurosphere formation and clinical outcome were analyzed retrospectively.
Results
Thirty-seven percent of all samples formed renewable neurospheres. Analysis of available clinical outcome data from 51 patients demonstrated significantly increased hazard ratios (HR) for both disease progression (HR=9.9, P < 0.001) and death (HR=16.6, P < 0.01) in the neurosphere forming group. Furthermore, neurosphere formation correlated with adverse progression free survival (PFS) in glial and embryonal tumors, but not in mixed glioneuronal tumors. Overall survival (OS) was significantly worse for neurosphere-forming patients with embryonal tumors, as a group and amongst the subgroup with medulloblastoma, but not in the glial group. Multivariate analysis showed that neurosphere formation was associated with diminished PFS and OS independent of age, gender, or treatment. Neurosphere formation was an independent predictor of diminished PFS of glial tumors after adjusting for grade. Multivariate analysis, adjusting for both Ki67 staining and neurosphere formation, demonstrated that neurosphere formation remained predictive of progression whereas Ki67 did not.
Conclusions
Neurosphere formation is more predictive of pediatric brain tumor progression than semi-quantitative Ki67 staining. Pediatric brain tumor derived neurospheres may provide a predictive model for preclinical explorations.
Keywords: brain, brain tumors, cancer biology, CNS tumors, neuro-oncology, outcomes research, tumors
INTRODUCTION
Pediatric brain tumors are a heterogeneous group of neoplasms with distinct clinical and pathological characteristics that compose 20–25% of pediatric cancer [1]. Clinical trials have resulted in considerable improvement in clinical outcome over the last few decades [1,2], however long-term cognitive and physical consequences of these therapies can be devastating and recurrent disease carries a dismal prognosis [3]. As pediatric tumors demonstrate properties distinct from adult tumors, further understanding of molecular pathogenesis and resistance to current therapies necessitate age directed research and the development of reliable preclinical models.
Patient-derived brain tumor cells can form spheres that can be repeatedly passaged (termed neurospheres [NS]) when placed in serum free media that are supplemented with epidermal and fibroblast growth factors [4]. The cells that give rise to neurospheres possess at least some of the characteristics of stemness: self renewal and multipotency, express neural stem cell-associated genes and, when xenotransplanted, confer in vivo tumorigenicity with recapitulation of the characteristics of parental tumors [5–7]. Because of these characteristics the terms “cancer stem cell” and “brain tumor stem cell” have been used for these cells. The cancer stem cell theory dictates that the stem cell-like cells, comprising a minority of the total cell population within the tumor are responsible for disease progression and resistance to therapy. Therefore, it would reason that the study of these cells would be highly relevant for the development of desperately needed novel therapies. However, the true clinical relevance of stem cell-like cells in brain tumors has not been fully elucidated.
In a previous study, we determined whether the ability to form multipassageable neurospheres is a reflection of the clinical aggressiveness of adult gliomas [6]. We found that those patients from whom we could derived neurospheres and maintain them in culture for at least three passages had worse outcomes, as measured by time until progression or death, than those from whom neurospheres could not be derived or maintained in culture. These findings held true even in the subgroup with glioblastoma and, thus, were not entirely dependent on grade. Nor were they dependent on other pathological measures, such as Ki67 staining [6]. Therefore, we concluded that, in adult glioma, the ability to form and maintain gliomas in culture reflected the biological nature of the tumor, indicating that the in vitro study of neurospheres and neurosphere-forming cells was a clinically relevant endeavor.
Because of the distinct nature and heterogeneity of pediatric brain tumors, we have now investigated the relationship between neurosphere formation and disease progression in pediatric brain tumor patients. As with adult gliomas, we have found that there is a general relationship between the ability to form multipassage neurospheres and patient outcome, in embyronal, including medulloblastoma, and glial tumors, but low grade, mixed glioneuronal tumors, mostly WHO grade I or II gangliogliomas, did not show a relationship. These data support for the hypothesis that the study of neurospheres derived from pediatric brain tumors is clinically relevant.
MATERIALS AND METHODS
Clinical Data and Tumor Collection
Informed consents were obtained from all patients and/or parents in accordance with UCLA Institutional Review Board. Resected brain tumor tissue and clinical follow-up data were collected from patients under 21 years of age treated for brain tumors between 2001 and 2009 at UCLA. Tissue diagnosis and grade were established by the neuropathologist in accordance with World Health Organization established guidelines [8]. This cohort included 42 primary and 14 recurrent tumors (for patient characteristics, see Table I). The percentage of tumor cells labeled by Ki67 immunostaining was estimated by two neuropathologists and the average value of the maximally stained areas was used for analysis in 48 cases.
TABLE I.
Patient Characteristics and Neurosphere Formation
| Characteristics | Total | Neurosphere forming patients | Neurosphere non-forming patients |
|---|---|---|---|
| Age (4 months to 20 years) | 7.4 ± 5.3a | 5.7 ± 4.4* | 8.5 ± 5.5* |
| Age groups | |||
| Less than 3 years old | 13 | 8 | 5 |
| 3–10 years old | 25 | 10 | 15 |
| More than 10 years old | 18 | 3 | 15 |
| Gender | |||
| Male | 35 | 15 | 20 |
| Female | 21 | 6 | 15 |
| Tumor type | |||
| Embryonal tumors | 14 | 8 | 6 |
| Medulloblastoma | 9 | 5 | 4 |
| PNETb | 3 | 2 | 1 |
| Pinealc | 2 | 1 | 1 |
| Glial tumors | 33 | 9 | 24 |
| High grade (HG) | 12 | 6 | 6 |
| Glioblastoma (GBM) | 4 | 2 | 2 |
| Anaplastic astrocytoma | 2 | 0 | 2 |
| HG oligodendroglial | 2 | 1 | 1 |
| Anaplastic Ependymomas | 4 | 3 | 1 |
| Low grade | 18 | 3 | 15 |
| Pilocytic astrocytoma | 15 | 3 | 12 |
| Other low grade gliomasd | 3 | 0 | 3 |
| Mixed glioneuronal tumors | 12 | 4 | 8 |
| Ganglioglioma | 7 | 3 | 4 |
| Gangliocytoma | 1 | 0 | 1 |
| DNET | 4 | 1 | 3 |
| Location | |||
| Supratentorial | 35 | 12 | 27 |
| Hemispheric | 30 | 10 | 20 |
| Right | 16 | 4 | 12 |
| Left | 12 | 5 | 7 |
| Bilateral | 2 | 1 | 1 |
| Midline | 5 | 2 | 3 |
| Pineal region | 3 | 2 | 1 |
| Suprasellar | 2 | 0 | 2 |
| Infratentorial | 21 | 9 | 13 |
| Cerebellar | 9 | 3 | 6 |
| Brainstem | 3 | 1 | 2 |
| Other posterior fossa | 9 | 5 | 4 |
| Total | 56 | 21 | 35 |
PNET, primitive neuroectodermal tumor; DNET, dysembryoplastic neuroepithelial tumor.
P = 0.045 (student t-test).
Mean ± SD in years.
One out of two supratentorial PNETs was associated with trilateral retinoblastoma.
A pineoblastoma and a pineocytoma.
Includes a pleomorphic xanthroastrocytoma and a subependymal giant cell astrocytoma.
Patient Cohort and Inclusion Criteria
Fifty-six patients with CNS tumors of glial and neural origin intrinsic to brain were included in this study. Cultures maintained for at least three passages were considered to be capable of renewable neurosphere formation. Twenty-one of the 56 cultures met this requirement. Clinical outcome data were available for 51 of 56 patients. Post-surgical care and follow-up of remaining five patients was carried out outside of UCLA, thus they were considered as loss to follow-up and excluded from survival analysis.
Cell Culture
Tumors were cultured as described previously [6] in DMEM-F12 medium (Invitrogen, Carlsabad, CA) supplemented with 1–100 B27 (Invitrogen), 20 ng/ml basic fibroblast growth factor (bFGF) (Peprotech, Rocky Hill, NJ), 50 ng/ml epidermal growth factor (EGF), penicillin/streptomycin (Invitrogen), L-glutamine (Invitrogen), and 5 µg/ml heparin (Sigma–Aldrich, St. Louis, MO). Heparin, bFGF, and EGF were added twice a week. Every 7–21 days, spheres were passaged into fresh media following either enzymatic dissociation with TrypLE and manual dissociation with small bore pipetting or chopping using an automatic chopper (Geneq).
Our group has previously reported this well established method of neurosphere culturing [5]. Fifty to seventy percent of the spheres in these cultures were about 150 µm (range 70–240 µm) when passaged to preserve viability of inner cells that may be in theoretical disadvantage of getting less nutrients and growth factors. In the early passages (<3) there were more irregularly shaped cellular aggregates, disorganized clusters and debris; however samples surviving all the way through the third passage consisted vastly of globularly shaped multi-cellular structures termed neurospheres, with rather morphologically uniform appearance (resembling those of their normal counterparts). Enzymatic dissociation followed by micro-pipette assisted dissociation was applied until passage three, and manual chopping was utilized only for consecutive passages, to standardize and eliminate potential artifact on selection of neurospherogenic cells. The unstructured cellular clamps, mostly two dimensional monolayers, and necrotic debris diminished significantly once passaged third time, leaving cultures with relatively monomorphic spherical structures and well organized three dimensional cellular compositions under light microscope, growing non-adherently. However, one of the medulloblastoma samples (desmoplastic) resulted in concomitant adherent growth.
This biologically vindicated threshold of three passages was shown to have statistically significant impact when applied to clinical outcome analyses of adult gliomas [6]. Based on our data, we strongly believe that this method allows enrichment of clinically relevant cells. However, about third of lines spontaneously expired by the fourth to fifth passages, and for the rest it was possible to propagate them longer, in general >7–10 passages. There was no aimed endpoint to access the maximum number of passages due to retrospective nature of this study, and most of the samples were terminated prior to passage 20, based on observations that in vivo tumorigenicity is best preserved for samples between passages 3 and 20, and because of concerns of in vitro artifact secondary to long-term passaging.
Statistical Analyses
As previously described [6], time to survival (TTS) was determined by the duration of life from the date of surgery using the death summaries or the social security death index, or, if alive, until the last follow-up date. Time to progression (TTP) was determined by the duration of progression-free survival from the date of surgery until recurrence, or, if progression-free, until the last follow-up date. Renewable neurosphere formation status and was defined as a binary variable as described later. TTS and TTP were related to the explanatory variables using a Cox proportional hazards model as implemented in Stata 8.0 (Stata Corp, College Station, Texas). This analysis was performed in the full cohort of 51 patients and within the subpopulations of glial, embryonal, or mixed glioneuronal tumors. Multivariate Cox regression models were performed to control for grade, age, Ki67 measurement values and treatment groups. The average of observed percentage of highest Ki67 staining areas was treated as a continuous variable. Treatment groups were used as categorical variables in multivariate analysis. All P values were two-sided and P < 0.05 was considered significant. To visualize the survival distribution, we used Kaplan–Meier method.
RESULTS
Patient characteristics are presented in Table I. Twenty-one of the 56 tumor samples formed renewable cultures under neurosphere conditions. One of five tumor samples from patients loss to follow-up formed renewable neurospheres in culture. Numbers of neurosphere forming versus non-forming tumors were calculated in each subgroup based on characteristics of patient age, patient gender, tumor type, and location (Table I). The average age of patients with neurosphere forming tumors was significantly younger compared to patients with tumors which neurosphere could not be propagated. When we examined neurosphere formation as a function of age, there was a significant correlation (Supplemental Fig. 1). Further analyses showed that three age groups (<3, 3–10, and >10–20 years old) had decreasingly lower proportion of high-grade lesions (77%, 48%, and 22%, respectively), associated with lower median Ki67 (35, 8, and 2.5, respectively) and less neurosphere formation (Table I). Mean Ki67 values were statistically different among three age groups (P = 0.0367, ANOVA).
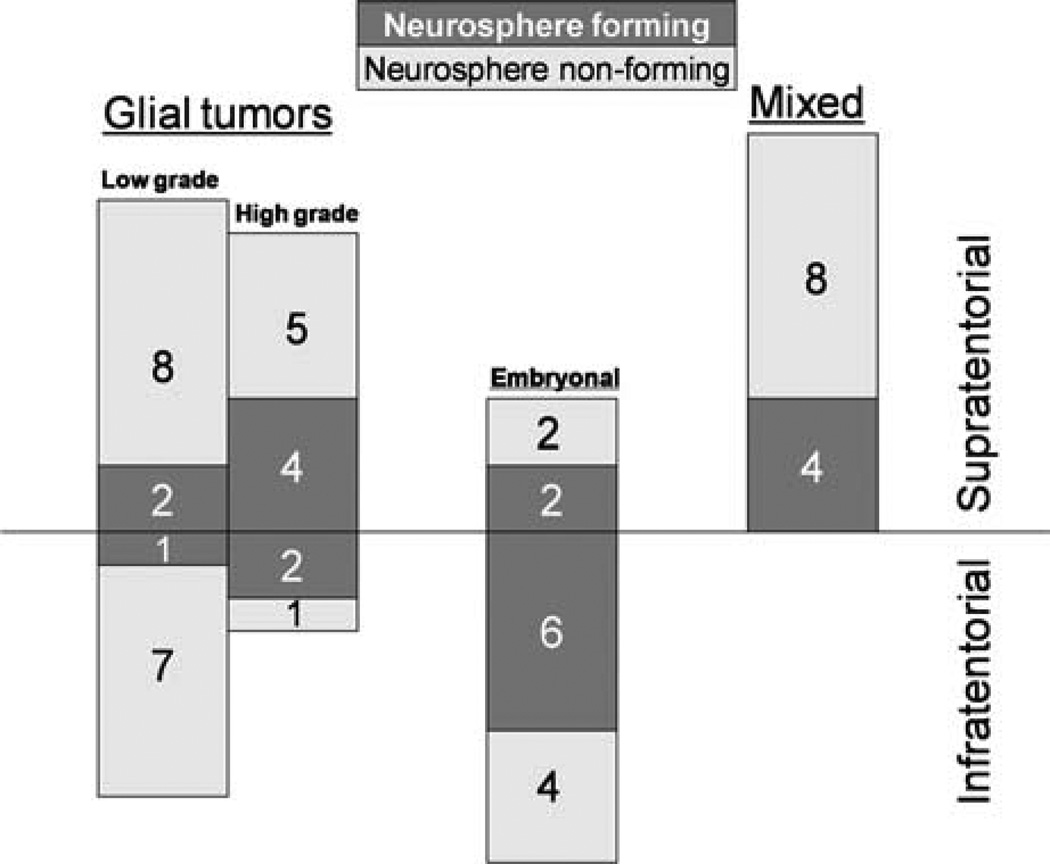
Thirty-four percent of supratentorial lesions and 43% of infratentorial lesions formed renewable neurospheres (Fig. 1). In mixed glioneuronal tumor group, 33% of samples formed renewable neurospheres. Only 17% of low grade glial tumors formed renewable neurospheres, whereas 50% of high grade glial tumors and 57% of embryonal tumors formed renewable neurospheres (Fig. 1). When high grade glial tumors were compared to low grade glial tumors, Pearson χ2 test resulted in 3.8 times higher probability of neurosphere formation (P = 0.051). Comparison of neurosphere formation between embryonal tumors and low grade glial tumors resulted in Pearson χ2 = 5.7 (P = 0.017), indicating that embryonal tumors are significantly more capable of generating renewable neurospheres.
Fig. 1.
Neurosphere formation by tumor location and tumor type in 56 pediatric brain tumor patients. Dark bars represent neurosphere forming tumors, and light bars stand for neurosphere non-forming tumors. Infratentorial and supratentorial locations are separated by horizontal line. Absolute numbers of tumors are illustrated in each group (neurosphere forming, in white; and neurosphere non-forming, in black). Right column designated as “Mixed” represents glioneuronal lesions.
To evaluate the relationship between in situ proliferation rates and neurosphere formation we examined neurosphere formation as a function of Ki67 staining in 48 patients with available Ki67 values. We found a significantly increased likelihood of neurosphere formation with higher Ki67 both in the whole cohort and glial sub-group (Supplemental Fig. 2A and B). Supplemental Figure 2C,D demonstrate these relationships of neurosphere formation as a function of Ki67, adjusted for age in the whole cohort and glial sub-group, accordingly.
Furthermore, we retrospectively sub-grouped patients according to combination of treatment modalities and analyzed renewable neurosphere formation in different treatment groups (Table II and Supplemental Table I). In patients, who received surgery only (n=32), 70% of lesions amenable to subtotal resection (STR) formed neurospheres, whereas only 14% of those with gross total resection (GTR) did so, indicating a possible invert relationship between the neurosphere forming capacity and tumor resectability. Neurosphere formation may recapitulate infiltrative nature and invasive growth of the lesions that were not totally resectable. For the whole cohort: only 6 of 30 (20%) GTR resulted in neurosphere forming samples, whereas 15 of 26 (57.7%) STR did so.
TABLE II.
Treatment Groups and Neurosphere Formation
| Treatment modality |
Number of patients |
Neurosphere forming patients (%) |
|---|---|---|
| Surgery only | 32b | 10 (31) |
| GTR | 22 | 3 (14) |
| STR | 10 | 7 (70) |
| Surgery and chemotherapya | 5c | 1 (20) |
| Surgery, chemo-, and radiotherapya | 11d | 5 (45) |
| Surgery, chemo-, and ASCRa | 8e | 5 (63) |
One patient per each group received brain tumor vaccine.
Five patients with loss of follow-up after surgery, eight had repeat resections.
One patient received antineoplastons in outside hospital.
One patient received postoperative radiation only.
One patient in ASCR group also received radiotherapy; ASCR, autologous stem cell rescue (after high dose chemotherapy); GTR, gross total resection; STR, sub-total resection.
Survival Analysis
In the cohort of 51 patients whose clinical follow up data were available, the mean follow up duration was 2 ± 1.8 years, the mean patient survival time was 762 days and the mean progression-free survival time was 739 days. Eight out of 51 patients died before the last date of analysis. The probability of 3-year PFS was 71 ± 7% (mean ± SE) and 3-year OS was 81 ± 6% in full population.
Cox proportional hazards regression was performed to evaluate potential differences in clinical outcome of patients with neurosphere forming tumors versus those not forming neurospheres (Table III). We evaluated these associations in the whole cohort of 51 patients with available clinical outcome data, and also in different subpopulations based on neuropathology subgroups, namely: in glial tumors (n=27), embryonal tumors (n=12), medulloblastomas amongst the embryonal subgroup (n=7), and mixed glioneuronal tumors (n=12). The hazard ratios of patient death and tumor progression for the full population and the three subgroups are shown in Table III. Analysis of the mixed glioneuronal tumors subpopulation (n=12) did not reveal significant associations. Renewable neurosphere formation was significantly associated with a 6.6 to > 10 hazard of progression (P < 0.01) in the remaining tumors (Table III). These associations were also significant for hazard of death (> 10) in the full population (P < 0.01), and in the subpopulations of embryonal and medulloblastoma tumors (P < 0.001). For glial tumors, we observed no statistically significant association between neurosphere formation and hazard of patient death.
TABLE III.
Cox Proportional Hazards Regressions
| Patient population | Variable | Hazard of patient death | Hazard of patient tumor progression |
|---|---|---|---|
| Full populationa (n = 51) | Neurosphere formation | 16.6, CI (1.93–142); P-value <0.010 | 9.94, CI (2.91–34.0); P-value <0.001 |
| Glial tumorsb (n = 27) | Neurosphere formation | 5.06 (NS), CI (0.48–53.4); P-value <0.177 | 6.62, CI (1.70–25.8); P-value 0.006 |
| Embryonalc (n = 12) | Neurosphere formation | >10, CI (>10 to >10) P-value <0.001 | >10, CI (>10 to >10); P-value <0.001 |
| Medulloblastomad (n = 7) | Neurosphere formation | >10, CI (>10 to >10); P-value <0.001 | >10, CI (>10 to >10); P-value <0.001 |
CI, 95% confidence interval; NS, not significant.
Mean age 8.4 years, 20/51 tumors with neurosphere formation.
Mean age 8.47 years, 9/27 tumors with neurosphere formation.
Mean age 5.56 years, 7/12 tumors with neurosphere formation.
Mean age 4.71 years, 4/7 tumors with neurosphere formation.
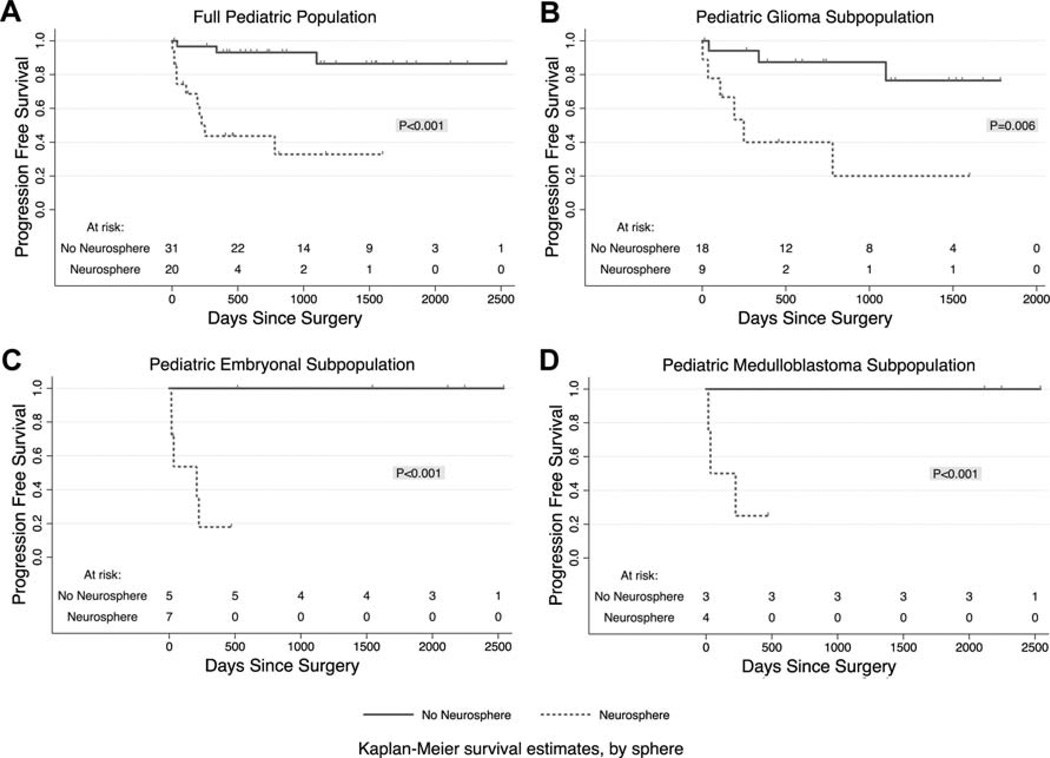
Kaplan–Meier curves displaying the proportion of progression free survival (PFS) as a function of days from surgery are demonstrated in Figure 2A–D. In the full cohort of 51 patients, the group with neurosphere formation had 33±13% 3-year PFS, whereas the group without neurosphere formation had 86 ± 7.8% 3-year PFS (P < 0.001). In the subpopulation of glial tumors, the group with neurosphere formation had only 20 ± 17% 3-year PFS, whereas the group without neurosphere formation had 76 ± 13.3% 3-year PFS (P < 0.006). In the embryonal group (n=12) and medulloblastoma subgroup (n=7) 1-year PFS for patients with neurosphere formation was drastically decreased to 18 ± 16% and 25 ± 22%, respectively, whereas patients without renewable neurosphere formation sustained 100% PFS for more than 5 years (P < 0.001).
Fig. 2.
Kaplan–Meier curves display the proportion of patients surviving without tumor progression as a function of time since surgery. Dotted lines donate groups with renewable neurosphere formation, and solid lines stand for patients whose tumors did not form renewable neurospheres. P values per Cox Regression analyses. A: The full cohort of pediatric CNS tumors (n = 51). B: The subpopulation of pediatric glioma (n = 27). C: The subpopulation of pediatric embryonal tumors (n = 12). D: The subpopulation of pediatric medulloblastoma (n = 7).
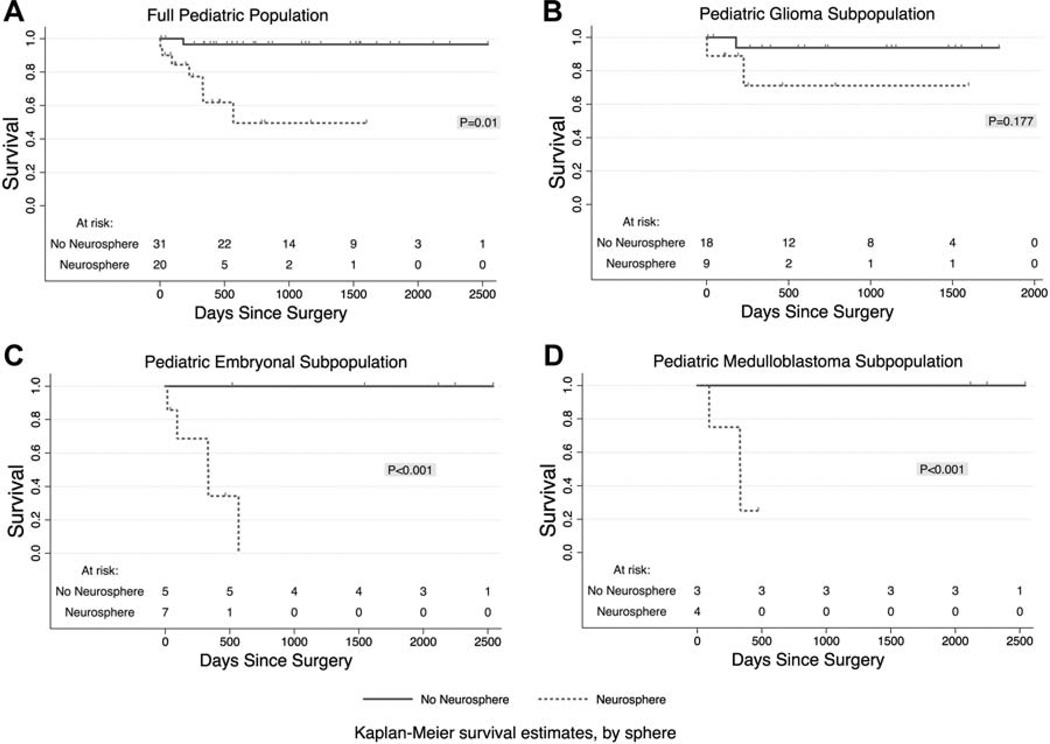
Figure 3A–D demonstrate Kaplan–Meier curves for overall survival (OS) as a function of days from surgery comparing groups with and without renewable neurosphere formation. After 3 years, in the full cohort of 51 tumors, the group with neurosphere formation had 50 ± 15% OS, whereas the group without neurosphere formation had 97 ± 3.4% OS (P = 0.01). For embryonal tumors, the probability of 3-year OS was 0% and 100% for groups with and without renewable neurosphere formation, respectively (P < 0.001). Accordingly, for 7 medulloblastoma cases, after 1-year follow up, the probability of OS was 25 ± 22% and 100% for neurosphere forming and non-forming groups, respectively (P < 0.001). For glial tumors, we observed no significant difference in OS (Fig. 3B, P = 0.177).
Fig. 3.
Kaplan–Meier curves display the proportion of patients surviving as a function of time since surgery. Dotted lines donate groups with renewable neurosphere formation, and solid lines stand for patients whose tumors did not form renewable neurospheres. P values per Cox Regression analyses. A: The full cohort of pediatric CNS tumors (n = 51). B: The subpopulation of pediatric glioma (n = 27). C: The subpopulation of pediatric embryonal tumors (n = 12). D: The subpopulation of pediatric medulloblastoma (n = 7).
Multivariate Cox proportional hazards logistic regression analysis showed that neurosphere formation remained a significant predictor of patient survival and tumor progression after adjusting for age (HR = 14, P < 0.03, and HR = 9, P = 0.002 respectively for OS and PFS), gender (HR = 16, P < 0.014, and HR = 10, P < 0.001) and treatment (HR = 15, P = 0.025, and HR = 9.1, P < 0.001) in the full population. Neurosphere formation status retained independent predictive value for PFS even when adjusted for grade and treatment in glial tumors (HR = 6.1, P = 0.018 and HR = 6.6, P = 0.022) or Ki67 in full population (HR = 4.2, P = 0.049), whereas Ki67 did not. Ninety-five percent confidence intervals of these hazard ratios are shown in Table IV. In summary, multivariate analysis showed that in this cohort of pediatric patients with CNS tumors neurosphere formation remained predictive of survival and progression, largely independent of Ki67 and treatment.
TABLE IV.
Multivariate Cox Proportional Hazards Regressions
| Patient population | Independent variable | Controlled variables | Hazard of patient death | Hazard of patient tumor progression |
|---|---|---|---|---|
| Full population (n = 43) | Neurosphere formation | Ki67, age, gender | 1.90 (not significant), CI (0.0051–594); P-value 0.8, ki67, and gender are independent | 4.23 (not significant), CI (0.80–22.3); P-value 0.089, no independence |
| Full population (n = 51) | Neurosphere formation | Age | 13.9, CI (1.29–150); P-value <0.030, sphere is independent of age | 8.92, CI (2.28–34.9); P-value 0.002, sphere is independent of age |
| Full populationa (n = 43) | Neurosphere formation | Ki67 | 3.81 (not significant), CI (0.311–46.7); P-value <0.296, sphere, and Ki67 are independent | 4.19, CI (1.01–17.4); P-value 0.049, sphere is independent of KI67 |
| Full population (n = 51) | Neurosphere formation | Gender | 15.9, CI (1.76–142); P-value <0.014, sphere is independent of gender | 9.90, CI (2.91–33.7); P-value <0.001, sphere is independent of gender |
| Full population (n = 51) | Neurosphere formation | Treatment (TX) | 15.1, CI (1.40–164); P-value 0.025, sphere is independent of TX | 9.10, CI (2.40–34.6); P-value <0.001, sphere is independent of TX |
| Glial tumors (n = 22) | Neurosphere formation | Ki67, grade | 0.154 (not significant), CI (0.0028–8.51); P-value 0.361, grade is independent | 4.64 (not significant), CI (0.277–77.7); P-value 0.286, no independence |
| Glial tumors (n = 27) | Neurosphere formation | Grade | 1.48 (not significant), CI (0.153–14.2); P-value 0.737, grade is independent of sphere | 6.10, CI (1.37–27.2); P-value 0.018, sphere is independent of grade |
| Glioma (n = 22) | Neurosphere formation | Ki67 | 0.177 (not significant), CI (0.000–552); P-value 0.673, no independence | 5.14 (not significant), CI (0.427–61.9); P-value 0.197, no independence |
| Glial tumors (n = 27) | Neurosphere formation | Treatment (TX) | 4.16 (not significant), CI (0.152–113); P-value 0.398, no independence | 6.58, CI (1.27–34.2); P-value 0.022, sphere is independent of TX |
CI, 95% confidence interval.
Ki67 and outcome information is available for 43 patients.
We also analyzed neurosphere formation amongst 14 recurrent patients at the time of study entry, who received chemotherapy and/or radiotherapy prior to neurosphere cultures. Patients with neurosphere forming recurrent high grade lesions experienced subsequent recurrence in 80% of cases (Supplemental Table III). All three patients who died in this group had chemo- and/or radiotherapy prior to neurosphere culturing. This further supports the theory that neurospheres may arise from subpopulation of highly malignant and tumorigenic cells resistant to current cytotoxic treatment.
DISCUSSION
Our results demonstrate significant correlations between renewable neurosphere formation and clinical outcome in certain pediatric CNS tumors. First, both PFS and OS were strongly associated with neurosphere growth in the whole cohort of 51 patients. Further analysis of the subpopulations suggests that this correlation is strongest for embryonal tumors including the subgroup of medulloblastoma patients, where the hazards of both disease progression and patient death were > 10 with P values of <0.001 in patients with neurosphere formation, despite the small sample sizes. In contrast, there was no correlation between neurosphere formation and clinical outcome in mixed glioneuronal tumors, all of which were low grade, located in hemispheres and primarily amenable to gross total resection (only 1 of 12 was partially resected). All these patients with mixed glioneuronal tumors were progression free and alive post-operatively. These findings concur with previously reported outcome data for pediatric gangliogliomas, in which cerebral hemisphere location and the achievement of total resection were associated with prolonged PFS [9]. These diverse findings probably reflect clinical-biological difference for various pediatric CNS tumors, showing that neurosphere formation significantly reflects the prognosis of high grade embryonal tumors, but not for totally respectable low grade mixed glioneuronal lesions.
In the heterogeneous glial subgroup, renewable neurosphere formation defined a subpopulation with a highly significant increased hazard of tumor progression. Four patients with NS forming and progressive glial tumors were salvaged by further therapies and were alive at the time of these analysis, resulting in no correlation between NS formation and overall survival. Thus, in this glial subgroup, grade remained independent of neurosphere formation for prediction of overall survival in multivariate analysis (Table IV). Of note, a report from the review of 6,212 cases of children with gliomas [10] also demonstrated that grade was the most significant independent prognostic factor for survival for children above 1 year of age.
However, when evaluating tumor progression, but not survival, in the glioma tumor subpopulation, neurosphere formation retained its significant predictive value, even when adjusted for grade of glial tumors. This property of neurosphere formation capacity to predict clinically relevant disease severity, independent of glioma grade, indicates that the neurosphere model harbors distinct prognostic value. In other words, while NS formation can be more predictive of pediatric glioma progression independent of grade, grade is more predictive of overall survival.
Our analyses demonstrated that both age and Ki67 were associated with neurosphere formation; however, age is not independent of Ki67 and these associations are reflective of the fact that younger patients had more high-grade lesions in our database. Although Ki67 immunoanalysis may serve as a valuable supplementary function in the diagnostic and prognostic evaluation of childhood malignant gliomas [11], astrocytomas [12] and ependymomas [13], for our 43 patients with Ki67 staining and outcome data, neurosphere formation remained a significant predictor of tumor progression, even after adjusting for Ki67. These relationships indicate that neurosphere formation may capture tumor aggressiveness more comprehensively than other, previously established methods such as Ki67 staining.
Other controlled variables, such as age and gender, were also included in our multivariate proportional hazard regression, because more tumors from males (43%) than females (29%) formed neurospheres, and younger age was associated with higher likelihood of neurosphere formation (Tables I and IV and Supplemental Fig. 1). In addition, historically age had different prognostic value for glial tumors depending on tumor grade [10,14,15]. Nevertheless, neurosphere formation retained its independent predictive value for both adverse PFS and OS when adjusting for both age and gender in full population (Table IV).
Although more patients in neurosphere forming group received most aggressive treatment combinations, their outcome still remained inferior to neurosphere non-forming patients (Supplemental Table II). Thus, treatment groups were also used as another controllable variable in multivariate analysis, which demonstrated that for the whole cohort, neurosphere formation was independent of the combination of treatment modalities in predicting both patient death and tumor progression (Table IV). This illustrates the limited role of radiotherapy and conventional alkylator-based cytotoxic chemotherapy intensification in severe disease, highlighting the necessity of new strategies to target cellular pathways in pediatric brain tumors [16].
Provided that neurosphere formation in this study may reflect the propagation of tumorigenic cell content of pediatric brain tumors [3], our results concur with the findings of previous studies, showing that the in vitro re-generation potential of glioblastoma cells and the presence of putative cancer stem cells are two considerable prognostic factors for disease progression and clinical outcome [6,17]. Neurospherogenic properties of brain tumor cells are not suitable for diagnostic purposes, and do not directly prove or disprove the hypothesis of brain tumor stem cells; nonetheless, further preclinical investigations are warranted to elucidate potential value of NS cultures to predict in vivo therapeutic response.
CONCLUSIONS
In vitro renewable neurosphere formation predicts the clinical course of pediatric glial and embryonal brain tumors with a unique ability that is largely independent of established prognostic indicators. Multivariate analysis of the full population, adjusting for both Ki67 and neurosphere formation, demonstrated that neurosphere formation remained predictive of progression, whereas Ki67 did not. This study confirms and extends previous assessments of neurosphere formation as predictor of clinical outcome [6,17]. Similar to malignant gliomas in adults, multi-passage neurosphere formation is prognostic of clinical severity for pediatric glial and embryonal tumors, confirming the validity of this culture system as a suitable robust in vitro model and comprehensive neurobiological tool for preclinical brain tumor research.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported in part by UCLA Tumor Cell Biology Training Program (Grant #T32 CA009056), which is funded by USHHS (NIH/NCI); UCLA Brain Tumor Translational Resource and Brain Tumor Funders’ Collaborative, Art of the Brain and NINDS R01NS052563.
Footnotes
Additional Supporting Information may be found in the online version of this article.
Conflict of interest: Nothing to declare.
REFERENCES
- 1.Mueller S, Chang S. Pediatric brain tumors: Current treatment strategies and future therapeutic approaches. Neurotherapeutics. 2009;6:570–586. doi: 10.1016/j.nurt.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kieran MW, Chi SN, Samuel D, et al. Pediatric brain tumors. In: Orkin SH, Fisher DE, Look T, editors. Oncology of infancy and childhood. Philadelphia: Elsevier, Inc.; 2009. pp. 623–681. [Google Scholar]
- 3.Lasky JL, III, Choe M, Nakano I. Cancer stem cells in pediatric brain tumors. Curr Stem Cell Res Ther. 2009;4:298–305. doi: 10.2174/157488809789649278. [DOI] [PubMed] [Google Scholar]
- 4.Lee J, Kotliarova S, Kotliarov Y, et al. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 2006;9:391–403. doi: 10.1016/j.ccr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 5.Hemmati HD, Nakano I, Lazareff JA, et al. Cancerous stem cells can arise from pediatric brain tumors. Proc Natl Acad Sci USA. 2003;100:15178–15183. doi: 10.1073/pnas.2036535100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laks DR, Masterman-Smith M, Visnyei K, et al. Neurosphere formation is an independent predictor of clinical outcome in malignant glioma. Stem Cells. 2009;27:980–987. doi: 10.1002/stem.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh SK, Hawkins C, Clarke ID, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 8.Kleihues P, Louis DN, Scheithauer BW, et al. The WHO classification of tumors of the nervous system. J Neuropathol Exp Neurol. 2002;61:215–225. doi: 10.1093/jnen/61.3.215. Discussion 226–219. [DOI] [PubMed] [Google Scholar]
- 9.El Khashab M, Gargan L, Margraf L, et al. Predictors of tumor progression among children with gangliogliomas. Clinical article. J Neurosurg Pediatr. 2009;3:461–466. doi: 10.3171/2009.2.PEDS0861. [DOI] [PubMed] [Google Scholar]
- 10.Qaddoumi I, Sultan I, Gajjar A. Outcome and prognostic features in pediatric gliomas: A review of 6212 cases from the Surveillance, Epidemiology, and End Results database. Cancer. 2009;4:298–305. doi: 10.1002/cncr.24663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pollack IF, Hamilton RL, Burnham J, et al. Impact of proliferation index on outcome in childhood malignant gliomas: Results in a multi-institutional cohort. Neurosurgery. 2002;50:1238–1244. doi: 10.1097/00006123-200206000-00011. Discussion 1244–1235. [DOI] [PubMed] [Google Scholar]
- 12.Torp SH. Diagnostic and prognostic role of Ki67 immunostaining in human astrocytomas using four different antibodies. Clin Neuropathol. 2002;21:252–257. [PubMed] [Google Scholar]
- 13.Wolfsberger S, Fischer I, Hoftberger R, et al. Ki-67 immunolabeling index is an accurate predictor of outcome in patients with intracranial ependymoma. Am J Surg Pathol. 2004;28:914–920. doi: 10.1097/00000478-200407000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Laithier V, Grill J, Le Deley MC, et al. Progression-free survival in children with optic pathway tumors: Dependence on age and the quality of the response to chemotherapy—Results of the first French prospective study for the French Society of Pediatric Oncology. J Clin Oncol. 2003;21:4572–4578. doi: 10.1200/JCO.2003.03.043. [DOI] [PubMed] [Google Scholar]
- 15.Sanders RP, Kocak M, Burger PC, et al. High-grade astrocytoma in very young children. Pediatr Blood Cancer. 2007;49:888–893. doi: 10.1002/pbc.21272. [DOI] [PubMed] [Google Scholar]
- 16.Herrington B, Kieran MW. Small molecule inhibitors in children with malignant gliomas. Pediatr Blood Cancer. 2009;53:312–317. doi: 10.1002/pbc.21950. [DOI] [PubMed] [Google Scholar]
- 17.Pallini R, Ricci-Vitiani L, Banna GL, et al. Cancer stem cell analysis and clinical outcome in patients with glioblastoma multiforme. Clin Cancer Res. 2008;14:8205–8212. doi: 10.1158/1078-0432.CCR-08-0644. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.