Abstract
BACKGROUND
Elevated blood pressure (BP), a heritable risk factor for many age-related disorders, is commonly investigated in population and genetic studies, but antihypertensive use can confound study results. Routine methods to adjust for antihypertensives may not sufficiently account for newer treatment protocols (i.e., combination or multiple drug therapy) found in contemporary cohorts.
METHODS
We refined an existing method to impute unmedicated BP in individuals on antihypertensives by incorporating new treatment trends. We assessed BP and antihypertensive use in male twins (n = 1,237) from the Vietnam Era Twin Study of Aging: 36% reported antihypertensive use; 52% of those treated were on multiple drugs.
RESULTS
Estimated heritability was 0.43 (95% confidence interval (CI) = 0.20–0.50) and 0.44 (95% CI = 0.22–0.61) for measured systolic BP (SBP) and diastolic BP (DBP), respectively. We imputed BP for antihypertensives by 3 approaches: (i) addition of a fixed value of 10/5mm Hg to measured SBP/DBP; (ii) incremented addition of mm Hg to BP based on number of medications; and (iii) a refined approach adding mm Hg based on antihypertensive drug class and ethnicity. The imputations did not significantly affect estimated heritability of BP. However, use of our most refined imputation method and other methods resulted in significantly increased phenotypic correlations between BP and body mass index, a trait known to be correlated with BP.
CONCLUSIONS
This study highlights the potential usefulness of applying a representative adjustment for medication use, such as by considering drug class, ethnicity, and the combination of drugs when assessing the relationship between BP and risk factors.
Keywords: antihypertensive, blood pressure, body mass index, heritability, hypertension, twins, VETSA.
Population and family studies are commonly used to investigate the relationship of blood pressure (BP) with traits or disease outcomes, and twin studies are a powerful approach for investigating the genetic and environmental determinants of BP. To the extent that individuals are treated for hypertension in older populations and unmedicated BP data are inaccessible, these studies are often confounded by antihypertensive treatment. There is little consensus on how to account for BP medication use. Some studies exclude medicated individuals,1,2 use BP measurements without adjusting for medication use,3 or adjust analyses by including medication use as a covariable.4 Such methods may alter the estimated effect of genetic or environmental determinants of BP, obscure the relationship of BP to other factors, or reduce statistical power.5–7 Further, when BP is the outcome variable, such as in genetic studies (e.g., genetic association, multivariable twin analyses) or studies identifying risk factors for BP, including an antihypertensive use variable as a predictor in a regression model is problematic because of the relationship between BP and antihypertensive use. In such cases, imputing unmedicated BP becomes relevant. Tobin et al. demonstrated by simulation and real sample data that predicting unmedicated BP based on clinical evidence is an effective strategy of imputing BP for genetic studies.6
Recent genetic studies have adopted an approach (“fixed addition”) in which a fixed value of 10/5mm Hg is added to the observed systolic (SBP)/diastolic (DBP) BP,8 but most studies do not report whether the adjustment altered the results.9–14 In a family study that also included twins, Cui et al. investigated the genetic and environmental components of BP variation in relation to body mass index (BMI) using fixed addition8 and, in a follow-up study, compared the results of genetic analysis using measured BP, fixed addition, as well as other approaches.15 They introduced a “stepped addition” approach in which increments of 8/4mm Hg, 14/10mm Hg, and 20/16mm Hg were added to measured SBP/DBP of treated subjects taking 1, 2, or 3 drug classes, respectively. They concluded that information was lost if treated individuals were excluded or if BP was not adjusted using imputation.
To reach optimal BP, a combination of drugs from different pharmaceutical classes is often required.16–18 Treatment response varies with drug class and by ethnicity.19–21 Recognizing that a refined approach integrating drug class and ethnicity information may better predict unmedicated BP, Wu et al. devised an algorithm to impute unmedicated BP based on drug class and ethnicity.22 Using published data from 137 clinical trials of monodrug therapies, Wu et al. reported the weighted average effect lowering of SBP and DBP in blacks and nonblacks for 6 major classes of antihypertensives. Using data from 28 clinical trials of combination drug therapies, they estimated the effects of the second medication.22 They suggested that accounting for these BP-lowering effects could be used to impute pretreatment BP levels to improve the power of studies confounded by BP medication. Adjustment based on drug class effects has been less applied than fixed addition, but its use in a large population study facilitated the identification of genetic risk factors for heart disease.23
Current therapy includes multiple (≥3) drugs,24–28 and fixed-dose combinations of antihypertensives in a single pill are now routinely prescribed to manage hypertension.27 Thus, to infer BP more closely reflecting pretreatment values, there is a need for approaches that (i) impute effects beyond 2 drug classes; (ii) incorporate information on newer drug classes; and (iii) account for combination drugs.
We extended the approach of Wu et al.22 to account for multiple drug therapies that reflect treatment scenarios found in recently ascertained cohorts. We applied this approach in an age-homogeneous sample comprised of middle-aged male twin pairs from the Vietnam Era Twin Study of Aging (VETSA) (age range = 51–60 years) in which hypertension is prevalent.29 We reasoned that our refined imputation approach based on the best available clinical trial evidence would yield a BP that is closer to a person’s “true” BP if they were unmedicated and represents the best estimate of true BP. To the extent that there is error contained in the imputations, they will deviate from the true BP. We examined the effect of this imputation approach on the results of genetic and population studies. We first compared the correlations between the imputed BP and BMI (a known correlate of BP)30 with correlations obtained with measured BP or BP imputed with a fixed or stepped addition. Because they reduce BP, antihypertensives also restrict the range of BP. Therefore, we predicted that correlations between drug class–adjusted BP and BMI would be higher than correlations with unadjusted measured BP because of the concomitant increased range of BP. Consistent with this idea, previous studies have shown that imputing BP by adding a clinically reasonable value to medicated BP increases the power to detect determinants of BP.6 We also investigated how the different imputation approaches affect estimation of heritability (i.e. the proportion of total variance in an observed trait that is due to genes31). We hypothesized that our imputation would result in higher heritability estimates for BP. There were 2 bases for this hypothesis. First, in reducing BP, antihypertensives may suppress the natural expression of genetic influences. By offsetting that suppression effect, the imputation might result in increased heritability estimates for BP. Second, medication adds an external environmental factor. Adding more environmental variance would reduce heritability, but the imputation would then offset that reduction. Heritabilities increased slightly, albeit nonsignificantly, after imputation based on drug class effects in a younger sample in which only 14.5% had hypertension.5 With our further refinement of the imputation and the higher prevalence of hypertension in our older sample, we hypothesized that the imputation might increase heritability.
METHODS
Study participants
Participants were from Wave 1 of VETSA, a longitudinal study of cognition and aging beginning in midlife,29 recruited from the Vietnam Era Twin Registry of male twin pairs who served in the US military sometime between 1965 and 1975.32,33 VETSA twins resemble the larger registry sample and are representative in demographic and health characteristics of the US population of similarly aged adult men based on US Census and Centers for Disease Control and Prevention data.34 Our sample (mean age = 55.4 years; age range = 51–60 years) consisted of 347 monozygotic (MZ) twin pairs, 267 dizygotic (DZ) twins pairs, and 9 unpaired twins. The study was approved by local institutional review boards at the University of California–San Diego and Boston University. Ethnicity was based on self-identification: black (n = 51), white (n = 1,110), and other (n = 76; including American Indian or Alaskan Native, Native Hawaiian or Pacific Islander, mixed ethnicity). Age, education, employment status, and ethnicity were similar between the MZ and DZ groups.35
BP and BMI
BP was measured in seated subjects using aneroid sphygmomanometry a total of 4 times, twice in the morning 5 minutes apart and twice in the afternoon 5 minutes apart. Participants rested for 5 minutes before the first BP reading, waited 1 minute, and had a second reading. Measured BP refers to the mean value of these 4 individual measurements. BMI was defined as weight in kilograms divided by height in meters squared. Height was measured using a wall-mounted stadiometer. Weight with light clothing was measured using a digital scale to the nearest 0.1 lb. BMI was available for 1,235 participants.
Medication
Medication use was assessed by a self-report structured interview design, which has previously been shown to be reliable when estimating recent use of cardiovascular drugs.36 Antihypertensives were classified into their pharmacologic classes (Table 1). Combination drugs were counted as 2 drug classes. Beta-blockers with BP-lowering effects prescribed for the treatment of cardiovascular diseases other than hypertension were included.
Table 1.
Participants per pharmacologic drug class
| Drug class | Unique drugs in drug class | Number of participants |
|---|---|---|
| Angiotensin-converting-enzyme inhibitors | 10 | 187 |
| α1-Blockers | 3 | 17 |
| α2-Blockers | 2 | 9 |
| β-Blockers | 7 | 181 |
| Calcium channel blockers | 6 | 71 |
| Diuretics, nonthiazide | 3 | 26 |
| Thiazides | 3 | 64 |
| Angiotensin receptor blocker | 5 | 57 |
| Combination | 12 | 71 |
| Total | 51 | 683 |
Three imputation approaches
For the fixed addition approach, 10/5mm Hg was added to the SBP/DBP of treated individuals.15 For the stepped addition approach, 8/4, 14/10, 20/16, 26/22, 32/28, or 38/34mm Hg was added to SBP/DBP of individuals on 1, 2, 3, 4, 5, or 6 drugs, respectively. For the drug class addition approach, addition of mm Hg was based on the weighted average effect (WAE) of drug class, number of drugs, and ethnicity, as described by Wu et al.22 The published WAEs of monodrug therapy categorized by drug class for blacks and nonblacks based on 165 clinical trials (n = 11,739) were used. The published WAE overall reported drug classes (15.4/11.2mm Hg for SBP/DBP in blacks and 15.7/10.5mm Hg for SBP/DBP in nonblacks) were used for the drug classes in our study that were not reported by Wu et al.22
For 2 drugs or combination drugs, the medication with the higher monodrug effect was designated as the first medication. The weighted effect of the second medication was calculated by the formula:
(WAE combination drug therapy − WAE 1st medication) / (WAE 2nd medication) x 100%. WAEs were based on published clinical trials. Weighted effects of the second medication were computed for combinations containing and not containing diuretics and considering ethnicity. The estimated effect on BP of the combination therapy was then calculated as: WAE1st drug + (WAE2nd drug x weighted effect2nd drug). Our study used this method for 2 drugs and combination drugs.
An algorithm for ≥3 drugs was not reported by Wu et al.22 Clinical trials are lacking and studies vary on the effect of additional drugs,37 although there are a few published studies evaluating the effect of adding a third drug, such as adding thiazide to an angiotensin receptor blocker/calcium channel blocker.38 Our imputation algorithm assumed additional drugs to have effects lower than its monodrug or second drug effect, as observed in published studies.38–41 We used the reported effect of approximately 0.5 of the effect for the second drug22 and conservatively added a smaller percentage effect of the third and subsequent drugs by decreasing the effect by 0.1 for each additional drug. The monodrug effect of the third drug was multiplied by 0.4, the monodrug effect of the fourth drug was multiplied by 0.3, the monodrug effect of the fifth drug was multiplied by 0.2, and the monodrug effect of the sixth drug was multiplied by 0.1. Proportions were totaled and added to the reported effects of the first and second drug class. The range of added mm Hg was 14–38/9–27 to measured SBP/DBP and is within the therapeutic range reported in clinical trials.42
The fixed, stepped, and drug class addition approaches progressively include more information in the imputation algorithm and are therefore considered in this study to be more refined in this sequential order.
Statistical analysis
Descriptive analysis was conducted using R v2.1.2.0. Linear mixed-effects models were used to test the significance of the differences in the mean measured BP and BMI between medicated and unmedicated individuals using the non-linear mixed effects model (NLME) package in R.43 By using family identification as a random effect in the model, we were able to account for the correlated nature of the twin data.
A 2-tailed significance test of the difference between dependent correlations using Fisher z prime transformation44 was used to assess whether the phenotypic correlation (rp) between BMI and imputed BP was significantly different from the phenotypic correlation of BMI and measured BP.44
Univariable twin model.
In the classical twin design, the variance of any trait is decomposed into the proportion attributed to additive genetic (A) influences (i.e., heritability), common environmental (C) influences (i.e., environmental factors that make members of a twin pair similar to one another), and unique environmental (E) influences (i.e., environmental factors that make members of a twin pair different from one another, including measurement error). This model is referred to as the ACE model (see Supplementary Information). Models were fit to raw data using the maximum-likelihood based structural equation modeling software Mx.45 Analyses were conducted with both age-unadjusted and age-adjusted BP measurements because there were small correlations (r < 0.1) between BP and age. However, results did not significantly differ after adjusting BP measurements for age, and age-unadjusted results are presented.
Bivariable twin model.
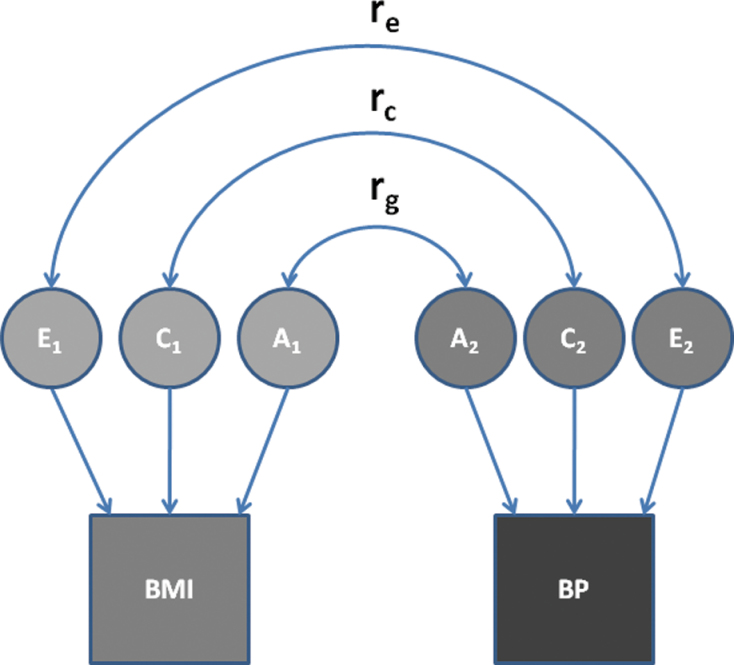
The bivariable extension of the ACE model, in which the covariance between traits is decomposed to derive genetic and environmental covariance estimates, was used to determine the degree of covariance between the A, C, and E contributions to BP and BMI (Figure 1) and to calculate phenotypic, genetic, and environmental correlations between BP and BMI. The genetic correlation between 2 phenotypes is equal to their genetic covariance divided by the square root of the product of their separate genetic variances.46 It represents the degree to which 2 traits share the same genetic influences.47 Environmental correlations are defined analogously.
Figure 1.
Bivariable models for genetic (A), common/shared environmental (C), and unique environmental (E) influences on the phenotypic correlation between body mass index (BMI) and blood pressure (BP). Abbreviations: rc, shared environment correlation; re, unique environment correlation; rg, genetic correlation.
RESULTS
Descriptive analyses
There were 445 (36%) participants on BP-lowering medication (Table 2). Of the 792 unmedicated individuals, 265 (21%) had SBP >140mm Hg or DBP >90mm Hg. The average age of the medicated and unmedicated groups was similar, but BMI was significantly higher in the medicated group (F = 67.42; P < 0.0001). Measured SBP and DBP were similar between the 2 groups (Table 3), but the imputed unmedicated SBP and DBP were significantly higher in the medicated group.
Table 2.
Participants on multiple drug classes
| No. of drug classes | Number of participants |
|---|---|
| 0 | 792 |
| 1 | 235 |
| 2 | 139 |
| 3 | 52 |
| 4 | 12 |
| 5 | 5 |
| 6 | 2 |
| Total | 1,237 |
Table 3.
Blood pressure in medicated vs. unmedicated participants
| Antihypertensive medication (n = 445) | No antihypertensive medication (n = 792) | ||||
|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | F (unadjusted for age) | P value (unadjusted for age) | P value (adjusted for age) | |
| Age | 55.8 (2.5) | 55.3 (2.5) | 0.46 | NS | NA |
| BMI | 31 (23) | 28 (18) | 67.42 | <0.0001 | <0.0001 |
| Measured SBP | 135 (16) | 133 (15) | 1.69 | NA | NS |
| Measured DBP | 84 (10) | 83 (9) | 0.26 | NS | NS |
Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; NA, not available; NS, not significant; SBP, systolic blood pressure.
Univariable twin analyses
Rates of self-reported antihypertensive use were not significantly different between MZ and DZ twins (χ 2=2.28; df=1; P = 0.13); 36% of MZs and 36% of DZs were taking antihypertensive medications. The MZ correlations for BP, BMI, and antihypertensive use were greater than the DZ correlations, suggesting genetic effects (Table 4). Genetic (A) and unique environmental (E) influences, but not common environmental influences (C), for BP and BMI accounted for the majority of the total variance (Table 4). Both the MZ and DZ correlations increased with more refined imputations. Because of an increase in total variance and a parallel increase in both MZ and DZ correlations after algorithm imputations, significant changes in heritability were not observed.
Table 4.
Estimates of additive genetic (A), common environmental (C), and unique environmental (E) influences on blood pressure and body mass index
| Trait | Variance components | MZ twins (n = 347 pairs) | DZ twins (n = 267 pairs) | ||
|---|---|---|---|---|---|
| A | C | E | rMZ | rDZ | |
| Measured SBP | 0.43 (0.20–0.50) | 0.00 (0.00–0.19) | 0.57 (0.50–0.66) | 0.43 | 0.21 |
| Fixed addition SBP | 0.47 (0.22–0.58) | 0.04 (0.00–0.24) | 0.49 (0.42–0.58) | 0.50 | 0.28 |
| Stepped addition SBP | 0.46 (0.22–0.61) | 0.08 (0.00–0.28) | 0.46 (0.39–0.53) | 0.52 | 0.33 |
| Drug class addition SBP | 0.45 (0.23–0.61) | 0.13 (0.00–0.32) | 0.43 (0.36–0.50) | 0.56 | 0.37 |
| Measured DBP | 0.44 (0.22–0.51) | 0.00 (0.00–0.17) | 0.56 (0.49–0.65) | 0.43 | 0.21 |
| Fixed addition DBP | 0.47 (0.21–0.56) | 0.03 (0.00–0.24) | 0.51 (0.44–0.59) | 0.48 | 0.29 |
| Stepped addition DBP | 0.42 (0.18–0.59) | 0.11 (0.00–0.31) | 0.47 (0.40–0.55) | 0.50 | 0.34 |
| Drug class addition DBP | 0.39 (0.16–0.60) | 0.16 (0.00–0.35) | 0.45 (0.38–0.52) | 0.54 | 0.37 |
| BMI | 0.58 (0.39–0.74) | 0.12 (0.00–0.30) | 0.30 (0.26–0.35) | 0.70 | 0.41 |
| Antihypertensive medication, yes/no | 0.37 (0.00–0.64) | 0.15 (0.00,0.50) | 0.48 (0.35–0.63) | 0.52a | 0.33a |
Variance component values represent the proportion of variance in blood pressure in our sample explained by the A, C, or E variance components. Values in parenthesis are 95% confidence intervals. Analyses shown are age-unadjusted; age-adjusted BP measurements yielded similar results.
Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; DZ, dizygotic; MZ, monozygotic; rDZ, DZ correlation; rMZ, MZ correlation; SBP, systolic blood pressure.
aTetrachoric correlation (dichotomous variable).
Relationship between BP and BMI
The phenotypic correlations between BMI and each imputed BP were significantly greater than the correlation between BMI and measured BP (Table 5). We observed a 61% increase in the correlation of BMI with the drug class addition imputation for SBP compared with measured SBP (from r = 0.18 to r = 0.29) and a 53% increase for DBP (from r = 0.21 to r = 0.32).
Table 5.
Phenotypic correlations (rp) for body mass index and blood pressure in all twin participants (n = 1,235)
| BP and BMI | rp | t a | P valuea |
|---|---|---|---|
| Measured SBP | 0.18 | -- | -- |
| Fixed addition SBP | 0.24 | −7.92 | <0.001 |
| Stepped addition SBP | 0.26 | −7.89 | <0.001 |
| Drug class addition SBP | 0.29 | −7.06 | <0.001 |
| Random addition of 14–38mm Hg to SBPb | 0.2785±0.006 (0.278–0.279) | -- | -- |
| Random addition of 14–50mm Hg to SBPb | 0.2784±0.008 (0.262–0.295) | -- | -- |
| Addition of 20mm Hg to all medicated SBPc | 0.275 | ||
| Measured DBP | 0.21 | -- | -- |
| Fixed addition DBP | 0.26 | −7.93 | <0.001 |
| Stepped addition DBP | 0.29 | −6.86 | <0.001 |
| Drug class addition DBP | 0.32 | −6.59 | <0.001 |
| Random addition of 9–27mm Hg to DBPb | 0.3046±0.006 (0.304–0.305) | -- | -- |
| Random addition of 9–40mm Hg to DBPb | 0.297±0.01 (0.279–0.318) | -- | -- |
| Addition of 14mm Hg to all medicated DBPc | 0.308 |
Data in parentheses are 95% confidence intervals.
Abbreviations: BMI, body mass index; BP, blood pressure; DBP, diastolic blood pressure; SBP, systolic blood pressure.
aThe rp of each imputed SBP and DBP was compared with the rp of the measured SBP and DPB, respectively; t statistic and P value from 2-tailed significance test of the difference between dependent correlations.
bAddition of mm Hg to SBP or DBP in medicated individuals. For these, the rp represents the mean phenotypic correlation of 500 simulations.
c The average mm Hg added to the DBP/SBP of medicated individuals in the drug class addition was 14mm Hg/20mm Hg.
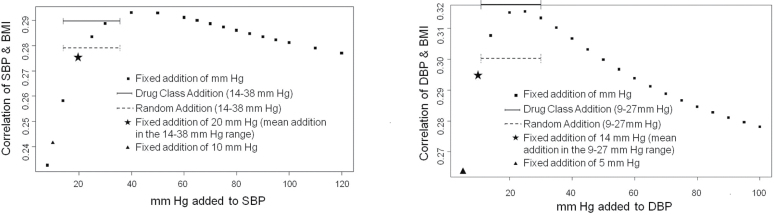
One might expect that any imputation that increases a substantial portion of BP values assigned to the medicated participants might increase the correlation between BMI and BP because it would increase the range of values. Thus, it could be that adding any value would have this effect. To address this question, we created a random addition correction that added a randomly generated integer between 9 and 27 and 14 and 38 to measured DBP and SBP, respectively, of all medicated individuals. This range of values covers the range of values in our drug class adjustment and is within the range of BP-lowering effect of antihypertensives observed in clinical trials. We created 500 sets of random addition DBPs and SBPs. We created a distribution of the 500 correlations between these sets of BPs and BMI. The mean phenotypic correlation between the randomly corrected SBP and BMI was 0.279 (95% confidence interval (CI) = 0.278–0.279) and mean phenotypic correlation between the randomly corrected DBP and BMI was 0.305 (95% CI =0.30–0.31). The correlation of BMI with the randomly corrected BP was greater than the phenotypic correlation between BMI and measured SBP (0.18) and DBP (0.21) (Table 5) but not greater than the correlation between the drug class addition SBP (0.29) and DBP (0.32) with BMI.
In addition we assessed the phenotypic correlation of SBP and DBP with BMI for a range of fixed addition values for the entire sample (Figure 2). The correlation peaked with the addition of 40mm Hg to SBP and 25mm Hg to DBP and steadily decreased with greater values. Thus, the addition of even greater values of mm Hg to medicated individuals did not significantly increase the correlation beyond that observed for the drug class adjustment. These results suggest that our imputation is meaningful and that randomly adding any value will not necessarily result in an increased BP–BMI correlation.
Figure 2.
Phenotypic correlation of systolic blood pressure (SBP) and diastolic blood pressure (DBP) with body mass index (BMI) for a range of fixed addition values. Fixed addition of mm Hg represents the range of fixed additions used (8–120 for SBP; 5–100 for DBP). Fixed addition of 10mm Hg is the fixed addition used for SBP in this study; 5mm Hg for DBP. Solid line shows the correlation for drug class/ethnicity addition, with values ranging from 14–38mm Hg for SBP and 9–27mm Hg for DBP. Dashed line shows the correlation for the random addition, with values ranging from 14–38mm Hg for SBP and 9–27mm Hg for DBP. Fixed addition of 20mm Hg was the mean addition when the drug class/ethnicity addition for SBP was used; 14mm Hg was the mean addition for DBP.
Bivariable twin analyses
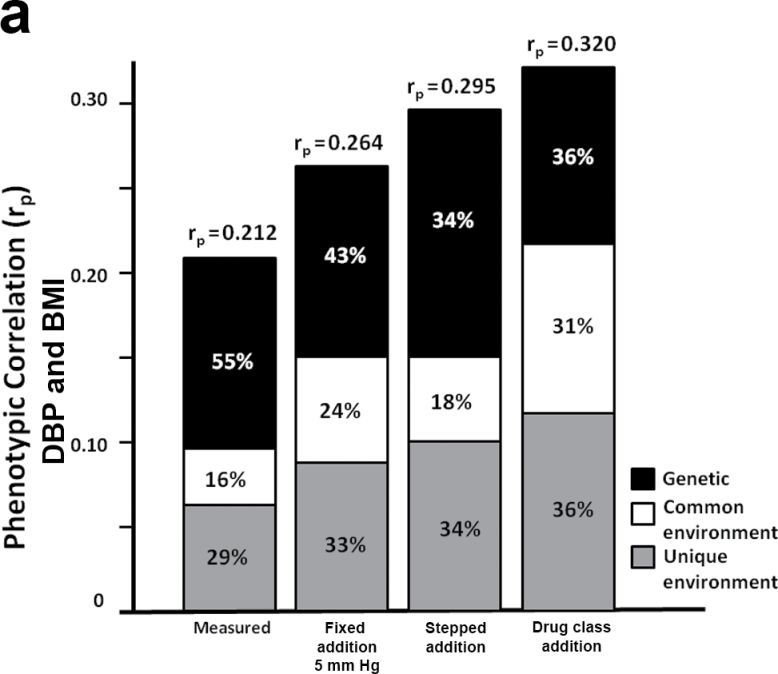
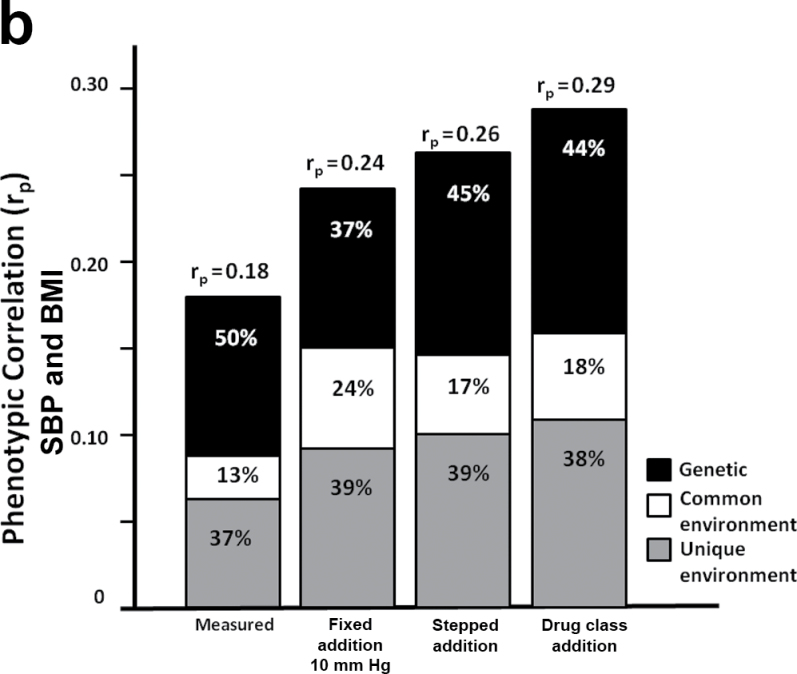
In parallel to our observation that heritability estimates of BP did not change significantly with the imputed values, the genetic correlation (rg) between BP and BMI also did not significantly change (SBP: 0.17 < rg< 0.29; DBP: 0.20< rg < 0.29), indicating that the proportion of genes shared between BP and BMI was not altered by adjustments to medicated individuals. However, the bivariable genetic analyses were useful in estimating the extent to which the phenotypic correlation between BP and BMI could be explained by genetic or environmental factors. Figure 3 shows the relative contribution of the genetic (A), common environmental (C), and unique environmental (E) influences to the phenotypic correlation between BMI and either DBP or SBP. These 2 sources of covariance combine to determine the phenotypic correlation, but it is only possible to definitively break down their relative contributions in a genetically informative study such as a twin study. Because we accounted for antihypertensives using more refined methods, the relative genetic influences on the correlation between BP and BMI tended to decrease while the environmental influences increased (Figure 3).
Figure 3.
Contribution of genetic, common environment, and unique environment to phenotypic correlation between body mass index (BMI) and (a) diastolic blood pressure (DBP) and (b) systolic blood pressure (SBP).
DISCUSSION
More than 30% of US adults have hypertension,48 and more than 60% of hypertensive adults are treated.49 Therefore, the majority of population-representative adult cohorts, particularly older cohorts, will have a large proportion of individuals on antihypertensives. Thus, the observed BP in these cohorts will be confounded by medication. Because unmedicated BP is rarely obtainable, we need to understand how imputing unmedicated BP impacts the outcome of population and genetic analyses.
We expanded an approach in which unmedicated BP is imputed based on published clinical data on drug response by ethnicity22 to include additional drug classes, combination drug therapy, and use of >2 drug classes. The more refined imputations increased the range and variance of the BP in the cohort with parallel increases in MZ and DZ twin pair correlations. In contrast to our expectation, heritability estimates did not increase significantly with any imputation. However, we observed increasingly higher correlations between BP and BMI with more refined approaches (Table 5).
The outcomes of studies investigating the impact of including BP-treated individuals in genetic studies vary depending on the age range, type of cohort, and the proportion of hypertensive and medicated individuals. Previous studies investigated BP in unrelated individuals or multigenerational families with a broad age range (typically on the order of 18–80 years).7,8,15,50 A large age range may obscure results. These results are difficult to interpret because BP changes with age and hypertension penetrance and treatment occur later in midlife. In the younger extreme, even if age is adjusted for statistically, if disease has not penetrated, genetic influences cannot be detected. If the genetic architecture differs with age, heritability may change with age. In the older extreme, bias may come from isolated systolic hypertension, which is more prevalent in the elderly,51,52 or loss of study participants due to the complications of elevated BP. Statistically adjusting for age as a covariable does not fully resolve the problem because this type of adjustment assumes a linear relationship of age with BP.
The lack of significantly increased heritability in our study is consistent with previous studies. Although the family/twin study of Cui et al.15 did not include the drug class approach, our results parallel theirs in that heritabilities did not increase significantly with the imputations. In the extended twin/family study of males (mean age = 31.3 years; SD = 11.2) by Kupper et al.,5 BP was imputed using reported drug class–specific treatment effect averages21 for ambulatory BP. They showed that heritability estimates increased slightly, but not significantly, when using imputed BPs. Our hypothesis that heritability would increase was not supported because the effects of the imputation were proportional in MZ and DZ twins.
The VETSA cohort has a narrow age range in which hypertension is prevalent.48,49 We observed little common environmental influences on BP. In contrast, some studies with wide age ranges tend to find that common environmental influences account for up to 20% of the BP variance. Studies using multigenerational family cohorts with a broad age range or small twin sample sizes may not have power to differentiate between common environmental and genetic determinants. The higher common environmental component in the studies with wide age ranges may be because of the high correlation of BP and age combined with the perfectly correlated age of members within a twin pair. With our narrow age range, BP was weakly correlated with age. Ongoing longitudinal assessment will enable us to determine whether there are age-associated changes in genetic or environmental influences on BP.
Furthermore, the distribution of risk factors for BP may be different across a broad age range. In a prior report, we observed a dramatic shift in BMI over a 28-year period; 75%–80% had normal (18.5–24.9kg/m2) BMIs at a mean age of 20, but 75%–80% had BMIs in the overweight (25–29.9kg/m2) or obese (≥30kg/m2) range in midlife.53 Consistent with high BMI being a known risk factor for elevated BP,54 in this study we found significantly higher phenotypic correlations with BMI after imputing unmedicated BP. The increased correlations are consistent with the notion that imputed BPs are more reflective of the underlying unmedicated BP. We observed the highest correlation with our drug class imputation approach, althought it was not significantly higher than the other imputation approaches. It also did not result in higher correlation with BMI compared with using a multiple linear regression approach in which BMI is the outcome variable predicted by measured BP and presence/absence of antihypertensives. However, when BP is the outcome predicted by potential risk factors (e.g., BMI, single nucleotide polymorphisms) or part of certain factor analytic analyses, including antihypertensive use as predictor is not appropriate. In such cases, an imputation approach such as our drug class imputation that predicts unmedicated BP is preferable. Although the difference in the magnitude of the correlations for the drug class approach and other less sophisticated imputation approaches were not statistically significant, it is the case that obtaining statistically significant correlations would require larger sample sizes for BP measures that used less information to predict unmedicated BP.
Strengths of our study include the age-homogeneous sample at an age at which hypertension prevalence begins to increase and an imputation algorithm that considers contemporary BP treatment strategies. Although the age-homogenous sample limits generalization to other age groups, ongoing assessments will provide longitudinal aging data.
A limitation of our study is that our algorithms are based on group mean data from other samples without accounting for individual variation. However, individualized data will be available only in rare cases, and the goal of this report is to assess how generalized imputation approaches affect results of studies to best predict unmedicated BP when individualized data are unavailable. As with many epidemiological studies, we relied on self-reported medication use, for which dosage and adherence to treatment were unknown; however, our method has been validated for estimating the use of cardiovascular drugs.36 Dosage information would provide an even more refined method for imputation. Despite similar limitations, Tobin et al. demonstrated that approaches in which a sensible constant was added to the observed BP of treated individuals performed better across a range of realistic settings than using nonimputed data or including medication use as a covariable.6
If common genetic factors contribute to the underlying BP and the disease, information may be lost if one considers only the observed BPs. When elevated BP is a risk factor for a disease, it might seem that unmedicated values would be less important because the observed, treated BP may contribute to the outcome rather than the underlying unmedicated BP. However, both pieces of information are important because being treated with antihypertensives, even if BP is normalized, is still associated with increased risk for cardiovascular disease. Our study suggests the potential value of applying an adjustment for medication use. We found a small, incremental advantage with a method that was representative of the effect of the therapeutic treatment, such as by considering drug class, ethnicity, and the combination of drugs, in imputation approaches when assessing the relationship of BP with BMI. Further studies are needed to replicate this finding in other samples, for other risk factors, and for other diseases that are confounded by therapeutic treatment.
SUPPLEMENTARY MATERIAL
Supplementary materials are available at American Journal of Hypertension (http://ajh.oxfordjournals.org).
DISCLOSURE
The authors declared no conflict of interest.
Supplementary Material
ACKNOWLEDGMENTS
The U.S. Department of Veterans Affairs has provided support for the development and maintenance of the Vietnam Era Twin Registry. Numerous organizations have provided invaluable assistance, including VA Cooperative Studies Program; Department of Defense; National Personnel Records Center, National Archives and Records Administration; the Internal Revenue Service; National Institutes of Health; National Opinion Research Center; National Research Council, National Academy of Sciences; the Institute for Survey Research, Temple University; and Schulman, Ronca, and Bucuvalas, Inc. Most importantly, we gratefully acknowledge the cooperation and participation of the members of the Vietnam Era Twin Registry and their families. Without their contribution this research would not have been possible. We also thank Jun Wu, Aldi T. Kraja and D.C. Rao for their advice. The VETSA project is supported by National Institutes of Health/National Institute on Aging (NIH/NIA) (K01 AG035031 to B.K.R.; R01 AG018386, R01 AG022381, and R01 AG022982 to W.S.K.; R01 AG018384 to M.J.L.).
REFERENCES
- 1. Vinck WJ, Fagard RH, Loos R, Vlietinck R. The impact of genetic and environmental influences on blood pressure variance across age-groups. J Hypertens 2001; 19:1007–1013 [DOI] [PubMed] [Google Scholar]
- 2. Fagard RH, Loos RJ, Beunen G, Derom C, Vlietinck R. Influence of chorionicity on the heritability estimates of blood pressure: a study in twins. J Hypertens 2003; 21:1313–1318 [DOI] [PubMed] [Google Scholar]
- 3. Schunkert H, Hense HW, Doring A, Riegger GA, Siffert W. Association between a polymorphism in the G protein beta3 subunit gene and lower renin and elevated diastolic blood pressure levels. Hypertension 1998; 32:510–513 [DOI] [PubMed] [Google Scholar]
- 4. Brand E, Wang JG, Herrmann SM, Staessen JA. An epidemiological study of blood pressure and metabolic phenotypes in relation to the Gbeta3 C825T polymorphism. J Hypertens 2003; 21:729–737 [DOI] [PubMed] [Google Scholar]
- 5. Kupper N, Willemsen G, Riese H, Posthuma D, Boomsma DI, de Geus EJ. Heritability of daytime ambulatory blood pressure in an extended twin design. Hypertension 2005; 45:80–85 [DOI] [PubMed] [Google Scholar]
- 6. Tobin MD, Sheehan NA, Scurrah KJ, Burton PR. Adjusting for treatment effects in studies of quantitative traits: antihypertensive therapy and systolic blood pressure. Stat Med 2005; 24:2911–2935 [DOI] [PubMed] [Google Scholar]
- 7. Hunt SC, Ellison RC, Atwood LD, Pankow JS, Province MA, Leppert MF. Genome scans for blood pressure and hypertension: the National Heart, Lung, and Blood Institute Family Heart Study. Hypertension 2002; 40:1–6 [DOI] [PubMed] [Google Scholar]
- 8. Cui J, Hopper JL, Harrap SB. Genes and family environment explain correlations between blood pressure and body mass index. Hypertension 2002; 40:7–12 [DOI] [PubMed] [Google Scholar]
- 9. Chen Y, Zhang K, Wen G, Rao F, Sanchez AP, Wang L, Rodriguez-Flores JL, Mahata M, Mahata SK, Waalen J, Ziegler MG, Hamilton BA, O’Connor DT. Human dopamine beta-hydroxylase promoter variant alters transcription in chromaffin cells, enzyme secretion, and blood pressure. Am J Hypertens 2011; 24:24–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Levy D, Ehret GB, Rice K, Verwoert GC, Launer LJ, Dehghan A, Glazer NL, Morrison AC, Johnson AD, Aspelund T, Aulchenko Y, Lumley T, Köttgen A, Vasan RS, Rivadeneira F, Eiriksdottir G, Guo X, Arking DE, Mitchell GF, Mattace-Raso FU, Smith AV, Taylor K, Scharpf RB, Hwang SJ, Sijbrands EJ, Bis J, Harris TB, Ganesh SK, O’Donnell CJ, Hofman A, Rotter JI, Coresh J, Benjamin EJ, Uitterlinden AG, Heiss G, Fox CS, Witteman JC, Boerwinkle E, Wang TJ, Gudnason V, Larson MG, Chakravarti A, Psaty BM, van Duijn CM. Genome-wide association study of blood pressure and hypertension. Nat Genet 2009; 41:677–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Taal HR, Verwoert GC, Demirkan A, Janssens AC, Rice K, Ehret G, Smith AV, Verhaaren BF, Witteman JC, Hofman A, Vernooij MW, Uitterlinden AG, Rivadeneira F, Ikram MA, Levy D, van der Heijden AJ, Cohort for Heart and Aging Research in Genome Epidemiology and Early Genetics and Lifecourse Epidemiology Consortia, Jaddoe VW, van Duijn CM. Genome-wide profiling of blood pressure in adults and children. Hypertension 2012; 59:241–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Salvi E, Kutalik Z, Glorioso N, Benaglio P, Frau F, Kuznetsova T, Arima H, Hoggart C, Tichet J, Nikitin YP, Conti C, Seidlerova J, Tikhonoff V, Stolarz-Skrzypek K, Johnson T, Devos N, Zagato L, Guarrera S, Zaninello R, Calabria A, Stancanelli B, Troffa C, Thijs L, Rizzi F, Simonova G, Lupoli S, Argiolas G, Braga D, D’Alessio MC, Ortu MF, Ricceri F, Mercurio M, Descombes P, Marconi M, Chalmers J, Harrap S, Filipovsky J, Bochud M, Iacoviello L, Ellis J, Stanton AV, Laan M, Padmanabhan S, Dominiczak AF, Samani NJ, Melander O, Jeunemaitre X, Manunta P, Shabo A, Vineis P, Cappuccio FP, Caulfield MJ, Matullo G, Rivolta C, Munroe PB, Barlassina C, Staessen JA, Beckmann JS, Cusi D. Genomewide association study using a high-density single nucleotide polymorphism array and case-control design identifies a novel essential hypertension susceptibility locus in the promoter region of endothelial NO synthase. Hypertension 2012; 59:248–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hottenga JJ, Whitfield JB, Posthuma D, Willemsen G, de Geus EJ, Martin NG, Boomsma DI. Genome-wide scan for blood pressure in Australian and Dutch subjects suggests linkage at 5P, 14Q, and 17P. Hypertension 2007; 49:832–838 [DOI] [PubMed] [Google Scholar]
- 14. Hottenga JJ, Boomsma DI, Kupper N, Posthuma D, Snieder H, Willemsen G, de Geus EJ. Heritability and stability of resting blood pressure. Twin Res Hum Genet 2005; 8:499–508 [DOI] [PubMed] [Google Scholar]
- 15. Cui JS, Hopper JL, Harrap SB. Antihypertensive treatments obscure familial contributions to blood pressure variation. Hypertension 2003; 41:207–210 [DOI] [PubMed] [Google Scholar]
- 16. Rabi DM, Daskalopoulou SS, Padwal RS, Khan NA, Grover SA, Hackam DG, Myers MG, McKay DW, Quinn RR, Hemmelgarn BR, Cloutier L, Bolli P, Hill MD, Wilson T, Penner B, Burgess E, Lamarre-Cliché M, McLean D, Schiffrin EL, Honos G, Mann K, Tremblay G, Milot A, Chockalingam A, Rabkin SW, Dawes M, Touyz RM, Burns KD, Ruzicka M, Campbell NR, Vallée M, Prasad GV, Lebel M, Campbell TS, Lindsay MP, Herman RJ, Larochelle P, Feldman RD, Arnold JM, Moe GW, Howlett JG, Trudeau L, Bacon SL, Petrella RJ, Lewanczuk R, Stone JA, Drouin D, Boulanger JM, Sharma M, Hamet P, Fodor G, Dresser GK, Carruthers SG, Pylypchuk G, Gilbert RE, Leiter LA, Jones C, Ogilvie RI, Woo V, McFarlane PA, Hegele RA, Poirier L, Tobe SW; Canadian Hypertension Education Program. The 2011 Canadian Hypertension Education Program recommendations for the management of hypertension: blood pressure measurement, diagnosis, assessment of risk, and therapy. Can J Cardiol 2011; 27:415–433 [DOI] [PubMed] [Google Scholar]
- 17. Mancia G, Grassi G. Impact of new clinical trials on recent guidelines on hypertension management. Ann Med 2011; 43:124–132 [DOI] [PubMed] [Google Scholar]
- 18. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ, Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003; 42:1206–1252 [DOI] [PubMed] [Google Scholar]
- 19. Koopmans RP, Insel PA, Michel MC. Pharmacogenetics of hypertension treatment: a structured review. Pharmacogenetics 2003; 13:705–713 [DOI] [PubMed] [Google Scholar]
- 20. Moser M. Black-white differences in response to antihypertensive medication. J Natl Med Assoc 1995; 87:612–613 [PMC free article] [PubMed] [Google Scholar]
- 21. Mancia G, Parati G. Office compared with ambulatory blood pressure in assessing response to antihypertensive treatment: a meta-analysis. J Hypertens 2004; 22:435–445 [DOI] [PubMed] [Google Scholar]
- 22. Wu J, Kraja AT, Oberman A, Lewis CE, Ellison RC, Arnett DK, Heiss G, Lalouel JM, Turner ST, Hunt SC, Province MA, Rao DC. A summary of the effects of antihypertensive medications on measured blood pressure. Am J Hypertens 2005; 18:935–942 [DOI] [PubMed] [Google Scholar]
- 23. Kraja AT, Huang P, Tang W, Hunt SC, North KE, Lewis CE, Devereux RB, de Simone G, Arnett DK, Rice T, Rao DC. QTLs of factors of the metabolic syndrome and echocardiographic phenotypes: the hypertension genetic epidemiology network study. BMC Med Genet 2008; 9:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Borghi C, Dormi A, D’Addato S, Gaddi A, Ambrosioni E. Trends in blood pressure control and antihypertensive treatment in clinical practice: the Brisighella Heart Study. J Hypertens 2004; 22:1707–1716 [DOI] [PubMed] [Google Scholar]
- 25. Stafford RS, Monti V, Furberg CD, Ma J. Long-term and short-term changes in antihypertensive prescribing by office-based physicians in the United States. Hypertension 2006; 48:213–218 [DOI] [PubMed] [Google Scholar]
- 26. Ma J, Lee KV, Stafford RS. Changes in antihypertensive prescribing during US outpatient visits for uncomplicated hypertension between 1993 and 2004. Hypertension 2006; 48:846–852 [DOI] [PubMed] [Google Scholar]
- 27. Gupta AK, Arshad S, Poulter NR. Compliance, safety, and effectiveness of fixed-dose combinations of antihypertensive agents: a meta-analysis. Hypertension 2010; 55:399–407 [DOI] [PubMed] [Google Scholar]
- 28. Byrd JB, Zeng C, Tavel HM, Magid DJ, O’Connor PJ, Margolis KL, Selby JV, Ho PM. Combination therapy as initial treatment for newly diagnosed hypertension. Am Heart J 2011; 162:340–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kremen WS, Jacobson KC, Xian H, Eisen SA, Waterman B, Toomey R, Neale MC, Tsuang MT, Lyons MJ. Heritability of word recognition in middle-aged men varies as a function of parental education. Behav Genet 2005; 35:417–433 [DOI] [PubMed] [Google Scholar]
- 30. Wu T, Snieder H, Li L, Cao W, Zhan S, Lv J, Gao W, Wang X, Ding X, Hu Y. Genetic and environmental influences on blood pressure and body mass index in Han Chinese: a twin study. Hypertens Res 2011; 34:173–179 [DOI] [PubMed] [Google Scholar]
- 31. Evans DM, Gillespie NA, Martin NG. Biometrical genetics. Biol Psychol 2002; 61:33–51 [DOI] [PubMed] [Google Scholar]
- 32. Eisen S, True W, Goldberg J, Henderson W, Robinette CD. The Vietnam Era Twin (VET) Registry: method of construction. Acta Genet Med Gemellol (Roma) 1987; 36:61–66 [DOI] [PubMed] [Google Scholar]
- 33. Henderson WG, Eisen S, Goldberg J, True WR, Barnes JE, Vitek ME. The Vietnam Era Twin Registry: a resource for medical research. Public Health Rep 1990; 105:368–373 [PMC free article] [PubMed] [Google Scholar]
- 34. Centers for Disease Control and Prevention. Health Data Interactive http://www.cdc.gov/nchs/hdi.htm
- 35. Panizzon MS, Lyons MJ, Jacobson KC, Franz CE, Grant MD, Eisen SA, Xian H, Kremen WS. Genetic architecture of learning and delayed recall: a twin study of episodic memory. Neuropsychology 2011; 25:488–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Glintborg B, Hillestrom PR, Olsen LH, Dalhoff KP, Poulsen HE. Are patients reliable when self-reporting medication use? Validation of structured drug interviews and home visits by drug analysis and prescription data in acutely hospitalized patients. J Clin Pharmacol 2007; 47:1440–1449 [DOI] [PubMed] [Google Scholar]
- 37. Swift PA, MacGregor GA. The frequent need for three or more drugs to treat essential hypertension. What evidence for optimal combinations? J Renin Angiotensin Aldosterone Syst 2002; 3:103–108 [DOI] [PubMed] [Google Scholar]
- 38. Volpe M, Christian Rump L, Ammentorp B, Laeis P. Efficacy and safety of triple antihypertensive therapy with the olmesartan/amlodipine/hydrochlorothiazide combination. Clin Drug Investig 2012; 32:649–664 [DOI] [PubMed] [Google Scholar]
- 39. Ferdinand KC, Weitzman R, Israel M, Lee J, Purkayastha D, Jaimes EA. Efficacy and safety of aliskiren-based dual and triple combination therapies in US minority patients with stage 2 hypertension. J Am Soc Hypertens 2011; 5:102–113 [DOI] [PubMed] [Google Scholar]
- 40. Murray AV, Koenig W, Garcia-Puig J, Patel S, Uddin A, Zhang J. Safety and efficacy of aliskiren/amlodipine/hydrochlorothiazide triple combination in patients with moderate to severe hypertension: a 54-week, open-label study. J Clin Hypertens (Greenwich) 2012; 14:821–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Huan Y, Townsend R. The single pill triple combination of aliskiren, amlodipine, and hydrochlorothiazide in the treatment of hypertension. Expert Opin Pharmacother 2012; 13:2409–2415 [DOI] [PubMed] [Google Scholar]
- 42. Laffer CL, Elijovich F. A critical appraisal of the clinical effectiveness of a fixed combination of valsartan, amlodipine, and hydrochlorothiazide in achieving blood pressure goals. Integr Blood Press Control 2011; 4:1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pinheiro J, Bates D, DebRoy S, Sarkar D, R Development Core Team. nlme: Linear and Nonlinear Mixed Effects Models. R package, 2012
- 44. Cohen J, Cohen P. Applied Multiple Regression/Correlation Analysis for the Behavioral Sciences. Halsted Press: New York, 1975 [Google Scholar]
- 45. Neale MC, Boker SM, Xie G, Maes HH. Mx: Statistical Modeling. 6th edn Department of Psychiatry, Medical College of Virginia: Richmond, 2004 [Google Scholar]
- 46. Neale MC, Cardon L.R. Methodology for Genetic Studies of Twins and Families. Kluwer Academic Publishers: Dordrecht, the Netherlands, 1992 [Google Scholar]
- 47. Carey G. Inference about genetic correlations. Behav Genet 1988; 18:329–338 [DOI] [PubMed] [Google Scholar]
- 48. Fields LE, Burt VL, Cutler JA, Hughes J, Roccella EJ, Sorlie P. The burden of adult hypertension in the United States 1999 to 2000: a rising tide. Hypertension 2004; 44:398–404 [DOI] [PubMed] [Google Scholar]
- 49. Cutler JA, Sorlie PD, Wolz M, Thom T, Fields LE, Roccella EJ. Trends in hypertension prevalence, awareness, treatment, and control rates in United States adults between 1988–1994 and 1999–2004. Hypertension 2008; 52:818–827 [DOI] [PubMed] [Google Scholar]
- 50. Ehret GB, Morrison AC, O’Connor AA, Grove ML, Baird L, Schwander K, Weder A, Cooper RS, Rao DC, Hunt SC, Boerwinkle E, Chakravarti A. Replication of the Wellcome Trust genome-wide association study of essential hypertension: the Family Blood Pressure Program. Eur J Hum Genet 2008; 16:1507–1511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kocemba J, Kawecka-Jaszcz K, Gryglewska B, Grodzicki T. Isolated systolic hypertension: pathophysiology, consequences and therapeutic benefits. J Hum Hypertens 1998; 12:621–626 [DOI] [PubMed] [Google Scholar]
- 52. Black HR. Isolated systolic hypertension in the elderly: lessons from clinical trials and future directions. J Hypertens 1999; 17:S49–54 [PubMed] [Google Scholar]
- 53. Franz CE, Grant MD, Jacobson KC, Kremen WS, Eisen SA, Xian H, Romeis J, Thompson-Brenner H, Lyons MJ. Genetics of body mass stability and risk for chronic disease: a 28-year longitudinal study. Twin Res Hum Genet 2007; 10:537–545 [DOI] [PubMed] [Google Scholar]
- 54. Dudina A, Cooney MT, Bacquer DD, Backer GD, Ducimetière P, Jousilahti P, Keil U, Menotti A, Njølstad I, Oganov R, Sans S, Thomsen T, Tverdal A, Wedel H, Whincup P, Wilhelmsen L, Conroy R, Fitzgerald A, Graham I, SCORE investigators. Relationships between body mass index, cardiovascular mortality, and risk factors: a report from the SCORE investigators. Eur J Cardiovasc Prev Rehabil 2011; 18:731–742 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.