Abstract
BACKGROUND
Although variations in plasma renin activity (PRA) and aldosterone have been examined in whites and blacks, the association of these hormones with blood pressure in multiethnic populations has not been described.
METHODS
We measured PRA and aldosterone in 1,021 participants in the Multi-Ethnic Study of Atherosclerosis not taking antihypertensives and examined the association between ethnicity and PRA/aldosterone and the association between PRA/aldosterone with systolic blood pressure (SBP).
RESULTS
Average age was 62 (SD = 9) years, and 49% of participants were women. Median PRA was 0.51 (interquartile range (IQR) = 0.29–0.87) ng/ml/hour, and median aldosterone was 12.6 (IQR = 9.1–17.1) ng/dl. After age and sex adjustment, compared with whites, blacks had 28% lower PRA and 17.4% lower aldosterone, and Hispanics had 20.1% higher PRA but similar aldosterone levels. After multivariable adjustment, compared with whites, only Hispanic ethnicity independently associated with higher PRA (0.18ng/ml/hour; 95% confidence interval (CI) = 0.06–0.31). Blacks had lower aldosterone (−1.7ng/dl; 95% CI = −3.2 to −0.2) compared with whites. After multivariable adjustment, PRA was associated with lower SBP in whites (−3.2mm Hg; 95% CI = −5.2 to −1.2 per standardized unit PRA), Chinese (−3.5mm Hg; 95% CI = −6.2 to −0.80 per standardized unit), and Hispanics (−2.3mm Hg; 95% CI = −4.1 to −0.6 per standardized unit) but not blacks. Aldosterone was associated with higher SBP only in Hispanics (2.5mm Hg; 95% CI = 0.4–4.5 per SD).
CONCLUSIONS
Compared with whites, blacks have lower aldosterone and Hispanics have higher PRA. Aldosterone had significant associations with higher SBP in Hispanics compared with other groups, a finding that may suggest a different mechanism of hypertension.
Keywords: black, blood pressure, Chinese, cross-sectional analysis, Hispanic, hypertension, white.
The renin-angiotensin-aldosterone system (RAAS) maintains extracellular volume in the setting of salt or volume loss through vasoconstriction and sodium retention. Although this system maintains blood pressure (BP) in pathologic states of effective volume depletion, renin and aldosterone are also believed to be major contributors to hypertension, atherosclerosis, and heart failure.1
Classically, renin release stimulates angiotensin activation and aldosterone release and is therefore considered to stimulate higher blood pressure. Despite this, several prior studies in community-living, nonhypertensive populations have demonstrated inverse relationships of plasma renin activity (PRA) and systolic BP (SBP). In addition, it is well recognized that there are forms of low-renin hypertension where aldosterone is elevated out of proportion to renin levels. Among hypertensives, this low-renin state is approximately twice as common in blacks versus whites;2–4 in these conditions, high aldosterone levels portend greater treatment resistance and end-organ damage.5 Although these hormones have been extensively studied in whites and blacks, little is known about variations in hormone levels for nonwhite, nonblack races/ethnicities.
The Multi-Ethnic Study of Atherosclerosis (MESA), a study of cardiovascular disease risk factors in whites, Chinese, blacks, and Hispanics, provides a unique opportunity to compare renin and aldosterone levels in individuals across these 4 races/ethnicities. Therefore, we investigated differences in PRA and aldosterone between races/ethnicities in individuals not treated for hypertension. Given the physiologic role of RAAS to maintain BP, we also examined the associations of PRA and aldosterone with BP in all races/ethnicities together and separately in each race/ethnicity. We hypothesized that among those not taking antihypertensive medications, PRA would be lower among blacks vs. other races/ethnicities and that Hispanics and Chinese would have similar patterns to whites in this regard.
METHODS
Study population
The MESA is a longitudinal cohort study of 6,814 men and women aged 45–84 years without evidence of clinical cardiovascular disease. Subjects were recruited between 1 August 2000 and 30 July 2002 from 6 US communities and had follow-up visits at approximately 1.5 years, 3 years, and 4.5 years after the initial intake. The study includes individuals who self-identified as black, Chinese American, Hispanic American, and non-Hispanic white based on the 2000 US Census questionnaire. Further details of the study design, including recruitment and sampling procedures, have been previously published.6
At clinic visits 2 and 3 (approximately 3 and 4.5 years after baseline, respectively), a random subsample of 1,960 MESA participants had PRA and aldosterone levels measured as part of an ancillary study investigating renal artery disease and were thus potentially eligible for this study. Participants underwent these measurements at either visit 2 or visit 3 based upon when they participated in the ancillary study; no individual participated in the ancillary study at both visits. Of the 1,960 subjects with PRA and aldosterone measured, participants were excluded if either PRA or aldosterone was missing (n = 54; 3%), if PRA or aldosterone was measured outside the detectable range (n = 132; 7%), or if the participant was taking antihypertensive medications (n = 753; 38%).
Health history, physical examination, and biomarker data were obtained from the same exam at which the participant had PRA and aldosterone measured, the only exception being the family history of coronary heart disease (CHD), which was collected only at clinic visit 2 and was therefore carried forward for participants who had PRA and aldosterone measured at visit 3.
Demographic, cardiovascular, laboratory, and other covariables
All MESA participants completed self-administered questionnaires at each visit and were interviewed by research staff about information pertaining to demographics, medical history, medication, and alcohol and tobacco use. Trained and certified clinic staff collected BP and anthropometric measurements on all MESA participants at each visit. After a 5-minute rest, BP was measured on seated subjects 3 times at 1-minute intervals using the Dinamap PRO 100 automated oscillometric device (Critikon, Tampa, FL) with the back and arm supported. The average of the second and third BP readings was used for analysis. Mean arterial pressure was calculated as (2(diastolic blood pressure (DBP)) + SBP)/3. With the participant wearing light clothing, body mass index was calculated as weight in kilograms divided by height in meters squared. Waist-to-hip ratio was calculated using measurements of waist and hip circumference with use of standard tape measure.
Family history of CHD was based on self-report by subjects at clinic visit 2. Alcohol consumption and smoking status were based on self-report and defined as a binary variable based on current status. Use of antihypertensive medication was based on self-report of use of medication from 1 of 6 common classes of antihypertensive medications (thiazide diuretics, angiotensin-converting enzyme inhibitors, angiotensin 2 receptor blocker, calcium channel blocker, β-blockers, and other (α-blockers, other peripheral vasodilators)). Use of estrogen was based on self-report of any form of postmenopausal hormone replacement therapy.
Blood was collected at each clinic visit after a suggested 12-hour fast and stored. Cholesterol and blood sugars were measured from the blood samples obtained at the time of the study visit after shipping to the Laboratory for Clinical Biochemistry Research (University of Vermont, Burlington, VT).7 Use of lipid medication was based on self-report of any lipid-lowering medication, including statins and niacin. Subjects were classified as having dyslipidemia if they were taking any lipid-lowering medication or if they had a cholesterol-to–high-density lipoprotein ratio >5. A diagnosis of diabetes was determined based on 2003 American Diabetes Association fasting blood sugar criteria of glucose ≥7mmol/L (126mg/dl) or current treatment with insulin or oral hypoglycemic medication. Creatinine was also measured, and estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation.8
PRA and aldosterone measurements
The MESA used a standardized collection and processing protocol initially developed for the Cardiovascular Health Study.7 Participant samples were originally collected in the morning, aliquoted into approximately 65 aliquots per participant, immediately placed on ice, flash frozen, and stored at −80 °C after processing. Both PRA and aldosterone assays were run in duplicate and averaged. PRA was measured after 3 freeze–thaw cycles; aldosterone was measured after 2 cycles.
Angiotensin I levels directly correlate with PRA. Therefore, PRA was measured using a radioimmunoassay of generated angiotensin I (GammaCoat Plasma Renin Activity 125I Kit; DiaSorin, Stillwater, MN). Plasma renin activity was defined as nanograms of angiotensin I generated per milliliter of sample per hour (ng/ml/hour). The assay range was 0.05–5.0ng/ml/hour. Intra-assay coefficients of variation ranged 6.89%–18.38%.
Aldosterone was measured using a competition-based radioimmunoassay (ALDOCTK-2; Diasorin, Stillwater, MN). Intra-assay coefficients of variation ranged 6.30%–8.87%. Aldosterone concentrations were divided by 10 to convert from units of pictograms per milliliter to clinical units of nanograms per deciliter. Aldosterone-to-renin ratio (ARR) was calculated by dividing aldosterone level by PRA with units of deciliter per milliliter per hour.
Statistical analysis
Means and SDs were used to summarize the characteristics of the various covariables across the 4 different racial/ethnic groups. Categorical and normally distributed continuous variables were compared with χ2 and analysis of variance tests, respectively. Plasma renin activity and aldosterone data were reviewed with histograms to assess normal distribution. The skewed continuous variables (PRA, aldosterone, and urine albumin-to-creatinine ratio) were compared across groups with the Kruskal–Wallis test. Because of the right skewed distribution of PRA and aldosterone, analyses were repeated using the log-scale; results were similar using either method, so linear scale results are reported here. Age- and sex-adjusted means of PRA and aldosterone were compared for each race/ethnicity. To better compare the strength of association that potential confounders had with PRA and aldosterone, these were converted to standardized units by dividing each by their respective SDs. This same standardization was also performed on waist-to-hip ratio. In addition, age, SBP, DBP, and eGFR variables were divided by 10 to create more relevant magnitudes.
To identify confounding variables, multivariable regression was performed with standardized PRA and aldosterone as dependent variables and the inclusion of any biologically plausible confounder as independent variables.
Linear regression was used to assess the contribution race/ethnicity had on PRA and aldosterone. This analysis was done with a series of models adjusting for relevant covariables with the white group as reference,= and the Chinese, black, and Hispanic groups as independent variables. We chose potential confounders of the relationship between race/ethnicity with PRA or aldosterone based on biological plausibility or on a P value of the beta coefficient for that potential confounder of 0.10 with either PRA or aldosterone. Model 1 adjusted for age and sex. Model 2 added SBP, DBP, the combined dyslipidemia variable defined above, current smoking status, current alcohol consumption, diabetes, family history of CHD, body mass index, waist-to-hip ratio, and estrogen treatment. Model 3 added eGFR to model 2, and model 4 mutually adjusted for aldosterone when PRA was the outcome or vice versa.
We also evaluated the association of PRA and aldosterone with SBP and DBP across the 4 racial/ethnic groups. In a univariable regression model with SBP or DBP as the outcome, we tested for effect modification by race/ethnicity for the association of PRA or aldosterone and SBP or DBP. We then compared the mean SBP and DBP for every quartile of PRA, aldosterone, or ARR between races/ethnicities. We used a series of nested models identical to models 1–3, with SBP and DBP as the outcome variable and PRA or aldosterone included as an independent variable, to fully evaluate differences across races/ethnicities.
All analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC). P < 0.05 was considered to be statistically significant for all analyses including interaction terms. The MESA study protocol was approved by institutional review boards at all 6 clinical sites, and all participants provided written informed consent. Data analysis was performed on deidentified data held at the University of California, San Diego.
RESULTS
The average age was 62 (SD = 9) years, and 49% of participants were women. Among the 1,021 study participants, 424 (42%) were white, 151 (15%) were Chinese, 157 (15%) were black, and 289 (28%) were Hispanic. The population median PRA was 0.51 (interquartile range (IQR) = 0.29–0.87) ng/ml/hour, and the median aldosterone was 12.6 (IQR = 9.1–17.1) ng/dl. Table 1 shows the characteristics of the study population categorized by race/ethnicity. Blacks had higher SBP, DBP, and mean arterial pressure compared with other races/ethnicities. Blacks and Hispanics had greater body mass indexes than whites and Chinese. Whites had greater alcohol consumption than the other 3 races/ethnicities. In unadjusted analysis, both PRA and aldosterone concentrations differed significantly by race/ethnicity; the most striking difference was markedly lower PRA and aldosterone levels in blacks relative to the other 3 racial/ethnic groups.
Table 1.
Baseline characteristics by ethnicity
| Characteristic | White (n = 424) | Chinese (n = 151) | Black (n = 157) | Hispanic (n = 289) |
|---|---|---|---|---|
| Age, y, mean (SD) | 63 (9) | 62 (10) | 62 (9) | 61 (9) |
| Female sex, % | 49.1 | 42.4 | 52.9 | 49.5 |
| SBP, mm Hg, mean (SD) | 117 (18) | 116 (17) | 125 (20) | 120 (19) |
| DBP, mm Hg, mean (SD) | 68 (9) | 69 (9) | 73 (10) | 69 (9) |
| MAP, mm Hg, mean (SD) | 84 (11) | 84 (11) | 90 (12) | 86 (11) |
| BMI, kg/m2, mean (SD) | 26.9 (4.6) | 23.5 (3.0) | 28.6 (4.8) | 29.1 (5.0) |
| DM, % | 4.5 | 7.3 | 10.2 | 12.5 |
| Current alcohol use, % | 71.6 | 36.4 | 48.4 | 43.4 |
| Current smoker, % | 13.9 | 4.6 | 17.2 | 13.2 |
| Family history of CHD, % | 11.7 | 4.0 | 18.5 | 15.9 |
| Waist-to-hip ratio, cm/cm, mean (SD) | 0.92 (0.08) | 0.91 (0.06) | 0.9 (0.08) | 0.96 (0.06) |
| Dyslipidemiaa, % | 34.2 | 27.2 | 19.1 | 40.1 |
| UACR, mg/g, median (IQR) | 4.9 (3.5–8.0) | 6.2 (3.8–11.2) | 4.4 (2.8–8.3) | 5.5 (3.7–10.1) |
| eGFR, ml/min/1.73m,2 mean (SD) | 76.3 (14.4) | 85.1 (12.7) | 86.3 (17.6) | 83.9 (14.4) |
| PRA, ng/ml/hour, median (IQR) | 0.52 (0.30–0.88) | 0.51 (0.30–0.87) | 0.31 (0.18–0.55) | 0.6 (0.35–1.04) |
| Aldosterone, ng/dl, median (IQR) | 12.8 (9.4–17.3) | 12.9 (9.6–16.9) | 10.5 (7.4–14.8) | 13 (9.5–17.8) |
All characteristics except sex and UACR were statistically different (P < 0.05) with appropriate χ2, analysis of variance, or Kruskal–Wallis test.
Abbreviations: BMI, body mass index; CHD, cardiovascular heart disease; DBP, diastolic blood pressure; DM, diabetes mellitus; eGFR: estimated glomerular filtration rate; MAP, mean arterial pressure; PRA: plasma renin activity; SBP, systolic blood pressure; UACR: urine albumin-to-creatinine ratio.
aDyslipidemia defined as cholesterol/high-density lipoprotein > 5 or taking lipid-lowering medication.
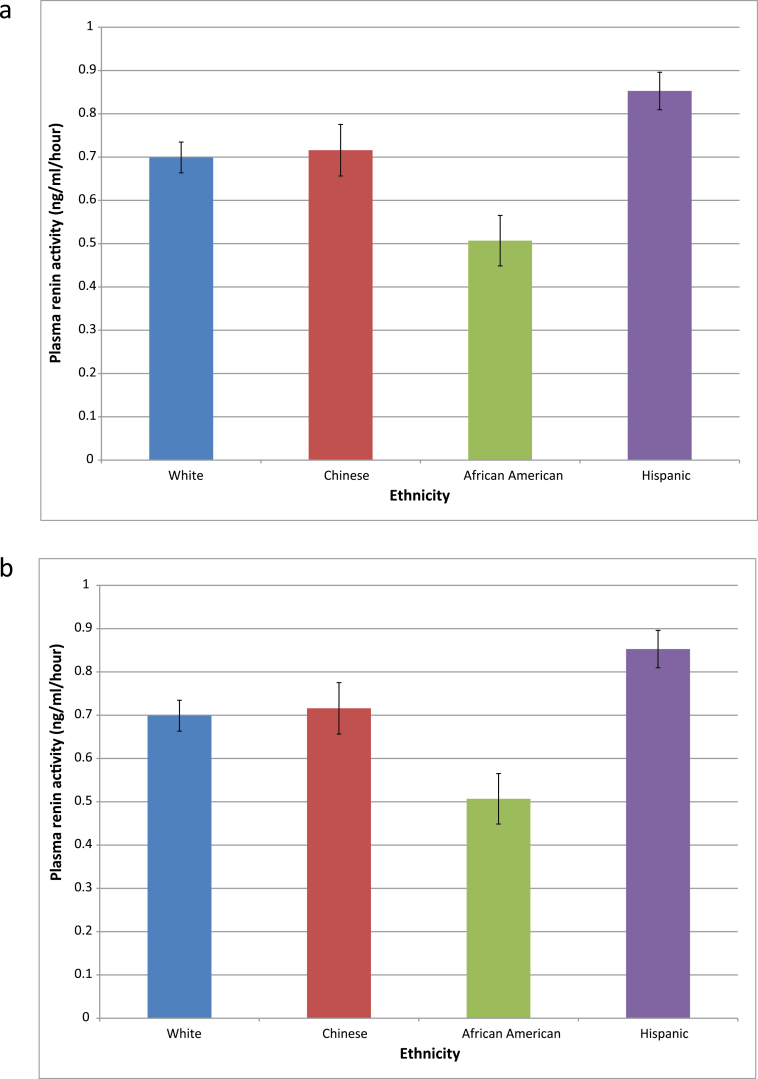
Figure 1 shows the age- and sex-adjusted means of PRA (Figure 1a) and aldosterone (Figure 1b) for each of the 4 race/ethnicities. After age and sex adjustment, blacks had significantly lower PRA and aldosterone than the other 3 races/ethnicities, whereas Hispanics had higher PRA than whites. PRA and aldosterone levels were similar in Chinese and white participants.
Figure 1.
Age- and sex-adjusted (a) plasma renin activity (PRA) and (b) aldosterone means by ethnicity. Error bars represent SE. P = 0.0001 for comparison of both PRA and aldosterone of blacks with those of all other ethnicities. P = 0.009 for comparison of PRA of Hispanic with that of whites.
Table 2 shows multivariable regression coefficients between demographic and clinical variables and both PRA and aldosterone levels (expressed per standardized unit of PRA or aldosterone). For PRA, the strongest associations were with sex and SBP. Men had higher PRA levels. Aldosterone had significant negative associations (P ≤ 0.10) with age and SBP with coefficients of −0.14 and −0.05, respectively. Aldosterone was also noted to have a significant association with DBP, dyslipidemia, waist-to-hip ratio, eGFR, estrogen replacement therapy, and alcohol. All of these, with the exception of eGFR, had positive coefficients (Table 2). Diabetes did not have a significant association with either PRA or aldosterone.
Table 2.
Multivariable regression coefficients between covariables and renin/aldosterone
| Variable | Renin (per SD) | Aldosterone (per SD) | ||||||
|---|---|---|---|---|---|---|---|---|
| Coefficient | 95% confidence limits | P value | Coefficient | 95% confidence limits | P value | |||
| Age, per 10 y | −0.08 | −0.16 | 0.01 | 0.07 | −0.14 | −0.23 | −0.06 | 0.001 |
| Male sex | 0.27 | 0.11 | 0.42 | 0.0007 | −0.13 | −0.29 | 0.02 | 0.09 |
| Systolic BP, per 10mm Hg | −0.09 | −0.14 | −0.04 | 0.0002 | −0.05 | −0.09 | 0.00 | 0.07 |
| Diastolic BP, per 10mm Hg | −0.01 | −0.10 | 0.09 | 0.92 | 0.16 | 0.06 | 0.25 | 0.001 |
| Hyperlipidemiaa | 0.03 | −0.11 | 0.16 | 0.67 | 0.19 | 0.06 | 0.33 | 0.006 |
| Diabetes | 0.14 | −0.09 | 0.37 | 0.24 | 0.06 | −0.17 | 0.30 | 0.60 |
| Waist-to-hip ratio, per SD | 0.02 | −0.06 | 0.10 | 0.60 | 0.09 | 0.01 | 0.17 | 0.03 |
| eGFR, per 10 | −0.02 | −0.06 | 0.03 | 0.53 | −0.08 | −0.13 | −0.03 | 0.001 |
| Estrogen replacement | 0.16 | −0.08 | 0.39 | 0.19 | 0.20 | −0.04 | 0.44 | 0.10 |
| Alcohol | 0.02 | −0.11 | 0.15 | 0.78 | 0.12 | −0.01 | 0.25 | 0.08 |
Coefficients reported are from multivariable regression with all covariables of model 3 included (see Methods).
aDefined as cholesterol/high-density lipoprotein >5 or taking lipid-lowering medication.
We next examined the effect of race/ethnicity on PRA and aldosterone (Table 3). In comparison with whites, those of Hispanic race/ethnicity had a 0.21 standardized unit higher level of PRA, which changed minimally with adjustment, and those of black race/ethnicity had a 0.26 standardized unit lower level of PRA than whites. This association was attenuated by adjustment for SBP in model 2. Black race/ethnicity also had a 0.35 standardized unit lower level of aldosterone after age and sex adjustment. This relationship persisted through model 3 before being attenuated by mutual adjustment by PRA.
Table 3.
Linear regression models
| Renin | ||||
|---|---|---|---|---|
| White | Chinese | Black | Hispanic | |
| Model 1 | — | 0.02 (−0.16 to 0.20) | −0.26 (−0.44 to −0.08) | 0.21 (0.06 to 0.35) |
| Model 2 | — | 0.05 (−0.14 to 0.25) | −0.17 (−0.36 to 0.02) | 0.24 (0.08 to 0.39) |
| Model 3 | — | 0.06 (−0.14 to 0.26) | −0.16 (−0.35 to 0.04) | 0.25 (0.09 to 0.41) |
| Model 4 | — | 0.06 (−0.13 to 0.24) | −0.09 (−0.27 to 0.10) | 0.23 (0.08 to 0.38) |
| Aldosterone | ||||
| White | Chinese | Black | Hispanic | |
| Model 1 | — | −0.08 (−0.27 to 0.10) | −0.35 (−0.53 to −0.17) | 0.0008 (−0.15 to 0.15) |
| Model 2 | — | −0.03 (−0.23 to 0.17) | −0.3 (−0.49 to −0.10) | 0.02 (−0.14 to 0.18) |
| Model 3 | — | 0.03 (−0.17 to 0.23) | −0.22 (−0.42 to −0.03) | 0.05 (−0.11 to 0.21) |
| Model 4 | — | 0.006 (−0.18 to 0.20) | −0.17 (−0.36 to 0.02) | −0.03 (−0.18 to 0.12) |
Model 1 includes age and sex. Model 2 includes model 1 plus systolic and diastolic blood pressure, dyslipidemia, smoking, alcohol, diabetes, family history of coronary heart disease, body mass index, waist-to-hip ratio, and estrogen therapy. Model 3 includes model 2 plus estimated glomerular filtration rate. Model 4 includes model 3 plus renin or aldosterone (mutually adjusted model).
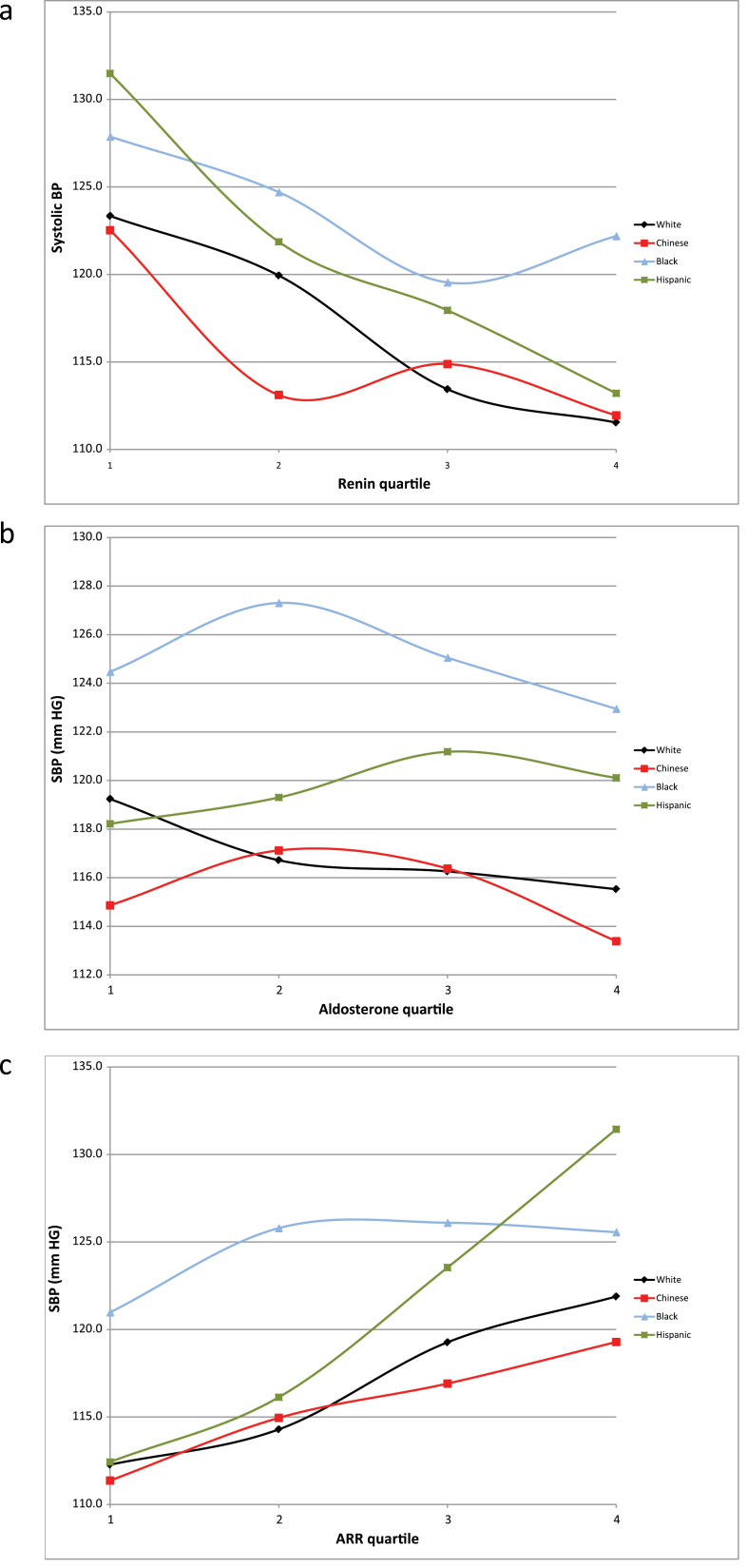
Figure 2 shows average SBP plotted by PRA quartile (Figure 2a), aldosterone quartile (Figure 2b), and ARR quartile (Figure 2c) for each race/ethnicity. Higher PRA quartiles were associated with lower SBP for all races/ethnicities, with a weaker relationship in blacks compared with other races/ethnicities (Figure 2a). Conversely, only a minimal association was seen between aldosterone quartiles and SBP (Figure 2b), with Hispanics having the only positive association. Across races/ethnicities, a positive correlation was seen with ARR and SBP (Figure 2c). These same analyses were repeated with DBP as the outcome variable, and we observed no significant associations in any racial/ethnic group (data not shown).
Figure 2.
Systolic blood pressure (SBP) mean plotted by (a) plasma renin activity (PRA) quartile, (b) aldosterone quartile, and (c) aldosterone-to-renin ratio (ARR) quartile.
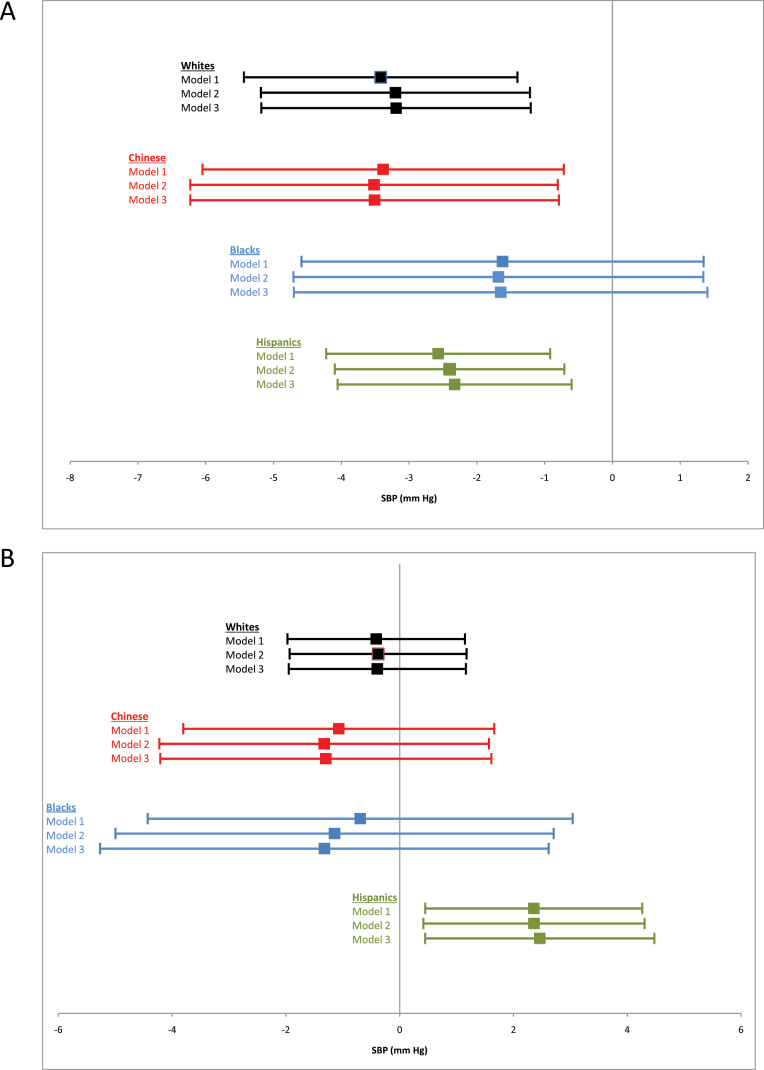
The association of PRA or aldosterone with SBP was further investigated with a series of linear regression models. Covariables were introduced in a stepwise fashion similar to models 1–3. Whites, Chinese, and Hispanics all had statistically significant inverse associations between PRA and SBP, whereas blacks did not (Figure 3a). When we repeated this analysis using aldosterone as the predictor variable, Hispanics were the only race/ethnicity to have a statistically significant association between aldosterone and SBP (Figure 3b). Formal testing for effect modification by race/ethnicity in the age- and sex-adjusted model did not show significant interaction for PRA (P = 0.71); for aldosterone, there was a borderline significant interaction only with the Hispanic race/ethnicity (vs. all others, P = 0.05).
Figure 3.
Results of ethnicity-specific multivariable regression of (a) plasma renin activity (PRA) and (b) aldosterone with systolic blood pressure (SBP). Model 1 adjusts for age and sex. Model 2 adjusts for age, sex, dyslipidemia, smoking, alcohol, diabetes, family history of coronary heart disease, body mass index, waist-to-hip ratio, and estrogen therapy. Model 3 adjusts for age, sex, systolic and diastolic blood pressure, dyslipidemia, smoking, alcohol, diabetes, family history of coronary heart disease, body mass index, waist-to-hip ratio, estrogen therapy, and estimated glomerular filtration rate.
DISCUSSION
In this study of community-dwelling individuals not treated for hypertension, without known cardiovascular disease, and compared with Caucasians, we found that blacks had lower PRA and aldosterone, whereas Hispanics had higher PRA, and PRA and aldosterone concentrations were similar in Chinese and white participants. The association between black race/ethnicity and lower PRA was, however, attenuated by adjustment for SBP. Additionally, we observed that higher PRA levels across all races/ethnicities except blacks were associated with lower SBP and that aldosterone was only positively associated with SBP in Hispanics, with minimal associations observed in the other races/ethnicities.
To our knowledge, this is the first description of race/ethnic variation in renin and aldosterone in a multiethnic population without hypertension. Our finding of higher PRA in Hispanics compared with whites is a relatively novel finding. To our knowledge, the only other studies of PRA in Hispanics were performed in a study of hypertensive individuals in the Kaiser Permanente Southern California (KPSC) health system.9,10 These KPSC studies looked at the association between PRA and antihypertensive drug use,10 as well as PRA and chronic kidney disease,9 rather than evaluating differences in hormone levels by race/ethnicity. In both of these studies, the distribution of PRA levels was higher in Hispanics than in whites.
Similarly, our finding that hormone levels in Chinese individuals are not significantly different from those in whites is also important and has been little studied. This suggests that a similar mechanism of hormonal regulation are operating in these 2 groups, despite the higher overall rate of hypertension noted among Chinese individuals in comparison with whites; other genetic, dietary, or environmental factors may be involved.11
Our findings also reinforce the previously established and well-known phenomenon whereby blacks have lower PRA than whites. This was first observed in the mid-1960s by Helmer2 who noted a higher frequency of reduced peripheral renin in black hypertensive patients (52%) compared with whites (31%) and has since been noted by many others in both black normotensives12–15 as well as hypertensives,12,13,16,17 with blacks having 25%12 to 77%13 of the renin level/PRA of whites. In our study, blacks had 72% of the PRA of whites. This difference in PRA has been proposed to contribute to the difference in the frequency of hypertension between blacks and whites.18 This is consistent with our finding showing that the negative association of black race/ethnicity with PRA was attenuated by adjustment with SBP. The difference in PRA has also been used to explain the differential response of hypertensive blacks to blood pressure–lowering therapy, with blacks more likely to be salt-sensitive and to respond to salt restriction or natriuresis.19–21
The lower aldosterone level we observed in blacks has been observed in some prior studies. Reports of normotensive black children22 and adults3,23,24 have noted lower3,22,23 aldosterone or no difference.24 However, a study in hypertensive blacks noted increased3 aldosterone. In a case–control study with blacks and French-Canadian normotensive and hypertensive individuals (off antihypertensive therapy for at least 1 week), Grim et al. reported elevated aldosterone despite suppressed PRA in the black hypertensive population.3 This finding led the authors to hypothesize that aldosterone-induced volume expansion is an important contributor to hypertension in this population and may in part be due to a subtle variant of primary aldosteronism.3 This has also been observed and noted by others.25,26 Despite the lower aldosterone levels among blacks in our study, the ARR remained elevated for this race/ethnicity as PRA was lowered disproportionately to aldosterone, thus consistent with this hypothesis. However, whereas Grim et al. noted a direct association of aldosterone with SBP and DBP, we saw an inverse association for blacks that did not reach statistical significance in our study.
Our finding that higher PRA is associated with lower SBP in a community-living sample without treated hypertension is consistent with prior literature, with higher PRA being an adaptive measure to raise or maintain BP and lower PRA being appropriate to higher BP with a normally functioning RAAS.15,27,28 This finding suggests that PRA serves as an index for long-term extracellular fluid status and, by extension, dietary salt intake, where increased salt and fluid intake may simultaneously elevate BP and suppress PRA. Recently, de Boer et al. investigated renin levels in the white, European cohort not on antihypertensive medications in the Prevention of Renal and Vascular End-Stage Disease (PREVEND) study. These investigators also reported an inverse relationship between renin and SBP. We observed similar relationships in all races/ethnicities except blacks; even those blacks in the highest quartile of PRA had higher SBP than their nonblack counterparts. This could be explained by a difference in the RAAS system among blacks, with 1 proposed mechanism being an increase in adrenal sensitivity to angiotensin II.25,26
A direct association between aldosterone and SBP was noted only within the Hispanic group. The association between aldosterone and SBP in Hispanics is, to our knowledge, a novel finding. Aldosterone has been linked with arteriolar inflammation, and given the differences in aldosterone’s association with SBP here, consideration might be given to race/ethnic differences in response to anti-inflammatory agents such as acetyl-salicylic acid.29 This direct association, together with the inverse association of PRA with SBP, resulted in a more pronounced direct association of ARR with SBP in Hispanics.
If our findings are confirmed and extended to cardiovascular disease outcomes, they could have important implications for hypertension and related outcomes, including stroke and cardiovascular disease. For example, the finding that Hispanics had higher PRA than whites could suggest that this population is more susceptible to cardiovascular morbidity and mortality because several recent studies have implicated renin level/PRA as an independent risk factor for myocardial infarction,17 heart failure,30 chronic kidney disease,9 and cardiovascular mortality.27,31–34 We also observed that the association of aldosterone with SBP was particularly strong in Hispanics. If confirmed in hypertensive populations, these findings may suggest that Hispanics may be particularly sensitive to antialdosterone agents for treatment of hypertension, an important hypothesis that could be tested in future randomized clinical trials. Additionally, the finding that Hispanics, blacks, and Chinese/white participants have different renin/aldosterone patterns might prompt preplanned subgroup analyses of these participants in studies of blockade of different aspects of the RAAS.
The strengths of our study include the large population of racially and ethnically diverse individuals from across the United States, the measurement of PRA and aldosterone in a central laboratory, and the careful assessment of covariables. We were able to include and adjust for a number of physiologically plausible covariables, including kidney function. Despite these and other strengths, our study also has important limitations. The PRA and aldosterone assays were performed on blood specimens obtained under casual conditions with the patient seated, rather than supine or under salt-loaded conditions, as in many of the original physiologic studies. Prorenin, which has been differentially correlated with outcomes in hypertension,35 was not available; information on genetic variation in ACE subtype was also not available for our study.36 Our exclusion of individuals on antihypertensive medications limited confounding because the majority of antihypertensive medications affect PRA and aldosterone and the use of medications may be affected by selection bias in terms of those who receive medication or do not because the use of medications is differentially distributed across the 4 racial/ethnic groups.11 However, this also means that our results are potentially not generalizable to hypertensive populations within each race/ethnicity and may exclude those whose physiology predisposes them to hypertension and is associated with PRA differently. Another potential limitation is the absence of home or ambulatory BP to better define hypertensive load.
In conclusion, in a large, racially and ethnically diverse cohort of community-living individuals without treatment for hypertension, we found that compared with community-living whites, blacks have lower aldosterone and Hispanics have higher PRA, whereas levels in Chinese are not significantly different for either measure. PRA had an inverse relationship with SBP in all races/ethnicities except for blacks, and aldosterone had a significant association with higher SBP in Hispanics compared with other races/ethnicities. If confirmed and extended to longitudinal health outcomes, these findings may have implications for racial/ethnic differences in diagnosis, treatment, and associated morbidity and mortality of cardiovascular disease.
DISCLOSURE
The authors declared no conflict of interest.
ACKNOWLEDGMENTS
M.A.A. was supported by NIDDK grant R01 DK080015; D.E.R. was supported by K23 DK091521.
REFERENCES
- 1. Franco V, Oparil S. Salt sensitivity, a determinant of blood pressure, cardiovascular disease and survival. J Am Coll Nutr 2006; 25:247S–255S [DOI] [PubMed] [Google Scholar]
- 2. Helmer OM. The renin-angiotensin system and its relation to hypertension. Prog Cardiovasc Dis 1965; 8:117–128 [DOI] [PubMed] [Google Scholar]
- 3. Grim CE, Cowley AW, Jr, Hamet P, Gaudet D, Kaldunski ML, Kotchen JM, Krishnaswami S, Pausova Z, Roman R, Tremblay J, Kotchen TA. Hyperaldosteronism and hypertension: ethnic differences. Hypertension 2005; 45:766–772 [DOI] [PubMed] [Google Scholar]
- 4. Sagnella GA. Why is plasma renin activity lower in populations of African origin? J Hum Hypertens 2001; 15:17–25 [DOI] [PubMed] [Google Scholar]
- 5. Pimenta E, Gaddam KK, Pratt-Ubunama MN, Nishizaka MK, Cofield SS, Oparil S, Calhoun DA. Aldosterone excess and resistance to 24-h blood pressure control. J Hypertens 2007; 25:2131–2137 [DOI] [PubMed] [Google Scholar]
- 6. Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Jr, Kronmal R, Liu K, Nelson JC, O’Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol 2002; 156:871–881 [DOI] [PubMed] [Google Scholar]
- 7. Cushman M, Cornell ES, Howard PR, Bovill EG, Tracy RP. Laboratory methods and quality assurance in the Cardiovascular Health Study. Clin Chem 1995; 41:264–270 [PubMed] [Google Scholar]
- 8. Stevens LA, Coresh J, Schmid CH, Feldman HI, Froissart M, Kusek J, Rossert J, Van Lente F, Bruce RD, Zhang YL, Greene T, Levey AS. Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3418 individuals with CKD. Am J Kidney Dis 2008; 51:395–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sim JJ, Shi J, Calara F, Rasgon S, Jacobsen S, Kalantar-Zadeh K. Association of plasma renin activity and aldosterone-renin ratio with prevalence of chronic kidney disease: the Kaiser Permanente Southern California cohort. J Hypertens 2011; 29:2226–2235 [DOI] [PubMed] [Google Scholar]
- 10. Sim JJ, Bhandari SK, Shi J, Kalantar-Zadeh K, Rasgon SA, Sealey JE, Laragh JH. Plasma renin activity (PRA) levels and antihypertensive drug use in a large healthcare system. Am J Hypertens 2012; 25:379–388 [DOI] [PubMed] [Google Scholar]
- 11. Kramer H, Han C, Post W, Goff D, Diez-Roux A, Cooper R, Jinagouda S, Shea S. Racial/ethnic differences in hypertension and hypertension treatment and control in the multi-ethnic study of atherosclerosis (MESA). Am J Hypertens 2004; 17:963–970 [DOI] [PubMed] [Google Scholar]
- 12. Levy SB, Lilley JJ, Frigon RP, Stone RA. Urinary kallikrein and plasma renin activity as determinants of renal blood flow. The influence of race and dietary sodium intake. J Clin Invest 1977; 60:129–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mitas JA I. RAcial analysis of the volume-renin relationship in human hypertension. Arch Intern Med 1979; 139:157–160 [PubMed] [Google Scholar]
- 14. James GD, Sealey JE, Müller F, Alderman M, Madhavan S, Laragh JH. Renin relationship to sex, race and age in a normotensive population. J Hypertens Suppl 1986; 4:S387–S389 [PubMed] [Google Scholar]
- 15. He J, Klag MJ, Appel LJ, Charleston J, Whelton PK. The renin-angiotensin system and blood pressure: differences between blacks and whites. Am J Hypertens 1999; 12:555–562 [DOI] [PubMed] [Google Scholar]
- 16. Urinary and serum electrolytes in untreated black and white hypertensives. J Chron Dis 1987; 40:839–847 [DOI] [PubMed] [Google Scholar]
- 17. Alderman MH, Ooi WL, Cohen H, Madhavan S, Sealey JE, Laragh JH. Plasma renin activity: a risk factor for myocardial infarction in hypertensive patients. Am J Hypertens 1997; 10:1–8 [DOI] [PubMed] [Google Scholar]
- 18. Centers for Disease Control and Prevention. Hypertension Among Adults in the United States, 2009–2010. http://www.cdc.gov/nchs/data/databriefs/db107.htm Accessed December 5, 2012
- 19. Moran A, Simon JA, Shiboski S, Pickering TG, Waters D, Rotter JI, Lyon C, Nickerson D, Yang H, Saad M, Hsueh W, Krauss RM. Differential effects of ramipril on ambulatory blood pressure in African Americans and Caucasians. Am J Hypertens 2007; 20:884–891 [DOI] [PubMed] [Google Scholar]
- 20. Materson BJ, Reda DJ, Cushman WC, Massie BM, Freis ED, Kochar MS, Hamburger RJ, Fye C, Lakshman R, Gottdiener J, Ramirez EA, Henderson WG. Single-drug therapy for hypertension in men—a comparison of six antihypertensive agents with placebo. New Engl J Med 1993; 328:914–921 [DOI] [PubMed] [Google Scholar]
- 21. Comparison of propranolol and hydrochlorothiazide for the initial treatment of hypertension. II. Results of long-term therapy. Veterans Administration Cooperative Study Group on Antihypertensive Agents. JAMA 1982; 248:2004–2011 [PubMed] [Google Scholar]
- 22. Pratt JH, Jones JJ, Miller JZ, Wagner MA, Fineberg NS. Racial differences in aldosterone excretion and plasma aldosterone concentrations in children. New Engl J Med 1989; 321:1152–1157 [DOI] [PubMed] [Google Scholar]
- 23. He FJ, Markandu ND, Sagnella GA, MacGregor GA. Importance of the renin system in determining blood pressure fall with salt restriction in black and white hypertensives. Hypertension 1998; 32:820–824 [DOI] [PubMed] [Google Scholar]
- 24. Kotchen TA, Guthrie GP, Cottrill CM, McKean HE, Kotchen JM. Low renin-aldosterone in “prehypertensive” young adults. JCEM 1982; 54:808–814 [DOI] [PubMed] [Google Scholar]
- 25. Griffing GT, Wilson TE, Melby JC. Alterations in aldosterone secretion and metabolism in low renin hypertension. J Clin Endocrinol Metab 1990; 71:1454–1460 [DOI] [PubMed] [Google Scholar]
- 26. Drayer JI, Weber MA, Sealey JE, Laragh JH. Low and high renin essential hypertension: a comparison of clinical and biochemical characteristics. Am J Med Sci 1981; 281:135–142 [DOI] [PubMed] [Google Scholar]
- 27. De Boer RA, Schroten NF, Bakker SJL, Mahmud H, Szymanski MK, van der Harst P, Gansevoort RT, van Veldhuisen DJ, van Gilst WH, Hillege HL. Plasma renin and outcome in the community: data from PREVEND. Eur Heart J 2012; 33:2351–2359 [DOI] [PubMed] [Google Scholar]
- 28. Meade TW, Imeson JD, Gordon D, Peart WS. The epidemiology of plasma renin. Clin Sci 1983; 64:273–280 [DOI] [PubMed] [Google Scholar]
- 29. Ishizuka T, Niwa A, Tabuchi M, Ooshima K, Higashino H. Acetylsalicylic acid provides cerebrovascular protection from oxidant damage in salt-loaded stroke-prone rats. Life Sci 2008; 82:806–815 [DOI] [PubMed] [Google Scholar]
- 30. Vergaro G, Emdin M, Iervasi A, Zyw L, Gabutti A, Poletti R, Mammini C, Giannoni A, Fontana M, Passino C. Prognostic value of plasma renin activity in heart failure. Am J Cardiol 2011; 108:246–251 [DOI] [PubMed] [Google Scholar]
- 31. Verma S, Gupta M, Holmes DT, Xu L, Teoh H, Gupta S, Yusuf S, Lonn EM. Plasma renin activity predicts cardiovascular mortality in the Heart Outcomes Prevention Evaluation (HOPE) study. Eur Heart J 2011; 32:2135–2142 [DOI] [PubMed] [Google Scholar]
- 32. Muhlestein JB, May HT, Bair TL, Prescott MF, Horne BD, White R, Anderson JL. Relation of elevated plasma renin activity at baseline to cardiac events in patients with angiographically proven coronary artery disease. Am J Cardiol 2010; 106:764–769 [DOI] [PubMed] [Google Scholar]
- 33. Brunner HR, Laragh JH, Baer L, Newton MA, Goodwin FT, Krakoff LR, Bard RH, Bühler FR. Essential hypertension: renin and aldosterone, heart attack and stroke. New Engl J Med 1972; 286:441–449 [DOI] [PubMed] [Google Scholar]
- 34. Alderman MH, Madhavan S, Ooi WL, Cohen H, Sealey JE, Laragh JH. Association of the renin-sodium profile with the risk of myocardial infarction in patients with hypertension. N Engl J Med 1991; 324:1098–1104 [DOI] [PubMed] [Google Scholar]
- 35. Jan Danser AH. Renin and prorenin as biomarkers in hypertension. Curr Opin Nephrol Hypertens 2012; 21:508–514 [DOI] [PubMed] [Google Scholar]
- 36. Duru K, Farrow S, Wang JM, Lockette W, Kurtz T. Frequency of a deletion polymorphism in the gene for angiotensin converting enzyme is increased in African-Americans with hypertension. Am J Hypertens 1994; 7:759–762 [DOI] [PubMed] [Google Scholar]