Abstract
Backgroundy
Percutaneous renal biopsy (PRB) is a safe and valuable procedure in the diagnosis of renal disease. The optimal duration of observation after a PRB has not been established, and patients are frequently hospitalized for overnight observation. We evaluated prospectively the safety of an outpatient PRB protocol at our institution.
Methods
During a 20-month period 100 patients underwent PRB using a standardized outpatient protocol. The biopsy was performed by 8 am by Nephrology Fellows under direct faculty supervision. All biopsies were done under real-time ultrasound with a 16-gauge spring-loaded biopsy gun, and followed immediately by color Doppler ultrasound to exclude active bleeding. Blood pressure and heart rate were monitored frequently. Hematocrit and hemoglobin were measured pre-biopsy, and 4, and 8 hours post-biopsy. If there were no complications, the patients were discharged 9 to 10 hours after the procedure.
Results
The patient demographics were as follow: 55% African Americans; 60% female; 17% diabetic; 65% hypertensive; mean BMI 29.8±7 (range, 17 to 51). The depth of the kidney from the skin was 6.8±2.3 cm (range, 3 to 13.5 cm). 53 patients required only 1 biopsy pass and 38 needed 2 passes, 5 needed 3 passes and 4 needed 4 passes, with 12.7±9.7 glomeruli obtained per patient. No major complications were encountered. A small perinephric hematoma (< 2×2 cm) was observed post-biopsy in 13 patients (13%). There was no need for vascular intervention or transfusion in any patient. Four patients (4%) were hospitalized for overnight observation due to a decrease in their hematocrit (> 4 from baseline), but none required a transfusion or intervention. No patient required subsequent hospitalization due to late biopsy-related complications.
Conclusions
Outpatient, real-time, ultrasound-guided percutaneous renal biopsy is safe and effective, and minimizes the need for post-biopsy hospitalization. Outpatient PRB can result in significant cost savings without exposing the patients to increased risk of complications.
Keywords: real-time ultrasound, percutaneous renal biopsy, outpatient, safety
Introduction
Percutaneous renal biopsy is a valuable procedure for the diagnosis of renal disease. It is helpful in guiding medical therapy, establishing prognosis and for research purposes. However, a renal biopsy may result in serious hemorrhagic complications, requiring additional diagnostic procedures, blood transfusion, vascular interventions, and prolongation of hospitalization (Donovan KL, Thomas DM). The optimal duration of observation after a percutaneous renal biopsy has not been established and patients are frequently hospitalized for overnight observation. When performing a renal biopsy, the operator would like to obtain the tissue required for accurate diagnosis, reduce the risk of major hemorrhagic complications and be able to offer a safe environment to the patient. By offering a safe and quality environment and with the present technology, we can provide an excellent service at a lower cost by minimizing the length of stay in the hospital. In most institutions practitioners perform percutaneous renal biopsies either blind or by ultrasound guided techniques, and admit their patients to the hospital for overnight observation. At our teaching institution, we perform renal biopsies under real-time ultrasound guidance with post-biopsy color Doppler evaluation for major bleedings as an outpatient procedure. Although it is often claimed that the major complications occur within the first 24 hours (Marwah DS, Korbet SM) (Whittier WL, Korbet SM), we think that if a complication is going to happen it would be within the first 8 hours post-biopsy.
During a twenty-month period at our teaching institution, one hundred (100) consecutive patients underwent a percutaneous renal biopsy using a standardized outpatient protocol. A prospective computerized database was used to enter the data. The goal of the present study was to evaluate if real-time ultrasound guided renal biopsies along with color Doppler surveillance was safe as an outpatient procedure.
Methods
Study Population
A computerized database was used to prospectively enter date on all outpatient native kidney biopsies performed by the Division of Nephrology at University of Alabama at Birmingham during the twenty-month period from February 1st, 2007 to September 30th, 2008. Biopsies of transplant kidneys and from the acute renal service were excluded from the analysis. Seven first-year nephrology fellows under the direct supervision of two nephrology faculty members performed all renal biopsies. A total of 100 renal biopsies were performed during the study period. All renal biopsy were performed under real-time ultrasound guidance along with post-biopsy color Doppler surveillance vs. blind procedure) was at the discretion of the faculty member, and was based on their past experience performing a given biopsy.
Renal biopsies were performed after verifying adequate blood pressure control and acceptable coagulation tests. Absolute contraindications to performing a percutaneous renal biopsy included a bleeding disorder, thrombocytopenia (platelet count < 50,000/mm3) or polycystic kidney disease. Uncontrolled hypertension (systolic blood pressure > 160 mm Hg or diastolic blood pressure > 100) was treated initially wit clonidine 0.1 mgs by mouth and in severe cases with hydralazine 10 mgs IV prior to the biopsy. Finally, aspirin and anti-platelet agents were withheld 7 days before the procedure.
Standardized outpatient protocol
Nephrologists from the either the UAB faculty or from surrounded facilities call the vascular access coordinator to schedule a renal biopsy. Patients are scheduled within 3 to 7 days from the day of call. They are instructed to come to the outpatient setting of interventional radiology/nephrology facility at the hospital by 6 am. Once there, they are admitted as outpatients to private rooms. A cell blood count (CBC), renal function panel, prothrombin time, partial thromboplastin time and a type and cross are obtained. Patients are taken to the nephrology ultrasound suite in the same pre-operative area at around 7:30 am. Their blood pressure, EKG and pulse-oximetry are monitored throughout the procedure. Patients are placed in a prone position on the table.
Real-time ultrasound-guided renal biopsy
Patients are placed in a prone position on the table. The nephrology fellow supervised by a nephrology attending performs an initial diagnostic bilateral renal ultrasound with and without color Doppler. Length and depth are measured and any abnormalities are dictated.
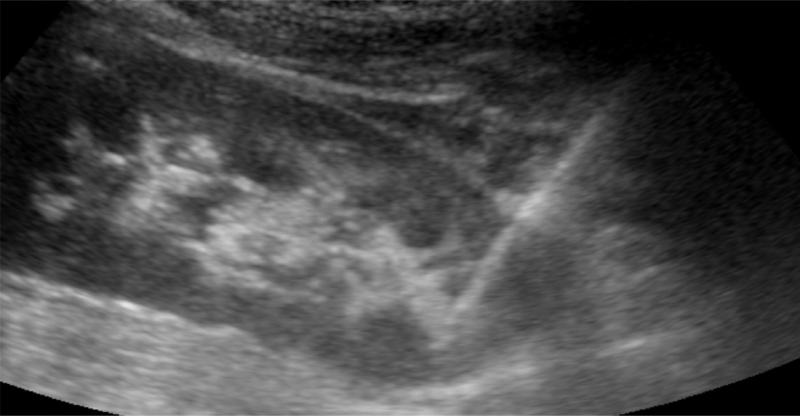
The skin is prepped with antiseptic solution and draped in the customary fashion. A sterile cover is placed over the ultrasound probe and the lower pole of the kidney visualized. The skin and subcutaneous tissue are anesthetized with lidocaine, and a small incision is made in the skin. A Nephrology Fellow performs the biopsy while the attending nephrologist holds the ultrasound probe for real-time guidance. The automated biopsy gun (Bard, 16 or 18 gauge (10, 16 or 20 cm)) with a sampling length of 22 mm is selected. The 16-gauge, 16 cm with 22 mm sample length is used most frequently. Under real-time ultrasound guidance the biopsy needle gun is advanced. Once it is close to the renal capsule, the gun is fired with the patient holding his or her breath (Figure 1). The biopsy needle is retrieved and the specimen placed in a media container and sent to surgical pathology. An average of one to two passes are performed per patient.
Figure 1.
Ultrasound image shows the biopsy needle going into the lower pole of the kidney.
Post-renal biopsy monitoring
A post-biopsy color Doppler ultrasound imaging is obtained immediately following for the biopsy to exclude active bleeding and at 5 minutes. Patients are taken and monitored in the pre-operative area and kept at bed rest for 8 hours, with blood pressure and heart rate recorded every 30 minutes. All voided urine samples are saved and inspected for gross hematuria. Hematocrit and hemoglobin values are measured at 4 and 8 hours following the biopsy. A second ultrasound or CT scan is obtained if the patient developed hypotension, gross hematuria or abdominal pain. If active bleeding is visualized, the patient undergoes selective renal artery embolization by Interventional Radiology. Blood transfusions were provided when clinically indicated. Patients are discharged after 8 hours of close monitoring and if their hematocrit and hemoglobin levels are stable. They are admitted for observation if a complication occurs. All of them are followed within 2 to 4 weeks by their nephrologists in the outpatient clinic.
Data Analysis
Approval was obtained from the UAB Institutional Review Board to review the patients’ medical records for research purposes. The prospective database included demographic and clinical features, laboratory values, biopsy complications, and diagnostic or therapeutic procedures to manage hemorrhagic complications. In addition, the surgical pathology reports were used to ascertain the adequacy of renal tissue and pathologic diagnosis.
Statistical analysis
Clinical characteristics and outcomes were recorded.
Results
The clinical and laboratory characteristics of the patients are in Table 1. The patient demographics were as follow: 55% African Americans; 60% female; 17% diabetic; 65% hypertensive; mean BMI 29.8±7 (range, 17 to 51). The outcomes are summarized in Table 2. The depth of the kidney from the skin was 6.8±2.3 cm (range, 3 to 13.5 cm). Fifty-three (53) patients required only 1 biopsy pass and 38 needed 2 passes, 5 needed 3 passes and 4 needed 4 passes, with 12.7±9.7 glomeruli obtained per patient, the tissue specimen was adequate in all patients undergoing a real-time ultrasound-guided renal biopsy. No major complications were encountered. A small perinephric hematoma (< 2×2 cm) was observed post-biopsy in 13 patients (13%), which goes along with the literature. There was no need for vascular intervention or transfusion in any patient. Four patients (4%) were hospitalized for overnight observation due to a decrease in their hematocrit (> 4 from baseline), but none required a transfusion or intervention. No patient required subsequent hospitalization due to late biopsy-related complications.
Table 1.
Clinical features
| N pts | 100 |
| Age, years | 41.93±15.37 |
| Sex, N (%) male | 40 (40%) |
| Race, N (%) white | 45 (45%) |
| Diabetes, N (%) | 17 (17%) |
| Hypertension, N (%) | 65 (65%) |
| Peripheral Vascular Disease, N (%) | 8 (8%) |
| Coronary Artery Disease, N (%) | 4 (4%) |
| Systolic BP (mmHg) | 132±15 |
| Diastolic BP (mmHg) | 85±10 |
| BMI (kg/m2) | 29.8 ±7 |
| Obesity (BMI >30 kg/ m2), N (%) | 43 (43%) |
| International normalized ratio (INR) | 0.99±0.08 |
| Pre-biopsy Platelets | 262,700±83,270 |
| Pre-biopsy hematocrit (%) | 36.02±6.3 |
| Pre-biopsy hemoglobin (g/dl) | 12.19±2.11 |
| Pre-biopsy serum creatinine (mg/dl) | 1.55±1.093 |
Table 2.
Outcomes
| N pts | 100 |
| Depth of kidney | 6.83±2.3 cm |
| Number of passes | 1.6±0.8 |
| Number of pieces | 1.24±0.5 |
| Number of glomeruli | 12.7±9.7 |
| Was tissue obtained in all patients? | 100 % |
| Patients admitted for observation | 4 (4%) Decrease in hct |
| Small perinephric hematomas | 13 (13%) |
| Large hematoma | 0 (0%) |
| Vascular intervention | 0 (0%) |
| Transfusion TX | 0 (0%) |
| Death | 0 (0%) |
Hematocrits were measured prior to each biopsy and at 4 and 8 hours post-biopsy. There was a drop in hematocrit from the baseline value from 36±6.3 to 34.2±5.8 at 4 hours but remained stable at 34.3±5.9 at 8 hours. The hemoglobin value dropped by 0.6 g/dL from 12.2±2.1 to 11.6±2.0 (Tables 3 and 4).
Table 3.
Hematocrit and hemoglobin follow-ups
| US-guided biopsy | |
|---|---|
| N pts | 100 |
| Pre-biopsy Hct | 36.02±6.31 |
| 4 hours post-biopsy | 34.21±5.82 |
| 8 hours post-biopsy | 34.34±5.94 |
| Pre-biopsy Hg | 12.19±2.11 |
| Final Hg | 11.65±1.96 |
Table 4.
Hematocrit and hemoglobin follow-ups
| Pre-biopsy Hct | 8 hours post-biopsy | P value |
|---|---|---|
| 36.02±6.31 | 34.34±5.94 | 0.054 |
| Pre-biopsy Hg | Final Hg | |
| 12.19±2.11 | 11.65±1.96 | 0.063 |
Discussion
The present study evaluates the outcomes of renal biopsies performed at a single institution under a strict standardized outpatient protocol. We found that real-time ultrasound-guided renal biopsies with a proper and rigorous outpatient protocol can be safely performed. Although the study was not randomized, it was performed prospectively with a proper follow-up of the patients.
The main reason for overnight stay in the hospital is basically as a safety net in case there is a major complication (Marwah DS, Korbet SM). The major complication, which one could encounter, is severe bleeding causing a large retroperitoneal hematoma. This complication can be catastrophic and should be addressed immediately by performing a selective renal arteriogram with embolization of the bleeding arteriole, which will infarct a small portion of the kidney. This complication is in the order of 0 to 6 % depending on the author (Marwah DS, Korbet SM) and (Whittier WL, Korbet SM) and (Maya ID Allon M) and (Doyle AJ, Gregory MC) and (Cozens NJ, Murchison JT) and (Hergessel O, Felten H) and (Burstein DM, Korbet SM); the reasons for these differences are not cleared but may be related to the technique used (blind vs. ultrasound guided biopsy), operator experience, gauge of the biopsy needle and the number of passes. We demonstrated lower frequency of hemorrhagic complications with real-time ultrasound-guided biopsies, as compared with blind biopsies (Maya ID, Allon M). Some authors believe that patients are still at risk for type complication beyond the 8 hours observation post-biopsy; we hypothesize that under a controlled environment (see standardized protocol) and a proper technique (real-time ultrasound) we can minimize this risk and be able to have the renal biopsy performed as an outpatient procedure. Also, the use of color Doppler post-biopsy surveillance enhances one's confidence that there is no major bleeding. In our institution, first-year fellows perform all renal biopsies (relatively inexperienced operators). Thus, one might expect a higher frequency of complications. However, that is not the case and it has been demonstrated not only on this study, but also in our prior study (Maya ID, Allon M). The use of the real-time ultrasound-guided technique minimizes the risk of major complications even in the hands of inexperienced operators. We documented that the lower risk of hemorrhagic complications with the ultrasound-guided technique may be attributable to the use of smaller biopsy needles (18-gauge), but in the present study the majority of the biopsies were performed with a 16-gauge needles with similar results.
Few published studies have addressed the important issue of safety and length of stay after a percutaneous renal biopsy. Some authors documented this procedure to be safe in the outpatient setting and some authors disagree. Marwak and Korbet reported on 394 patients and concluded that less than 8-hour period observation can miss up to 20% of complications (Marwah DS, Korbet SM). In this study all complications (minor and major) were accounted together, only 42% of the patients had the biopsy performed with an automated gun, the rest were performed with a manual biopsy device and all biopsies were performed with 14-gauge needles and there was no report on how many passes were performed. They timed the major complications, which accounted for 24 out of a total of 394 biopsies (6.6%), and reported that 19 of them were observed before the 8-hour mark. Thus, only 5 major complications were captured after 8 hours of observation. Whittier and Korbet re-evaluated the data and reported a series of 750 patients, in which they added the patients from the prior study (Whittier WL, Korbet SM). This time, they concluded that less than 8-hour period of observation was not optimal and they reported that it could miss up to 33% of complications. Again, all complications (minor and major) were placed in the same category. Out of 750 biopsies, 45 had a major complication (6.6%). Thirty of them were diagnosed before 8-hours of observation, the other 15 were diagnosed between 9 to 24 hours. On the contrary, there at least four studies showing different results. Farazier and Fairley reported only minor complications in a series of 118 patients (only 2 patients) (Fraser IR, Fairley KF). Oviasu E and ugdodaga P, from Nigeria reported in no complications in a series of 20 patients (Oviasu E, Ugbodaga P). Murphy BF et al, had similar data (Murphy BF, MacIsaac A). Bairy M et al, reported on 178 outpatient renal biopsies and reported no major complications with only 13.2% of minor complications to include 4 patients with gross hematuria, 16 patients with small perinephric hematomas and 3 with both hematuria and hematoma (Bairy M AJKD 2008). No interventions were needed and only two patients stayed over night. The current study shows similar results.
Minor complications including small perinephric hematomas and transient gross hematuria are more frequent. Most perinephric hematomas resolve within weeks, the produce no symptomatology and they do not need any intervention. The present study shows that 13% of the patients developed a small hematoma (< 2 by 2 cm) at the time of the biopsy, none of them had transient gross hematuria. The hematomas were observed almost immediately or at the time of the color Doppler ultrasound surveillance exam. None of them required further intervention.
It is a fact that the more passes on the average, the higher the risk for active bleeding. We averaged 1.6±0.8 passes with good adequate renal tissue yield and 100% accuracy. Renal adequate tissue by real-time ultrasound runs between 93 to 100% as compared with a 79% frequency with the blind technique. In our study we obtain 100% adequate tissue. The findings of the current study are consistent with those reported by other investigators. It has been reported that smaller gauge needle sizes (16 or 18) and the use of automated biopsy guns decrease the rate of major complications.
In conclusion, outpatient, real-time, ultrasound-guided percutaneous renal biopsy is safe and effective, and minimizes the need for post-biopsy hospitalization. Outpatient percutaneous renal biopsy can result in significant cost savings without exposing the patients to increased risk of complications.
Acknowledgments
This study was sponsored in part by a NKF Young Investigator Award.
Footnotes
Disclaimer: Portion of this study was presented in the form of a poster at the American Society of Nephrology (ASN) meeting held in Philadelphia November 2008.
References
- Fraser IR, Fairley KF. Renal biopsy as an outpatient procedure. Am J Kidney Dis. 1995;25:876–878. doi: 10.1016/0272-6386(95)90569-3. [DOI] [PubMed] [Google Scholar]
- Oviasu E, Ugbodaga P. Evaluation of percutaneous renal biopsy as a daycase procedure: experience from Nigeria. J Nephrol. 1998;11:246–248. [PubMed] [Google Scholar]
- Murphy BF, MacIsaac A. Percutaneous renal biopsy as a day-patient procedure. Am J Kidney Dis. 1989;14:77. doi: 10.1016/s0272-6386(89)80100-3. [DOI] [PubMed] [Google Scholar]
- Whittier WL, Korbet SM. Timing of complications in percutaneous renal biopsy. J Am Soc Nephrol. 2004;15:142–147. doi: 10.1097/01.asn.0000102472.37947.14. [DOI] [PubMed] [Google Scholar]
- Marwah DS, Korbet SM. Timing of complications in percutaneous renal biopsy: what is the optimal period of observation? Am J Kidney Dis. 1996;28:47–52. doi: 10.1016/s0272-6386(96)90129-8. [DOI] [PubMed] [Google Scholar]
- Cozens NJ, Murchison JT, Allan PL, Winney RJ. Conventional 15 G needle technique for renal biopsy compared with ultrasound-guided spring-loaded 18 G needle biopsy. Br J Radiol. 1992;65:594–597. doi: 10.1259/0007-1285-65-775-594. [DOI] [PubMed] [Google Scholar]
- Burstein DM, Korbet SM, Schwartz MM. The use of the automatic core biopsy system in percutaneous renal biopsies: A comparative study. Am J Kidney Dis. 1993;22:545–552. doi: 10.1016/s0272-6386(12)80927-9. [DOI] [PubMed] [Google Scholar]
- Dowd PE, Mata JA, Crow A, Culkin DJ, Venable DD. Ultrasound guided percutaneous renal biopsy using an automatic core biopsy system. J Urol. 1991;146:1216–1217. doi: 10.1016/s0022-5347(17)38049-7. [DOI] [PubMed] [Google Scholar]
- Doyle AJ, Gregory MC, Terreros DA. Percutaneous native renal biopsy: comparison of 1.2 mm spring-driven system with a traditional 2-mm hand-driven system. Am J Kidney Dis. 1994;23:498–503. doi: 10.1016/s0272-6386(12)80370-2. [DOI] [PubMed] [Google Scholar]
- Hergessel O, Felten H, Andrassy K, Kuhn K, Ritz E. Safety of ultrasound-guided percutaneous renal biopsy - retrospective analysis of 1090 consecutive vases. Nephrol Dial Transplant. 1998;13:975–977. doi: 10.1093/ndt/13.4.975. [DOI] [PubMed] [Google Scholar]
- Donovan KL, Thomas DM, Wheeler DC, Macdougall IC, Williams JD. Experience with a new method for percutaneous renal biopsy. Nephrol Dial Transplant. 1991;6:731–733. doi: 10.1093/ndt/6.10.731. [DOI] [PubMed] [Google Scholar]
- Maya ID, Madella P, Barker J, Allon M. Percutaneous renal biopsy: comparison of blind and real-time ultrasound-guided technique. Seminars in dialysis. Jul-Aug. 2007;20(4):355–8. doi: 10.1111/j.1525-139X.2007.00295.x. [DOI] [PubMed] [Google Scholar]