Abstract
STUDY QUESTION
Can human Sertoli cells cultured in vitro and that have formed an epithelium be used as a model to monitor toxicant-induced junction disruption and to better understand the mechanism(s) by which toxicants disrupt cell adhesion at the Sertoli cell blood–testis barrier (BTB)?
SUMMARY ANSWER
Our findings illustrate that human Sertoli cells cultured in vitro serve as a reliable system to monitor the impact of environmental toxicants on the BTB function.
WHAT IS KNOWN ALREADY
Suspicions of a declining trend in semen quality and a concomitant increase in exposures to environmental toxicants over the past decades reveal the need of an in vitro system that efficiently and reliably monitors the impact of toxicants on male reproductive function. Furthermore, studies in rodents have confirmed that environmental toxicants impede Sertoli cell BTB function in vitro and in vivo.
STUDY DESIGN, SIZE AND DURATION
We examined the effects of two environmental toxicants: cadmium chloride (0.5–20 µM) and bisphenol A (0.4–200 µM) on human Sertoli cell function. Cultured Sertoli cells from three men were used in this study, which spanned an 18-month period.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Human Sertoli cells from three subjects were cultured in F12/DMEM containing 5% fetal bovine serum. Changes in protein expression were monitored by immunoblotting using specific antibodies. Immunofluorescence analyses were used to assess changes in the distribution of adhesion proteins, F-actin and actin regulatory proteins following exposure to two toxicants: cadmium chloride and bisphenol A (BPA).
MAIN RESULTS AND THE ROLE OF CHANCE
Human Sertoli cells were sensitive to cadmium and BPA toxicity. Changes in the localization of cell adhesion proteins were mediated by an alteration of the actin-based cytoskeleton. This alteration of F-actin network in Sertoli cells as manifested by truncation and depolymerization of actin microfilaments at the Sertoli cell BTB was caused by mislocalization of actin filament barbed end capping and bundling protein Eps8, and branched actin polymerization protein Arp3. Besides impeding actin dynamics, endocytic vesicle-mediated trafficking and the proper localization of actin regulatory proteins c-Src and annexin II in Sertoli cells were also affected. Results of statistical analysis demonstrate that these findings were not obtained by chance.
LIMITATIONS, REASONS FOR CAUTION
(i) This study was done in vitro and might not extrapolate to the in vivo state, (ii) conclusions are based on the use of Sertoli cell samples from three men and (iii) it is uncertain if the concentrations of toxicants used in the experiments are reached in vivo.
WIDER IMPLICATIONS OF THE FINDINGS
Human Sertoli cells cultured in vitro provide a robust model to monitor environmental toxicant-mediated disruption of Sertoli cell BTB function and to study the mechanism(s) of toxicant-induced testicular dysfunction.
Keywords: human Sertoli cell, testis, F-actin, cell adhesion protein, spermatogenesis
Introduction
About 15% of couples in the USA are infertile (http://www.mayoclinic.com/health/infertility/DS00310), and about 48.5 million couples worldwide are also infertile (Mascarenhas et al., 2012). Among these couples, men are the sole or contributory infertility factor in 50% of cases (Smith et al., 2010). It is increasingly clear that environmental toxicants could be one of the major causes of infertility in both men and women (Hunt et al., 2009; Cheng et al., 2011; Marques-Pinto and Carvalho, 2013) and are implicated in declining male fertility (Benoff et al., 2000; Cheng et al., 2011; Mruk and Cheng, 2011b; Marques-Pinto and Carvalho, 2013). Recent reports that track semen quality (e.g. sperm concentrations) among men in multiple countries for decades and/or years have demonstrated a declining trend in both developing and developed countries (Nordkap et al., 2012; Rolland et al., 2013), suggesting that toxicants may be one of the contributing factors. However, public concern about the disruptive effects of environmental chemicals (e.g. pesticides, polychlorinated biphenyls, food additives) on male reproductive health is not supported by available data in humans (Swan et al., 2000; Bonde et al., 2008; Sharpe, 2010; Toft et al., 2012). It has also been shown that environmental toxicants that induce male reproductive dysfunction exert their effects at multiple levels (Heindel, 2006; Hauser, 2008; Hunt et al., 2009; Siu et al., 2009a; Mruk and Cheng, 2011b; Wong and Cheng, 2011; Wan et al., 2013e). Indeed, Sertoli cells, in particular testis-specific cell junctions at the Sertoli-Sertoli and Sertoli-germ cell interface, are an emerging target for toxicant effects (Wong et al., 2010; Mruk and Cheng, 2011b; Wong and Cheng, 2011; Qiu et al., 2013; Wan et al., 2013c). Since virtually all earlier studies that examined the impact of environmental toxicants on male reproductive functions were based on in vivo or in vitro rodent models (Siu et al., 2009a; Skinner et al., 2010; Cheng et al., 2011; Vandenberg et al., 2012; Sengujpta, 2013; Wan et al., 2013c), these findings may not be directly applicable to humans. In fact, a number of significant differences between species have been documented including variation in the timing of Sertoli cell proliferation, the effects of androgen suppression, seasonal variability and the responses to toxins (Brown et al., 1995; Apostoli et al., 1998; Young and Nelson, 2001; Sharpe et al., 2003). Sertoli cell proliferation is thought to occur in two periods in all species, one during fetal and neonatal life, and a second in the peripubertal period. However, in some species, one period is most important, such as in rhesus monkeys where proliferation mainly occurs in the peripubertal period and in the rat where proliferation in the neonatal period (the timing of which overlaps with the peripubertal period) predominates. Both periods are important in humans where they are separated by more than a decade (Sharpe et al., 2003). In mammals, the efficacy of spermatogenesis is vastly different between species. For instance, only ∼3 spermatids are formed per differentiated A1 spermatogonium in humans versus 97, 51 and 39 in rats, dogs and rabbits, respectively (Ehmcke and Schlatt, 2006; Hess and de Franca, 2008). Also, there are data showing that the correlation of male reproductive toxicity in animals and in humans is often poor. Moreover, widely varying sensitivities to male reproductive toxins have been reported even within the same species, for example, in different strains of rats to effects of lead exposure (Apostoli et al., 1998). No mixture of cultured cells can replicate the biochemical and physiologic processes of a whole body but due to the ethical limitations of testing on human volunteers, animals are the only option for testing in whole organisms. To increase the accuracy of toxicology testing the use of ex-vivo or 3-dimensional in vitro model systems composed of normal human cells, particularly human stem cells, has been proposed (Trosko, 2010). Thus, there is a need to develop in vitro methods, such as a reliable human Sertoli cell in vitro culture system, to study testicular toxicity and to detect Sertoli cell toxicants in a way that is relevant to their effects in vivo.
A population of human Sertoli cells isolated from deceased normal males remains proliferative when cultured in vitro if fetal bovine serum (FBS) is included in the medium (Ahmed et al., 2009; Chui et al., 2011). Importantly, this population of cells has been extensively characterized with specific Sertoli cell markers to positively identify putative and highly purified human Sertoli cells via flow cytometry, electron microscopy and fluorescence microscopy. Functionally, the ability of Sertoli cells to form tight junction (TJ)-permeability barriers that mimic the Sertoli cell blood–testis barrier (BTB) in vivo has also been reported (Chui et al., 2011). We sought to examine whether human Sertoli cells cultured in vitro can be used to assess the effects of two environmental toxicants cadmium and bisphenol A (BPA) on the integrity of cell junctions at the Sertoli cell–cell interface. We also provide a mechanistic basis for how these two toxicants impede Sertoli cell junction integrity. Since a similar in vitro system using rodent Sertoli cells has been extensively characterized (Byers et al., 1986; Janecki et al., 1991, 1992; Grima et al., 1992; Lui et al., 2001; Kaitu'u-Lino et al., 2007; Yan et al., 2008; Nicholls et al., 2009; Siu et al., 2009b; Lie et al., 2012; Su et al., 2012c), and reproduces studies performed in vivo (Su et al., 2012b,c), we reasoned that this model is a physiologically relevant one.
Materials and Methods
Human Sertoli cell cultures
Human Sertoli cells obtained from 3 different deceased donors (12-, 23- and 36-year old) as previously described (Chui et al., 2011) were from MandalMed (San Francisco, CA, USA), which also offers the cells for research through Lonza (Walkersville, MD, USA). Human Sertoli cells were shipped on dry ice and stored in liquid nitrogen upon arrival. Cryopreserved cells were thawed in a 37°C water bath and transferred immediately to 100 mm culture dishes. All culture dishes were coated with 2 μg/cm2 human fibronectin (BD Biosciences). Fibronectin was prepared as a 1 mg/ml stock in sterile deionized water and diluted in PBS [10 mM NaH2PO4 containing 0.15 M NaCl, pH 7.4 at 22°C] according to the instructions supplied by the manufacturer, as described previously (Chui et al., 2011). Sertoli cells were propagated in Dulbecco's Modified Eagle's Medium/Ham's F12 Nutrient Mixture (DMEM/F12) (Sigma-Aldrich) supplemented with 5% (v/v) fetal bovine serum (FBS, Invitrogen) and penicillin (100 units/ml)/streptomycin (100 μg/ml) (Sigma-Aldrich), and incubated at 35°C in a CO2-incubator with 5% CO2–95% air (v/v) in a humidified atmosphere. The growth medium was changed every 3–4 days. When required for further use, such as subculture or cryopreservation, cells about to reach 70–80% confluence were removed from the culture substrate by treatment with trypsin (0.05%)-EDTA (0.02%) solution (Sigma-Aldrich) for 2 min so that human Sertoli cells were re-suspended in medium and were collected (100 g, 5 min, room temperature). Human Sertoli cells cultured in the presence of 5% FBS in F12/DMEM remained mitotically active as described (Ahmed et al., 2009; Chui et al., 2011), and the cells used for all the experiments reported herein were from the fourth or fifth passage to ensure reproducibility. Sertoli cells were plated at an initial density at about 25% of the targeted cell density on fibronectin-coated coverslips (targeted cell density 5 × 104 cells/cm2) or 12- or 24-well dishes (targeted cell density 1 × 105 cells/cm2), respectively, to be used for immunofluorescence analysis or immunoblotting. Cell density was monitored and assessed by cell counts using a hematocytometer following trypsinization in a control well or coverslip in duplicates. Under these culture conditions, the doubling time of human Sertoli cells is ∼4 days in F12/DMEM with 5% FBS and penicillin/streptomycin. It routinely took ∼10 days to reach subculture readiness for specific experiments. For cryopreservation, human Sertoli cells cultured in a 100-mm dish to ∼70% confluence were trypsinized, suspended in 9-ml F12/DMEM, washed three times (100 g, 5 min each at room temperature) and pelleted at 100 g (5 min). Next, the cells were resuspended in 1 ml of freezing medium (5% DMSO, 25% F12/DMEM, 70% FBS, no antibiotics) in a cryovial. Cryovials were placed immediately in an isopropanol chamber and stored at −80°C overnight, before transfer to liquid nitrogen for long-term storage.
Treatment of human Sertoli cell cultures with environmental toxicants
When Sertoli cells reached 70–80% confluence, cells were serum-starved for 5 h to quench signaling pathways before treatment. Thereafter, cells were treated with vehicle control (0.1% ethanol), 0.5–20 μM CdCl2 (i.e. 0.09–3.7 µg/ml) or 0.4–200 μM BPA (i.e. 0.09–46 µg/ml) in F12/DMEM supplemented with 5% FBS and antibiotics. These selected concentrations of CdCl2 and BPA were based on initial pilot experiments in which a phenotype was detected after exposure. Observed changes were: (i) localization and/or distribution of integral membrane proteins at the Sertoli cell–cell interface, (ii) organization of F-actin in Sertoli cells and/or (iii) steady-state levels of proteins of the adhesion protein complexes. It is noted that human serum/plasma levels of cadmium (Pollack et al., 2011; Chen et al., 2013) and BPA (Sprague et al., 2013; Wan et al., 2013a) are <1 ng/ml and ∼1 ng/ml, respectively. It is estimated that human daily intake (orally, via food and drink) of BPA and cadmium is 34 ng/kg body weight and 1.06 µg/kg body weight, respectively (Wan et al., 2013e). While the doses used herein are acute so that phenotypes were detected based on pilot experiments for this mechanistic study, however, the half-life of cadmium and BPA in humans are >20 years and 2 h, respectively (Wan et al., 2013e). As such, high and toxic level of toxicants, in particular cadmium, can be accumulated in specific organs (e.g. the testis) over an extended period (Siu et al., 2009a; Wan et al., 2013e). Furthermore, evidence is emerging that challenges the assumption that humans metabolize BPA with a half-life of under 2 h, quickly enough to render it undetectable in blood and therefore decrease its harmful effects (Vandenberg et al., 2013). For instance, a high serum level of BPA has been reported to be associated with elevated breast density, an index of breast cancer risk, in post-menopausal women (Sprague et al., 2013).
RT–PCR and immunoblot analysis
For RNA extraction and protein lysate preparation, human Sertoli cell cultures (density ∼1 × 105 cells/cm2) on fibronectin-coated 12- or 24-well dishes (each well contained 2.5- or 1.5-ml medium, respectively) were terminated following exposure to a specified toxicant for 2 days. For RNA extraction, cells were harvested in TRIzol® Reagent (Invitrogen) according to manufacturer's instructions. For protein lysate preparation, media were removed and cells were treated with lysis buffer (10 mM Tris, pH 7.4 at 22°C containing 0.15 M NaCl, 1% NP-40 [v/v] and 10% glycerol [v/v]. Protease and phosphatase inhibitors were added immediately before use at a 1:100 dilution in a final volume of ∼200–300 µl. RT–PCR was carried out as described earlier (Xiao et al., 2011). Two primer pairs (Gene Link) were used for detection of the occludin gene in cultured human Sertoli cells (Table I). For immunoblotting, total protein concentration was determined using the Bio-Rad DC protein assay kit. Immunoblot analysis was performed using ∼15–25 µg protein per lane as described previously (Xiao et al., 2011, 2013b) using a Fujifilm LAS-4000 mini imaging system, and a chemiluminescence detection kit prepared in our laboratory (Mruk and Cheng, 2011a). Table II lists the antibodies and conditions used for immunoblotting experiments in this study.
Table I.
Human occludin-specific primers used for RT–PCRa.
| Primer sequence | Orientation | Position | Size (bp) | Tm (°C) | Reference |
|---|---|---|---|---|---|
| 5′-GACCCAAGAGCAGCAA-3′ | Sense | 833–848 | 688 | 50.5 | NC_018921.2/NM_001205254.1 |
| 5′-CATCCACAGGCGAAGT-3′ | Antisense | 1505–1520 | |||
| 5′-TTTGTGGGACAAGGAACACA-3′ | Sense | 1273–1292 | 312 | 53.8 | NC_018921.2/NM_001205255.1 |
| 5′-GCAGGTGCTCTTTTTGAAGG-3′ | Antisense | 1565–1584 |
aRT–PCR was performed by using a GeneAmp 2400 PCR system (Applied Biosystems, Inc.), Tm (°C), annealing temperature and 30 cycles.
Table II.
Antibodies used for different experiments in this report.
| Antibody | Host species | Vendor | Catalog number | Application(s)/dilution(s) |
|---|---|---|---|---|
| ZO-1 | Rabbit | Invitrogen | 61-7300 | IB (1:250), IF (1:100) |
| N-Cadherin | Rabbit | Santa Cruz Biotechnology | sc-7939 | IB (1:200), IF (1:100) |
| Mouse | Invitrogen | 33-3900 | IB (1:250) | |
| β-Catenin | Rabbit | Invitrogen | 71-2700 | IB (1:250), IF (1:100) |
| Actin | Goat | Santa Cruz Biotechnology | sc-1616 | IB (1:200) |
| Arp3 | Mouse | Sigma-Aldrich | A5979 | IB (1:3000), IF (1:200) |
| Src | Mouse | Santa Cruz Biotechnology | sc-8056 | IB (1:200), IF (1:100) |
| Annexin II | Rabbit | Santa Cruz Biotechnology | sc-9061 | IB (1:200) |
| Mouse | BD Transduction laboratories | 610068 | IB (1:1000), IF (1:100) |
IB, immunoblotting; IF, immunofluorescence analysis.
It is noted from the manufacturers that these antibodies cross-reacted with the corresponding proteins in humans.
Immunofluorescence analysis
For immunofluorescence analysis, Sertoli cells were plated at a density of ∼2.5–5 × 104 cells/cm2 on fibronectin-coated round glass coverslips (18-mm, diameter) in 12-well dishes with each well containing 2-ml F12/DMEM with 5% FBS. After 2 days of exposure to a specified toxicant, immunofluorescence analysis was performed (Xiao et al., 2011, 2012a) by using Alexa Fluor® Dyes 488- or 555-conjugated secondary antibodies (Invitrogen). Sertoli cells at ∼0.025–0.05 × 106 cells/cm2 on fibronectin-coated coverslips were fixed in methanol at −20°C for 5 min. For F-actin staining, cells were fixed in 4% paraformaldehyde (w/v) in PBS at room temperature for 10 min, followed by incubation with rhodamine-phalloidin (Invitrogen, Eugene, OR, USA). Images were captured using a Nikon Ds-Qi1Mc-U2 camera in a Nikon 90i motorized fluorescence microscope, and acquired using the NIS-Elements AR software (v3.2; Nikon Instruments, Inc.), and compiled in Adobe Photoshop in Adobe Creative Suite CS 3.0 (Adobe Systems). Table II lists the antibodies and conditions used for all immunofluorescence-staining experiments in the study. The immunofluorescence analysis was repeated at least three times using different batches of human Sertoli cells. Results were similar each time and data from a representative experiment are presented. Pilot experiments that were used to establish the necessary experimental conditions including changes in phenotypes were excluded from this analysis. All the samples used to compare the treatment groups versus the corresponding controls were processed in a single experimental session to avoid inter-experimental variation.
Cytotoxicity assay
Cells were seeded at a density of 0.5–1 × 105 cells/cm2 in 96-well plates and treated with the corresponding toxicant at specified concentrations for 1, 2 or 3 day(s). Cell proliferation kit II (XTT, sodium 3′-[1-(phenylaminocarbonyl)-3,4-tetrazolium]-bis (4-methoxy-6-nitro) benzene sulfonic acid hydrate) (Roche) was used for quantification of cytotoxicity as described (Li et al., 2009). The XTT solution with a final concentration 0.3 mg/ml was added to each well 24 h prior to absorption measurements, which were done with microplate reader (Model 680, Bio-Rad) at a wavelength of 450 nm with the reference wavelength set at 655 nm.
Image analysis
Immunoblot data were quantified by using Scion Image (v4.0.3.2; Scion Corporation; http://scion-image.software.informer.com/) as described (Xiao et al., 2011). To obtain semi-quantitative data from immunofluorescence images for analysis, fluorescence signals: (i) in human Sertoli cells such as red fluorescence for F-actin visualized by rhodamine-phalloidin, or (ii) at the Sertoli cell–cell interface such as tight junction adaptor protein ZO-1 and basal ectoplasmic specialization (ES) proteins N-cadherin and β-catenin, were quantified by ImageJ (Version 1.45, U.S. National Institutes of Health, Bethesda, MD, USA; http://rsbweb.nih.gov/ij) in micrographs without the DAPI overlay to avoid interference. The fluorescence intensity of a target protein in control cells was arbitrarily set at one against which statistical analysis was performed. Comparisons among multiple experimental groups were performed by two-way analysis of variance (ANOVA), using the repeated measures model followed by Dunnett's test to compare changes between treatment groups and their corresponding controls, and P-value of <0.05 was taken as statistically significant. About 50 cells were randomly selected in each coverslip, and at least two coverslips were examined in each experiment; and data from three experiments were pooled and analyzed with n = 300 cells.
Statistical analysis
Each experiment used human Sertoli cells from a specific donor (Table III). In each experiment, triplicate dishes (e.g. for immunoblotting, XTT cytotoxicity assay) or microscopic slides (e.g. for immunofluorescence analysis) were used to collect data. Each data point expressed as a mean ± SD of n = 3 independent experiments, and each experiment equates to a different Sertoli cell donor (see Table III). It is noted that pilot experiments performed to optimize the experimental conditions were not included in our statistical analysis. For image analysis of fluorescence signals, at least 300 cells were scored, and for cell adhesion proteins (e.g. N-cadherin, β-catenin, ZO-1), at least two panels per pair of adjacent cells were analyzed to assess changes in protein localization as illustrated by the white rectangles shown in Fig. 1B. For each experiment, data in treatment groups were normalized against the corresponding control, which was arbitrarily set at 1. As such, no error bars were present in controls. Two-way analysis of variance (ANOVA) using the repeated measures model followed by Dunnett's test was performed to compare changes between treatment groups and their corresponding controls using the GB-STAT statistical analysis software package (Version 7.0; Dynamic Microsystems, Silver Spring, MD, USA). This thus assessed within-experiment effects which were the focus of the analysis. P-value of <0.05 was taken as statistically significant. A bar without any annotation indicated that treatment group was not statistically significant different from its corresponding control.
Table III.
Typical conditions used for culturing the three human Sertoli cell samples for experiments reported in this study.
| Donor age (years) | Thawing of cells | Time needed to reach ∼70–80% confluency (days) |
|||||
|---|---|---|---|---|---|---|---|
| P1 | P2 | P3 | P4 | P5 | |||
| 36 | Time 0 | 16 | 24 | 8 | 63 | 14 | No FBS |
| 23 | 8 | 9 | 16 | 24 | With FBS | ||
| 12 | 11 | 14 | 21 | 53 | |||
P, passage number. For all the experiments reported herein, human Sertoli cells were used on the fourth or fifth passage. In the absence of FBS, human Sertoli cells were also proliferative, but it took longer culture for them to reach ∼70–80% confluence. Sertoli cells obtained from the 12-year-old subject are differentiated since human Sertoli cells cease to divide in vivo by puberty at 12 years of age (Sharpe et al., 2003) without exposure to serum, and Sertoli cells from this subject displayed similar morphological characteristics as of the other two subjects, and the responses of Sertoli cells from this subject to Cd and BPA were also similar to the other two subjects as reported herein (see Figs 1, 3 and 4).
Figure 1.
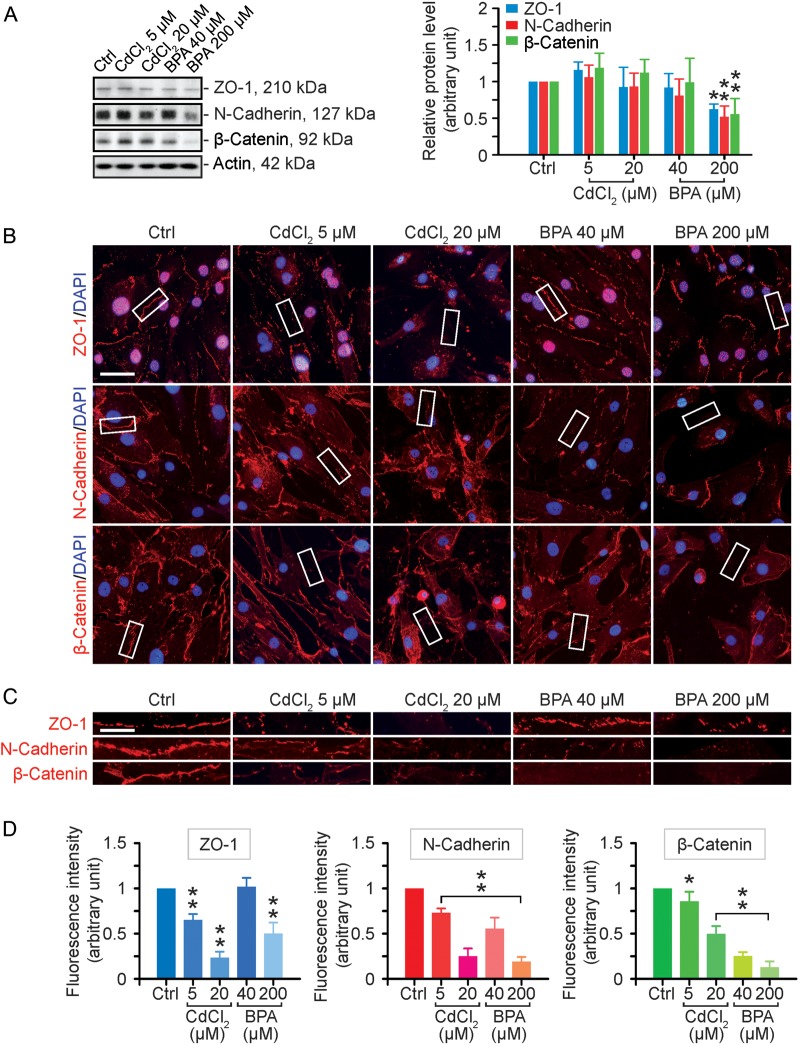
A study to assess the effects of cadmium chloride (CdCl2) and bisphenol A (BPA) on the expression and localization of cell adhesion proteins in human Sertoli cells. (A) Human Sertoli cells were cultured on fibronectin-coated dishes and exposed to CdCl2 (5 and 20 µM) or BPA (40 and 200 µM) for 2 days. Thereafter, cells were harvested for lysate preparation and for immunoblotting (left panel). The histogram in the right panel summarizes the immunoblotting data. Protein bands were densitometrically scanned, and normalized against β-actin; each bar is a mean ± SD of n = 3 independent experiments. Controls were normalized to 1.0. *P < 0.05; **P < 0.01 with treatment versus corresponding control (Ctrl) group. (B) Immunofluorescence analysis of some blood–testis barrier (BTB)-associated proteins (all in red fluorescence): zonula occludens-1 (ZO-1, a tight junction adaptor), N-cadherin (a basal ectoplasmic specialization (ES) integral membrane protein) and β-catenin (a basal ES adaptor). Cell nuclei were visualized by DAPI (4′,6-diamidino-2-phenylindole) staining. Scale bar = 40 µm. (C) Enlarged images corresponding to the white boxes in (B) of ZO-1, N-cadherin and β-catenin at the human Sertoli cell–cell interface, illustrating changes in their localization following exposure to toxicants. Scale bar = 15 µm. (D) Image analysis of the changes in the fluorescence intensity of cell adhesion proteins at the human Sertoli cell–cell interface. Each bar is a mean ± SD of three experiments including ∼100 cells per experiment. Controls were normalized to 1.0 in each experiment. *P < 0.05; **P < 0.01 with treatment versus corresponding control (Ctrl) group.
Results
Maintenance of human Sertoli cells in vitro
Nineteen experiments were performed using human Sertoli cells from three subjects (see Table III which listed 13 experiments), including 6 pilot experiments to optimize Sertoli cell culture conditions. The initial cell density in each subculture was at ∼0.01–0.2 × 105 cells/cm2, and when cells reached confluence of ∼70–80% via proliferation in ∼8–63 days, a subculture was performed, such as in P1 (Table III). In all experiments reported herein, we used cells from the fourth pass (P4) for the 23- and 12-year-old donors and fifth pass (P5) for the 36-year-old donor. Notably, even in the absence of FBS in F12/DMEM, human Sertoli cells remained proliferative, but required more time to reach ∼70–80% confluence versus cells cultured in medium with 5% FBS (Table III).
Cadmium and BPA perturb human Sertoli cell junction integrity
When human Sertoli cells were exposed to CdCl2 for 2 days, no apparent effects were found on the expression of several BTB-associated proteins in cell lysates examined by immunoblot analysis: ZO-1 (a tight junction (TJ) adaptor), N-cadherin (a basal ES integral membrane protein) and β-catenin (a basal ES adaptor protein). On the contrary, BPA at higher doses down-regulated the expression of these proteins (Fig. 1A). Since these BTB-associated proteins usually localize to the Sertoli cell–cell interface and constitute the Sertoli BTB (Cheng and Mruk, 2009, 2010), we next examined the localization pattern and/or distribution at the human Sertoli cell–cell interface following exposure to toxicants (Fig. 1B–D). While these toxicants did not perturb the expression of these proteins, they significantly altered protein localization at the Sertoli cell BTB (Fig. 1B), which can be better visualized in the enlarged images shown in Fig. 1C. In control cells, ZO-1, N-cadherin and β-catenin were localized distinctively to the human Sertoli cell–cell interface, but treatment of these cells with CdCl2 or BPA, even at low doses, induced their redistribution, mostly via re-localization from the cell surface to the cell cytosol (Fig. 1B and C). This loss of fluorescence at the cell–cell interface was semi-quantified and is shown in Fig. 1D. These findings support the notion that there are considerable changes in the localization and/or distribution of these proteins at the human Sertoli cell BTB following exposure to two environmental toxicants. Data shown in Fig. 1B suggest that cells exposed to a high dose of either toxicant appeared to be retracting their cytoplasmic processes, and thus perturbing Sertoli cell barrier function. Also, while occludin has been detected at the tight junction in human blastocysts (Ghassemifar et al., 2003), human intestinal epithelial T84 cells (Ando-Akatsuka et al., 1996) and cervical epithelial cells (Zeng et al., 2004), it was not detected in human Sertoli cells when two different primer pairs (see Table I) were used for RT–PCR amplification. The lack of occludin in human Sertoli cells reported herein is consistent with an earlier report that human occludin was not found at the Sertoli cell tight junction in human testes (Moroi et al., 1998), thus occludin was not included in our analysis.
Cytotoxicity of cadmium and BPA in human Sertoli cells
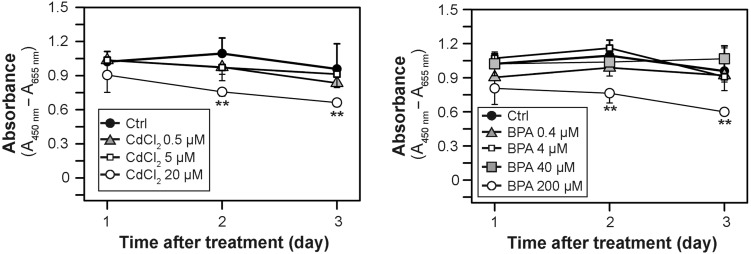
To assess whether changes observed in the expression, localization or distribution of BTB-associated proteins in the human Sertoli cell epithelium following exposure to toxicants were caused simply by cell cytotoxicity, we assessed cytotoxicity using an XTT assay. As shown in Fig. 2, following exposure of human Sertoli cells to high doses of CdCl2 and BPA at 20 µM and 200 µM, respectively, for 2 or 3 days, (but not for 1 day), mild but statistically significant cytotoxicity was noted.
Figure 2.
Cytotoxicity assay. Human Sertoli cells cultured for 1, 2 and 3 days in the presence of either CdCl2 (0.5, 5 and 20 µM) or BPA (0.4, 4 40, and 200 µM) or in the absence of both toxicants (control, Ctrl) were subjected to a cytotoxicity assay based on the reduction of the tetrazolium dye XTT. Each data point is a mean ± SD of n = 3 experiments.**P < 0.01.
Cadmium and BPA induce truncation and depolymerization of F-actin in human Sertoli cell epithelium
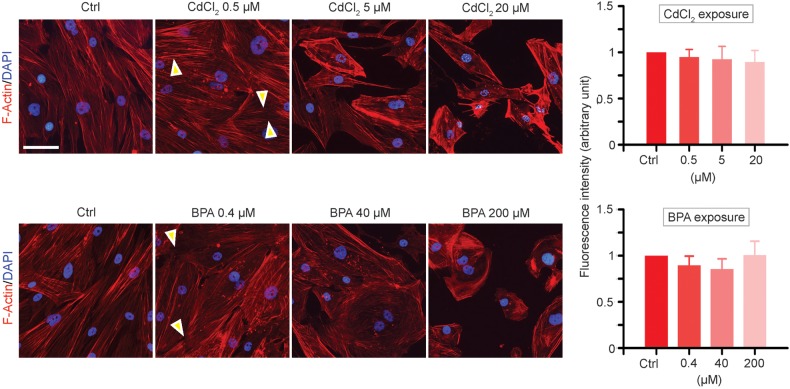
We examined changes in the distribution of the F-actin network, following exposure of human Sertoli cells to CdCl2 or BPA, by using rhodamine-phalloidin to visualize the microfilaments. In human Sertoli cells under control conditions, microfilaments of F-actin were properly aligned along the cell cytosol (Fig. 3), similar to rat Sertoli cells (Siu et al., 2009c; Xiao et al., 2011). Following exposure to 0.5 and 5 µM CdCl2 or 0.4 and 40 µM BPA, doses where no cytotoxicity was detected (Fig. 2), truncation and/or de-polymerization of actin microfilaments was clearly noted (Fig. 3). Also more F-actin was observed near the Sertoli cell surface, and localized more closely to the cell nuclei (Fig. 3), despite the fact that overall fluorescence signals of F-actin were similar in all treatment groups versus controls (Fig. 3). These findings support the notion that disorganization of F-actin in Sertoli cells in the epithelium impedes the attachment of the adhesion protein complex (e.g. N-cadherin- β-catenin) to the underlying actin-based cytoskeleton (Fig. 1B). Through this perturbation in proper localization of F-actin, an impairment of Sertoli cell BTB integrity could result.
Figure 3.
A study to assess changes in the F-actin organization in human Sertoli cells following exposure to CdCl2 or BPA. Cells were exposed to each toxicant at specified concentrations for 2 days, fixed and stained for F-actin using rhodamine-phalloidin (red fluorescence) as shown on the left panel. Following exposure to toxicants, actin microfilaments were found to be truncated and depolymerized (annotated by ‘yellow’ arrowheads). Furthermore, actin microfilaments were shown to be retrieved into the cell cytosol instead of stretching out across the cell cytosol as shown in normal control cells. At these doses, no cytotoxicity was detected at up to 3 days as shown in Fig. 2. Cell nuclei were visualized by DAPI. On the right panel based on image analysis of fluorescence signals, the overall levels of F-actin in the cells in both treatment groups versus controls were not significantly different except there were changes in distribution and/or localization as illustrated on the left panel. Scale bar = 40 µm, which applies to all other micrographs.
Cadmium and BPA induce changes in the localization and/or distribution of F-actin regulatory proteins in Sertoli cell epithelium
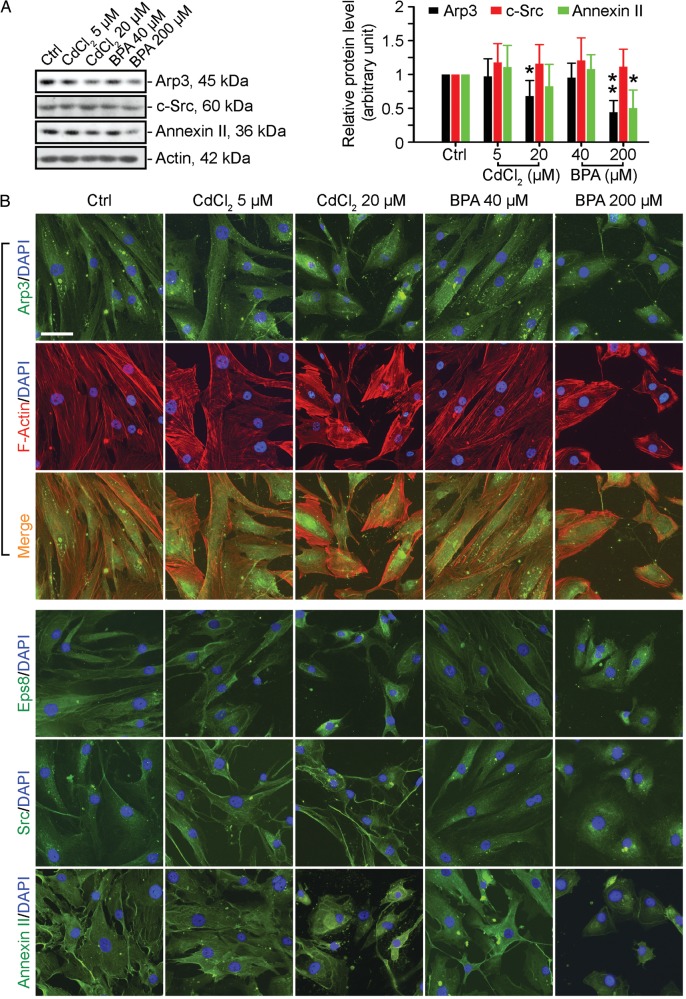
We examined the underlying mechanism by which these toxicants impede the actin-based cytoskeleton in Sertoli cells. Both ZO-1- and β-catenin-based integral membrane proteins at the cell–cell interface adhere to actin for their attachment. Earlier studies have shown that an extensive network of microfilaments creates arrays of actin bundles at the basal ES, which confer unusually high adhesive strength to the BTB. In addition, the conversion of ‘bundled’ to ‘un-bundled/branched’ configurations that likely facilitate the transport of preleptotene spermatocytes across the BTB at stage VIII of the epithelial cycle is a result of the concerted activity of the actin barbed end capping and bundling protein Eps8 (epidermal growth factor receptor pathway substrate 8) (Lie et al., 2009) and actin barbed end polymerization protein Arp3 (actin-related protein 3) (Lie et al., 2010, 2012). Furthermore, c-Src (a nonreceptor protein tyrosine kinase) and annexin II (also known as annexin A2, a putative substrate of c-Src) that are known to be involved in F-actin dynamics in mammalian epithelial cells and tissues (Frame, 2004; Tobe, 2010; Grieve et al., 2012; Xiao et al., 2012b) are also found in the testis and expressed predominantly by Sertoli cells (Lee and Cheng, 2005; Xiao et al., 2013a). Treatment of human Sertoli cells with both toxicants for 2 days had relatively little effect on the expression of these proteins (except at high doses) (Fig. 4A). However, CdCl2 at 5 and 20 µM and BPA at 200 µM caused the proteins to be re-distributed in human Sertoli cells from the cell periphery to the nuclei, with associated retraction of cytoplasmic processes (Fig. 4B) clearly illustrated in Arp3 immunostained cells dual-labeled with rhodamine-phalloidin (Fig. 4B). It is likely that the mislocalization and/or re-distribution of actin regulatory proteins perturbed the organization of F-actin in Sertoli cells (Fig. 3), thereby impeding the proper localization of cell adhesion protein complexes at the cell–cell interface (Fig. 1). Based on these findings, we propose a hypothetical model shown in Fig. 5, illustrating the likely events that lead to disruption of cell adhesion in human Sertoli cells following exposure to CdCl2 or BPA. In brief, toxicants induce disorganization of actin microfilaments, making them incapable of assuming a ‘bundled’ configuration via changes in the localization of actin bundling protein Eps8 and branched actin polymerization protein Arp3 (Fig. 5). As such, adhesion protein complexes can no longer confer proper cell adhesion, leading to BTB disruption.
Figure 4.
A study to assess changes in the localization and/or distribution of actin regulatory proteins in human Sertoli cell following exposure to CdCl2 or BPA. (A) The expression of several actin regulatory proteins, such as Apr3, c-Src and annexin II, examined by immunoblotting of lysates of human Sertoli cells was not significantly affected by CdCl2 or BPA unless a high dose was used (see left panel). These immunoblotting data are summarized in the histogram shown on the right panel. Each bar is a mean ± SD of n = 3 independent experiments. *P < 0.05; **P < 0.01 with treatment versus corresponding control (Ctrl) group. (B) Sertoli cells were exposed to either CdCl2 (5 or 20 µM) or BPA (40 or 200 µM) for 2 days before being fixed and processed for immunofluorescence analysis to visualize the distribution and/or localization of Arp3, F-actin, Eps8, Src and annexin II. Cell nuclei were visualized by DAPI staining. Scale bar = 40 µm, which applies to all other micrographs.
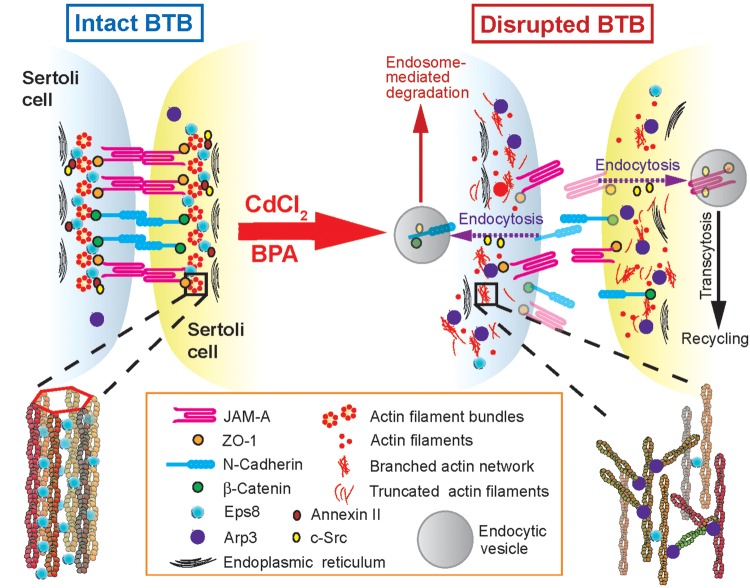
Figure 5.
A schematic drawing illustrating a cascade of events leading to the mislocalization of adhesion protein complexes at the human Sertoli cell–cell interface following exposure to the environmental toxicants CdCl2 or BPA. On the left panel, the intact BTB between adjacent Sertoli cells is maintained by a network of actin filament bundles, which is created by the actin barbed end capping and bundling protein Eps8; both c-Src and annexin II also promote the integrity of actin filament bundles, so that adhesion protein complexes such as JAM-A-ZO-1 and N-cadherin-β-catenin can properly localize to the Sertoli cell–cell interface (Fig. 1). However, following the exposure of human Sertoli cells to toxicants such as CdCl2 or BPA, there are changes in the localization and/or distribution of the actin regulating proteins Arp3, Eps8, c-Src and annexin II. These changes promote the conversion of F-actin from a ‘bundled’ to a ‘de-bundled/branched’ configuration in which microfilaments are retrieved from near the cell surface, become truncated and depolymerized, and are no longer capable of supporting the proper localization of ZO-1- and β-catenin-based adhesion protein complexes that normally confers cell adhesion between Sertoli cells, and BTB disruption results. The precise mechanism(s) and/or pathway(s) by which c-Src and/or annexin II regulates actin microfilaments remain unknown. Recent studies have shown that they may be involved in regulating the events of endocytic vesicle-mediated protein trafficking (Xiao et al., 2011) as hypothesized here (right panel).
Discussion
Human Sertoli cells, when cultured in vitro, establish a functional epithelium that is associated with a physiological, tight junction-permeability barrier that mimics the Sertoli cell BTB in vivo (Chui et al., 2011). These findings are analogous to primary rat or mouse Sertoli cells cultured in vitro which also establish a functional tight junction barrier that mimics the BTB in vivo, and this system has been widely used to study Sertoli cell BTB function in vitro (Janecki et al., 1991, 1992; Grima et al., 1992; Gye, 2003; Lui et al., 2003a; Siu et al., 2003; Chen et al., 2012; Cheng and Mruk, 2012; Du et al., 2013; Puri and Walker, 2013; Qiu et al., 2013). More important, many earlier data obtained from this system have now been expanded and confirmed in subsequent studies in vivo that ravel the biology of the BTB (Lui et al., 2003b; Wong et al., 2004; Su et al., 2012b; Wan et al., 2013b). Herein, we have shown that BTB-associated proteins, such as ZO-1, N-cadherin and β-catenin, also assemble at the human Sertoli cell–cell interface to confer cell adhesion, analogous to rodent Sertoli cells cultured in vitro (Su et al., 2011; Lie et al., 2012; Xiao et al., 2012a, 2013b). As found in the rat in which environmental toxicants, such as CdCl2, BPA and PFOS (perfluorooctanesulfonate), at non-cytotoxic concentrations were shown to induce reversible Sertoli cell tight junction disruption (Janecki et al., 1992; Siu et al., 2009c; Su et al., 2012a; Qiu et al., 2013; Wan et al., 2013d), human Sertoli cells are also susceptible to treatment with either CdCl2 or BPA.
It was long thought that mammalian Sertoli cells do not divide after puberty. For instance, Sertoli cells in rats are differentiated by ∼15–17-day post-partum (dpp) (Orth, 1982) when they cease to divide at the time the BTB begins to assemble a functional immunological barrier, coinciding with the occurrence of the first meiosis I (Bergmann and Dierichs, 1983; Russell et al., 1989; Mok et al., 2011). In humans, Sertoli cells are differentiated and cease to divide at puberty at ∼12 years of age (Sharpe et al., 2003). Thus, Sertoli cells isolated from 20-day-old rat testes are differentiated and cease to divide when cultured in chemically defined serum-free medium (Mather and Sato, 1979; Mruk and Cheng, 2011c). Recently this view has been challenged by results from several studies. Proliferation was observed in Sertoli cells in adult Djungarian hamsters that were exposed to short day photoperiod to suppress gonadotrophins and then treated with FSH, suggesting that the cells were not terminally differentiated but existed in a transitional state, exhibiting both undifferentiated and differentiated features (Tarulli et al., 2006). In mice, the proliferation of adult Sertoli cells in vitro in the absence of specific hormonal supplementation was found to be associated with a 70% decrease in expression of the cell cycle inhibitor CDKN1B (P27kip1), and a 2-fold increase in the levels of the proliferation inducer ID2 (inhibitor of DNA binding/differentiation) (Ahmed et al., 2009). We are able to isolate routinely a small number of human adult Sertoli cells from normal adult testis from cadaveric tissue that actively divide in culture in the absence of specific hormone treatment, possess immunoregulatory activity, and are able to form tight junctions (a property unique to Sertoli cells in the testes) (Chui et al., 2011). However, it is noted that fetal bovine serum was included in the culture medium used in these earlier studies (Ahmed et al., 2009; Chui et al., 2011). Nonetheless, the fact that we can isolate only a relatively small number of cells that possess this ability from the testes suggests that they represent a subpopulation of Sertoli cell-progenitor cells as has been suggested (Hayrabedyan et al., 2012). However, it is not clear if this is the case or if adult Sertoli cells in general can resume robust proliferation in vitro in the absence of specific hormone supplementation but with fetal bovine serum in the culture medium. Fluorescence-assisted cell sorting (FACS) revealed that proliferative human Sertoli cells and mesenchymal stem cells express a number of the same cell surface antigens, suggesting that the ability of this population of adult human Sertoli cells to actively divide may be another characteristic that is shared with mesenchymal stem cells (Chui et al., 2011). Recently, it was shown that knockdown of the expression of c-myc reduced WNT/β-catenin-mediated stimulation of the proliferation of adult human Sertoli cells (Li et al., 2012). Interestingly, proliferation of human umbilical cord blood-derived mesenchymal stem cells by treatment with prostaglandin E(2) was also induced by β-catenin-mediated c-Myc and VEGF expression (Jang et al., 2012). Needless to say, these findings illustrate that it may be feasible to use human Sertoli cells for mechanistic and functional studies without the need for fresh primary cultures from human testes. Herein, we also show that even in the absence of FBS, human adult Sertoli cells, unlike rat neonatal Sertoli cells, remain proliferative, but require twice the time for plated cells to reach ∼70–80% confluence. This observation is also consistent with a recent study that reported that differentiated and non-dividing post-pubertal rat Sertoli cells resumed proliferation after transplantation (Mital et al., 2013), illustrating differentiated Sertoli cells from both humans and rodents can be de-differentiated and mitotically active, and can even proliferate slowly, perhaps in response to ‘stress’ conditions which may be a necessary physiological response for species survival. Collectively, these findings illustrate that the human Sertoli cell in vitro system as characterized herein is a useful system to study BTB function, and it is physiologically relevant to the BTB in humans in vivo.
Using Sertoli cells obtained from three different human donors, we observed that exposure to CdCl2 or BPA at doses considered non-cytotoxic induces mislocalization of adhesion proteins at the cell–cell interface. This in turn is mediated via changes in F-actin organization in which microfilaments became truncated and defragmented, retracting from the cell–cell interface. As a consequence, cell adhesion protein complexes such as N-cadherin- β-catenin fail to anchor onto the actin-based cytoskeleton, redistribute from the cell–cell interface into the cell cytosol and perturb Sertoli cell adhesion. These toxicant-induced changes in actin microfilament organization at the Sertoli cell BTB are mediated, in part, by changes in the localization and/or distribution of actin barbed end capping and bundling protein Eps8 and branched actin polymerization protein Arp3. In vivo rat studies have shown that the restrictive spatiotemporal expression of these two proteins during the epithelial cycle plays a crucial role in regulating the conversion between the ‘bundled’ and ‘de-bundled/branched’ configuration of the actin microfilaments at the Sertoli cell BTB (Lie et al., 2009, 2010). For instance, Eps8 is highly expressed at the Sertoli cell BTB at stage V–VII of the epithelial cycle to maintain the integrity of the actin filament bundles, but Eps8 is considerably diminished at stage VIII of the cycle to allow actin microfilament restructuring to accommodate the transport of preleptotene spermatocytes at the BTB (Lie et al., 2009). In contrast, Arp3 that induces barbed end branched actin nucleation and polymerization (Cheng and Mruk, 2011; Rotty et al., 2013), effectively converting bundled actins to a branched network, and destabilizing the basal ES at the BTB, is weakly expressed at the basal ES/BTB at stage V-VII (Lie et al., 2010). But Arp3 is intensively expressed at the basal ES/BTB in stage VIII of the epithelial cycle (Lie et al., 2010). Thus, the concerted efforts of Eps8 and the Arp2/3 complex confer plasticity to the actin cytoskeleton in Sertoli cells during the epithelial cycle, which in turn facilitate BTB restructuring and to accommodate the transport of preleptotene spermatocytes at the BTB at stage VIII of the epithelial cycle (Cheng et al., 2013).
In brief, changes in the localization and/or distribution of Arp3 and Eps8 perturb the homeostasis of actin microfilament organization. In turn, there is perturbation of the anchorage of adhesion protein complexes to the underlying actin-based cytoskeleton, causing junction disruption at the Sertoli cell–cell interface. These changes are accompanied by redistribution of the c-Src and annexin II which are the proteins that play a role in actin dynamics and endocytic vesicle-mediated trafficking in epithelial cells (Grieve et al., 2012; Xiao et al., 2012b). For instance, it is known that annexin 2, a putative substrate of c-Src, is involved in remodeling of actin microfilaments via its effects on barbed end capping (Hayes et al., 2006), and also activation of cofilin (de Graauw et al., 2008), which is an actin-binding and depolymerization protein that induces actin filament disassembly by severing microfilament at its minus end (Bravo-Cordero et al., 2013). c-Src, on the other hand, is a crucial regulator of endocytic vesicle-mediated trafficking between the plasma membrane and late endosomes and lysosomes (Tsutsumi et al., 2008; Sato et al., 2009). It is also increasingly clear that annexin II is involving in Rab11a-mediated protein recycling (Lock and Stow, 2005; Bryant et al., 2010). Thus, it is tempting to speculate that the toxicant-induced changes in the localization and/or distribution of c-Src and annexin II may impede actin microfilament dynamics by enhancing cofilin-mediated actin cleavage and also by disrupting endocytic vesicle-mediated protein trafficking. As noted in Fig. 5, we provide a hypothetic model based on these data, which could form the basis of future functional studies of human Sertoli cells cultured in vitro to examine the mechanisms by which environmental toxicants perturb human reproductive function.
Authors' roles
C.Y.C. conceptualized and designed research; X.X. and E.I.T. performed research and acquired data; C.Y.C., X.X. and D.D.M. analyzed and interpreted data; X.X. and C.Y.C. drafted the article; C.M.J., P.J.T., C.K.C.W., W.M.L., B.S. and C.Y.C. revised article critically for its intellectual content; X.X., D.D.M., E.I.T., C.K.C.W., W.M.L., C.M.J., P.J.T., B.S. and C.Y.C. were involved in final approval of the version to be published.
Funding
This work was supported by grants from the National Institutes of Health (NICHD R01 HD056304 to C.Y.C.; U54 HD029990 Project 5 to C.Y.C.; NIEHS R43 ES019824 to C.M.J. and P.J.T.), National Science Foundation of China (NSFC 31371176 to X.X.), NSFC/Research Grants Council (RGC) of Hong Kong Joint Research Scheme (N_HKU 717/12 to W.M.L.), Committee on Research and Conference Grants (CRCG) Seed Funding, University of Hong Kong (to W.M.L.), and Hong Kong Baptist University (HKBU) Strategic Development Fund (SDF11-1215-P07, to C.K.C.W.).
Conflict of interest
C.M.J. has equity in MandalMed which has a pending patent on proliferative human Sertoli cells. All other authors have no competing interest.
References
- Ahmed EA, Barten-van Rijbroek AD, Kal HB, Sadri-Ardekani H, Mizrak SC, van Pelt AM, de Rooij DG. Proliferative activity in vitro and DNA repair indicate that adult mouse and human Sertoli cells are not terminally differentiated, quiescent cells. Biol Reprod. 2009;80:1084–1091. doi: 10.1095/biolreprod.108.071662. [DOI] [PubMed] [Google Scholar]
- Ando-Akatsuka Y, Saitou M, Hirase T, Kishi M, Sakakibara A, Itoh M, Yonemura S, Furuse M, Tsukita S. Interspecies diversity of the occludin sequence: cDNA cloning of human, mouse, dog, and rat-kangaroo homologoues. J Cell Biol. 1996;133:43–47. doi: 10.1083/jcb.133.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostoli P, Kiss P, Porru S, Bonde JP, Vanhoorne M. Male reproductive toxicity of lead in animals and humans. ASCLEPIOS Study Group. Occup Environ Med. 1998;55:364–374. doi: 10.1136/oem.55.6.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoff S, Jacob A, Hurley IR. Male infertility and environmental exposure to lead and cadmium. Hum Reprod Update. 2000;6:107–121. doi: 10.1093/humupd/6.2.107. [DOI] [PubMed] [Google Scholar]
- Bergmann M, Dierichs R. Postnatal formation of the blood-testis barrier in the rat with special reference to the initiation of meiosis. Anat Embryol. 1983;168:269–275. doi: 10.1007/BF00315821. [DOI] [PubMed] [Google Scholar]
- Bonde JP, Toft G, Rylander L, Rignell-Hydbom A, Giwercman A, Spano M, Manicardi GC, Bizzaro D, Ludwicki JK, Zvyezday V, et al. Fertility and markers of male reproductive function in Inuit and European populations spanning large contrasts in blood levels of persistent organochlorines. Environ Health Perspect. 2008;116:269–277. doi: 10.1289/ehp.10700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo-Cordero JJ, Magalhaes MA, Eddy RJ, Hodgson L, Condeelis J. Functions of cofilin in cell locomotion and invasion. Nat Rev Mol Cell Biol. 2013;14:405–415. doi: 10.1038/nrm3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown NA, Spielmann H, Bechter R, Flint OP, Freeman SJ, Jelinek RJ, Koch E, Nau H, Newall DR, Palmer AK, et al. Screening chemicals for reproductive toxicity: the current alternatives. The Report and Recommendations of an ECVAM/ETS Workshop (ECVAM Workshop 12) Altern Lab Anim. 1995;23:868–882. [Google Scholar]
- Bryant DM, Datta A, Rodriguez-Fraticelli AE, PeraCurrency Signnen J, Martin-Belmonte F, Mostov KE. A molecular network for de novo generation of the apical surface and lumen. Nat Cell Biol. 2010;12:1035–1045. doi: 10.1038/ncb2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers S, Hadley MA, Djakiew D, Dym M. Growth and characterization of epididymal epithelial cells and Sertoli cells in dual environment culture chambers. J Androl. 1986;7:59–68. doi: 10.1002/j.1939-4640.1986.tb00871.x. [DOI] [PubMed] [Google Scholar]
- Chen A, Kim SS, Chung E, Dietrich KN. Thyroid hormones in relation to lead, mercury, and cadmium exposure in the National Health and Nutrition Examination Survey, 2007–2008. Environ Health Perspect. 2013;121:181–186. doi: 10.1289/ehp.1205239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Fok KL, Chen H, Zhang XH, Xu WM, Chan HC. Cryptorchidism-induced CFTR down-regulation results in disruption of testicular tight junctions through up-regulation of NF-κB/COX-2/PGE2. Hum Reprod. 2012;27:2585–2597. doi: 10.1093/humrep/des254. [DOI] [PubMed] [Google Scholar]
- Cheng CY, Mruk DD. An intracellular trafficking pathway in the seminiferous epithelium regulating spermatogenesis: a biochemical and molecular perspective. Crit Rev Biochem Mol Biol. 2009;44:245–263. doi: 10.1080/10409230903061207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CY, Mruk DD. A local autocrine axis in the testes that regulates spermatogenesis. Nat Rev Endocrinol. 2010;6:380–395. doi: 10.1038/nrendo.2010.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CY, Mruk DD. Regulation of spermiogenesis, spermiation and blood-testis barrier dynamics: novel insights from studies on Eps8 and Arp3. Biochem J. 2011;435:553–562. doi: 10.1042/BJ20102121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CY, Mruk DD. The blood-testis barrier and its implication in male contraception. Pharmacol Rev. 2012;64:16–64. doi: 10.1124/pr.110.002790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CY, Wong EWP, Lie PPY, Li MWM, Su L, Siu ER, Yan HHN, Mannu J, Mathur PP, Bonanomi M, et al. Environmental toxicants and male reproductive function. Spermatogenesis. 2011;1:2–13. doi: 10.4161/spmg.1.1.13971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CY, Lie PPY, Wong EWP, Mruk DD, et al. Focal adhesion kinase and actin regulatory/binding proteins that modulate F-actin organization at the tissue barrier. Lesson from the testis. Tissue Barriers. 2013;1:e24252. doi: 10.4161/tisb.24252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chui K, Trivedi A, Cheng CY, Cherbavaz DB, Dazin PF, Huynh ALT, Mitchell JB, Rabinovich GA, Noble-Haeusslein LJ, John CM. Characterization and functionality of proliferative human Sertoli cells. Cell Transplant. 2011;20:619–635. doi: 10.3727/096368910X536563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Graauw M, Tijdens I, Smeets MB, Hensbergen PJ, Deelder AM, Van de Water B. Annexin A2 phosphorylation mediates cell scattering and branching morphogenesis via cofilin activation. Mol Cell Biol. 2008;28:1029–1040. doi: 10.1128/MCB.01247-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du M, Young J, De Asis M, Cipollone J, Roskelley C, Takai Y, Nicholls PK, Stanton PG, Deng W, Finlay BB, et al. A novel subcellular machine contributes to basal junction remodeling in the seminiferous epithelium. Biol Reprod. 2013;88:60. doi: 10.1095/biolreprod.112.104851. [DOI] [PubMed] [Google Scholar]
- Ehmcke J, Schlatt S. A revised model for spermatogonial expansion in man: lessons from non-human primates. Reproduction. 2006;132:673–680. doi: 10.1530/rep.1.01081. [DOI] [PubMed] [Google Scholar]
- Frame MC. Newest findings on the oldest oncogene: how activated src does it. J Cell Sci. 2004;117:989–998. doi: 10.1242/jcs.01111. [DOI] [PubMed] [Google Scholar]
- Ghassemifar MR, Eckert JJ, Houghton FD, Picton HM, Leese HJ, Fleming TP. Gene expression regulating epithelial intercellular junction biogenesis during human blastocyst development in vitro. Mol Hum Reprod. 2003;9:245–252. doi: 10.1093/molehr/gag033. [DOI] [PubMed] [Google Scholar]
- Grieve AG, Moss SE, Hayes MJ. Annexin A2 at the interface of actin and membrane dynamics: a focus on its roles in endocytosis and cell polarization. Int J Cell Biol. 2012:852430. doi: 10.1155/2012/852430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grima J, Pineau C, Bardin CW, Cheng CY. Rat Sertoli cell clusterin, α2-macroglobulin, and testins: biosynthesis and differential regulation by germ cells. Mol Cell Endocrinol. 1992;89:127–140. doi: 10.1016/0303-7207(92)90219-v. [DOI] [PubMed] [Google Scholar]
- Gye MC. Changes in the expression of claudins and transepithelial electrical resistance of mouse Sertoli cells by Leydig cell coculture. Int J Androl. 2003;26:271–278. doi: 10.1046/j.1365-2605.2003.00423.x. [DOI] [PubMed] [Google Scholar]
- Hauser R. Urinary phthalate metabolites and semen quality: a review of a potential biomarker of susceptibility. Int J Androl. 2008;31:112–117. doi: 10.1111/j.1365-2605.2007.00844.x. [DOI] [PubMed] [Google Scholar]
- Hayes MJ, Shao D, Bailly M, Moss SE. Regulation of actin dynamics by annexin 2. EMBO J. 2006;25:1816–1826. doi: 10.1038/sj.emboj.7601078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayrabedyan S, Todorova K, Pashova S, MNollova M, Fernandez N. Sertoli cell quiescence—new insights. Am J Reprod Immunol. 2012;68:451–455. doi: 10.1111/j.1600-0897.2012.01137.x. [DOI] [PubMed] [Google Scholar]
- Heindel JJ. Role of exposure to environmental chemicals in the developmental basis of reproductive disease and dysfunction. Semin Reprod Med. 2006;24:156–167. doi: 10.1055/s-2006-944423. [DOI] [PubMed] [Google Scholar]
- Hess RA, de Franca LR. Spermatogenesis and cycle of the seminiferous epithelium. Adv Exp Med Biol. 2008;636:1–15. doi: 10.1007/978-0-387-09597-4_1. [DOI] [PubMed] [Google Scholar]
- http://www.mayoclinic.com/health/infertility/DS00310 .
- Hunt PA, Susiarjo M, Rubio C, Hassold TJ. The bisphenol A experience: a primer for the analysis of environmental effects on mammalian reproduction. Biol Reprod. 2009;81:807–813. doi: 10.1095/biolreprod.109.077008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janecki A, Jakubowiak A, Steinberger A. Regulation of transepithelial electrical resistance in two-compartment Sertoli cell cultures: in vitro model of the blood-testis barrier. Endocrinology. 1991;129:1489–1496. doi: 10.1210/endo-129-3-1489. [DOI] [PubMed] [Google Scholar]
- Janecki A, Jakubowiak A, Steinberger A. Effect of cadmium chloride on transepithelial electrical resistance of Sertoli cell monolayers in two-compartment cultures—a new model for toxicological investigations of the ‘blood-testis’ barrier in vitro. Toxicol Appl Pharmacol. 1992;112:51–57. doi: 10.1016/0041-008x(92)90278-z. [DOI] [PubMed] [Google Scholar]
- Jang MW, Yun SP, Park JHR, Ryu JM, Lee JH, Han HJ. Cooperation of Epac1/Rap1/Akt and PKA in prostaglandin E(2)-induced proliferation of human umbilical cord blood derived mesencymal stem cells: Involvement of c-Myc and VEGF expression. J Cell Physiol. 2012;227:3756–3767. doi: 10.1002/jcp.24084. [DOI] [PubMed] [Google Scholar]
- Kaitu'u-Lino TJ, Sluka P, Foo CF, Stanton PG. Claudin-11 expression and localisation is regulated by androgens in rat Sertoli cells in vitro. Reproduction. 2007;133:1169–1179. doi: 10.1530/REP-06-0385. [DOI] [PubMed] [Google Scholar]
- Lee NPY, Cheng CY. Protein kinases and adherens junction dynamics in the seminiferous epithelium of the rat testis. J Cell Physiol. 2005;202:344–360. doi: 10.1002/jcp.20119. [DOI] [PubMed] [Google Scholar]
- Li MWM, Mruk DD, Lee WM, Cheng CY. Disruption of the blood-testis barrier integrity by bisphenol A in vitro: is this a suitable model for studying blood-testis barrier dynamics? Int J Biochem Cell Biol. 2009;41:2302–2314. doi: 10.1016/j.biocel.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Gao Q, Yin G, Ding X, Hao J. WNT/β-catenin-signaling pathway stimulates the proliferation of cultured adult human Sertoli cells via upregulation of c-Myc expression. Reprod Sci. 2012;19:1232–1240. doi: 10.1177/1933719112447126. [DOI] [PubMed] [Google Scholar]
- Lie PPY, Mruk DD, Lee WM, Cheng CY. Epidermal growth factor receptor pathway substrate 8 (Eps8) is a novel regulator of cell adhesion and the blood-testis barrier integrity in the seminiferous epithelium. FASEB J. 2009;23:2555–2567. doi: 10.1096/fj.06-070573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie PPY, Chan AYN, Mruk DD, Lee WM, Cheng CY. Restricted Arp3 expression in the testis prevents blood-testis barrier disruption during junction restructuring at spermatogenesis. Proc Natl Acad Sci USA. 2010;107:11411–11416. doi: 10.1073/pnas.1001823107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie PPY, Mruk DD, Mok KW, Su L, Lee WM, Cheng CY. Focal adhesion kinase-Tyr407 and -Tyr397 exhibit antagonistic effects on blood-testis barrier dynamics in the rat. Proc Natl Acad Sci USA. 2012;109:12562–12567. doi: 10.1073/pnas.1202316109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock JG, Stow JL. Rab11 in recycling endosomes regulates the sorting and basolateral transport of E-cadherin. Mol Biol Cell. 2005;16:1744–1755. doi: 10.1091/mbc.E04-10-0867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui WY, Lee WM, Cheng CY. Transforming growth factor-β3 perturbs the inter-Sertoli tight junction permeability barrier in vitro possibly mediated via its effects on occludin, zonula occludens-1, and claudin-11. Endocrinology. 2001;142:1865–1877. doi: 10.1210/endo.142.5.8116. [DOI] [PubMed] [Google Scholar]
- Lui WY, Lee WM, Cheng CY. Transforming growth factor-β3 regulates the dynamics of Sertoli cell tight junctions via the p38 mitogen-activated protein kinase pathway. Biol Reprod. 2003a;68:1597–1612. doi: 10.1095/biolreprod.102.011387. [DOI] [PubMed] [Google Scholar]
- Lui WY, Wong CH, Mruk DD, Cheng CY. TGF-β3 regulates the blood-testis barrier dynamics via the p38 mitogen activated protein (MAP) kinase pathway: an in vivo study. Endocrinology. 2003b;144:1139–1142. doi: 10.1210/en.2002-0211. [DOI] [PubMed] [Google Scholar]
- Marques-Pinto AM, Carvalho D. Human infertility: are endocrine disruptors to blame? Endocr Connect. 2013;2:R15–R29. doi: 10.1530/EC-13-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascarenhas MN, Flaxman SR, Boerman T, Vanderpoel S, Stevens GA. National, regional, and global trends in infertility prevalence since 1990: A systematic analysis of 277 health surveys. PLoS Med. 2012;9:e1001356. doi: 10.1371/journal.pmed.1001356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather JP, Sato GH. The use of hormone-supplemented serum-free media in primary cultures. Exp Cell Res. 1979;124:215–221. doi: 10.1016/0014-4827(79)90271-4. [DOI] [PubMed] [Google Scholar]
- Mital P, Kaur G, Bowlin B, Paniagua NJ, Korbutt GS, Dufour JM. Nondividing, post-pubertal rat Sertoli cells resumed proliferation after transplantation. Biol Reprod. 2014;90:13. doi: 10.1095/biolreprod.113.110197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok KW, Mruk DD, Lee WM, Cheng CY. A study to assess the assembly of a functional blood-testis barrier in developing rat testes. Spermatogenesis. 2011;1:270–280. doi: 10.4161/spmg.1.3.17998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroi S, Saitou M, Fujimoto K, Sakakibara A, Furuse M, Yoshida O, Tsukita S. Occludin is concentrated at tight junctions of mouse/rat but not human/guinea pig Sertoli cells in testes. Am J Physiol Cell Physiol. 1998;274:C1708–C1717. doi: 10.1152/ajpcell.1998.274.6.C1708. [DOI] [PubMed] [Google Scholar]
- Mruk DD, Cheng CY. Enhanced chemiluminescence (ECL) for routine immunoblotting. An inexpensive alternative to commercially available kits. Spermatogenesis. 2011a;1:121–122. doi: 10.4161/spmg.1.2.16606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mruk DD, Cheng CY. Environmental contaminants. Is male reproductive health at risk? Spermatogenesis. 2011b;1:283–290. doi: 10.4161/spmg.1.4.18328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mruk DD, Cheng CY. An in vitro system to study Sertoli cell blood-testis barrier dynamics. Methods Mol Biol. 2011c;763:237–252. doi: 10.1007/978-1-61779-191-8_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls PK, Harrison CA, Gilchrist RB, Farnworth PG, Stanton PG. Growth differentiation factor 9 is a germ cell regulator of Sertoli cell function. Endocrinology. 2009;150:2481–2490. doi: 10.1210/en.2008-1048. [DOI] [PubMed] [Google Scholar]
- Nordkap L, Joensen UN, Blomberg JM, Jorgensen N. Regional differences and temporal trends in male reproductive health disorders: semen quality may be a sensitive marker of environmental exposures. Mol Cell Endocrinol. 2012;355:221–230. doi: 10.1016/j.mce.2011.05.048. [DOI] [PubMed] [Google Scholar]
- Orth JM. Proliferation of Sertoli cells in fetal and postnatal rats: a quantitative autoradiographic study. Anat Rec. 1982;203:485–492. doi: 10.1002/ar.1092030408. [DOI] [PubMed] [Google Scholar]
- Pollack AZ, Schisterman EF, Goldman LR, Mumford SL, Albert PS, Jones RL, Wactawski-Wende J. Cadmium, lead, and mercury in relation to reproductive hormones and anovulation in premenopausal women. Environ Health Perspect. 2011;119:1156–1161. doi: 10.1289/ehp.1003284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri P, Walker WH. The tyrosine phosphatase SHP2 regulates Sertoli cell junction complexes. Biol Reprod. 2013;88:59. doi: 10.1095/biolreprod.112.104414. [DOI] [PubMed] [Google Scholar]
- Qiu L, Zhang X, Zhang X, Zhang Y, Gu J, Chen M, Zhang Z, Wang X, Wang SL. Sertoli cell is a potential target for perfluorooctane sulfonate-induced reproductive dysfunction in male mice. Toxicol Sci. 2013;135:229–240. doi: 10.1093/toxsci/kft129. [DOI] [PubMed] [Google Scholar]
- Rolland M, Le Moal J, Wagner V, Royere D, De Mouzon J. Decline in semen concentration and morphology in a sample of 26,609 men close to general population between 1989 and 2005 in France. Hum Reprod. 2013;28:462–470. doi: 10.1093/humrep/des415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotty JD, Wu CC, Bear JE. New insights into the regulation and cellular functions of the ARP2/3. Nat Rev Mol Cell Biol. 2013;14:7–12. doi: 10.1038/nrm3492. [DOI] [PubMed] [Google Scholar]
- Russell LD, Bartke A, Goh JC. Postnatal development of the Sertoli cell barrier, tubular lumen, and cytoskeleton of Sertoli and myoid cells in the rat, and their relationship to tubular fluid secretion and flow. Am J Anat. 1989;184:179–189. doi: 10.1002/aja.1001840302. [DOI] [PubMed] [Google Scholar]
- Sato I, Obata Y, Kasahara K, Nakayama Y, Fukumoto Y, Yamasaki T, Yokoyama KK, Saito T, Yamaguchi N. Differential trafficking of Src, Lyn, Yes and Fyn is specified by the state of palmitoylation in the SH4 domain. J Cell Sci. 2009;122:965–975. doi: 10.1242/jcs.034843. [DOI] [PubMed] [Google Scholar]
- Sengujpta P. Environmental and occupational exposure of metals and their role in male reproductive functions. Drug Chem Toxicol. 2013;36:353–368. doi: 10.3109/01480545.2012.710631. [DOI] [PubMed] [Google Scholar]
- Sharpe RM, McKinnell C, Kivlin C, Fisher JS. Proliferation and functional maturation of Sertoli cells, and their relevance to disorders of testis function in adulthood. Reproduction. 2003;125:769–784. doi: 10.1530/rep.0.1250769. [DOI] [PubMed] [Google Scholar]
- Sharpe RM. Environmental/lifestyle effects on spermatogenesis. Phil Trans R Soc B Biol Sci. 2010;365:1697–1712. doi: 10.1098/rstb.2009.0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu ER, Mruk DD, Porto CS, Cheng CY. Cadmium-induced testicular injury. Toxicol Appl Pharmacol. 2009a;238:240–249. doi: 10.1016/j.taap.2009.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu ER, Wong EWP, Mruk DD, Porto CS, Cheng CY. Focal adhesion kinase is a blood-testis barrier regulator. Proc Natl Acad Sci USA. 2009b;106:9298–9303. doi: 10.1073/pnas.0813113106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu ER, Wong EWP, Mruk DD, Sze KL, Porto CS, Cheng CY. An occludin-focal adhesion kinase protein complex at the blood-testis barrier: a study using the cadmium model. Endocrinology. 2009c;150:3336–3344. doi: 10.1210/en.2008-1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu MKY, Lee WM, Cheng CY. The interplay of collagen IV, tumor necrosis factor-α, gelatinase B (matrix metalloprotease-9), and tissue inhibitor of metalloprotease-1 in the basal lamina regulates Sertoli cell-tight junction dynamics in the rat testis. Endocrinology. 2003;144:371–387. doi: 10.1210/en.2002-220786. [DOI] [PubMed] [Google Scholar]
- Skinner MK, Manikkam M, Guerrero-Bosagna C. Epigenetic transgenerational actions of endocrine disruptors. Reprod Toxicol. 2010;31:337–343. doi: 10.1016/j.reprotox.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JF, Eisenberg ML, Millstein SG, Nachtigall RD, Shindel AW, Wing H, Cedars M, Pasch L, Katz PP. Infertility outcomes program project group. The use of complementary and alternative fertility treatment in couples seeking fertility care: data from a prospective cohort in the United States. Fertil Sertil. 2010;93:2169–2174. doi: 10.1016/j.fertnstert.2010.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague BL, Trentham-Dietz A, Hedman CJ, Wang J, Hemming JDC, Hampton JM, Buist DSM, Bowles EJA, Sisney GS, Brunside ES. Circulating serum xenoestrogens and mammographic breast density. Breast Cancer Res. 2013;15:R45. doi: 10.1186/bcr3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su L, Mruk DD, Cheng CY. Regulation of drug transporters in the testis by environmental toxicant cadmium, steroids and cytokines. Spermatogenesis. 2012a;2:285–293. doi: 10.4161/spmg.22536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su L, Mruk DD, Lie PPY, Silvestrini B, Cheng CY. A peptide derived from laminin-γ3 reversibly impairs spermatogenesis in rats. Nat Commun. 2012b;3:1185. doi: 10.1038/ncomms2171. doi:1110.1038/ncomms2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su L, Mruk DD, Lui WY, Lee WM, Cheng CY. P-glycoprotein regulates blood-testis barrier dynamics via its effects on the occludin/zonula occludens 1 (ZO-1) protein complex mediated by focal adhesion kinase (FAK) Proc Natl Acad Sci USA. 2011;108:19623–19628. doi: 10.1073/pnas.1111414108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su WH, Wong EWP, Mruk DD, Cheng CY. The Scribble/Lgl/Dlg polarity protein complex is a regulator of blood-testis barrier dynamics and spermatid polarity during spermatogenesis. Endocrinology. 2012c;153:6041–6053. doi: 10.1210/en.2012-1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan SH, Elkin EP, Fenster L. The question of declining sperm density revisited: an analysis of 101 studies published 1934–1996. Environ Health Perspect. 2000;108:961–966. doi: 10.1289/ehp.00108961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarulli GA, Stanton PG, Lerchl A, Meachem SJ. Adult Sertoli cells are not terminally differentiated in the Djungarian hamster: effect of FSH on proliferation and juncitkon protein organization. Biol Reprod. 2006;74:798–806. doi: 10.1095/biolreprod.105.050450. [DOI] [PubMed] [Google Scholar]
- Tobe T. Cytoskeleton-modulating effectors of enteropathogenic and enterohemorrhagic Escherichia coli: role of EspL2 in adherence and an alternative pathway for modulating cytoskeleton through Annexin A2 function. FEBS J. 2010;277:2403–2408. doi: 10.1111/j.1742-4658.2010.07654.x. [DOI] [PubMed] [Google Scholar]
- Toft G, Jonsson BA, Lindh CH, Giwercman A, Spano M, Heederik D, Lenters V, Vermeulen R, Rylander L, Pedersen HS, et al. Exposure to perfluorinated compounds and human semen quality in Arctic and European populations. Hum Reprod. 2012;27:2532–2540. doi: 10.1093/humrep/des185. [DOI] [PubMed] [Google Scholar]
- Trosko JE. Commentary on ‘Toxicity testing in the 21st century: a vision and a strategy’: stem cells and cell-cell communication as fundamental targets in assessing the potential toxicity of chemicals. Hum Exp Toxicol. 2010;29:21–29. doi: 10.1177/0960327109354663. [DOI] [PubMed] [Google Scholar]
- Tsutsumi K, Tomomura M, Furuichi T, Hisanaga S. Palmitoylation-dependent endosomal localization of AATYK1A and its interaction with Src. Genes Cells. 2008;13:949–964. doi: 10.1111/j.1365-2443.2008.01219.x. [DOI] [PubMed] [Google Scholar]
- Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DRJ, Lee DH, Shioda T, Soto AM, Vom Saal FS, Welshons WV, et al. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr Rev. 2012;33:378–455. doi: 10.1210/er.2011-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Hunt PA, Myers JP, Vom Saal FS. Human exposures to bisphenol A: mismatches between data and assumptions. Rev Environ Health. 2013;28:37–58. doi: 10.1515/reveh-2012-0034. [DOI] [PubMed] [Google Scholar]
- Wan HT, Leung PY, Zhao YG, Wei X, Wong MH, Wong CK. Blood plasma concentrations of endocrine disrupting chemicals in Hong Kong populations. J Hazard Mater. 2013a;261:769–769. doi: 10.1016/j.jhazmat.2013.01.034. [DOI] [PubMed] [Google Scholar]
- Wan HT, Mruk DD, Li SYT, Mok KW, Lee WM, Wong CKC, Cheng CY. p-FAK-Tyr397 regulates spermatid adhesion in the rat testis via its effects on F-actin organization at the ectoplasmic specialization. Am J Physiol Endocrinol Metab. 2013b;305:E687–E699. doi: 10.1152/ajpendo.00254.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan HT, Mruk DD, Wong CKC, Cheng CY. The apical ES-BTB-BM functional axis is an emerging target for toxicant-induced infertility. Trends Mol Med. 2013c;19:396–405. doi: 10.1016/j.molmed.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan HT, Mruk DD, Wong CKC, Cheng CY. Perfluorooctanesulfonate (PFOS) perturbs male rat Sertoli cell blood-testis barrier function by affecting F-actin organization via p-FAK-Tyr407—and in vitro study. Endocrinology. 2013d;155:249–262. doi: 10.1210/en.2013-1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan HT, Mruk DD, Wong CKC, Cheng CY. Targeting testis-specific proteins to inhibit spermatogenesis—lession from endocrine disrupting chemicals. Expert Opin Ther Targets. 2013e;17:839–855. doi: 10.1517/14728222.2013.791679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CH, Mruk DD, Lui WY, Cheng CY. Regulation of blood-testis barrier dynamics: an in vivo study. J Cell Sci. 2004;117:783–798. doi: 10.1242/jcs.00900. [DOI] [PubMed] [Google Scholar]
- Wong EWP, Cheng CY. Impacts of environmental toxicants on male reproductive dysfunction. Trends Pharmacol Sci. 2011;32:290–299. doi: 10.1016/j.tips.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong EWP, Yan HHN, Li MWM, Lie PPY, Mruk DD, Cheng CY. Cell junctions in the testis as targets for toxicants. In: Hoyer PB, Richburg JH, editors. Comprehensive Toxicology. 2nd edn. Vol. 11. Oxford: Academic Press, Elsevier; 2010. pp. 167–188. McQueen, CA, Ed; Series Editor Reproductive and Endocrine Toxicology. [Google Scholar]
- Xiao X, Cheng CY, Mruk DD. Intercellular adhesion molecule (ICAM)-1 is a regulator of blood-testis barrier function. J Cell Sci. 2012a;125:5677–5689. doi: 10.1242/jcs.107987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X, Mruk DD, Cheng FL, Cheng CY. c-Src and c-Yes are two unlikely partners of spermatogenesis and their roles in blood-testis barrier dynamics. Adv Exp Med Biol. 2012b;763:295–317. doi: 10.1007/978-1-4614-4711-5_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X, Cheng CY, Mruk DD. Intercellular adhesion molecule-2 is involved in apical ectoplasmic specialization dynamics during spermatogenesis in the rat. J Endocr. 2013a;216:73–86. doi: 10.1530/JOE-12-0434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X, Mruk DD, Cheng CY. c-Yes regulates cell adhesion at the apical ectoplasmic specialization-blood-testis barrier axis via its effects on protein recruitment and distribution. Am J Physiol Endocrinol Metab. 2013b;304:E145–E159. doi: 10.1152/ajpendo.00422.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X, Mruk DD, Lee WM, Cheng CY. c-Yes regulates cell adhesion at the blood-testis barrier and the apical ectoplasmic specialization in the seminiferous epithelium of rat testes. Int J Biochem Cell Biol. 2011;43:651–665. doi: 10.1016/j.biocel.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan HHN, Mruk DD, Wong EWP, Lee WM, Cheng CY. An autocrine axis in the testis that coordinates spermiation and blood-testis barrier restructuring during spermatogenesis. Proc Natl Acad Sci USA. 2008;105:8950–8955. doi: 10.1073/pnas.0711264105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young K, Nelson RJ. Mediation of seasonal testicular regression by apoptosis. Reproduction. 2001;122:677–685. doi: 10.1530/rep.0.1220677. [DOI] [PubMed] [Google Scholar]
- Zeng R, Li X, Gorodeski GI. Estrogen abrogates transcervical tight junctional resistance by acceleration of occludin modulation. J Clin Endocrinol Metab. 2004;89:5145–5155. doi: 10.1210/jc.2004-0823. [DOI] [PubMed] [Google Scholar]