Abstract
Macrophage migration inhibitory factor (MIF) is an upstream immunoregulatory cytokine associated with the pathogenesis of autoimmune inflammatory diseases. There is evidence that MIF functions in a positive feedback loop with TNF-α that could perpetuate the inflammatory process in systemic lupus erythematosus (SLE). In this case-control study we investigated whether commonly occurring functional MIF polymorphisms are associated with SLE as well as with MIF and TNF-α serum levels in a Mexican-Mestizo population. Genotyping of the -794CATT5- 8(rs5844572) and -173G>C(rs755622) MIF polymorphisms was performed by PCR and PCR-RFLP respectively in186 SLE patients and 200 healthy subjects. MIF and TNF-α serum levels were determined by ELISA. A significant increase of MIF and TNF-α levels was found in SLE patients. According to a genetic model, we found a significant association of genotypes carrying the -794CATT7 and -173*C risk alleles with susceptibility to SLE and with a significant increase of TNF-α . In conclusion, MIF gene polymorphisms are associated with SLE susceptibility and with an increase of TNF-α serum levels in a Mexican-Mestizo population.
Keywords: Systemic lupus erythematosus, association, macrophage migration inhibitory factor, tumor necrosis factor alpha, polymorphism
1. Introduction
Systemic lupus erythematosus (SLE) is an autoimmune disease that leads to progressive end-organ damage and is characterized by the presence of autoantibodies directed against nuclear and cytoplasmic antigens [1–3]. Globally, the incidence rate of SLE varies from 1-10 per 100,000 person-years and the prevalence rate varies from 20-70 per 100,000 person-years [4]. SLE mainly affects women of reproductive age in a 1:10 ratio [1].
The phenotypic expression of the disease varies between individuals from different populations and its development is influenced by factors such as ethnicity, genetic susceptibility, environment and gender. In particular, genetic predisposition influences the development of SLE ~15% [2,5,6].
SLE is characterized by a significant humoral response with altered proinflammatory cytokine production, suggesting a critical role for cytokines in its pathogenesis [5,7–11]. The cytokine macrophage migration inhibitory factor (MIF) is distinguished functionally by its ability to counter-regulate glucocorticoid immunosuppression and sustain pro-inflammatory activation by inhibiting activation-induced apoptosis [12]. MIF further co-stimulates T and B lymphocytes and upregulates the production of interleukin-6, interferon-γ, and tumor necrosis factor alpha (TNF-α ) by a feed-forward, positive feedback loop [13–15]. Notably, these is evidence that TNF-α levels correlate with the exacerbation of the inflammatory response, with subsequent tissue damage and SLE disease activity [16]. Ten polymorphic sites have been described within the MIF gene [17] . Of these, only two polymorphisms identified in the promoter region appear to have functional importance, the first is the short tandem repeat (STR) −794 CATT5-8 MIF (rs5844572) which is a microsatellite repetition of cytosine-adenine-thyminethymine (CATT) at position −794 bp, in which the repeat length (5 to 8 repetitions) correlates with increased gene expression and with serum MIF circulation levels [18,19]. The second polymorphism is a single nucleotide polymorphism (SNP) -173 G>C MIF (rs755622) at position −173 of the MIF gene in which there is a change from guanine (G) by cytosine (C). The −173*C allele is associated with increased MIF levels in circulation [18,20] in several populations, most likely by linkage disequilibrium with the −794 CATT7 high expression allele [18,21,22]. Both polymorphisms also occur commonly in different populations, with minor allele frequencies of > 5% [23]. Previously, both functional MIF polymorphisms have been associated with several diseases with an autoimmune component [24-30].
Based on this knowledge, polymorphisms in the MIF locus are candidate genetic determinants that could contribute to the susceptibility or clinical severity of SLE. Therefore, we designed this study to investigate the association of −794 CATT5-8 and −173 G>C MIF polymorphisms with SLE susceptibility and clinical variables as well as with MIF and TNF-α serum levels in a Mexican-Mestizo population.
2. Material and methods
2.1. Subjects
A case-control study was conducted with two study groups; the first group consisted of 186 SLE patients classified according to the 1982 American College of Rheumatology (ACR) criteria for SLE [31] and enrolled from the Rheumatology Department of the Hospital General de Occidente in Zapopan, Jalisco, Mexico. Mexican-Systemic Lupus Erythematosus-Disease Activity Index (Mex-SLEDAI) and Systemic Lupus International Collaborating Clinics (SLICC) indexes were applied to patients [32,33]. For the control study group, 200 healthy subjects identified by self-report and recruited from the general population in the same geographic area were matched for analysis. All subjects were from an unrelated Mexican-Mestizo population with a family history of ancestors, at least back to the third generation.
2.2. Ethical considerations
Informed written consent was obtained from all patients and subjects before enrollment to the study, according to the ethical guidelines of the 2008 Declaration of Helsinki and the investigation was approved by the ethical, investigation, and biosecurity committee of the Centro Universitario de Ciencias de la Salud, Universidad de Guadalajara (C.I.084-2012).
2.3. Quantification of MIF and TNF-α serum levels
Serum was obtained from all individuals at the time of inclusion, cytokine levels were quantified in a subset of 135 SLE patients that were not being treated with glucocorticoids and matched by age with 200 control subjects. The determination of MIF and TNF-α serum levels was performed by commercial ELISA kits (RayBio®, USA and Invitrogen™, USA, respectively) according to manufacturer's instructions. The MIF assay sensitivity was 6 pg/mL and the TNF-α assay sensitivity was 1.7 pg/mL.
2.4. Genotyping of -794 CATT5-8 and -173 G>C MIF polymorphisms
Total genomic DNA (gDNA) was isolated from peripheral blood leukocytes by the salting out method [34]. The −794 CATT5–8 MIF polymorphism was analyzed by conventional polymerase chain reaction (PCR) and polyacrylamide gel electrophoresis using the primers reported by Radstake et al [18]. Cycling conditions were: initial denaturing 95°C for 4 min followed by 35 cycles of 30s at 95°C, 30s at 60°C and 30s at 72°C, then a final extension of 2 min at 72°C. Amplification products were further electrophoresed on a 29:1 10% polyacrylamide gel at 120V during 16h and stained with 0.02% AgNO3.
The −173G>C MIF polymorphism was genotyped by the PCR- restriction fragment length polymorphism (RFLP) technique. Amplification of the polymorphic fragment was done using the primers reported by Makhija et al [35]; 35 cycles and an annealing temperature of 60°C were used. The 366 bp fragment obtained was further digested with the Alu I restriction endonuclease (New England Biolabs, Ipswich, MA, USA) by overnight incubation at 37°C. Finally, the digestion was resolved on a 29:1 6% polyacrylamide gel stained with 0.02% AgNO3. The −173*G allele resulted in a 268 bp and a 98 bp fragment while the 173*C allele was represented by 206 bp, 98 bp and 62 bp fragments. To confirm the results, genotyping of both polymorphisms was done in duplicate in all cases and confirmed by automatized sequencing of a randomly selected subset of −794 CATT5-8 and −173 G>C MIF genotypes (Applied Biosystems, USA).
2.5. Immunoassay
dsDNA, Sm and Sm/RNP antibodies IgG type were measured in serum samples from SLE patients by ELISA kits (The Binding Site Ltd, Birmingham, UK). The cutoff level was of >10 U/mL for Sm and Sm/RNP antibodies and >75 U/mL for dsDNA antibody. The Immunoassays were performed following the instructions of manufacturer.
2.6. Statistical analysis
Statistical analysis was performed using the statistical software STATA v 9.2 and GraphPad Prism v 5.0. The statistical power was evaluated according to the calculation of sample size using the Kelsey's formula for proportions in case-control studies[36]. For the descriptive analysis, nominal variables were expressed as frequencies, continuous variables with nonparametric distribution were expressed as medians, percentile 5-95 and interquartile ranges 25-75. We determined genotype and allele frequencies for the polymorphisms −794 CATT5-8 and −173 G>C MIF gene by direct counting. We performed chi-square test to compare proportions between groups, to compare the genotype and allele frequencies and to evaluate the Hardy-Weinberg equilibrium. To compare nonparametric quantitative determinations we used the U Mann-Whitney test, Odds ratio (OR) and 95% confidence interval (95% CI) were used to analyze the risk for SLE associated with the MIF gene polymorphisms. In order to evaluate the effect of both polymorphisms on SLE and clinical variables we performed dominant inheritance genetic models as well as linear regression models adjusted by gender. For correlation analysis of continuous variables with nonparametric distribution we used the Spearman correlation test. Differences were considered significant at p<0.05.
3. Results
3.1. Clinical and demographic characteristics
The clinical characteristics of the 186 SLE patients in this study are shown in Table 1. The median age of patients was 33 years; 94% were female and 6% male. The average evolution time of the disease was 5 years, and the subjects had moderate activity and chronicity scores evaluated by Mex-SLEDAI and SLICC-ACR indexes, respectively. The control group included 200 healthy subjects comprised of 65% women and 35% men with a median age of 30 (19-55) years.
Table 1.
Clinical features of SLE patients.
| Variable | n=186 |
|---|---|
| Demographics | |
| Age (years) a | 33 (17-65) |
| Gender % (n) | |
| Male b | 6 (11) |
| Female b | 94 (175) |
| Disease status | |
| Disease evolution (years) a | 5(0.5-17) |
| Clinical assessment | |
| Mex-SLEDAIa | 2 (0-9) |
| SLICC-ACRa | 1 (0-2) |
| Renal activity % (n) | 5 (9) |
| CRF % (n) | 4 (7) |
| Autoantibodies % (n) | |
| ANAsb | 49 (92) |
| Anti-dsDNA b | 33 (62) |
| Anti-La b | 4 (8) |
| Anti-Ro b | 13 (25) |
| Anti-Sm b | 6 (11) |
| Anti-RNP b | 12 (23) |
| Treatment | |
| NSAIDs b | 28 (53) |
| Glucocorticoids b | |
| Prednisone | 27 (51) |
| DMARDSb | |
| Azathioprine | 27 (51) |
| Chloroquine | 20 (38) |
| Methotrexate | 6 (12) |
Data provided in median (p5-p95).
Data provided in percentages and n. ANAs= anti-nuclear antibodies; CRF=chronic renal failure , NSAIDs= non-steroidal anti-inflammatory drugs , DMARDS=disease modifying antirheumatic drugs.
3.2. MIF and TNF-α serum levels
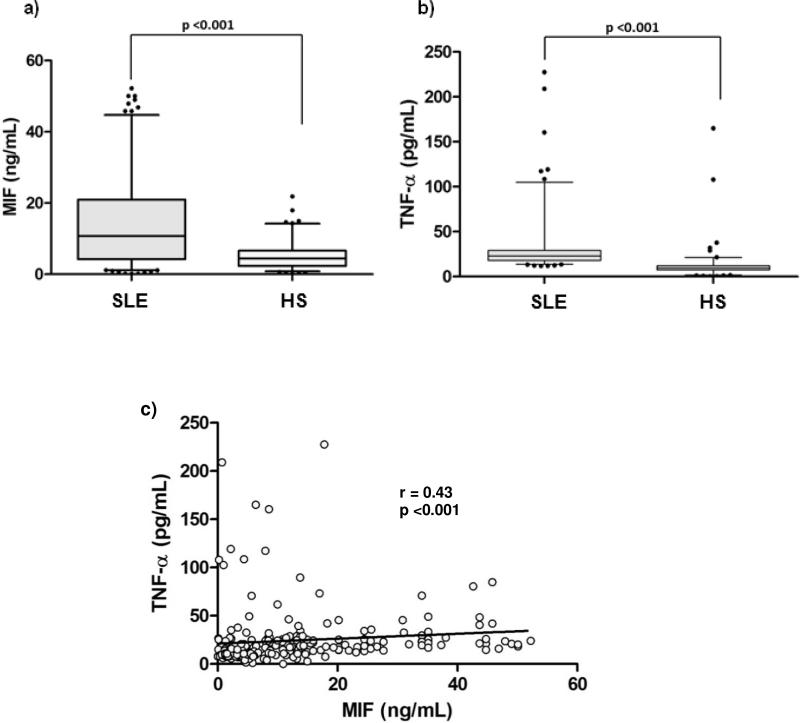
A significant increase in MIF (10.74 vs 4.47 ng/mL; p <0.001; Fig. 1a) and TNF-α serum levels (23.01 vs 9.75 pg/mL, p <0.01; Fig. 1b) was found in the SLE group compared with control subjects.
Figure 1.
Serum levels by study groups and correlation of MIF and TNF-α. 1a) Comparison of MIF serum levels by study groups; 1b) comparison of TNF-α serum levels by study groups. Data provided in median (p5-p95). U Mann-Whitney test. 1c) Positive correlation of serum levels of MIF and soluble TNF-α . Spearman's correlation test.
Previous experimental studies have reported MIF's role in enhanced TNF-α expression and a positive feedback loop between these two cytokines [37]; accordingly, we determined the correlation between MIF and TNF-α serum levels in our study groups. Fig. 1c shows a positive correlation between serum levels of MIF and TNF- (r = 0.43, p <0.001) for both study groups.
Regarding the relationships between MIF or TNF- serum levels with SLE clinical activity, we did not find a significant correlation with the evolution time of the disease, activity and chronicity as evaluated by Mex-SLEDAI and SLICC indexes, respectively (data not shown).
3.3. Distribution of −794 CATT5-8 and −173 G>C MIF polymorphisms in SLE patients and healthy subjects
In this study, both MIF promoter polymorphisms evaluated were in Hardy-Weinberg equilibrium in the control group (−794 CATT5-8 p=0.47 and −173 G>C p= 1.0). The distribution of −794 CATT5-8 and −173 G>C MIF polymorphisms in SLE patients and control subjects are shown in Table 2.
Table 2.
Genotype and allele frequencies of -794 CATT5-8 and -173 G>C MIF polymorphisms in cases and controls
| Polymorphism | SLE %(n =186) | CS %(n =200) | p value* | OR (CI 95%) | p value* |
|---|---|---|---|---|---|
| -794 CATT5-8 MIF | |||||
| Genotype | 0.02 | ||||
| 5,5 | 4 (7) | 2 (4) | 2.36 (0.56-11.6) | 0.17 | |
| 5,6 | 17 (31) | 27 (54) | 0.78 (0.42-1.44) | 0.40 | |
| 5,7 | 13 (24) | 8 (16) | 2.03 (0.92-4.56) | 0.05 | |
| 6,6§ | 26 (48) | 33 (65) | 1 | - | |
| 6,7 | 35 (66) | 24 (49) | 1.83 (1.04-3.19) | 0.02 | |
| 7,7 | 5 (10) | 6 (12) | 1.13 (0.4-3.11) | 0.79 | |
| Allele | 0.06 | ||||
| 5 | 18 (69) | 20 (78) | 1.06 (0.72-1.60) | 0.73 | |
| 6§ | 52 (193) | 58 (233) | 1 | - | |
| 7 | 30 (110) | 22 (89) | 1.5 (1.05-2.13) | 0.02 | |
| Genetic model | |||||
| Do | < 0.01 | ||||
| -,-§ | 46 (86) | 62 (123) | 1 | - | |
| -,7 + 7,7 | 54 (100) | 38 (77) | 1.86 (1.22-2.84) | <0.01 | |
| -173 G>C MIF | |||||
| Genotype | 0.08 | ||||
| GG§ | 43 (80) | 53 (106) | 1 | - | |
| GC | 49 (91) | 43 (85) | 1.42 (0.92-2.19) | 0.09 | |
| CC | 8 (15) | 4 (9) | 2.2 (0.85-6.01) | 0.07 | |
| Allele | 0.03 | ||||
| G§ | 67 (251) | 74 (297) | 1 | - | |
| C | 33 (121) | 26 (103) | 1.40 (1.01-1.93) | 0.03 | |
| Genetic model | |||||
| Do | 0.01 | ||||
| GG§ | 43 (80) | 53 (106) | 1 | - | |
| GC+ CC | 57 (116) | 47 (94) | 1.64 (1.08-2.48) | 0.01 | |
Chi square test χ2; OR: odds ratio; CI: confidence interval; Do: dominant inheritance genetic model (-,- = genotypes without risk allele ; -,7 = heterozygous genotypes with allele risk)
: reference category.
We found significant differences in the distribution of genotype frequencies of the -794 CATT5-8 MIF polymorphism by study group (p = 0.02) with an OR of 1.83 (CI 1.04-3.19, p = 0.02) for the 6,7 repeat heterozygote genotype and an OR of 1.5 (CI 1.05-2.13, p = 0.02) for the 7 repeat allele (−794 CATT7), indicating that the −794 CATT7 allele carriers have a 1.5 fold greater susceptibility to present SLE. For the 6,7 repeats heterozygote genotype carriers, the susceptibility increases to 1.83 fold.
Applying a genetic model of dominant inheritance where the genotypes that contain the −794 CATT7 risk allele were grouped, we found significant differences in the comparison of genotypic proportions by study group (p <0.01). We also determined an OR of 1.86 (CI 1.22-2.84, p <0.01) for genotypes carrying −794 CATT7 risk allele (-, 7 +7.7) which indicates that subjects who are carriers of genotypes that contain the −794 CATT7 risk allele have 1.86 fold more susceptibility to present SLE compared with subjects who are carriers of genotypes without the −794 CATT7 risk allele.
For the −173 G>C MIF polymorphism, no significant differences in the distribution of the genotype frequencies by study group were observed, nevertheless a significant difference in the distribution of allele frequencies was found (p = 0.03). We determined an OR of 1.40 (CI 1.01-1.93, p = 0.03) for the −173*C allele, which indicates that subjects who are −173*C allele carriers have 1.40 fold more susceptibility to present SLE.
Following a similar genetic model of dominant inheritance we found a significant difference in the comparison of genotypic proportions by study group (p = 0.01); we also determined an OR of 1.64 (CI 1.08-2.48, p = 0.01) for the −173*C risk allele (GC+CC) carriers which indicates that subjects who are carriers of genotypes that contain the −173*C risk allele have 1.64 folds more susceptibility to present SLE compared with subjects who are GG genotype carriers (Table 2).
3.4. Association of TNF-α serum levels with MIF promoter polymorphisms in SLE
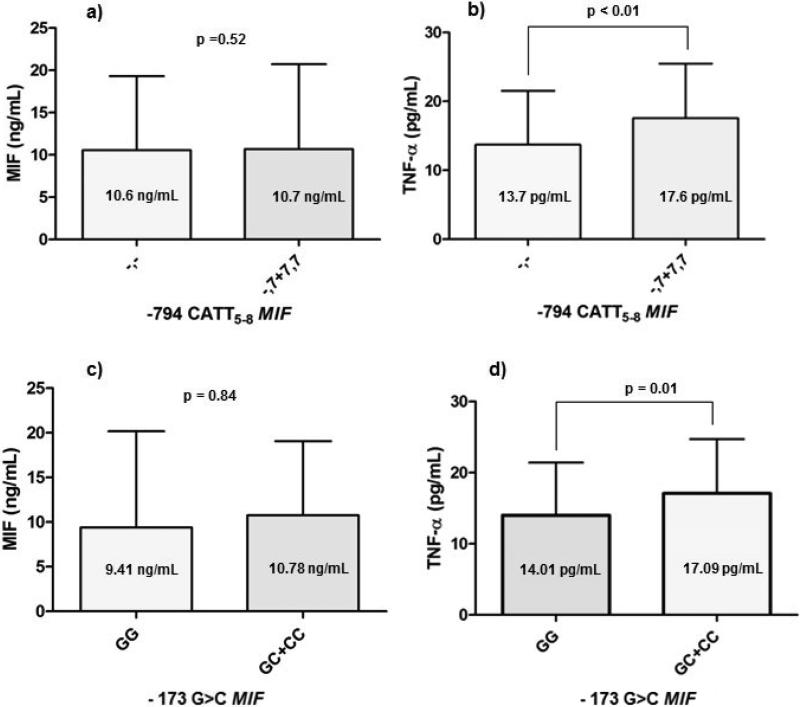
MIF and TNF-α serum levels were compared by genotypes grouped according to the dominant genetic model proposed for each polymorphism (Fig. 2).
Figure 2. MIF and TNF-α concentrations by −794 CATT5-8 −173 G>C MIF genotypes according to a dominant genetic model.
. a) MIF serum levels 10.6 ng/mL (4.80-19.32) vs. 10.7 ng/mL (4.06-20.18) by −794 CATT5-8 MIF genotypes b) TNF-α serum levels 13.7 pg / mL (8.90-21.51) vs 17.6 pg/mL (10.7-25.48) by −794 CATT5-8 MIF genotypes. c) MIF serum levels 9.41 ng/mL (4.85-20.18) vs 10.78 ng/mL (4.27-18.81) by −173 G>C MIF genotypes d) TNF-α serum levels 14.01 pg/mL (8.78-21.32) vs 17.09 pg/mL (10.64-24.64) by 173 G>C MIF genotypes. Data provided in median (p25-p75). U Mann-Whitney test.
For STR −794 CATT5-8 MIF, SLE patients with genotypes without the −794 CATT7 risk allele (-,-) had 10.6 ng/mL of MIF while patients carrying genotypes with −794 CATT7 allele risk (-, 7 +7,7) showed a slight increase of 10.7 ng/mL of MIF, this difference was not significant (p = 0.52) (Fig. 2a). Regarding the levels of TNF-α , SLE patients with genotypes without the −794 CATT7 risk allele (-,-) showed lower levels of TNF-α compared to genotypes with −794 CATT7 allele risk (-,7+7.7), which showed a significant increase of TNF-α (13.7 vs 17.6 pg/mL, p <0.01) (Fig. 2b).
In the case of SNP -173 G>C MIF, SLE patients with GG genotype had 9.41 ng/mL of MIF while patients carrying genotypes with the −173*C risk allele carriers (GC+CC) had a slight increase of 10.78 ng/mL, this difference was not significant (p = 0.84) (Fig. 2C). Regarding the levels of TNF-α , SLE patients with GG genotype showed lower TNF-α serum levels compared to genotypes of −173*C risk allele carriers (GC+CC) which had a significant increase of TNF-α (14.01 vs 17.09 pg/mL, p <0.01) (Fig. 2d).
To estimate the contribution of both MIF polymorphisms to serum levels of TNF-α, multiple linear regression models were used. After adjustment by gender, it was determined that the genotype −173 GC MIF was associated with a significant increase of TNF-α (β1=9.35; IC95%=1.55-17.14; R2 = 0.04; p= 0.01). However, we did not find a relationship of the −794 CATT5-8 MIF genotype with TNF-α (data not shown).
4. Discussion
In the present study we evaluated the association of the functionally relevant −794 CATT5-8 and -173 G>C MIF polymorphisms with SLE as well as with MIF and TNF- α serum levels in Mexican Mestizo patients. Regarding the genotype and allele frequencies, both polymorphisms were distributed similarly to those reported in our previous study of a Mexican-Mestizo population by Llamas-Covarrubias et al. [22] Unlike this previous study, in the case of the −794 CATT5-8 MIF polymorphism we did not observe the presence of genotypes with the −794 CATT8 high expression allele which was reported in low frequency (1%) in Mexican Mestizo patients with rheumatoid arthritis (RA). When we compared genotype and allele frequencies of both polymorphisms with frequencies reported in European Caucasians populations in the Netherlands [18], a Spanish population, [27] Caucasian Americans and African Americans populations [28] we observed that the genotype frequencies in our population were distributed similarly to those reported in these previous studies. However, in our study we observed a higher frequency of −794 CATT6,−794 CATT7, and −173*C MIF alleles.
The differences in the distribution of allele frequencies in both MIF gene polymorphisms may be attributed to the sample size and inclusion criteria in each study; besides these differences may be attributed to the racial influence, which is important in the global population stratification of the MIF locus [23]. It is known that the Mexican population originated from a mixture of European (60-64%), African (15%), and Amerindian groups (21-25%), giving origin to the Mexican-Mestizo population which has a higher genetic diversity in the distribution of these and other polymorphisms [38]. It has also been determined in previous studies that the STR −794 CATT5-8 MIF is distributed in relationship to the migration patterns of human populations, with an increase in high-expressing alleles accordingly as human populations become more diverse [23,39], which may explain the observed differences in the present study.
As an important finding in our study we found that the −794 CATT7 allele carriers are 1.5 fold more susceptible to present with SLE and for 6,7 repeats genotype carriers the susceptibility increases 1.83 fold.
By applying a dominant inheritance genetic model, these associations were confirmed, with an OR of 1.86 for genotypes carrying the −794 CATT7 risk allele for SLE compared with subjects who are carriers of genotypes without the risk allele. In the case of −173 G>C MIF polymorphism significant differences in the distribution of allele frequencies were found, −173*C allele carriers present 1.40 fold more susceptibility to SLE. By applying a similar genetic model of dominant inheritance we highlighted differences by study group, finding an OR of 1.64 for the −173*C risk allele carriers to present with SLE.
Nevertheless, the associations found in our study differ from those reported by Sreih et al in Caucasians and African Americans with SLE [28], because in these populations a protective effect of −794 CATT7 and −173*C high expression alleles in the incidence of SLE has been attributed. In this study, the results shown in Caucasian and African Americans populations highlight the dual effect of MIF in the pathogenesis of SLE, where it has been proposed that high expression alleles are associated with reduced susceptibility to SLE in early stages of the disease, possibly by increasing the activity of macrophages in the clearance of infectious agents or immune complexes, but once the disease is established the low expression alleles protect against organ damage and the presence of high expression alleles are pathogenic and contribute to the manifestation of the disease at inflammation sites [28,39].
Several studies support the role of the −794 CATT7 and −173*C high expression alleles in the pathogenesis of autoimmune diseases and in genetic studies with autoimmune diseases, the high expression alleles has been associated with the severity of the clinical manifestations of diseases such as scleroderma, rheumatoid arthritis and psoriasis [18,29,40]. In our previous study by Llamas-Covarrubias et al, the association of the −794 CATT7 and −173*C high expression alleles with early onset of RA and disease activity was reported in a Mexican-Mestizo population, where both high expression alleles presented a strong linkage disequilibrium (LD = 0.87, p <0.001) which indicates that both alleles are segregated in block from one generation to another and may confer a similar risk [22]. In a Spanish population with SLE, Sánchez et al also reported the association of −794 CATT7 and −173*C high expression alleles with increased susceptibility to present SLE (OR = 2, p <0.01) [27]. This association is similar to that reported in our study in SLE, which differs significantly with the reported for Caucasians and African Americans in the study of Sreih et al [28]. These differences between populations highlight the role of racial influence in the susceptibility to SLE, and the role of genetic factors in the predisposition to autoimmunity.
Experimental studies have reported the functional effect of polymorphisms in the MIF promoter. With respect to the −794CATT5-8 polymorphism gene reporter assays have demonstrated that the CATT5 allele has the lowest transcriptional activity [30] while carriers of higher repeats alleles (6 to 8 repeats) show higher MIF levels [18–20]. These findings provide a biological support to the associations observed in our study.
MIF is functionally distinguished from other cytokines by its upstream immunoregulatory action on immune cells and to the positive feedback loop between MIF and pro-inflammatory cytokine TNF-α , which is a primary mediator of damage to vascular endothelial cells of organs [4]. In our study, serum levels of TNF-α (23.01 pg/mL) and MIF (10.74 ng/mL) were significantly increased in the group of patients with SLE and a positive correlation was observed between serum levels of these two cytokines (r = 0.43); this finding also was consistent with previously reported studies [22,28,42].
The increase of TNF-α in SLE patients was consistent with the reports in Mexican-Mestizo, Caucasian and African American populations [22,28]. In other Caucasian populations, elevated MIF levels have been correlated with indices of organ damage in patients with SLE [42], however we could not replicate these associations in our population.
Serum MIF levels in SLE patients were found to be above the normal values reported for clinically healthy subjects (2-6 ng/mL) [43]. In our previous study in a Mexican-Mestizo population Llamas-Covarrubias et al reported values of 9.5 ng/mL of MIF in RA patients [41] which is consistent with our results in SLE patients. However, the results of our study group contrast with those reported in Caucasian American and African Americans with SLE which on average show normal values of MIF (4.6 and 5.6 ng/mL, respectively) [28]. The factors by which these variations in MIF serum levels between populations could be attributed to racial differences in additional risk loci and differences in the clinical characteristics of the studied groups, including the duration of disease, disease activity, and the effects of immunosuppressive therapy including glucocorticoid administration [42].
To evaluate the association of MIF polymorphisms with MIF and TNF-α levels, we stratified our data according to the dominant inheritance genetic models proposed for both MIF polymorphisms. However both polymorphisms did not show significant differences with MIF levels. This differs with reports in European, Caucasian American and African Americans populations [18,19,28,44]. In the previous study by our research group in RA [22] and in the present study in SLE, we were not able to replicate the association of MIF polymorphisms with MIF serum levels, possibly due to differences in the genetic structure of our population which may influence activity at the MIF gene locus. Recently, it was demonstrated that the transcriptional repressor HBP1 inhibits MIF gene transcription by interacting with −811 to −792 bp of the MIF promoter [44], which coincides with the polymorphic region for the CATT repeat. Nevertheless, the effect of this transcriptional repressor in the −794 CATT5-8 MIF alleles has not been evaluated and further studies are required to demonstrate the functional regulation of the MIF gene in different immunologic contexts.
An important finding in SLE patients with genotypes without the −794 CATT7 risk allele are lower levels of TNF-α compared with genotypes with the −794 CATT7 risk allele (13.7 vs 17.6 pg/mL, respectively). In the case of SNP -173 G>C, a similar pattern was observed, SLE patients with the −173 GG genotype showed lower levels of TNF-α compared with genotypes with the −173*C risk allele (14.01 vs 17.09 pg/mL, respectively) and the −173 GC genotype containing the −173*C risk allele was associated with an increase of 9.35 pg/mL of TNF-α. Accordingly, 4% of the variability in TNF- serum levels may be attributed to the presence of GC genotype.
We describe for the first time in a Mexican-Mestizo population that MIF polymorphism may contribute to a significant increase of TNF-α serum levels. A possible explanation for this finding could be the well-documented positive feedback loop between MIF and pro-inflammatory cytokine such as TNF-α reported in several studies [18,22,28,29,40,42]. In murine models, it has been reported that immunoneutralization of MIF decreases the production of TNF-α [45–47] and in vitro studies have shown that MIF is a potent inducer of TNF-α in macrophages [13,37,48].
The relationship between genotypes carrying the −794 CATT7 and −173*C high expression alleles with TNF-α serum levels is partly explained by the positive feedback loop between MIF and TNF-α . SLE is mainly mediated by immune complexes; the role of MIF in the pathogenesis of SLE could include activation and migration of macrophages at sites of inflammation [15]. Deposition of immune complexes in the microvasculature tends to induce the production of high levels of TNF-α in macrophages, which promotes inflammation and subsequent tissue damage [16]. The presence of the −794 CATT7 and −173*C high expression alleles therefore may contribute to exacerbate this inflammatory response.
One of the limitations in our study was heterogeneity in terms of therapy, and the duration and stage of disease evolution in patients with SLE. Furthermore, the quantification of cytokines in serum by ELISA may not accurately reflect the increased expression of MIF in inflammation sites, so these finding must be interpreted with caution.
In conclusion, our findings in a Mexican-Mestizo population provide evidence that MIF polymorphisms are associated with susceptibility to SLE and MIF serum levels may contribute to disease persistence by the induction of TNF-α to promote inflammatory responses that are characteristic of SLE.
Acknowledgments
This study was supported by Grant No. 180663 (JFMV) from the National Council of Science and Technology (CONACYT Ciencia Básica-Universidad de Guadalajara) and in part by Grant NIH AR-050498 (RB). The funding source had no involvement on any steps of the study.
Abbreviations
- SLE
Systemic lupus erythematosus
- MIF
macrophage migration inhibitory factor
- TNF-α
tumor necrosis factor alpha
- STR
short tandem repeat
- SNP
single nucleotide polymorphism
- bp
base pair
- ACR
American College of Rheumatology
- Mex-SLEDAI
Mexican-systemic lupus erythematosus-disease activity index
- SLICC
systemic lupus international collaborating clinics
- gDNA
genomic DNA
- PCR
polymerase chain reaction
- RFLP
restriction fragment length polymorphism
- AgNO3
silver nitrate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Manson JJ, Rahman A. Systemic lupus erythematosus. Orphanet J Rare Dis. 2006;1:6. doi: 10.1186/1750-1172-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsokos GC. Systemic lupus erythematosus. N Engl J Med. 2011;365:2110–21. doi: 10.1056/NEJMra1100359. [DOI] [PubMed] [Google Scholar]
- 3.Rahman A, Isenberg DA. Systemic lupus erythematosus. N Engl J Med. 2008;358:929–39. doi: 10.1056/NEJMra071297. [DOI] [PubMed] [Google Scholar]
- 4.Pons-Estel GJ, Alarcón GS, Scofield L, Reinlib L, Cooper GS. Understanding the epidemiology and progression of systemic lupus erythematosus. Semin Arthritis Rheum. 2010;39:257–68. doi: 10.1016/j.semarthrit.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crispín JC, Liossis S-NC, Kis-Toth K, Lieberman LA, Kyttaris VC, Juang Y-T, et al. Pathogenesis of human systemic lupus erythematosus: recent advances. Trends Mol Med. 2010;16:47–57. doi: 10.1016/j.molmed.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moser KL, Kelly JA, Lessard CJ, Harley JB. Recent insights into the genetic basis of systemic lupus erythematosus. Genes Immun. 2009;10:373–9. doi: 10.1038/gene.2009.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krishnamurthy S, Mahadevan S. Systemic Lupus Erythematosus: Recent Concepts in Genomics, Pathogenetic Mechanisms, and Therapies. ISRN Immunol. 2011;2011:1–7. [Google Scholar]
- 8.Yap DYH, Lai KN. Cytokines and their roles in the pathogenesis of systemic lupus erythematosus: from basics to recent advances. J Biomed Biotechnol. 2010;2010:365083. doi: 10.1155/2010/365083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu L-J, Yang X, Yu X-Q. Anti-TNF-alpha therapies in systemic lupus erythematosus. J Biomed Biotechnol. 2010;2010:465898. doi: 10.1155/2010/465898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kariuki SN, Niewold TB. Genetic regulation of serum cytokines in systemic lupus erythematosus. Transl Res J Lab Clin Med. 2010;155:109–17. doi: 10.1016/j.trsl.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohl K, Tenbrock K. Inflammatory cytokines in systemic lupus erythematosus. J Biomed Biotechnol. 2011;2011:432595. doi: 10.1155/2011/432595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calandra T, Roger T. Macrophage migration inhibitory factor: a regulator of innate immunity. Nat Rev Immunol. 2003;3:791–800. doi: 10.1038/nri1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aeberli D, Leech M, Morand EF. Macrophage migration inhibitory factor and glucocorticoid sensitivity. Rheumatol Oxf Engl. 2006;45:937–43. doi: 10.1093/rheumatology/kel142. [DOI] [PubMed] [Google Scholar]
- 14.Bacher M, Metz CN, Calandra T, Mayer K, Chesney J, Lohoff M, et al. An essential regulatory role for macrophage migration inhibitory factor in T-cell activation. Proc Natl Acad Sci U S A. 1996;93:7849–54. doi: 10.1073/pnas.93.15.7849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Denkinger CM, Metz C, Fingerle-Rowson G, Denkinger MD, Forsthuber T. Macrophage migration inhibitory factor and its role in autoimmune diseases. Arch Immunol Ther Exp (Warsz) 2004;52:389–400. [PubMed] [Google Scholar]
- 16.Postal M, Appenzeller S. The role of Tumor Necrosis Factor-alpha (TNF- ) in the pathogenesis of systemic lupus erythematosus. Cytokine. 2011;56:537–43. doi: 10.1016/j.cyto.2011.08.026. [DOI] [PubMed] [Google Scholar]
- 17.Yende S, Angus DC, Kong L, Kellum JA, Weissfeld L, Ferrell R, et al. The influence of macrophage migration inhibitory factor gene polymorphisms on outcome from community-acquired pneumonia. FASEB J Off Publ Fed Am Soc Exp Biol. 2009;23:2403–11. doi: 10.1096/fj.09-129445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Radstake TRDJ Sweep FCGJ, Welsing P, Franke B, Vermeulen SHHM, Geurts-Moespot A, et al. Correlation of rheumatoid arthritis severity with the genetic functional variants and circulating levels of macrophage migration inhibitory factor. Arthritis Rheum. 2005;52:3020–9. doi: 10.1002/art.21285. [DOI] [PubMed] [Google Scholar]
- 19.Renner P, Roger T, Bochud P-Y, Sprong T, Sweep FCGJ, Bochud M, et al. A functional microsatellite of the macrophage migration inhibitory factor gene associated with meningococcal disease. FASEB J Off Publ Fed Am Soc Exp Biol. 2012;26:907–16. doi: 10.1096/fj.11-195065. [DOI] [PubMed] [Google Scholar]
- 20.Donn R, Alourfi Z, De Benedetti F, Meazza C, Zeggini E, Lunt M, et al. Mutation screening of the macrophage migration inhibitory factor gene: positive association of a functional polymorphism of macrophage migration inhibitory factor with juvenile idiopathic arthritis. Arthritis Rheum. 2002;46:2402–9. doi: 10.1002/art.10492. [DOI] [PubMed] [Google Scholar]
- 21.Donn R, Alourfi Z, Zeggini E, Lamb R, Jury F, Lunt M, et al. A functional promoter haplotype of macrophage migration inhibitory factor is linked and associated with juvenile idiopathic arthritis. Arthritis Rheum. 2004;50:1604–10. doi: 10.1002/art.20178. [DOI] [PubMed] [Google Scholar]
- 22.Llamas-Covarrubias MA, Valle Y, Bucala R, Navarro-Hernández RE, Palafox-Sánchez CA, Padilla-Gutiérrez JR, et al. Macrophage migration inhibitory factor (MIF): Genetic evidence for participation in early onset and early stage rheumatoid arthritis. Cytokine. 2013;61:759–65. doi: 10.1016/j.cyto.2012.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhong X, Leng L, Beitin A, Chen R, McDonald C, Hsiao B, et al. Simultaneous detection of microsatellite repeats and SNPs in the macrophage migration inhibitory factor (MIF) gene by thin-film biosensor chips and application to rural field studies. Nucleic Acids Res. 2005;33:e121. doi: 10.1093/nar/gni123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oliver J, Márquez A, Gómez-Garcia M, Martinez A, Mendoza JL, Vilchez JR, et al. Association of the macrophage migration inhibitory factor gene polymorphisms with inflammatory bowel disease. Gut. 2007;56:150–1. doi: 10.1136/gut.2006.107649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bossini-Castillo L, Simeon CP, Beretta L, Vonk MC, Callejas-Rubio JL, Espinosa G, et al. Confirmation of association of the macrophage migration inhibitory factor gene with systemic sclerosis in a large European population. Rheumatol Oxf Engl. 2011;50:1976–81. doi: 10.1093/rheumatology/ker259. [DOI] [PubMed] [Google Scholar]
- 26.Núñez C, Rueda B, Martínez A, López-Nevot MA, Fernández-Arquero M, de la Concha EG, et al. Involvement of macrophage migration inhibitory factor gene in celiac disease susceptibility. Genes Immun. 2007;8:168–70. doi: 10.1038/sj.gene.6364365. [DOI] [PubMed] [Google Scholar]
- 27.Sánchez E, Gómez LM, Lopez-Nevot MA, González-Gay MA, Sabio JM, Ortego-Centeno N, et al. Evidence of association of macrophage migration inhibitory factor gene polymorphisms with systemic lupus erythematosus. Genes Immun. 2006;7:433–6. doi: 10.1038/sj.gene.6364310. [DOI] [PubMed] [Google Scholar]
- 28.Sreih A, Ezzeddine R, Leng L, LaChance A, Yu G, Mizue Y, et al. Dual effect of the macrophage migration inhibitory factor gene on the development and severity of human systemic lupus erythematosus. Arthritis Rheum. 2011;63:3942–51. doi: 10.1002/art.30624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Donn RP, Plant D, Jury F, Richards HL, Worthington J, Ray DW, et al. Macrophage migration inhibitory factor gene polymorphism is associated with psoriasis. J Invest Dermatol. 2004;123:484–7. doi: 10.1111/j.0022-202X.2004.23314.x. [DOI] [PubMed] [Google Scholar]
- 30.Baugh JA, Chitnis S, Donnelly SC, Monteiro J, Lin X, Plant BJ, et al. A functional promoter polymorphism in the macrophage migration inhibitory factor (MIF) gene associated with disease severity in rheumatoid arthritis. Genes Immun. 2002;3:170–6. doi: 10.1038/sj.gene.6363867. [DOI] [PubMed] [Google Scholar]
- 31.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–7. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 32.Guzmán J, Cardiel MH, Arce-Salinas A, Sánchez-Guerrero J, Alarcón-Segovia D. Measurement of disease activity in systemic lupus erythematosus. Prospective validation of 3 clinical indices. J Rheumatol. 1992;19:1551–8. [PubMed] [Google Scholar]
- 33.Gladman D, Ginzler E, Goldsmith C, Fortin P, Liang M, Urowitz M, et al. The development and initial validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index for systemic lupus erythematosus. Arthritis Rheum. 1996;39:363–9. doi: 10.1002/art.1780390303. [DOI] [PubMed] [Google Scholar]
- 34.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Makhija R, Kingsnorth A, Demaine A. Gene polymorphisms of the macrophage migration inhibitory factor and acute pancreatitis. JOP J Pancreas. 2007;8:289–95. [PubMed] [Google Scholar]
- 36.Dean A, Sullivan K, Soe M. OpenEpi: Open Source Epidemiologic Statistics for Public Health. [ http://www.openepi.com]
- 37.Calandra T, Bernhagen J, Mitchell RA, Bucala R. The macrophage is an important and previously unrecognized source of macrophage migration inhibitory factor. J Exp Med. 1994;179:1895–902. doi: 10.1084/jem.179.6.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rangel-Villalobos H, Muñoz-Valle JF, González-Martín A, Gorostiza A, Magaña MT, Páez-Riberos LA. Genetic admixture, relatedness, and structure patterns among Mexican populations revealed by the Y-chromosome. Am J Phys Anthropol. 2008;135:448–61. doi: 10.1002/ajpa.20765. [DOI] [PubMed] [Google Scholar]
- 39.Bucala R. MIF, MIF Alleles, and Prospects for Therapeutic Intervention in Autoimmunity. J Clin Immunol. 2012;33:72–8. doi: 10.1007/s10875-012-9781-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu S-P, Leng L, Feng Z, Liu N, Zhao H, McDonald C, et al. Macrophage migration inhibitory factor promoter polymorphisms and the clinical expression of scleroderma. Arthritis Rheum. 2006;54:3661–9. doi: 10.1002/art.22179. [DOI] [PubMed] [Google Scholar]
- 41.Llamas-Covarrubias MA, Valle Y, Navarro-Hernández RE, Guzmán-Guzmán IP, Ramírez-Dueñas MG, Rangel-Villalobos H, et al. Serum levels of macrophage migration inhibitory factor are associated with rheumatoid arthritis course. Rheumatol Int. 2012;32:2307–11. doi: 10.1007/s00296-011-1951-6. [DOI] [PubMed] [Google Scholar]
- 42.Foote A, Briganti EM, Kipen Y, Santos L, Leech M, Morand EF. Macrophage migration inhibitory factor in systemic lupus erythematosus. J Rheumatol. 2004;31:268–73. [PubMed] [Google Scholar]
- 43.Donn RP, Ray DW. Macrophage migration inhibitory factor: molecular, cellular and genetic aspects of a key neuroendocrine molecule. J Endocrinol. 2004;182:1–9. doi: 10.1677/joe.0.1820001. [DOI] [PubMed] [Google Scholar]
- 44.Chen YC, Zhang XW, Niu XH, Xin DQ, Zhao WP, Na YQ, et al. Macrophage migration inhibitory factor is a direct target of HBP1-mediated transcriptional repression that is overexpressed in prostate cancer. Oncogene. 2010;29:3067–78. doi: 10.1038/onc.2010.97. [DOI] [PubMed] [Google Scholar]
- 45.Leech M, Metz C, Santos L, Peng T, Holdsworth SR, Bucala R, et al. Involvement of macrophage migration inhibitory factor in the evolution of rat adjuvant arthritis. Arthritis Rheum. 1998;41:910–7. doi: 10.1002/1529-0131(199805)41:5<910::AID-ART19>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 46.Santos L, Hall P, Metz C, Bucala R, Morand EF. Role of macrophage migration inhibitory factor (MIF) in murine antigen-induced arthritis: interaction with glucocorticoids. Clin Exp Immunol. 2001;123:309–14. doi: 10.1046/j.1365-2249.2001.01423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Onodera S, Ohshima S, Tohyama H, Yasuda K, Nishihira J, Iwakura Y, et al. A novel DNA vaccine targeting macrophage migration inhibitory factor protects joints from inflammation and destruction in murine models of arthritis. Arthritis Rheum. 2007;56:521–30. doi: 10.1002/art.22407. [DOI] [PubMed] [Google Scholar]
- 48.Calandra T, Bernhagen J, Metz CN, Spiegel LA, Bacher M, Donnelly T, et al. MIF as a glucocorticoid-induced modulator of cytokine production. Nature. 1995;377:68–71. doi: 10.1038/377068a0. [DOI] [PubMed] [Google Scholar]