Summary
Background
Spontaneous “miniature” transmitter release takes place at low rates at all synapses. Long thought as an unavoidable leak, spontaneous release has recently been suggested to be mediated by distinct pre- and post-synaptic molecular machineries and to have a specialized role in setting up and adjusting neuronal circuits. It remains unclear how spontaneous and evoked transmission are related at individual synapses, how they are distributed spatially when an axon makes multiple contacts with a target and whether they are commonly regulated.
Results
Electrophysiological recordings in the Drosophila larval neuromuscular junction, in the presence of the use-dependent glutamate receptor (GluR) blocker Philanthotoxin, indicated that spontaneous and evoked transmission employ distinct sets of GluRs. In vivo imaging of transmission using synaptically-targeted GCaMP3 to detect Ca2+ influx through the GluRs revealed little spatial overlap between synapses participating in spontaneous and evoked transmission. Spontaneous and evoked transmission were oppositely correlated with presynaptic levels of the protein Brp: synapses with high Brp favored evoked transmission, whereas synapses with low Brp were more active spontaneously. High frequency stimulation did not increase the overlap between evoked and spontaneous transmission, and instead decreased the rate of spontaneous release from synapses that were highly active in evoked transmission.
Conclusions
While individual synapses can participate in both evoked and spontaneous transmission, highly-active synapses show a preference for one mode of transmission. The presynaptic protein Brp promotes evoked transmission and suppresses spontaneous release. These findings suggest the existence of presynaptic mechanisms that promote synaptic specialization to either evoked or spontaneous transmission.
Introduction
Neuronal synapses are specialized sites for fast information transfer, poised to release neurotransmitter within a millisecond of arrival of the action potential. In addition to this evoked transmission, synapses also release small packets of neurotransmitter spontaneously [1]. Classically, presynaptic vesicles, their release sites and the cluster of postsynaptic receptors that respond to the transmitter were taken to be the same for evoked and spontaneous transmission, permitting the calculation of quantal content from the ratio of amplitudes of evoked to spontaneous currents [2]. Spontaneous transmission does not appear to carry information equivalent to that of evoked transmission, because it elicits such a small postsynaptic response, lacks coordination across synapses, and occurs at a very low rate. Originally dismissed as unavoidable random leakage, spontaneous transmission has been re-examined in recent years due to emerging evidence that it could have specialized physiological roles in the development and function of neuronal circuits, and could be mediated by pre- and postsynaptic machinery distinct from that of evoked transmission. [3–5]. A central issue that is brought up by these investigations is the question of how, with specialized synaptic function and transmission proteins, evoked and spontaneous transmission can co-exist side by side in the same neuronal compartments.
Here, we employed electrophysiological recording and quantal-resolution postsynaptic Ca2+ imaging of synaptic transmission in the Drosophila larval neuromuscular junction (NMJ) to ask to what degree spontaneous and evoked transmission employ the same set of synapses. We found that a use-dependent blocker of GluRs inhibits spontaneous transmission independently of evoked transmission, indicating that the two modes of transmission rely on different sets of GluRs. Quantal-resolution imaging showed that individual synapses can participate in both evoked and spontaneous transmission, but the most active synapses have a propensity for one of these transmission modes.
Searching for a possible molecular basis for this type of synaptic specialization, we compared single-synapse transmission properties to presynaptic levels of the scaffolding protein Brp. We found that synapses with high presynaptic Brp levels are more active under evoked transmission, while synapses with low Brp levels favor spontaneous transmission. In mutants where Brp is absent or not fully functional, spontaneous miniature release frequency is increased. These findings suggest that, in addition to its established role in promoting evoked release, Brp functions to suppress spontaneous release.
Results
The activity-dependent GluR blocker PhTox blocks spontaneous miniature EPSPs independently of evoked EPSPs
The classical model of synaptic transmission holds that spontaneous transmission and action-potential evoked transmission use a common set of transmitter-loaded vesicles, occur at the same synapses and activate the same postsynaptic receptors, so that the spontaneous miniature excitatory postsynaptic potential (mEPSP) is one and the same as the unitary evoked excitatory postsynaptic potential (EPSP). We reasoned that, if this model were correct, then mEPSPs and EPSPs should be inhibited to the same degree and over the same time course by blockers. To test this model, we employed the use-dependent GluR blocker Philanthotoxin-433 (PhTox) [6–8], which was demonstrated to produce stable inhibition of postsynaptic responses in the standard Drosophila larval NMJ preparation [9].
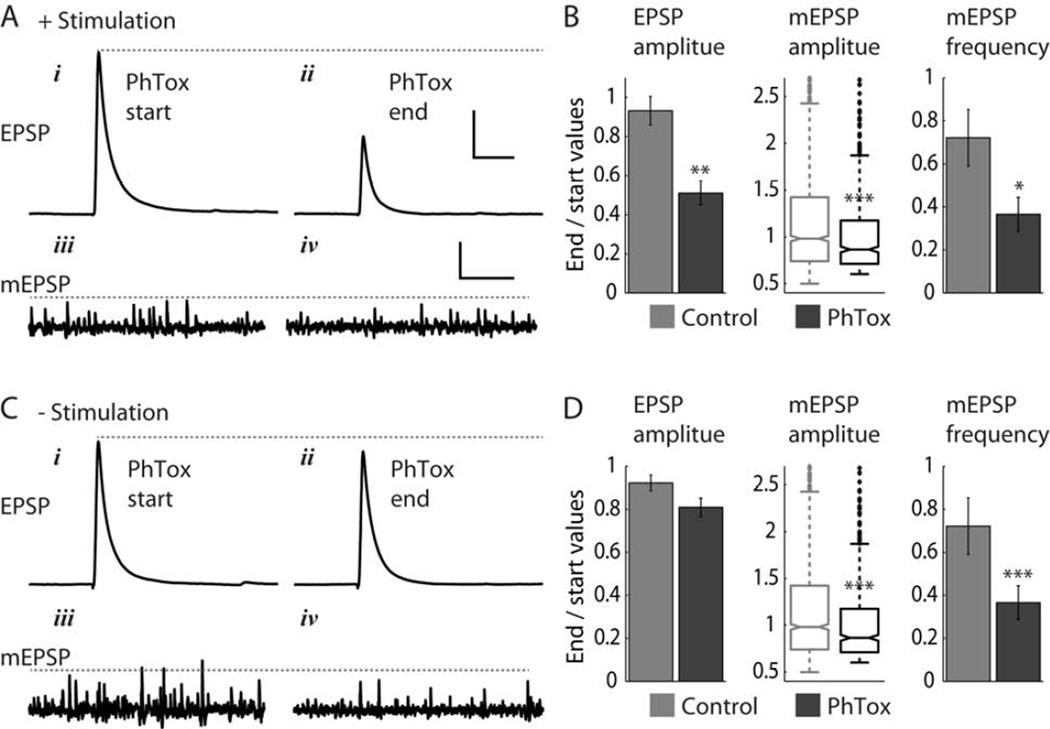
Application of PhTox (final concentration 4μM) reduced EPSP amplitude, mESPS amplitude and mEPSP frequency. At 0.1 Hz nerve stimulation and in the presence of PhTox EPSP amplitude was reduced by approximately half (to 0.51 ± 0.06 of initial values) over 25 minutes (Figure 1A – B, Figure S1A, D). In contrast, in absence of PhTox, the amplitude of EPSPs evoked at 0.1 Hz dropped to 0.93 ± 0.07 of initial amplitude (Student’s t-test p < 0.003, n = 5 NMJs for both PhTox and control experiments). mEPSPs recorded during the 0.1 Hz nerve stimulation in PhTox also decreased in amplitude, but by a smaller average amount (Figure 1A – B, Figure S1B, C, E, F). Median mEPSP amplitude dropped to 0.86 of the initial baseline amplitude (with corresponding relative quartiles Q1 = 0.71 and Q3 = 1.17), compared to a drop to 0.98 of initial median value (with Q1 = 0.74, Q3 = 1.42) in the absence of PhTox (Wilcoxon rank-sum test p < 2×10−5, n = 5 NMJs for both PhTox and control experiments). This reduction in mEPSP amplitude in PhTox was accompanied by a steep drop in mEPSP frequency (to 0.36 ± 0.08 of initial frequency; Figure 1B, Figure S1G), compared to a smaller drop in mEPSP frequency in the absence of PhTox (to 0.72 ± 0.13 of initial frequency), but both drops in mEPSP frequency were significant (Student’s t-test p < 0.05, n = 5 NMJs). We note that PhTox is expected to reduce both mEPSP amplitude and frequency: the degree of block during each transmission event will depend on the percentage of GluRs that open in each GluR postsynaptic cluster and on the concentration dependent entry of PhTox into the open channel, and mEPSP frequency will drop as mEPSPs fall below the detection threshold.
Figure 1. PhTox blocks spontaneous mEPSPs independently of evoked EPSPs.
(A, B) PhTox block during 0.1 Hz nerve stimulation. (Ai – iv) Representative experiment showing EPSP and mEPSPs traces at start of PhTox application and 25 minutes later.
(B) Quantification of block of EPSP amplitude, mEPSP amplitude, and mEPSP frequency. PhTox n = 5 NMJs, control n = 5 NMJs.
(C, D) PhTox block without nerve stimulation (brief 0.1 Hz stimulation was applied only at start and end of experiments). Panels are as in (A, B). PhTox n = 8 NMJs, control n = 7 NMJs.
Scale bars in (A, C): 100 ms and 10 mV (EPSP traces), 2 s and 0.5mV (mEPSP traces). Values reported in (B, D) are mean ± SEM (EPSP amplitude and mESPS frequency) and median and first and third quartiles (mEPSP amplitude). Statistical significance tests are Student’s t-test (EPSP amplitude and mEPSP frequency) and Wilcoxon rank sum test (mEPSP amplitude). Asterisks mark significance values: * p < 0.05, ** p < 0.003, *** p < 0.0002.
See also Figure S1.
In absence of nerve stimulation PhTox only produced a small, non-significant, decrease in EPSP amplitude (Figure 1C – D; End/Start amplitude = 0.81 ± 0.04, compared to control ratio of 0.92 ± 0.04; Student’s t-test p = 0.07, n = 8 and 7 for PhTox and control experiments, respectively), consistent with the lack of evoked activation of GluRs. Nevertheless, mEPSP amplitude and frequency decreased to the same extent as when stimulation was applied (Figure 1C – D, Figure S1G), with median amplitude dropping to 0.81 of initial value (Q1 = 0.67, Q3 = 1.11), compared to control drop to 0.92 of initial value (Q1 = 0.72, Q3 = 1.24; Wilcoxon rank-sum test p < 2×10−11, n = 8 and 7 for PhTox and control experiments, respectively) and mEPSP frequency dropping to 0.43 ± 0.03 of initial value (compared to control drop to 0.79 ± 0.07 of initial frequency, Student’s t-test p < 0.0002).
Thus, the decline in EPSP amplitude depended on stimulation but that of mEPSP amplitude did not, indicating that GluRs that are activated by evoked transmission are distinct from those that are activated by spontaneous transmission. This could arise in at least two distinct ways. One possibility is that these two types of synaptic transmission modes activate different types of GluRs within the same postsynaptic GluR cluster, and that these are therefore blocked by PhTox to different degrees. Indeed, this was the conclusion of earlier studies that employed use-dependent block with MK-801 or PhTox to compare the suppression of evoked and spontaneous miniature transmission in hippocampal neurons [10, 11]. Another possible explanation, also considered in [10], and which we investigate further below, is that evoked and spontaneous transmission employ separate sets of synapses.
Quantal imaging shows little overlap between evoked and spontaneous transmission
The above electrophysiological recordings revealed a divergence between evoked and spontaneous transmission events. However, EPSPs and mEPSPs reflect transmission pooled from hundreds of synapses that are distributed over the entire NMJ. To compare evoked and spontaneous transmission at individual synapses, we turned to optical imaging of transmission with a postsynaptically targeted, genetically-encoded, Ca2+ sensing fluorescent protein, which detects Ca2+ influx through GluRs. We previously showed that this method enables detection of both evoked and spontaneous miniature transmission events with single-synapse quantal resolution [12]. Here we employed a targeted sensor based on GCaMP3 [13], which we refer to as SynapGCaMP3. It is an improvement over its predecessor, SynapGCaMP2 [12], retaining the ability to detect synaptic transmission with quantal resolution (Figure S2) while providing a substantially better signal to noise ratio (Figure S3) and enabling a more accurate analysis of transmission properties.
We used SynapGCaMP3 to monitor the transmission statistics of synapses that participate in spontaneous miniature transmission and evoked transmission in the NMJs of wild-type larvae. To record basal levels of evoked responses (avoiding facilitation or depression), we stimulated the motor nerve once every 15 seconds. Spontaneous miniature transmission events were monitored in the intervals between nerve-stimulations. Activity maps for evoked and spontaneous transmission were compiled from multiple stimuli and intervals, respectively (typically > 50 trials for each NMJ, see Supplemental experimental procedures, Movie S1, and Figure S4).
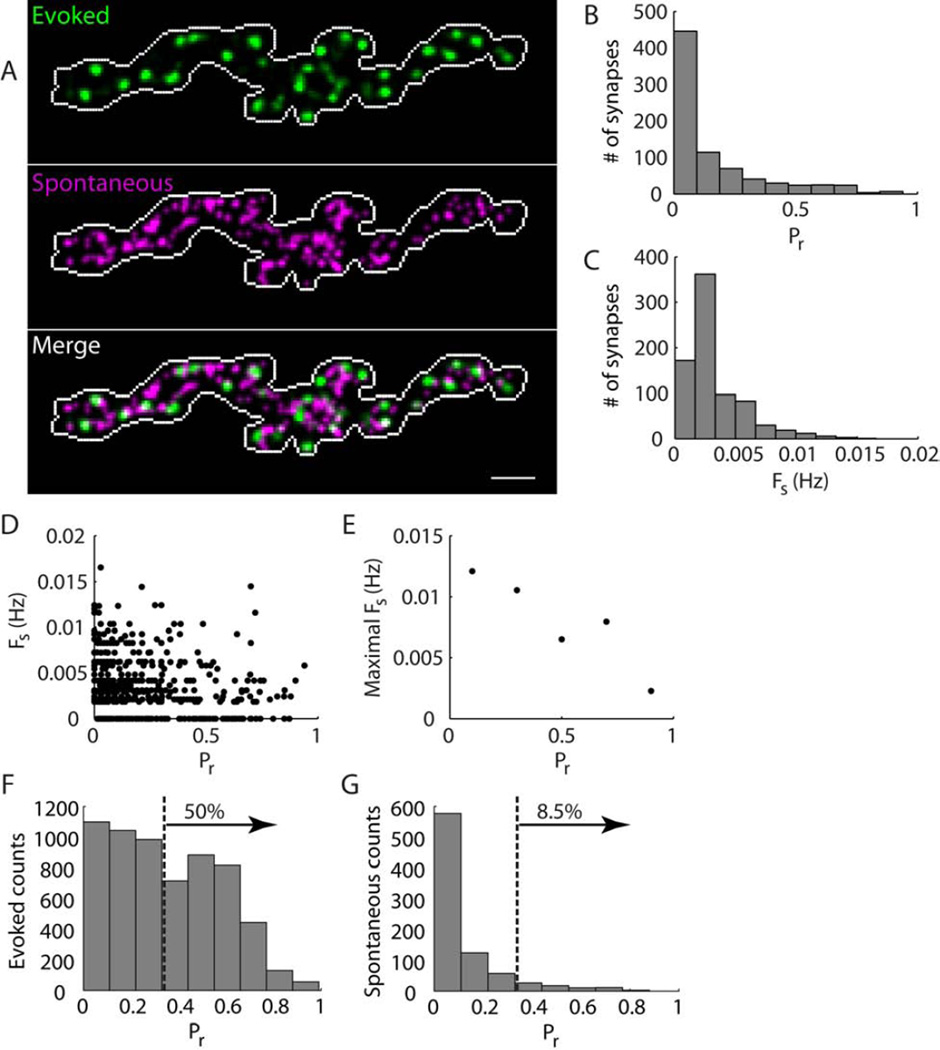
Example wild-type activity maps are shown in Figure 2A – B. The evoked activity map (Figure 2A, green) shows the distribution of probabilities of dozens of synapses in an NMJ to respond to nerve-stimulation, while the spontaneous activity map (Figure 2A, magenta) shows the rate of spontaneous transmission at each synapse. Here, we define a synapse as any location in the NMJ that showed a spontaneous or evoked transmission event. As we showed previously, individual synapses participate in evoked transmission with a wide range of probabilities [12]. In the current experiments on wild-type NMJs, evoked response probability (Pr) ranged from 0 to 0.55, with a median of 0.04 (Figure 2C), similar to our earlier results [12]. Spontaneous miniature transmission rate (Fs) also varied between synapses, ranging from 0 to 0.036 Hz with a median of 0.003 Hz (Figure 2D).
Figure 2. Contrast between evoked and spontaneous transmission in wild-type NMJs.
(A) Activity maps showing the probability of synapses to participate in evoked transmission (green) and the rate of spontaneous miniature transmission (magenta) across a wild-type NMJ. Overlap between the two patterns of activity shows in white color in the merged activity map. Maximal observed values for the NMJ shown were Pr = 0.31 and Fs = 0.023 Hz. Correlation coefficient between the activity patterns was 0.32.
(B) Blowup of activity maps for the two boutons boxed in (A). Yellow arrows point to locations that show overlap between evoked and spontaneous activity patterns.
(C, D) Histograms of Pr (C) and Fs (D) values in wild-type NMJs.
(E) Plot of Fs versus Pr for all synapses examined.
(F) Plot of maximal observed Fs over three Pr ranges. Maximal values were obtained by dividing Pr to bins of size 0.2 and calculating the mean of the top 10 Fs values for each bin.
(G, H) Histograms showing counts of evoked (G) and spontaneous (H) transmission events versus Pr. Dashed line marks the median Pr for evoked counts, Pr = 0.07. 50% of evoked and 20% of spontaneous miniature transmission events occurred at synapses with Pr > 0.07.
(C – H) Histograms show data pooled from 957 synapses at 5 wild-type NMJs.
Scale bar in (A), 5 μm.
See also Figures S2, S3, S4, and Movie S1.
The arrangement of evoked and spontaneous transmission events across the NMJ showed little overlap (Figure 2A – B). Cross-correlation between the two types of events is 0.35 ± 0.06 (n = 5 NMJs), a value that is larger than that of random arrangements but smaller than what would be expected if the two transmission modes followed the same statistics in spatial distribution (see calculations in Supplemental information). In other words, individual synapses can participate in both types of transmission, but they appear to do so with different probabilities. We next tested this interpretation with a quantitative analysis of transmission statistics to compare evoked and spontaneous miniature transmission at individual synapses.
Individual synapses can be highly active in either evoked or spontaneous transmission, but not both
To compare the levels of evoked and spontaneous activity at individual synapses, we plotted Fs versus Pr for each synapse (Figure 2E). The plot shows that the majority of synapses participate in both evoked and spontaneous miniature transmission, albeit with low levels of activity. Strikingly, synapses that are highly active show a tradeoff between the two types of transmission: synapses with high Pr tend to have low Fs and synapses with high Fs tend to have low Pr (Figure 2E – F). Another way of viewing this behavior is that while 50% of evoked release events occur in synapses with Pr > 0.07, the same set of synapses supports only 20% of spontaneous miniature release events, the remaining 80% of spontaneous events taking place at lower-Pr synapses (Figure 2G – H). Thus, while not completely segregated, evoked and spontaneous transmission rely on divergent sets of synapses: low-Pr synapses support more spontaneous than evoked transmission, while high-Pr synapses support more evoked than spontaneous transmission.
Evoked and spontaneous transmission segregate further in a mutant with sparse evoked transmission and elevated Pr
To further explore the possibility that evoked and spontaneous transmission rely on different (or partially different) sets of synapses, we turned to a mutant of the small vesicle-associated GTPase Rab3, in which evoked transmission is limited to a subset of synapses. In the rab3 mutant, the presynaptic protein Brp is concentrated in a subset of active zones in the NMJ so that about a third of active zones contain higher than normal levels of Brp, while others have little or none [14]. This is in contrast to wild-type NMJs, where almost every active zone contains Brp [15]. A consequence of this redistribution is that in rab3-mutant NMJs evoked transmission is almost entirely limited to the Brp-rich synapses [12]. We examined evoked and spontaneous transmission in rab3-mutant NMJs and asked whether spontaneous transmission is limited to the same set of synapses that participate in evoked transmission. This could be the case if, for example, synapses that are highly active in evoked transmission are also more active spontaneously, or if the altered presynaptic organization in the mutant NMJs renders the Brp-lacking synapses incapable of any type of transmission. On the other hand, if evoked and spontaneous transmission do not rely on the same set of synapses and do not require the presence of the same presynaptic machinery then spontaneous transmission may be found at sites that lack Brp.
In rab3-mutant NMJs the sites of spontaneous transmission were arrayed at a much higher density than the sites of evoked transmission, with little overlap between them (Figure 3A). Indeed, whereas the density of evoked transmission sites in the rab3 mutant was about half that of wild-type (0.29 ± 0.03 sites/µm2 in wild-type, n = 5; 0.15 ± 0.01 sites/µm2 in rab3 mutant, n = 6; Student’s t-test p < 0.0007), spontaneous transmission site density did not differ significantly between wild-type and the rab3 mutant (0.42 ± 0.05 sites/µm2 in wild-type, n = 5; 0.35 ± 0.04 sites/µm2 in rab3 mutant, n = 6; Student’s t-test p = 0.31). The divergence between the spatial distributions of sites of evoked and spontaneous transmission in the rab3 mutant was also evident in the low correlation values between the two types of activity maps (Figure 3A). The mutant correlation value was 0.16 ± 0.02, about half of the wild-type correlation value of 0.35 ± 0.06 (n = 6 and 5 NMJs for rab3-mutant and wild-type, respectively, Student’s t-test p < 0.02).
Figure 3. Contrast between evoked and spontaneous transmission in rab3-mutant NMJs.
(A) Activity maps showing the probability of synapses to participate in evoked transmission (green) and the rate of spontaneous miniature transmission (magenta) across a rab3-muatnt NMJ. Overlap between the two patterns of activity shows in white color in the merged activity map. Maximal observed values for the NMJ shown were Pr = 0.9 and Fs = 0.017 Hz. Correlation coefficient between the activity patterns was 0.19.
(B, C) Histograms of Pr (B) and Fs (C) values in rab3-mutant NMJs.
(D) Plot of Fs versus Pr for all synapses examined.
(E) Plot of maximal observed Fs over five Pr ranges. Maximal values were obtained by dividing Pr to bins of size 0.2 and calculating the mean of the top 10 Fs values for each bin.
(F, G) Histograms showing counts of evoked (F) and spontaneous (G) transmission events versus Pr. Dashed line marks the median Pr for evoked counts, Pr = 0.33. 50% of evoked and 8.5% of spontaneous miniature transmission events occurred at synapses with Pr > 0.33.
(B – G) include data pooled from 785 synapses at 6 rab3-NMJs.
Scale bar in (A), 5 μm.
Compared to wild-type NMJs, rab3-mutant synapses have a wider range of evoked probability (Figure 3B; Pr = 0 – 0.94, median 0.07), and a narrower range of spontaneous transmission rate (Figure 3C; Fs = 0 – 0.017 Hz, median 0.002 Hz). Nevertheless, individual synapses show statistics similar to those of wild-type synapses with respect to their participation in evoked and/or spontaneous transmission. As in wild-type, rab3-mutant synapses can participate in both modes of transmission, however the most active synapses show a preference for either evoked or spontaneous transmission (Figure 3D – E); and, as in wild-type NMJs, the majority of spontaneous transmission events in the rab3 mutant take place at low-Pr synapses — in this case only 8.5% of spontaneous miniature events occur at high-Pr synapses (Pr > 0.33), which carry 50% of evoked release events (Figure 3F – G). In comparison to wild-type, rab3-mutant NMJs show a more extreme divergence between synapses that support evoked and spontaneous transmission: mutant NMJs contain more high-Pr synapses, which carry more evoked events and a smaller percentage of spontaneous transmission events (compare Figure 2G – H to Figure 3F – G). Thus, the rab3 mutant confirms the wild-type results and provides an example of even more extreme contrast between evoked and spontaneous transmission.
Evoked and spontaneous transmission oppositely correlated with levels of presynaptic Brp
Having observed segregation between synapses that participate in evoked transmission and those that participate in spontaneous transmission, we asked whether this specialization has a molecular basis. In rab3-mutant NMJs most evoked transmission is concentrated at Brp-positive synapses. This made us ask whether presynaptic Brp levels are related to the preferred transmission mode of individual synapses. We started by comparing the locations of transmission events, determined by in vivo imaging, to locations of Brp puncta, determined by fixing and antibody staining of the preparations immediately after in vivo imaging. For each rab3-mutant recording we marked the locations of evoked and spontaneous transmission events on top of the corresponding Brp staining image and found that evoked transmission events are clustered at Brp puncta (Figure 4A and Figure S5A – B). Spontaneous transmission events are often situated away from Brp puncta (Figure 4A and Figure S5B), but occur at locations that contain the postsynaptic GluRIIC subunit (Figure S5C), indicating that spontaneous transmission takes place mostly at synaptic sites that lack presynaptic Brp. Consistent with this, at individual synapses evoked and spontaneous transmission were both correlated with presynaptic Brp level, but in opposite ways: Pr increased with increasing levels of Brp, in agreement with earlier reports [12, 14], while Fs decreased with increasing Brp level (Figure 4B). These data provide an independent confirmation of the preference of individual synapses for either evoked or spontaneous transmission and suggest that Brp is a good predictor of a synapse’s preferred transmission mode.
Figure 4. Evoked and spontaneous transmission are oppositely correlated with levels of the presynaptic protein Brp.
(A) Comparison of Brp staining to locations of evoked and spontaneous transmission in the NMJ. Locations where transmission was detected during live imaging are marked in white on top of a Brp staining image. Note that each marked location typically experienced multiple transmission events over the time course of the experiment.
(B) Correlation of single-synapse presynaptic Brp levels with Pr (left) and Fs (right). Reported values are mean ± SEM. Figures include data from 729 synapses (showing either evoked or spontaneous transmission, or presynaptic Brp with no transmission) at 6 rab3-mutant NMJs (Student’s t-test, ** p < 0.006).
Scale bar in (A), 5 µm.
See also Figure S5.
Increased rate of spontaneous miniature release in brp mutants
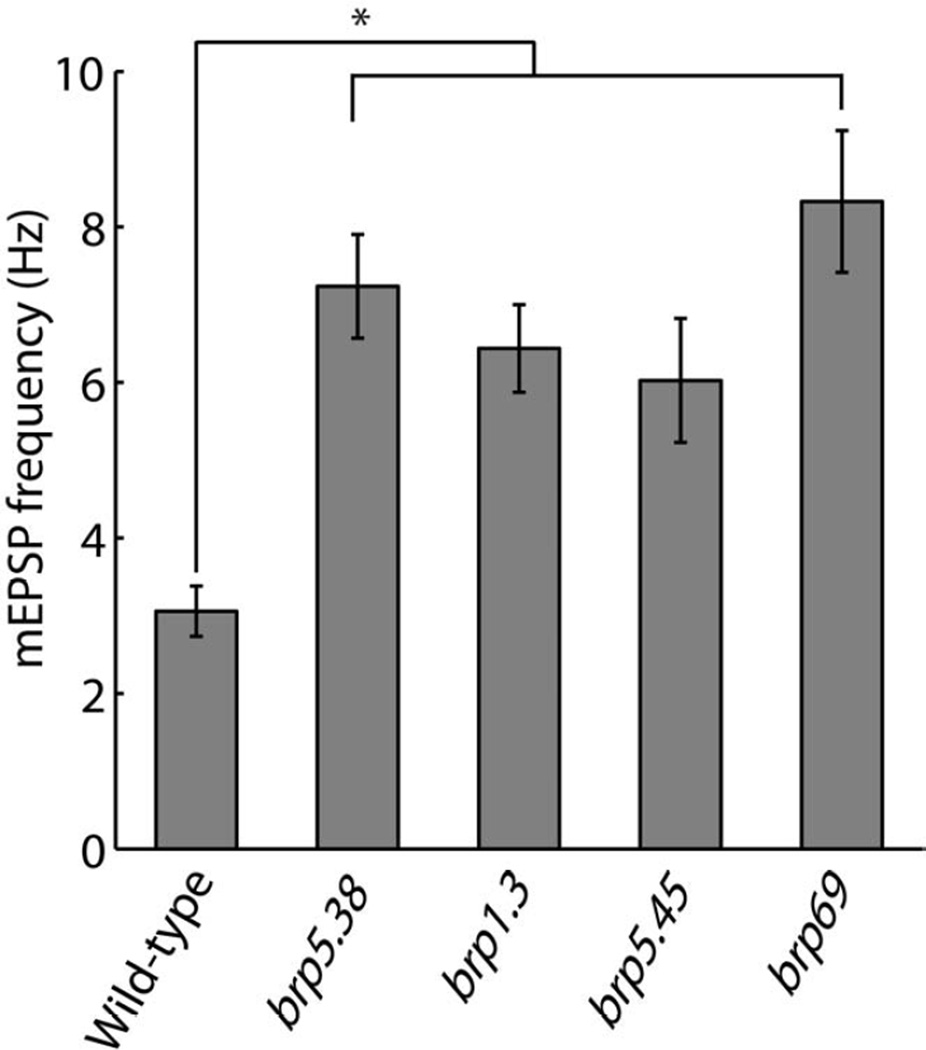
The opposite correlation of Brp level with evoked and spontaneous transmission raises the question of whether, in addition to its function in promoting active zone assembly and evoked release [12, 16], Brp also functions to suppress spontaneous release. To address this question we compared spontaneous mEPSP frequency in wild-type and brp-mutant NMJs. We examined four different brp mutant alleles which are missing progressively larger parts of the C-terminal of the Brp protein, resulting in progressively larger disruptions in presynaptic active-zone ultrastructure and synaptic function [15–17]. brp5.38 mutants are missing 17 amino acids of the Brp C-terminal (~1% of the protein), brp1.3 and brp5.45 mutants are missing ~30% and ~50% of the Brp protein, respectively, and brp69 is missing most of the Brp protein, and is functionally and structurally equivalent to a null mutant [16, 17]. All mutants showed an increase in mEPSP frequency, to more than twice the mEPSP frequency in wild-type NMJs (Figure 5, Figure S6A). The elevated spontaneous release frequency was also evident in SynapGCaMP3 imaging of brp69-mutnat NMJs, and analysis of single-synapse properties indicated that this was due to an elevated spontaneous release rate at individual synapses (Movies S2 and S3, Figure S6B).
Figure 5. Increased mEPSP frequency in brp mutants.
mEPSP frequency in wild-type and brp-mutant NMJs. All brp mutants had one copy of the indicated mutant allele over the corresponding second-chromosome deficiency BSC29. All brp mutants show elevated mEPSP frequency significantly higher than wild-type values.
Reported values are mean ± SEM. n = 16, 12, 10, 10 and 8 for wild-type, brp5.38, brp1.3, brp5.45 and brp69 NMJs, respectively. One-way ANOVA p < 1.1×10−7. Asterisk marks pairwise significance from post-hoc comparison with Bonferroni criterion (* p < 0.01).
An earlier study reported that spontaneous release frequency is not enhanced in the brp69 mutant [15]. We note that in this study wild-type spontaneous release frequency was 1.55 Hz, almost two times lower than our wild-type frequency of 3.1 Hz, and brp69 spontaneous frequency was 1.87 Hz – higher than the wild-type value but not statistically significant. The differences in findings between the current and earlier study could originate from differences in experimental conditions (see experimental details in Supplemental experimental procedures), such as muscle type (muscle 4 in the current study vs. muscle 6 in [15]), external [Ca2+] in recording solution (1.5 mM vs. 1 mM), electrophysiology recording mode (Bridge vs. TEVC), genetic background of flies (w1118 with SynapGCaMP3 vs. Gal4 driver elav), or other conditions which could result in a different baseline wild-type spontaneous release frequency. We find significantly elevated brp69-mutant mEPSP frequency in the same recording solution and muscle as in [15] (Figure S6C), indicating that these two factors are not responsible for the differences between the two studies.
Thus, we find that synapses spontaneously release neurotransmitter with a higher than normal frequency when Brp is absent or when Brp function is impaired, indicating that the presynaptic presence of functional Brp suppresses spontaneous release. It is striking that mEPSP frequency is elevated even in the brp5.38 mutant, where only a small part of the Brp C-terminal is missing. In this mutant, synapses have a normal presynaptic ultrastructure, with the exception that synaptic vesicles are not tethered to the presynaptic T-bar density as they are in wild-type synapses [17]. This suggests that the tethering of synaptic vesicles by Brp is sufficient to suppress spontaneous miniature release.
High frequency stimulation does not change overlap between evoked and spontaneous transmission
So far we have examined the spatial distribution and statistics of evoked transmission under basal conditions, as induced by motor nerve stimulation at low frequency. However, normal larval crawling typically involves bursts of neuronal activity, with a presynaptic action potential frequency of ~10 Hz or higher [18]. We wondered if synapses that appear silent under basal conditions would become active during a burst of action potentials and thereby change the statistics of evoked transmission and its overlap with spontaneous transmission. To investigate this possibility we compared, within the same preparations, evoked and spontaneous transmission properties following a single stimulation pulse or a burst of stimulation pulses (five pulses at 20 Hz).
We quantified evoked transmission in response to a stimulation burst as the probability of release in response to one or more of the five stimulation pulses in the burst (zero corresponding to no release during the burst, 1 corresponding to one or more release events), a value that we report as Pr(5) (see Supplemental experimental procedures). We found that a stimulation burst did not significantly change the way evoked transmission is distributed across synapses. Activity maps of evoked transmission following a stimulation burst were very similar to those obtained following a single stimulation pulse (Figure 6A; single-pulse responses were recorded in separate, single-pulse stimulation trials), with a correlation of 0.83 ± 0.01 (n = 5 NMJs) between the single- and five-pulse activity maps. Almost all synapses had Pr(5) values that were higher than the single-pulse probability Pr(1) (Figure 6B), as expected for the larger number of stimulation pulses. However it was rare for low-Pr(1) synapses to turn into high-Pr(5) synapses: 35 ± 4 % of synapses had Pr(1) < 0.05 (in n = 5 NMJs), and 7 ± 2 % of these (equivalent to 3 ± 1 % of all synapses) had Pr(5) larger than the mean Pr(5) of 0.3.
Figure 6. High-frequency stimulation does not alter overlap between evoked and spontaneous transmission.
(A) Merged activity map showing the probability that synapses distributed throughout a wild-type NMJ participate in evoked transmission triggered either by one nerve stimulation (green), five stimulations (magenta), or both (white). Maximal observed values for this NMJ were Pr(1) = 0.7 and Pr(5) = 0.94. The correlation coefficient between these activity patterns was 0.84.
(B) Plot of Pr(5) versus Pr(1) for all synapses in the NMJ of (A). Solid line indicates no change in probability (i.e. Pr(5) = Pr(1)).
(C) Merged activity maps for the NMJ of (A), showing the probability that synapses participate in either evoked (green) or spontaneous (magenta) transmission or both (white). Right and left panels correspond to one and five nerve stimulations, respectively. For this NMJ, correlation values between the evoked and spontaneous activity maps were 0.36 and 0.24 for one and five nerve stimulations, respectively.
Scale bar in (A), 5 µm.
See also Figure S7.
Importantly, the correlation between the spatial distribution of sites of evoked and spontaneous transmission did not change following a stimulation burst (Figure 6C): the correlation values were 0.24 ± 0.04 and 0.25 ± 0.02 for single-pulse and five-pulse activity maps, respectively (n = 5 NMJs, Student’s paired t-test p = 0.74). As in wild-type, transmission statistics and overlap between evoked and spontaneous transmission also did not change with high-frequency stimulation in rab3-mutant NMJs (Figure S7). In conclusion, high-frequency stimulation increases overall evoked release but does not change the statistics of evoked and spontaneous transmission and does not result in a change in the overlap between the two types of transmission.
Drop in counts of spontaneous miniature release events immediately after high-frequency stimulation
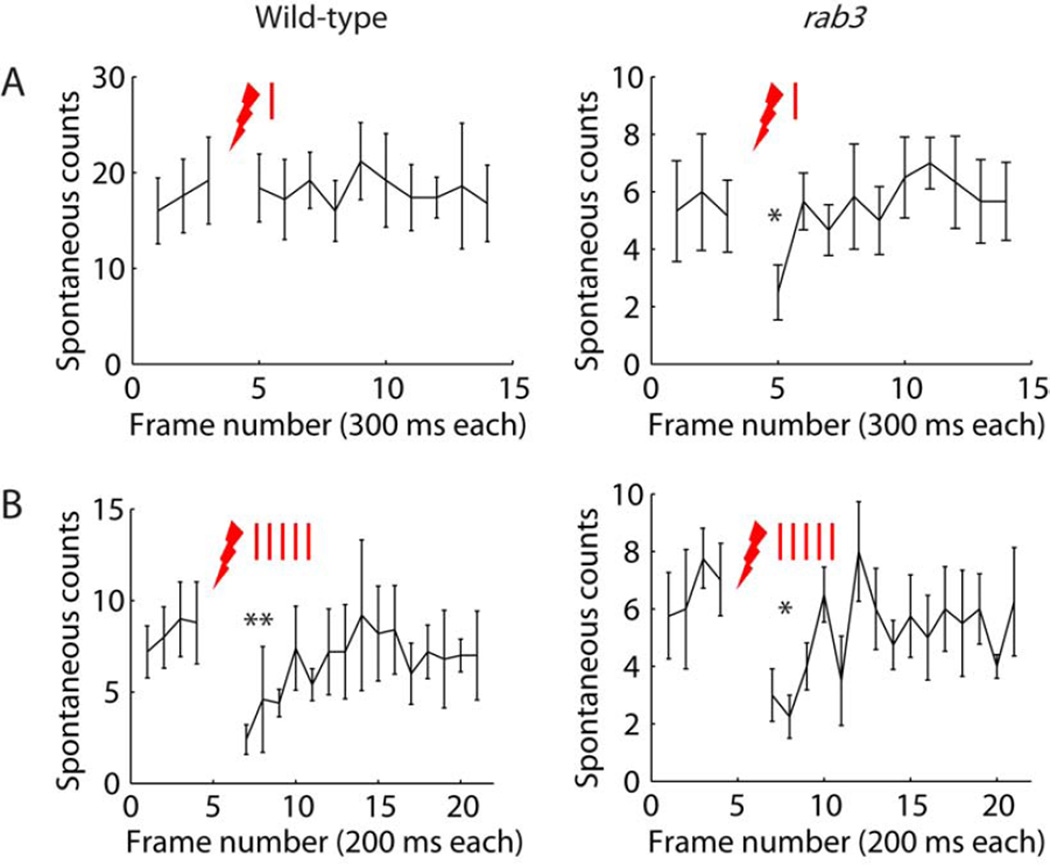
Multiple studies have reported an increase or decrease in the frequency of spontaneous miniature release after presynaptic stimulation [19–21]. We examined spontaneous miniature event counts before and after nerve stimulation. In wild-type, a single nerve stimulation did not affect spontaneous miniature counts (Figure 7A, left; 19 ± 4 and 18 ± 4 counts in one imaging frame before and after stimulation, respectively; Student’s paired t-test p = 0.8, n = 5 NMJs). However, in rab3-mutant NMJs there was a significant drop in spontaneous event counts in the imaging frame immediately following a single nerve stimulation, corresponding to a time of 300 ms after stimulation (Figure 7A, right; 5 ± 1 and 2 ± 1 counts in one imaging frame before and after stimulation, respectively; Student’s paired t-test p < 0.05, n = 6 NMJs). Following five stimulation pulses at 20 Hz, both wild-type and rab3-mutant NMJs showed a decrease in spontaneous miniature counts (Figure 7B). Wild-type counts were 9 ± 2 and 4 ± 1 for an average of three frames (corresponding to a time of 600 ms) before and after stimulation, respectively (Student’s paired t-test p < 0.002, n = 5 NMJs). At corresponding imaging frames rab3-mutant counts were 7 ± 2 and 3 ± 1 (Student’s paired t-test p < 0.02, n = 4 NMJs). These findings suggest a link between an increase in evoked transmission (high Pr values and/or more presynaptic activity) and a decrease in spontaneous miniature release.
Figure 7. Counts of spontaneous miniature events before and after nerve stimulation.
Plots showing the number of spontaneous miniature events identified at each SynapGCaMP3 imaging frame versus frame number. Red lightning bolts mark time of nerve stimulation.
(A) Single nerve stimulation. Wild-type (left, n = 5) and rab3-mutant (right, n = 6) NMJs. Time between start of consecutive frames was 300 ms. The single nerve stimulation was applied after frame number 3.
(B) Five nerve stimulations at 20 Hz. Wild-type (left, n = 5) and rab3-mutant (right, n = 4) NMJs. Time between start of consecutive frames was 200 ms. The train of five nerve stimulations was applied after frame number 4.
Reported values are mean ± SEM. Student’s paired t-tests were used to compare spontaneous counts before and after nerve stimulation: (A) comparing one frame (~ 300 ms) before to one frame after nerve stimulation, (B) comparing three frames (~600 ms) before to three frames after nerve stimulation. Asterisks mark significance values: * p < 0.05, ** p < 0.002.
We next examined the locations of spontaneous miniature events before and after nerve stimulation, to determine whether the drop in spontaneous counts is divided equally among all synapses or occurs mainly in a subset of synapses. We asked whether there is a difference in evoked transmission probability Pr(5) between sites of spontaneous transmission that took place before and after nerve stimulation. Our analysis was limited to experiments with five stimulation pulses (Figure 7B), as in these recordings the drop in spontaneous counts extends over a longer time period (~600 ms), providing us with higher numbers of spontaneous events and more robust statistics. In wild-type preparations Pr(5) at sites of spontaneous transmission was 0.26 ± 0.02 and 0.22 ± 0.02 before and after nerve stimulation, respectively — a small but non-significant decrease (Student’s paired t-test p = 0.1, n = 5 NMJs, 129 spontaneous events before and 57 after nerve stimulation). In the rab3 mutant, Pr(5) at sites of spontaneous transmission showed a significant decrease at the time of decreased spontaneous counts, dropping from 0.33 ± 0.03 before nerve stimulation to 0.19 ± 0.03 after stimulation (Student’s paired t-test p < 0.003, n = 4 NMJs, 83 spontaneous events before and 37 after nerve stimulation). Thus, the results indicate that, in the ~ 600 ms following high-frequency nerve stimulation, spontaneous miniature counts drop below basal values and that in the rab3 mutant this decrease takes place mostly in synapses that have a high Pr(5).
Discussion
PhTox block shows that evoked and spontaneous transmission employ different sets of postsynaptic GluRs
Our electrophysiological measurements show that the activity-dependent GluR antagonist PhTox blocks spontaneous transmission independently of evoked transmission. During nerve stimulation, EPSPs are reduced by about half and mEPSPs show a shift in distribution towards lower values and a decrease in frequency. Without nerve stimulation, EPSPs decline very slightly, while mEPSPs are blocked to the same degree as when nerve stimulation was applied. Thus, these modes of transmission appear to employ distinct sets of GluRs. Similar results were obtained earlier in hippocampal neurons for both NMDA receptors, using the blocker MK-801 [10], and AMPA receptors, using PhTox [11]. In the case of the hippocampal neurons, the two sets of receptors were proposed to be primarily situated in neighboring micro-domains of the same postsynaptic density because imaging of presynaptic vesicle fusion indicated that ~ 80% of synapses participated in both modes of transmission [10]. The remaining ~ 20% of synapses, however, could support mainly one mode of transmission.
High-activity synapses show a preference for either evoked or spontaneous transmission
There has been a debate in the field as to whether evoked and spontaneous modes of transmission use the same set of synapses and the same pool of synaptic vesicles [3–5]. Here, we used postsynaptic Ca2+ imaging with single-synapse quantal resolution to examine how evoked and spontaneous transmission are distributed between synapses in the NMJ. We found that synapses can participate in both evoked and spontaneous transmission, but to varying degrees. The majority of synapses show a low level of activity in both modes of transmission, but high activity synapses tend to have either a high probability of evoked transmission or a high rate of spontaneous transmission. A similar conclusion was reported recently using an analogous approach [22].
To test the robustness of these properties, we examined synaptic transmission in two different sets of experiments which increased Pr levels and created more demanding conditions at synapses that participated in evoked transmission – the rab3 mutant and high frequency stimulation. In rab3-mutant NMJs, evoked transmission is limited to a Brp-rich subset of synapses. In contrast, we find that spontaneous transmission is seen at sites lacking Brp, and, when Brp is present, then the more Brp the lower the rate of spontaneous transmission. This demonstrates that spontaneous transmission is not constrained by the mechanisms that alter the distribution of evoked transmission, and also recapitulates our wild-type findings of a preference for spontaneous transmission to take place at locations that have less evoked transmission.
Under high-frequency stimulation, synapses were more likely to participate in evoked transmission, in both wild-type and rab3-mutant NMJs. However, there was no significant change in the distribution of evoked and spontaneous transmission among synapses and the low levels of overlap between the two modes of transmission remained unchanged. High-frequency stimulation did affect the rate of spontaneous transmission: in both wild-type and rab3-mutant NMJs (and in rab3-mutant NMJs after a single stimulation pulse) there was a decrease in spontaneous release rate for a time extending up to 600 ms (300 ms for a single stimulation pulse) after nerve stimulation. In the rab3 mutant, this decrease in the rate of spontaneous transmission occurred mainly at high- Pr synapses. This indicates that the evoked and spontaneous modes of transmission at least partially share molecular machinery or cellular resources needed for neurotransmitter release, and that these resources are depleted after evoked release. Thus, high-frequency stimulation can lead to higher levels of evoked release and suppression of spontaneous release at the high-Pr synapses, resulting in an additional contrast between evoked and spontaneous transmission.
Brp promotes evoked transmission and suppresses spontaneous transmission
Brp, a Drosophila protein with homology to ELKS/CAST [15, 23, 24], is present in almost all presynaptic release sites. It arrives at release sites at a late stage of development, after other scaffolding components such as DSyd-1 and DLiprin-α [25]. It is part of the presynaptic T-bar structure, and is necessary for the recruitment of Ca2+ channels and synaptic vesicles to the release site [15, 17]. Functionally, low levels of presynaptic Brp are linked with delayed transmission and low evoked release probability [15], while high levels of Brp result in elevated evoked probabilities [12, 14]. Our measurements in the rab3-mutant show that evoked and spontaneous transmission are oppositely correlated with Brp levels: evoked transmission is concentrated at Brp-rich sites, while spontaneous transmission is more likely to occur at Brp-poor sites. This suggests that in addition to its known function in promoting evoked transmission, Brp could also function to suppress spontaneous transmission.
To address this possibility, we examined spontaneous transmission in brp mutants, where Brp function is disrupted across all synapses. In four brp mutant alleles we observed an increase in mEPSP frequency to about twice the wild-type frequency, supporting the hypothesis that presynaptic presence of Brp suppresses spontaneous release. Strikingly, the effect of brp5.38, which is missing only 17 amino acids at the C-terminal of the protein, and has a defect in the tethering of synaptic vesicles to the presynaptic T-bar [17], was as extreme as a null mutant. This suggests that the tethering of synaptic vesicles makes them less likely to undergo spontaneous release.
Release sites in vertebrate sensory neurons have electron dense presynaptic projections (synaptic ribbons), which are thought to function to tether synaptic vesicles at the release site and may play a homologous role to Brp [26]. In goldfish retinal bipolar cells, evoked vesicle fusion is more likely to occur near synaptic ribbons, while spontaneous vesicle fusion is more likely to occur far (> 700 nm away) from ribbons [27]. In this case it is not clear if the distinction is between separate release sites (with or without a synaptic ribbon) or between synaptic and extra-synaptic release. Still, this provides an additional demonstration that the presence of presynaptic dense projections, such as Brp, promotes evoked transmission and suppresses spontaneous transmission.
As Brp is an essential presynaptic component of release sites, it stands to reason that it would promote evoked transmission. Its function in suppressing spontaneous transmission is less obvious, and could be a separate functional role or a more indirect result - the function or presence of Brp could affect other presynaptic proteins which in turn change evoked and/or spontaneous transmission properties. For example, disruptions to the function of the presynaptic Ca2+ sensitive proteins Syt1 and Syt2 [28–32], as well as complexin [33, 34], also result in reduced evoked transmission and enhanced frequency of spontaneous miniature release. Additionally, the level of presynaptic Brp at a release site likely also reflects the levels of other presynaptic proteins essential for release, such as RIM-binding protein [35] and voltage-gated Ca2+ channels [15, 23]. The opposite correlations we find for evoked and spontaneous transmission with Brp level will have to be further examined with regard to the levels of additional active zone cytomatrix proteins to examine their possible roles, independently or as part of an interaction with Brp, in the regulation of synaptic transmission.
Implications for the use of quantal analysis and the understanding of plasticity experiments
A basic element of quantal analysis is that the spontaneous miniature unitary event represents a quantum and that transmission evoked by an action-potential is the synchronous release of multiple quanta, enabling quantal content to be determined by dividing the amplitude of the evoked event by that of the spontaneous event. Our results do not preclude this type of analysis, as we have previously demonstrated that the magnitude of postsynaptic response (ΔF/F) is the same for spontaneous and evoked release events at the single synapse level [12]. However, the fact that the two types of transmission take place largely at different sets of synapses has potential consequences for the understanding of synaptic plasticity experiments. When an experimental manipulation results in a change in transmission properties, it is generally assumed that those changes are identical across synapses and for spontaneous and evoked transmission. Our findings indicate a need to reevaluate such assumptions – a change in spontaneous miniature release amplitude or frequency does not necessarily reflect a change in evoked transmission properties, and vice versa.
Conclusions
Our findings point to the co-existence in one axon of three different kind of excitatory synapses: a majority of undifferentiated weak synapses that have low probability of evoked release and low frequencies of spontaneous release, and then two minority subsets of synapses that disproportionately contribute to transmission: a set that has a high probability for evoked release and low frequency spontaneous release, and another set that has a low probability of evoked release and a high rate of spontaneous release. We propose that distinct regulatory processes may underlie differentiation from the majority weak synapses into one of the strong sets and that Brp is part of this regulatory pathway.
Supplementary Material
Highlights.
Evoked and spontaneous transmission employ distinct sets of GluRs
Evoked and spontaneous transmission rely on divergent sets of synapses
Highly-active synapses show a preference for evoked or spontaneous transmission
Brp appears to promote evoked transmission and suppress spontaneous release
Acknowledgements
We thank members of the Isacoff Lab for helpful discussions and support, Grant Kauwe and Tracey Kim for generating the SynapGCaMP3 fly line, and Robert S. Zucker for comments on an earlier version of this manuscript. We also thank Aaron DiAntonio and Stephan Sigrist for gifts of fly strains and antibodies and Loren Looger for gift of the GCaMP3 construct. This work was supported by the National Science Foundation (FIBR 0623527) and by the National Science Foundation Graduate Research Fellowship (DGE 1106400, Z.L.N.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental information
Supplemental information includes seven figures, three movies, supplemental experimental procedures, and supplemental references.
References
- 1.Fatt P, Katz B. Spontaneous subthreshold activity at motor nerve endings. J Physiol. 1952;117:109–128. [PMC free article] [PubMed] [Google Scholar]
- 2.Del Castillo J, Katz B. Quantal components of the endplate potential. J Physiol. 1954;124:560–573. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zucker RS. Minis: whence and wherefore? Neuron. 2005;45:482–484. doi: 10.1016/j.neuron.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Kavalali ET, Chung C, Khvotchev M, Leitz J, Nosyreva E, Raingo J, Ramirez DM. Spontaneous neurotransmission: an independent pathway for neuronal signaling? Physiology (Bethesda) 2011;26:45–53. doi: 10.1152/physiol.00040.2010. [DOI] [PubMed] [Google Scholar]
- 5.Ramirez DM, Kavalali ET. Differential regulation of spontaneous and evoked neurotransmitter release at central synapses. Curr Opin Neurobiol. 2011;21:275–282. doi: 10.1016/j.conb.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eldefrawi AT, Eldefrawi ME, Konno K, Mansour NA, Nakanishi K, Oltz E, Usherwood PN. Structure and synthesis of a potent glutamate receptor antagonist in wasp venom. Proc Natl Acad Sci U S A. 1988;85:4910–4913. doi: 10.1073/pnas.85.13.4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karst H, Piek T. Structure-activity relationship of philanthotoxins--II. Effects on the glutamate gated ion channels of the locust muscle fibre membrane. Comp Biochem Physiol C. 1991;98:479–489. doi: 10.1016/0742-8413(91)90237-n. [DOI] [PubMed] [Google Scholar]
- 8.Karst H, Piek T, Van Marle J, Lind A, Van Weeren-Kramer J. Structure-activity relationship of philanthotoxins--I. Pre- and postsynaptic inhibition of the locust neuromuscular transmission. Comp Biochem Physiol C. 1991;98:471–477. doi: 10.1016/0742-8413(91)90236-m. [DOI] [PubMed] [Google Scholar]
- 9.Frank CA, Kennedy MJ, Goold CP, Marek KW, Davis GW. Mechanisms underlying the rapid induction and sustained expression of synaptic homeostasis. Neuron. 2006;52:663–677. doi: 10.1016/j.neuron.2006.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Atasoy D, Ertunc M, Moulder KL, Blackwell J, Chung C, Su J, Kavalali ET. Spontaneous and evoked glutamate release activates two populations of NMDA receptors with limited overlap. J Neurosci. 2008;28:10151–10166. doi: 10.1523/JNEUROSCI.2432-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sara Y, Bal M, Adachi M, Monteggia LM, Kavalali ET. Use-dependent AMPA receptor block reveals segregation of spontaneous and evoked glutamatergic neurotransmission. J Neurosci. 2011;31:5378–5382. doi: 10.1523/JNEUROSCI.5234-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peled ES, Isacoff EY. Optical quantal analysis of synaptic transmission in wild-type and rab3-mutant Drosophila motor axons. Nat Neurosci. 2011;14:519–526. doi: 10.1038/nn.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tian L, Hires SA, Mao T, Huber D, Chiappe ME, Chalasani SH, Petreanu L, Akerboom J, McKinney SA, Schreiter ER, Bargmann CI, Jayaraman V, Svoboda K, Looger LL. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat Methods. 2009;6:875–881. doi: 10.1038/nmeth.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graf ER, Daniels RW, Burgess RW, Schwarz TL, DiAntonio A. Rab3 dynamically controls protein composition at active zones. Neuron. 2009;64:663–677. doi: 10.1016/j.neuron.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kittel RJ, Wichmann C, Rasse TM, Fouquet W, Schmidt M, Schmid A, Wagh DA, Pawlu C, Kellner RR, Willig KI, Hell SW, Buchner E, Heckmann M, Sigrist SJ. Bruchpilot promotes active zone assembly, Ca2+ channel clustering, and vesicle release. Science. 2006;312:1051–1054. doi: 10.1126/science.1126308. [DOI] [PubMed] [Google Scholar]
- 16.Fouquet W, Owald D, Wichmann C, Mertel S, Depner H, Dyba M, Hallermann S, Kittel RJ, Eimer S, Sigrist SJ. Maturation of active zone assembly by Drosophila Bruchpilot. J Cell Biol. 2009;186:129–145. doi: 10.1083/jcb.200812150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hallermann S, Kittel RJ, Wichmann C, Weyhersmuller A, Fouquet W, Mertel S, Owald D, Eimer S, Depner H, Schwarzel M, Sigrist SJ, Heckmann M. Naked dense bodies provoke depression. J Neurosci. 2010;30:14340–14345. doi: 10.1523/JNEUROSCI.2495-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cattaert D, Birman S. Blockade of the central generator of locomotor rhythm by noncompetitive NMDA receptor antagonists in Drosophila larvae. J Neurobiol. 2001;48:58–73. doi: 10.1002/neu.1042. [DOI] [PubMed] [Google Scholar]
- 19.Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]
- 20.Eliot LS, Kandel ER, Hawkins RD. Modulation of spontaneous transmitter release during depression and posttetanic potentiation of Aplysia sensory-motor neuron synapses isolated in culture. J Neurosci. 1994;14:3280–3292. doi: 10.1523/JNEUROSCI.14-05-03280.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoshihara M, Adolfsen B, Galle KT, Littleton JT. Retrograde signaling by Syt 4 induces presynaptic release and synapse-specific growth. Science. 2005;310:858–863. doi: 10.1126/science.1117541. [DOI] [PubMed] [Google Scholar]
- 22.Melom JE, Akbergenova Y, Gavornik JP, Littleton JT. Spontaneous and evoked release are independently regulated at individual active zones. J Neurosci. 2013;33:17253–17263. doi: 10.1523/JNEUROSCI.3334-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sigrist SJ, Schmitz D. Structural and functional plasticity of the cytoplasmic active zone. Curr Opin Neurobiol. 2011;21:144–150. doi: 10.1016/j.conb.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 24.Wagh DA, Rasse TM, Asan E, Hofbauer A, Schwenkert I, Durrbeck H, Buchner S, Dabauvalle MC, Schmidt M, Qin G, Wichmann C, Kittel R, Sigrist SJ, Buchner E. Bruchpilot, a protein with homology to ELKS/CAST, is required for structural integrity and function of synaptic active zones in Drosophila. Neuron. 2006;49:833–844. doi: 10.1016/j.neuron.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 25.Owald D, Fouquet W, Schmidt M, Wichmann C, Mertel S, Depner H, Christiansen F, Zube C, Quentin C, Korner J, Urlaub H, Mechtler K, Sigrist SJ. A Syd-1 homologue regulates pre- and postsynaptic maturation in Drosophila. J Cell Biol. 2010;188:565–579. doi: 10.1083/jcb.200908055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhai RG, Bellen HJ. The architecture of the active zone in the presynaptic nerve terminal. Physiology (Bethesda) 2004;19:262–270. doi: 10.1152/physiol.00014.2004. [DOI] [PubMed] [Google Scholar]
- 27.Zenisek D. Vesicle association and exocytosis at ribbon and extraribbon sites in retinal bipolar cell presynaptic terminals. Proc Natl Acad Sci U S A. 2008;105:4922–4927. doi: 10.1073/pnas.0709067105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Littleton JT, Stern M, Schulze K, Perin M, Bellen HJ. Mutational analysis of Drosophila synaptotagmin demonstrates its essential role in Ca(2+)-activated neurotransmitter release. Cell. 1993;74:1125–1134. doi: 10.1016/0092-8674(93)90733-7. [DOI] [PubMed] [Google Scholar]
- 29.Broadie K, Bellen HJ, DiAntonio A, Littleton JT, Schwarz TL. Absence of synaptotagmin disrupts excitation-secretion coupling during synaptic transmission. Proc Natl Acad Sci U S A. 1994;91:10727–10731. doi: 10.1073/pnas.91.22.10727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DiAntonio A, Schwarz TL. The effect on synaptic physiology of synaptotagmin mutations in Drosophila. Neuron. 1994;12:909–920. doi: 10.1016/0896-6273(94)90342-5. [DOI] [PubMed] [Google Scholar]
- 31.Pang ZP, Sun J, Rizo J, Maximov A, Sudhof TC. Genetic analysis of synaptotagmin 2 in spontaneous and Ca2+-triggered neurotransmitter release. Embo J. 2006;25:2039–2050. doi: 10.1038/sj.emboj.7601103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu J, Pang ZP, Shin OH, Sudhof TC. Synaptotagmin-1 functions as a Ca2+ sensor for spontaneous release. Nat Neurosci. 2009;12:759–766. doi: 10.1038/nn.2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huntwork S, Littleton JT. A complexin fusion clamp regulates spontaneous neurotransmitter release and synaptic growth. Nat Neurosci. 2007;10:1235–1237. doi: 10.1038/nn1980. [DOI] [PubMed] [Google Scholar]
- 34.Maximov A, Tang J, Yang X, Pang ZP, Sudhof TC. Complexin controls the force transfer from SNARE complexes to membranes in fusion. Science. 2009;323:516–521. doi: 10.1126/science.1166505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu KS, Siebert M, Mertel S, Knoche E, Wegener S, Wichmann C, Matkovic T, Muhammad K, Depner H, Mettke C, Buckers J, Hell SW, Muller M, Davis GW, Schmitz D, Sigrist SJ. RIM-binding protein, a central part of the active zone, is essential for neurotransmitter release. Science. 2011;334:1565–1569. doi: 10.1126/science.1212991. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.