Abstract
Early studies reported preserved formulaic language in left hemisphere damaged subjects and reduced incidence of formulaic expressions in the conversational speech of stroke patients with right hemispheric damage. Clinical observations suggest a possible role also of subcortical nuclei. This study examined formulaic language in the spontaneous speech of stroke patients with left, right, or subcortical damage. Four subjects were interviewed and their speech samples compared to normal speakers. Raters classified formulaic expressions as speech formulae, fillers, sentence stems, and proper nouns. Results demonstrated that brain damage affected novel and formulaic language competence differently, with a significantly smaller proportion of formulaic expressions in subjects with right or subcortical damage compared to left hemisphere damaged or healthy speakers. These findings converge with previous studies that support the proposal of a right hemisphere/subcortical circuit in the management of formulaic expressions, based on a dual-process model of language incorporating novel and formulaic language use.
Keywords: Aphasia, formulaic language, basal ganglia
Introduction and rationale
Current views of human language processing have diverged from the standard generative model, which positioned the newly created, novel sentence at the center of native competence. Unitary utterances, such as speech formulae and idioms, were dismissed as a peripheral, limited set of ‘lexical items’ which should be allocated in the grammar to look-up lists. The change in view has arisen from studies that reveal extensive incidence and communicative importance for formulaic expressions in actual language use (Schiffrin, 1987; Pawley, 2007). Furthermore, studies of brain processing of novel and formulaic language reveal neurological dissociations as well.
As an approach to linguistic analysis, studies of formulaic language belong to ‘pragmatics’, which has its focus on utterances in actual language usage. For this approach, discourse, including conversation, story-telling, narratives, picture description, and interviews are examined in content and form. Studies of discourse reveal that formulaic expressions are common and frequent, and that they vary in category and amount with type of discourse. Conversation includes a characteristic repertory of near-mandatory opening and closing speech formulae (‘how are you’, ‘have a good day’, ‘see you later’). Other formulaic expressions are idioms (‘He spilled the beans’), expletives (‘Oh, heck’), and a rather large array of conventional expressions which can be variously categorized (Wray, 2002; Van Lancker Sidtis, 2004). These differ from novel expressions in that they all have stereotyped form (certain words in a certain order) and conventionalized meanings (social or contextual meanings not strictly derivable from dictionary lexical representations). Formulaic expressions are known to a language community (Kitzinger, 2000), and are used to achieve special purposes in communication (Tannen and Öztek, 1981; Wray and Perkins, 2000), such as structuring talk (Jucker, 1993; Fox Tree, 2006); negotiating complaints (Drew and Holt, 1988); partnership solidarity (Bell and Healey, 1992; Bruess and Pearson, 1993), maintaining fluency in various contexts such as sport, weather forecasting, horse races, and auctions (Kuiper, 2004), and generally sounding like a native speaker of the language (Fillmore, 1979; see review in Van Lancker Sidtis, 2004).
While many interesting theoretic-linguistic differences between formulaic and novel expressions can be and have been enumerated, an even more compelling suggestion has arisen that these two kinds of language are mediated by different neurological structures. Clinical impressions of stroke patients abound with impressions that pragmatics of communication are differentially affected by left and right brain damage. For well over a century, aphasiologists from Europe and America have observed that persons with aphasia following left hemisphere damage have preserved ‘automatic speech’, which includes various kinds of formulaic language. It is further known that aphasic patients utilize these expressions to communicate when novel language is severely deficient (Hughlings Jackson,1874; Gloning, Gloning, and Hoff, 1963; Critchley, 1970; Espir and Rose, 1970; Van Lancker, 1973; 1988; 1993). This observation was more recently systematically supported by utilizing clinical surveys (Code, 1989; Blanken and Marini, 1997), which document types of formulaic expressions observed in severe aphasia in English and German. Similar results were later seen for Chinese speakers (Chung, Code, and Ball, 2004).
As an extreme example of this clinical presentation, records exist of an otherwise normally developing, right handed adult who underwent left-sided hemispherectomy for treatment of brain cancer (Smith, 1966). This individual lost all novel speech, but preserved a near normal ability to express speech formulae (‘oh, yes’), expletives (‘goddammit’), pause fillers (‘uh’, ‘ah’), sentence stems (‘I can’t’), and discourse elements (‘well’) (Van Lancker and Cummings, 1999). In this case, the preserved communication function must be attributed to an intact right hemisphere-subcortical circuit. Lum and Ellis (1994) designed a controlled study comparing formulaic with novel speech in persons with aphasia, finding that sentence completion tasks did reveal a different competence for the two modes. For physical evidence that ‘automatic speech’ is differently controlled in the brain, greater left-sided mouth openings were measured for automatic tasks, while greater right-sided openings were seen for propositional speech tasks in stroke subjects (Graves and Landis, 1985). Similar mouth opening asymmetries were reported for singing with words versus singing without words in normal subjects (Cadalbert, Landis, Regard, and Graves, 1994), and for babbling (syllabic production with consonant-vowel alternations) compared with smiling in babies between 5–12 months (Holowka and Petitto, 2002). On the other side of the cerebral coin, the spontaneous speech of persons with right hemisphere damage, although not grammatically, phonologically, or lexically deficient, reveals pragmatic deficits of various kinds (Kaplan, Brownell, Jacobs, and Gardner, 1990; Brownell and Joanette, 1993; Myers, 1998, Cheang and Pell, 2006; for review see Van Lancker, 1997), such as turn-taking and topic maintenance in conversation and appreciation of humor and non-literal meanings. The communicative incompetence observed clinically in right hemisphere damage may arise at least in part from faulty use of formulaic expressions.
Functional imaging studies addressing questions about brain structures underlying automatic speech have yielded equivocal results. Early reports of bilateral regional cortical blood flow using the xenon inhalation method during performance of automatic speech tasks (Larsen, Skinhøj, and Lassen, 1978; Ryding, Bradvik, and Ingvar, 1987) suggested support of the prevailing model of the time, which posited that automatic speech production is modulated by both hemispheres. However, subsequent functional imaging studies reported bilateral hemispheric signal for virtually all speech and language tasks, undermining the interpretability of the early xenon series (Van Lancker Sidtis, 2006a; Sidtis, 2007). Using PET imaging, two automatic speech production tasks (months of the year and the Pledge of Allegiance) showed activation in traditional language areas, failing to reveal unique brain substrates for these tasks (Bookheimer, Zeffiro, Blaxton, Gaillard, and Theodore, 2000). In Blank, Scott, Murphy, Warburton, and Wise (2002), bilateral and left hemisphere brain signal using fMRI technology was reported for both novel speech (propositional task) and for nursery rhymes and counting (non-propositional tasks) in various brain sites, depending upon the contrast analysis, but consistent patterning between the two kinds of speech did not emerge. In a PET study conducted with normal and aphasic subjects, two verbal production tasks, animal name generation and counting, were utilized and compared to non-verbal vocalization. As was expected, examination of the behavioral data indicated that normal and aphasic groups did not differ in counting or non-verbal vocalizations, but did differ significantly in word generation. Because these tasks did not lend themselves to subtraction techniques, a partial least squares analysis was used, which yields latent variables associating tasks with brain sites (Van Lancker Sidtis, McIntosh, and Grafton, 2003). The first and second latent variables identified naming and vocalizing with bilateral anterior and temporal areas, with left predominating over right. In the third (only marginally significant) variable, which was associated with counting, right and subcortical sites predominated, suggesting that counting and naming are processed differently in the brain. Thus functional imaging studies have yielded a range of results that do not yet lead to clear interpretation of cerebral structures underlying novel and formulaic language.
Clear findings emerged from a recent study of subjects with lateralized brain lesions (Van Lancker Sidtis and Postman, 2006). This study examined discourse by left- and right- hemisphere damaged subjects, compared with age- and education-matched normal control subjects, to examine incidence of formulaic expressions in spontaneous speech obtained from these subjects. Transcriptions of talk describing family and work produced by five members in each of the three study groups (left-damaged, right-damaged, and normal- control) were analysed for incidence of the following categories: (1) conversational speech formulae (e.g. ‘first of all’, ‘right’); (2) idioms (e.g. ‘We see eye to eye’); (3) conventional expressions (e.g. ‘and so forth’); (4) expletives (e.g. ‘gosh’); (5) sentence stems (e.g. ‘I guess’); (6) discourse particles (e.g. ‘well’), and (7) pause fillers (e.g. ‘uh’). Following findings by Code (1989) for production and Van Lancker and Klein (1990) for comprehension, Category 8, familiar proper nouns (those personally known to the speaker) were also identified. Results documented significantly fewer formulaic expressions in persons with right hemisphere damage than the normal control group, while the left- hemisphere damaged subjects produced significantly more formulaic expressions than both other groups (Van Lancker Sidtis and Postman, 2006). These findings indicating an effect of side of damage in production of formulaic language, although provocative, were limited by the use of written transcripts, and did not include persons with damaged restricted to subcortical nuclei.
There are suggestions in the clinical literature and arising from our clinical observations that, in addition to a right cortical hemisphere influence, the basal ganglia contribute significantly to production of formulaic (and ‘automatic’) expressions. A case study by Speedie, Wertman, T’air, and Heilman (1993) reported diminution of expressive formulaic language following damage to right-sided subcortical nuclei. A second case study described an intrusive syllable, /sis/ as a result of brain damage, probably in the basal ganglia, which occurred significantly more frequently on formulaic than novel expressions (Van Lancker, Bogen, and Canter, 1983). Other suggestive reports arise from observations in Parkinson’s subjects, who have diminished basal ganglia function, revealing reduced formulaic language expressions when speaking (Illes, Metter, Hanson, and Iritani, 1988). Further evidence of a subcortical role in formulaic expression production comes from stimulation studies and observations of increased incidence. During stereotaxic surgery, formulaic expressions are sometimes elicited when subcortical structures are stimulated (Schaltenbrand, 1965; Petrovici, 1980). Hyperactivation of one type of formulaic expression, cursing, is seen in many cases of Tourette’s syndrome, a disease which is believed to arise from subcortical dysfunction (Van Lancker and Cummings, 1999). These experimental results and clinical observations have led to a model that attributes production of formulaic expressions to a right hemisphere-subcortical circuit, through which the configurational abilities of the right hemisphere and the motor-organizational functions of subcortical systems facilitate production of these over-learned vocalizations.
In this study, we undertook to examine the spontaneous speech of individuals with documented single-episode brain lesions occurring in the left hemisphere, right hemi- sphere, or basal ganglia. The purpose is to further investigate the hypothesis that spontaneous speech in left hemisphere damage contains an abnormal abundance of formulaic expressions, while right hemisphere damage is associated with reduced formulaic language. In addition, we undertook to investigate formulaic language production in two persons with neurological lesions confined to subcortical nuclei. This allowed us to more specifically address the model of a right hemisphere-subcortical circuit.
To overcome limitations of the previous study where only written transcripts and the most essential medical information were available, we utilized recorded, spoken discourse from patients with full neurological, neurobehavioural, and language evaluations for this study. Knowing that different types of discourse may elicit different linguistic features, we formulated a structured interview for the normal-control speech samples that matched the discourse structures utilized in the test subjects on essential parameters for three of the subjects. For one of these subjects (Case 1 below), a pre-morbid speech sample was available in an interview setting matching the sample used in this study. Use of these materials was approved by the University Committee on Activities Involving Human Subjects at our institution.
Method
Procedure
Audio and video recordings of subjects in interviews with a clinician were obtained for three subjects. The fourth subject provided video recordings of interviews conducted previous to and following his stroke utilizing the identical discourse format. These samples of spontaneous speech were transcribed and entered into a protocol designed to document the presence of formulaic expressions. The method for identifying and classifying formulaic expressions has been described previously (Van Lancker Sidtis and Rallon, 2004; Van Lancker Sidtis and Postman, 2006). For each transcript, three trained independent raters identified and classified formulaic expressions, placing them into these categories: speech formulae (‘don’t be silly’), idioms (‘killed two birds with one stone’), conventional expressions (‘in the meantime’), sentence stems (‘I think’), discourse particles (‘well’), pause fillers (‘uh’, ‘um’), and proper nouns. Method of classification included formal and functional criteria as well as native speakers’ intuitions as evidence (Devitt, 2006). Policies and guidelines were established to ensure consistency of rating throughout the study and all raters were trained on these guidelines. In all cases, identification and classification were consensual across raters following discussion and consultation.
To calculate proportions of words in formulaic expressions, total word counts were obtained for each corpus. To compare numbers of formulaic expressions across subjects, each corpus was truncated at 500 words for a count of expressions in corpora matched for length.
Normal-control speech samples were obtained utilizing a structured interview that was designed to resemble the discourse settings of the test subjects. The examiner asked questions about medical history, interests, hobbies, and concerns, and each interviewee answered in free form. The interviews were tape recorded using a mounted Koss microphone attached to a Marantz professional tape recorder. For clinical cases and normal control subjects, recorded interviews were transcribed exactly and the transcriptions were proofed by two other listeners in order to ensure overall accuracy and to validate that all pause fillers and other pragmatic elements were faithfully transcribed.
Subjects
Four subjects who sustained brain lesions due to a single cerebral vascular accident as documented by radiographic imaging were studied. Brain imaging was performed for all subjects, and results were interpreted by a radiologist and a behavioural neurologist. All subjects were right-handed, had no prior neurological or psychiatric history, and were born and educated in the US (see Table I). All received extensive language, speech, and cognitive testing appropriate to the presenting complaint. Protocols selected varied for each subject; reported below are those that reveal clinical profiles relevant to this study. For one subject (Subject 1), lengthy pre-morbid and post-morbid speech samples were available on videotape in comparable discourse formats—an interview setting similar to that utilized for the other three subjects. For the subjects with right hemisphere and subcortical damage, language evaluation revealed normal performance. Three of these subjects (Subjects 1–3) were referred to the Speech Clinic because their speech was severely dysprosodic. The left hemisphere-damaged subject (Subject 4) was referred for language evaluation. For all subjects, data were collected between 7 and 24 months following injury. Detailed medical and clinical information are given below and representative speech samples are provided in the Appendix.
Table 1.
Age, education, general area of damage (SC = subcortical, RH = right hemisphere, LH = left hemisphere, Bi = bilateral), and localization of damage (GP = globus pallidus, FTP = frontal-temporal-parietal, F-P = frontoparietal). Mean age and years of education are given for four test subjects and 10 normal-control (N-C) subjects.
| Subjects | Age | Education | Damage | Cerebral Site |
|---|---|---|---|---|
| Patient 1 | 48 | 20 | SC | R putamen, GP, IC |
| Patient 2 | 50 | 14 | RH | FTP lobes |
| Patient 3 | 36 | 16 | SC | Bi putamen, GP, caudate |
| Patient 4 | 66 | NA* | LH | F-P |
| Mean of four cases | 50 | 16.7 | ||
| Mean of N-C subjects | 41.6 | 16.4 |
Due to Patient 4’s severe language deficit and lack of family contacts, this information was not obtained. Records indicated that the patient was an affluent businessman and had served as the vice president of a merchandising company.
Patient 1 is a 48-year-old Caucasian male who sustained a haemorrhagic infarct in the right basal ganglia, specifically the right putamen, globus pallidus, and posterior limb of the internal capsule. Following the stroke, speech and language assessment revealed normal language, with a decrease in expressiveness and a monotonous and breathy vocal pattern. His Wechsler Adult Intelligence Scale [WAIS-R] (Wechsler, 1981) verbal IQ was 121 with a Full-Scale IQ of 118. He performed below normal on verbal fluency tests but normally on the Boston Naming Test (Kaplan, Goodglass, and Weintraub, 1983). His performance on the Formulaic and Novel Language Comprehension Test [FANL-C] (Kempler and Van Lancker, 1985) was flawless on both sub-tests. Complete test and clinical data are given in a previous study (Van Lancker Sidtis, Pachana, Cummings, and Sidtis, 2006). According to his report and that of close acquaintances, following his injury he interrupted others more often than before and sounded ‘irritable’ in conversation. Pre-morbid speech was available for this subject; we analysed a sample containing 1248 words. The word count of his post-morbid speech sample was 1043.
Patient 2 is a 50-year-old, Caucasian male, who sustained a large right-sided infarct in the distribution of the middle cerebral artery to frontal, parietal, and temporal lobes following a single cerebral vascular accident. Patient 2 received a broad range of clinical evaluation protocols following the stroke. He performed normally (29/30) on the Minimental State Examination (Folstein, Folstein, and McHugh, 1975). His performance on the Western Aphasia Battery (Kertesz, 1982) yielded normal performance, with an Aphasia Quotient of 98.6/100; his score was 99/100 on the Reading Comprehension Battery for Aphasia (LaPointe and Horner, 1979), and 100% on the Kempler Syntax Comprehension Test (Kempler, 1986). In contrast, performance on The Affective- Linguistic Prosody Test was deficient: for linguistic prosody, the production sub-test yielded 13/24; the comprehension sub-test 18/24; for affective prosody, production ability was 0, and comprehension 12/16 (Van Lancker and Sidtis, 1992). Results on the MiniInventory for Right Brain Injury (Pimental and Kingsbury, 1989) were 35/43, indicating ‘mild severity’. On the Formulaic and Novel Language Comprehension Test (Kempler, Van Lancker, Marchman, and Bates, 1999), he performed more poorly on the formulaic portion (17/20) than the novel portion (19/20). This is the communication profile of a right hemisphere damaged individual: normal propositional language, abnormal prosody, and impaired formulaic language comprehension. He had been employed as a salesman and described himself as a gregarious and talkative person prior to the cerebral incident. However, at clinical evaluation, this subject (Patient 2) demonstrated a terse, unnatural quality to his spontaneous speech, which we suspected to be attributed, at least in part, to a paucity of formulaic expressions. The speech sample used in this analysis contained 1249 words.
Patient 3 is a 36-year-old, African-American female. Language function was clinically normal. Her WAIS-R Verbal IQ was 90, with a Full Scale IQ of 83. Verbal fluency tests were normal, while naming performance (Boston Naming Test) was below average. Her performance on formulaic language comprehension was defective, with a score of 13/20 on the formulaic portion of the FANL-C compared to 18/20 on the novel sentence portion. CT scan revealed bilateral basal ganglia infarcts, probably due to hypoxia. T2-weighted and proton-density MRI images taken 2 months post-injury revealed bilateral involvement of the globus pallidus and medial putamen. A PET study performed 2 years following the injury revealed hypometabolism in the caudate and putamen bilaterally, but more marked on the right side. Returning to work 6 months following the incident, Subject 3 noted that the most substantial change since her injury was alteration of her speech, including dysprosody, which has been described elsewhere (Van Lancker Sidtis et al., 2006). In conversation, spontaneous speech seemed terse and unnatural. She insightfully volunteered the information that, in normal everyday interactions, she used fewer greetings and everyday common expressions, stating that ‘They just don’t occur to me’. The speech sample of patient 3 contained 1237 words.
Patient 4 is a 66-year-old male diagnosed with a left frontoparietal embolic stroke, which resulted in a language deficit and right hemiplegia. His speech was fluent but lacked meaningful communication. He had severely impaired comprehension and naming, and preserved repetition. His clinical presentation and his diagnosis on the Western Aphasia Battery was transcortical sensory aphasia (Kertesz, 1982; see also Berthier, 1999). On the Western Aphasia Battery, Patient 4 scored 3.6/10 on language comprehension (severe range), and correctly named only 3/60 items on the naming sub-test. His speech was fluent but consisted of frequent formulaic expressions (‘What else can I say?’; ‘How would I call it’) with normal articulation and prosody. Formulaic expressions along with intact repetition abilities were frequently utilized to result in fluent spontaneous speech. Because of the severe semantic impairment of this subject, the conversational speech sample utilized for Patient 4 contained a smaller amount: 507 words.
Normal-control subjects, three male and seven female adults, recruited following IRB guidelines utilizing a public flier, were interviewed with their informed consent. These subjects were between ages 30 and 58 (mean age 41.6) and were all native speakers of American English with no history of neurological or psychiatric disease. Education ranged between 16 and 20 years (mean 16.4). Normal-control subjects were interviewed using a structured format similar to that utilized for the neurological subjects, and their speech was transcribed and analysed using the same methods. The mean word count of normal-control speech samples was 1941 words.
Results
Proportions of words in formulaic expressions
Total proportions of words comprising formulaic expressions in their respective speech samples were calculated for four clinical subjects and compared with proportions obtained for the normal-control group (including the pre-morbid speech sample for Patient 1). This allowed us to determine incidence of formulaic language in different size corpora. In addition, pre- and post-morbid samples for Subject 1 were compared. Mean proportions of words in formulaic expressions for individual normal control (N-C) subjects are shown alongside means for each study case in Figure 1. The three subjects with subcortical or right cortical damage had a lower proportion of words in formulaic language than the N-C group, while the subject with left hemisphere damage had a higher portion (see Figure 2). These differences were significant in comparison to the normal-control group using a t- distribution procedure. Mean proportion of formulaic words for the subcortical subject (Patient 1) in the pre-stroke speech sample was not significantly different from normal.
Figure 1.
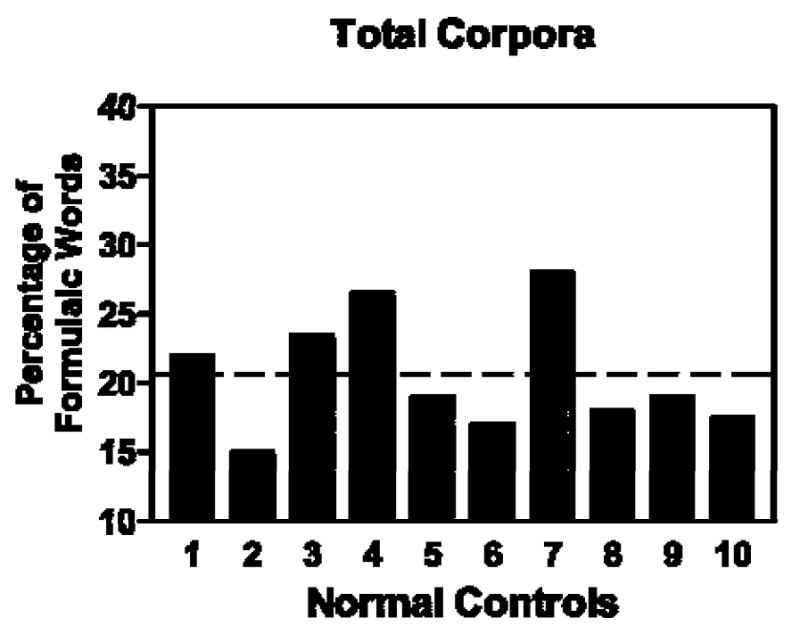
Proportion of words in formulaic expressions measured in spontaneous speech samples of 10 normal-control (N-C) subjects. The dotted line represents the mean for the N-C group (20.5%).
Figure 2.
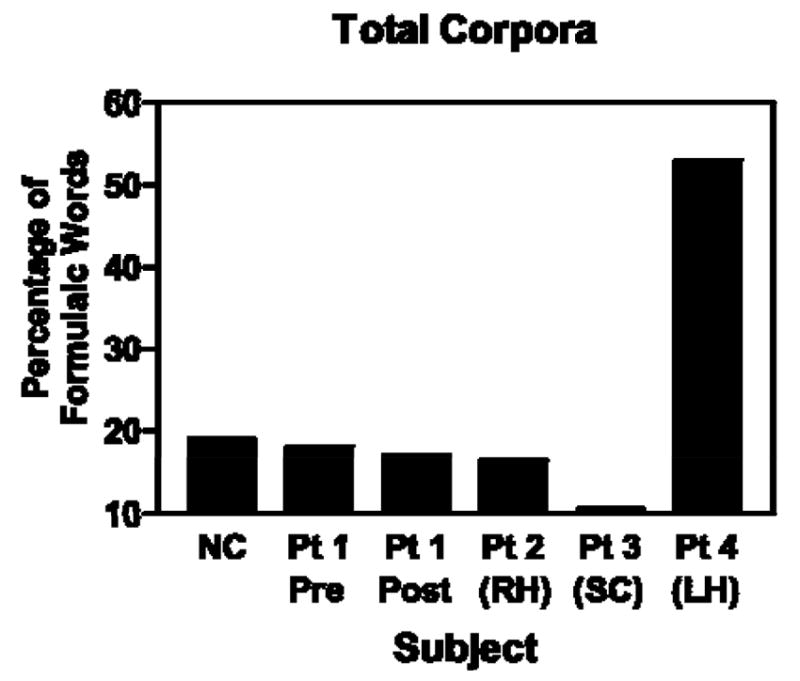
Proportion of words in formulaic expressions measured in spontaneous speech samples of test subjects compared to the mean of 10 normal-control (N-C) subjects (SC = subcortical; RH = right hemisphere; LH = left hemisphere).
The overall normal-control (N-C) mean, represented by the dotted horizontal line on the chart in Figure 2, was 20.5 (SD=4.14). Using the 95% confidence interval based on a t- distribution, mean proportions of words in formulaic expressions differed significantly from the N-C for the four cases with brain damage. For Patient 1, the post-morbid speech sample revealed a proportion of 16.9 comprising words in formulaic expressions; this is significantly different from normal at p<.05; for Patient 2, the incidence was 16.1, which is significantly different form normal at p<.025; for Patient 3, the incidence was 11.0, which is significantly different at p<.025; and Patient 4, with left hemisphere damage, the proportion of 53.8 of words in formulaic expressions achieved significance at p<.025. Patient 1’s pre-morbid tally of formulaic expressions, 18.3, was not significantly different from normal performance by this procedure.
Numbers and types of formulaic expressions
As stated above, we wished to compare numbers and categories of formulaic expressions in the samples. To achieve this, the samples were equated in word count by truncating each sample at 500 words. This allowed us to observe absolute numbers of formulaic expressions across subjects, and to gain an idea of how types of formulae were distributed. These numbers are limited for statistical comparison. We present these data as suggestive for future studies.
Following initial analysis of the original categories of formulaic expressions the seven categories were condensed into four: Formulae (consisting of idioms, speech formulas, expletives, and conventional expressions), Fillers (consisting of single-word pause fillers and discourse particles), Sentence Stems (SS), and Proper Nouns. Collapsing of categories was practical in these single case studies as some speech samples contained no examples of a given category, such as expletives, or very few, such as idioms. Usage of the four categories was examined for patterns. First we note that by this measure, formulaic expressions and fillers comprise most of the types of formulaic language in the normal-control sample. When compared to other subjects and to normal controls, Patient 4, the left hemisphere damaged subject with transcortical sensory aphasia, demonstrated a very high proportion of speech formulae. This measure fits well with our clinical impression and experience with this patient. Further examination revealed that, of the four categories, this subject’s speech contained a preponderance of multi-word formulaic expressions, contributing in a major way to the high proportion shown for this subject in Figure 3. Fillers are also prominent across most of the samples, reflecting their importance in speech, as recently described in sociolinguistic studies (Aijmer, 1996; Fox Tree and Schrock, 1999; Clark and Fox Tree, 2006). Patients 1 and 3, those with exclusively subcortical damage, differ in counts and distribution. For these two subjects, the site of subcortical damage also differs. Further studies of the selective effects on the production of formulaic expressions brought about by damage to specific basal ganglia would seem to be indicated by these results.
Figure 3.

In speech samples equated for word count, absolute numbers of four groupings of formulaic expressions for subjects with subcortical (SC), right hemisphere (RH), and left hemisphere (LH) damage, and the mean of the normal control group. Formulaic expressions in the matched pre-morbid (pre) speech for Patient 1 are given in the first record and compared to the post-morbid (post) speech count (SS: Sentence stems).
Discussion
A remarkable role of certain kinds speech performance referred to as ‘automatic speech’ in aphasic presentation has long been recognized and anecdotally included in descriptions of aphasia (Hughlings Jackson, 1874). The underlying assumption was that left hemisphere damage interfered less with production of these utterances than with the problem of newly- created language. These clinical impressions have more recently been supported and augmented by surveys (Code, 1989; Blanken and Marini, 1997), and controlled group studies (Graves and Landis, 1985; Lum and Ellis, 1994; Van Lancker Sidtis and Postman, 2006). Single case reports (Speedie et al., 1993; Van Lancker Sidtis et al., 2006) have associated damaged subcortical nuclei, especially referring to right-sided subcortical damage, with diminished or impaired formulaic language production.
The study of four single cases reported in this article supports previous conclusions (Van Lancker Sidtis and Postman, 2006) that an intact right hemisphere is required for normal production of formulaic language. The lower proportion of formulaic expressions following right hemisphere damage is concordant with other known specialties of the right hemisphere, particularly emotional experiencing and processing of social and verbal context (Bloom, Borod, Obler, and Koff, 1990; Brownell and Martino, 1997; Borod, Zgaljardic, Tabert, and Koff, 2001). Formulaic expressions naturally carry attitudinal and emotional nuances, and they function in enabling social interaction (see Tannen, 1989; Wray, 2002; Van Lancker Sidtis, 2008). Thus, the formulaic/propositional distinction parallels another dichotomy, modal versus referential speech (Nespoulous, Code, Virbel, and Lecours, 1998), which has been proposed to explain observations in aphasic speech. Using modal speech, the patient communicates expressions of feeling and attitude, a major function of formulaic language, especially as represented in sentence stems (SS) (e.g. ‘I want …’, ‘I think’). Some expressions can be used for either modalizing or propositionalizing functions, and this is especially true for sentence stems, which, syntactically, often introduce complement clauses, and which can also be used propositionally. It is possible that the isolated appearance of sentence stems in jargon aphasia, which is otherwise unintelligible, represents isolated outcroppings of modalizing expression (Buckingham, Avakian-Whitaker, and Whitaker, 1975). Other implications for lexical access and linguistic planning, following in the tradition of Goldman-Eisler (1968) and Butterworth (1974), and lately elaborated in sociolinguistic studies by Clark and Fox Tree (2006) and others, remain to be examined using larger subject samples, in which subcategories of formulaic utterances can be compared It is likely that differential incidence of such formulaic subcategories in neurological populations might shed some light on cerebral involvement in lexical access and linguistic planning.
The study took a further step by uncovering evidence that damage restricted to subcortical nuclei interfered significantly with formulaic language production, with focus on right-sided subcortical damage. A qualitative look at relative incidence of speech formulae, sentence stems, fillers, and proper nouns revealed an overall prominence of speech formulae and fillers across all subjects. The striking paucity of proper nouns in Patient 2 (right hemisphere damage) is consistent with several studies demonstrating that the right hemisphere stores and processes personally familiar names (Van Lancker and Klein, 1990). The high incidence of formulaic expressions in Patient 4 (left hemisphere damage) matches our clinical impression that this person utilized formulaic language (along with repetition) to maintain constant verbal fluency, actually masking, in large measure, his severe novel language deficit. The low incidence of formulaic expressions for subcortically damaged subjects (1 and 3) also corresponds with clinical experience with their conversational behaviours and with their self-report.
We recognize certain limitations of our study. First, results from four individual cases bear the burden of proof that they can be generalized, as always in single case studies. We have addressed this limitation by sampling a relatively large normal control group using the same discourse format and by utilizing statistical comparisons where possible. Further, the concordance of the results with previous group studies provides some assurance that we are on the right track. Obtaining measures from spontaneous speech contains well-known challenges because of individual variability in speech patterns, and identifying and classifying linguistic entities is not always straightforward. This is no less true for identifying formulaic expressions. To minimize threats to validity from these sources, we employed trained raters and used the same method of analysis throughout these and previous studies. Our findings for relative incidence of formulaic expressions in persons with localized damage correspond well both to long-standing clinical observations and to previous experimental studies and case reports. The results, clearly showing a reduction of formulaic language in right hemisphere and subcortical impairment, lend support to the existence of a right hemisphere/right subcortical circuit in modulation of formulaic expressions, and to the dual process model of language competence (Wray and Perkins, 2000; Van Lancker Sidtis, 2008). In this model, novel and formulaic language are differently learned, stored, and processed in the brain and are differentially affected by brain damage. This model draws on findings from studies of the basal ganglia, which suggest that these nuclei modulate procedural and configurational action programs (Mishkin, Malamut, and Bachevalier, 1984; Graybiel, 1998). This allows for the likelihood that routinized vocal behaviours are managed through a right hemisphere/subcortical circuit with minimal required input from left hemisphere language areas. The dual process model (Wray and Perkins, 2000; Van Lancker Sidtis, 2004; 2006b; 2008) posits that both kinds of language, novel and propositional, are ‘volitionally’ produced using both pyramidal and extrapyr- amidal systems, but that they are stored and processed differently from each other and utilize different cerebral resources. While it has long been observed that the intact left hemisphere can perform all kinds of linguistic processing well, these studies suggest that formulaic language can also be modulated by structures involving intact right hemisphere and/or subcortical nuclei.
The model benefits from recent advances in describing and modeling formulaic language in normal speech (Wray, 2002; Van Lancker Sidtis, 2004), which continue to depict these kinds of expressions as qualitatively different from novel expressions on a large number of parameters. These advances have allowed us to begin to paint a profile of formulaic language processing in the brain using data from a range of experimental approaches, especially lesion studies, leading to the dual process model. In the dual process model, normal language competence consists of creative interplay between these two modes (Van Lancker Sidtis, 2006b).
These findings have implications for evaluation and treatment of language disorders. Although the study of the principles of language use—pragmatics—has become an important field in the language sciences, deficits in pragmatics of communication are often very difficult to precisely characterize in the clinical setting. Impressionistically, the conversation of a person with right hemisphere dysfunction seems problematic, but exactly in what way the speech is non-normal may be elusive. The work described in this paper has clinical relevance because it is part of a study program that is, first, moving toward establishing quantity and quality of normal use of formulaic expressions in various discourse settings, and, secondly, aiming to more precisely describe deviance, due to brain damage, from normal pragmatic practices. Once an accurate evaluation is made, it is likely that therapeutic approaches could be directed toward remediation of scarcity or over- abundance of formulaic expressions. Further, it is important to our understanding of aphasia and useful in applied clinical service to be clear about recovery of language in rehabilitation from aphasia (Van Lancker Sidtis, 2008). In some cases, formulaic expressions become relatively more fluently available to the subject, while novel language production remains impaired. Clinical insight into what kind of competence is being reinstated could well assist in designing and directing therapy programs.
Appendix
Fifty-word excerpts, chosen to be typical, from study subjects. Formulaic expressions are underlined.
Patient 1
And so you would see some of the same kinds of aesthetic sensibility there because of the Muslim faith, but it, it stands on its own as a very unique combination of works of art produced by very interesting and unique people.
Patient 2
I’ll be able to uh t-to go when where I want to when I want to and not have to wait on someone else. Depend on someone else.
Um. It’s funny when I work on things like this. It seems that the long sentences give me trouble. When I’m reading uh sentences … um I know I can read, but I just get tongue-twisted.
Patient 3
Well, I woke up and I was in unfamiliar surroundings, I was in the hospital and I didn’t know what happened, and I was depressed because I couldn’t move my left side of my leg, and my arm was hurting and I had a sore in the palm of my hand, I guess …
Patient 4
I … I remember it very well …L-l-let me tell you something. I understand what I’m talking about … Uh, I-I … I know what you’re thinking. It’s just that it’s, it’s, it’s, it’s. How can I … It just takes time. It doesn’t happen over night. Oh you’re kidding … Absolutely. I hope so, I hope so, I hope so.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Aijmer K. Conversational routines in English. London, New York: Longman; 1996. [Google Scholar]
- Bell RA, Healey JG. Idiomatic communication and interpersonal solidarity in friends’ relational cultures. Human Communication Research. 1992;14:47–67. [Google Scholar]
- Berthier M. Transcortical aphasias. East Sussex, UK: Psychology Press; 1999. [Google Scholar]
- Blank SC, Scott SK, Murphy K, Warburton W, Wise R. Speech production: Wernicke, Broca and beyond. Brain. 2002;125:1829–1838. doi: 10.1093/brain/awf191. [DOI] [PubMed] [Google Scholar]
- Blanken G, Marini V. Where do lexical speech automatisms come from? Journal of Neurolinguistics. 1997;10:19–31. [Google Scholar]
- Bloom RL, Borod JC, Obler LK, Koff E. A preliminary characterization of lexical emotional expression in right and left brain-damaged patients. International Journal of Neuroscience. 1990;55:71–80. doi: 10.3109/00207459008985952. [DOI] [PubMed] [Google Scholar]
- Bookheimer SY, Zeffiro TA, Blaxton TA, Gaillard PW, Theodore WH. Activation of language cortex with automatic speech tasks. Neurology. 2000;55:1151–1157. doi: 10.1212/wnl.55.8.1151. [DOI] [PubMed] [Google Scholar]
- Borod JC, Zgaljardic D, Tabert M, Koff E. Asymmetries of emotional perception and expression in normal adults. In: Gainotti G, editor. Handbook of neuropsychology. 2. Vol. 5. Amsterdam: Elsevier; 2001. pp. 181–205. [Google Scholar]
- Brownell H, Martino G. Deficts in inference and social cognition: the effects of right hemisphere brain damage on discourse. In: Beeman M, Chiarello C, editors. Right hemisphere language comprehension: Perspectives from cognitive science. Mahwah, NJ: Lawrence Erlbaum; 1997. pp. 309–328. [Google Scholar]
- Brownwell HH, Joanette Y. Narrative discourse in neurological impaired and normal aging adults. San Diego, CA: Singular Publishing Company; 1993. [Google Scholar]
- Bruess CJ, Pearson JC. ‘Sweet pea’ and ‘pussy cat’: an examination of idiom use and marital satisfaction over the life cycle. Journal of Social and Personal Relationships. 1993;10:609–615. [Google Scholar]
- Buckingham HW, Avakian-Whitaker H, Whitaker HA. Linguistic structures in stereotyped aphasic speech. Linguistics. 1975;154-155:5–13. [Google Scholar]
- Butterworth B. Hesitation and semantic planning in speech. Journal of Psycholinguistic Research. 1974;4:75–87. [Google Scholar]
- Cadalbert A, Landis T, Regard N, Graves RE. Singing with and without words: Hemispheric asymmetries in motor control. Journal of Clinical and Experimental Neuropsychology. 1994;16:664–670. doi: 10.1080/01688639408402679. [DOI] [PubMed] [Google Scholar]
- Cheang HS, Pell MD. A study of humor and communicative intention following right hemisphere stroke. Clinical Linguistics and Phonetics. 2006;20:447–462. doi: 10.1080/02699200500135684. [DOI] [PubMed] [Google Scholar]
- Chung KKH, Code C, Ball MJ. Lexical and non-lexical speech automatisms in aphasic Cantonese speakers. Journal of Multilingual Communication Disorders. 2004;2:32–42. [Google Scholar]
- Clark CH, Fox Tree JE. Using uh and um in spontaneous speaking. Cognition. 2006;84:73–111. doi: 10.1016/s0010-0277(02)00017-3. [DOI] [PubMed] [Google Scholar]
- Code C. Speech automatisms and recurring utterances. In: Code C, editor. The characteristics of aphasia. London: Taylor & Francis; 1989. pp. 155–177. [Google Scholar]
- Critchley M. Aphasiology and other aspects of language. London: Edward Arnold, Ltd; 1970. [Google Scholar]
- Devitt M. Intuitions in linguistics. British Journal of Philosophical Science. 2006;57:481–513. [Google Scholar]
- Drew P, Holt E. Complainable matters: the use of idiomatic expressions in making complaints. Social Problems. 1988;35:398–417. [Google Scholar]
- Espir L, Rose F. The basic neurology of speech. Oxford: Blackwell Scientific Publications; 1970. [Google Scholar]
- Fillmore C. On fluency. In: Fillmore CJ, Kempler D, Wang WS-Y, editors. Individual differences in language ability and language behavior. London: Academic Press; 1979. pp. 85–102. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. ‘Mini-Mental State’: a practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fox Tree JE. Placing like in telling stories. Discourse Studies. 2006;8:723–743. [Google Scholar]
- Fox Tree JE, Schrock JC. Discourse markers in spontaneous speech: oh what a difference an o h makes. Journal of Memory and Language. 1999;40:280–295. [Google Scholar]
- Gloning I, Gloning K, Hoff H. Aphasia—a clinical syndrome. In: Halpern L, editor. Problems of dynamic neurology. Jerusalem: Hebrew University Press; 1963. pp. 63–70. [Google Scholar]
- Goldman-Eisler F. Psycholinguistics: experiments in spontaneous speech. London; New York: Academic Press; 1968. [Google Scholar]
- Graves R, Landis T. Hemispheric control of speech expression in aphasia. Archives of Neurology. 1985;42:249–251. doi: 10.1001/archneur.1985.04060030067011. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. The basal ganglia and chunking of action repertoires. Neurobiology of Learning and Memory. 1998;70:119–136. doi: 10.1006/nlme.1998.3843. [DOI] [PubMed] [Google Scholar]
- Holowka S, Pettito LA. Left hemisphere cerebral specialization for babies while babbling. Science. 2002;297:1515. doi: 10.1126/science.1074941. [DOI] [PubMed] [Google Scholar]
- Hughlings Jackson J. On the nature of the duality of the brain. In: Taylor J, editor. Selected writings of John Hughlings Jackson. Vol. 2. London: Hodder and Stoughton; 1874. pp. 129–145. [Google Scholar]
- Illes J, Metter EJ, Hanson WR, Iritani S. Language production in Parkinson’s disease: acoustic and linguistic considerations. Brain and Language. 1988;33:146–160. doi: 10.1016/0093-934x(88)90059-4. [DOI] [PubMed] [Google Scholar]
- Jucker AH. The discourse marker ‘well’: relevance theoretical account. Journal of Pragmatics. 1993;19:435–452. [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. The Boston Naming Test. Philadelphia: Lea & Ferbinger; 1983. [Google Scholar]
- Kaplan JA, Brownell HH, Jacobs JR, Gardner H. The effects of right hemisphere damage on the pragmatic interpretation of conversational remarks. Brain and Language. 1990;38:122–134. doi: 10.1016/0093-934x(90)90117-y. [DOI] [PubMed] [Google Scholar]
- Kempler D. Kempler Syntax Comprehension Test. 1986 Copyright. [Google Scholar]
- Kempler D, Van Lancker D. The Familiar and Novel Language Comprehension Test (FANL-C) 1985 Copyright. [Google Scholar]
- Kempler D, Van Lancker D, Marchman V, Bates E. Idiom comprehension in children and adults with unilateral brain damage. Developmental Neuropsychology. 1999;15:327–349. [Google Scholar]
- Kertesz A. The Western Aphasia Battery. New York: Grune & Stratton Inc; 1982. [Google Scholar]
- Kitzinger C. How to resist an idiom. Research on Language and Social Interaction. 2000;33:121–154. [Google Scholar]
- Kuiper K. Formulaic performance in conventionalized varieties of speech. In: Schmitt N, editor. Formulaic sequences: Acquisition, processing and use. Amsterdam: John Benjamin; 2004. pp. 37–53. [Google Scholar]
- LaPointe LL, Horner J. Reading Comprehension Battery for Aphasia. Tegoid, OR: C.C. Publications; 1979. [Google Scholar]
- Larsen B, Skinhøj E, Lassen HA. Variations in regional cortical blood flow in the right and left hemispheres during automatic speech. Brain. 1978;10:193–200. doi: 10.1093/brain/101.2.193. [DOI] [PubMed] [Google Scholar]
- Lum CC, Ellis AW. Is ‘nonpropositional’ speech preserved in aphasia? Brain and Language. 1994;46:368–391. doi: 10.1006/brln.1994.1020. [DOI] [PubMed] [Google Scholar]
- Mishkin M, Malamut B, Bachevalier J. Memories and habits: two neural systems. In: Lynch G, McGaugh JL, Weinberger NM, editors. Neurobiology of Learning and Memory. New York: The Guilford Press; 1984. pp. 65–67. [Google Scholar]
- Myers P. Right hemisphere damage: Disorders of communication and cognition. San Diego: Singular Press; 1998. [Google Scholar]
- Nespoulous J-L, Code C, Virbel J, Lecours AR. Hypotheses in the dissociation between ‘referential’ and ‘modalizing’ verbal behavior in aphasia. Applied Psycholinguistics. 1998;19:311–331. [Google Scholar]
- Pawley A. Developments in the study of formulaic language since 1970: a personal view. In: Skandera P, editor. Phraseology and culture in English. Berlin: Mouton de Gruyter; 2007. pp. 3–34. [Google Scholar]
- Pawley A, Syder FH. Two puzzles for linguistic theory: nativelike selection and nativelike fluency. In: Richards JC, Schmidt R, editors. Language and communication. London: Longman; 1983. pp. 191–225. [Google Scholar]
- Petrovici J-N. Speech disturbances following stereotaxic surgery in ventrolateral thalamus. Neurosurgical Review. 1980;3:189–195. doi: 10.1007/BF01647128. [DOI] [PubMed] [Google Scholar]
- Pimental P, Kingsbury NA. Mini inventory of right brain injury. Austin, TX: Pro-Ed; 1989. [Google Scholar]
- Ryding E, Bradvik B, Ingvar DH. Changes of regional cerebral blood flow measured simultaneously in the right and left hemisphere during automatic speech and humming. Brain. 1987;110:1345–1358. doi: 10.1093/brain/110.5.1345. [DOI] [PubMed] [Google Scholar]
- Schaltenbrand G. The effects of stereotactic electrical stimulation in the depth of the brain. Brain. 1965;88:835–840. doi: 10.1093/brain/88.4.835. [DOI] [PubMed] [Google Scholar]
- Schiffrin D. Discourse markers. New York: Cambridge University Press; 1987. [Google Scholar]
- Sidtis JJ. Some problems for representations of brain organization based on ‘activation’. Brain and Language. 2007;102:130–140. doi: 10.1016/j.bandl.2006.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. Speech and other functions after left (dominant) hemispherectomy. Journal of Neurology, Neurosurgery and Psychiatry. 1966;29:467–471. doi: 10.1136/jnnp.29.5.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speedie LJ, Wertman E, T’air J, Heilman KM. Disruption of automatic speech following a right basal ganglia lesion. Neurology. 1993;43:1768–1774. doi: 10.1212/wnl.43.9.1768. [DOI] [PubMed] [Google Scholar]
- Tannen D. Talking voices: Repetition, dialogue, and imagery in conversational discourse. Cambridge: Cambridge University Press; 1989. [Google Scholar]
- Tannen D, Öztek PC. Health to our mouths: formulaic expressions in Turkish and Greek. In: Coulmas F, editor. Conversational routine. The Hague: Mouton; 1981. pp. 37–54. [Google Scholar]
- Van Lancker D. Language lateralization and grammars. In: Kimball J, editor. Studies in syntax and semantics. II. New York: Academic Press; 1973. pp. 197–204. [Google Scholar]
- Van Lancker D. Nonpropositional speech: neurolinguistic studies. In: Ellis AE, editor. Progress in the psychology of language. III. Hillsdale, NJ: Lawrence Erlbaum; 1988. pp. 49–118. [Google Scholar]
- Van Lancker D. Nonpropositional speech in aphasia. In: Blanken G, Dittmann J, Grimm J, Marshall JC, Wallesch C-W, editors. Linguistic disorders and pathologies, An international handbook. Berlin: Walter de Gruyter; 1993. pp. 215–225. [Google Scholar]
- Van Lancker D. Rags to riches: our increasing appreciation of cognitive and communicative abilities of the human right cerebral hemisphere. Brain and Language. 1997;57:1–11. doi: 10.1006/brln.1997.1850. [DOI] [PubMed] [Google Scholar]
- Van Lancker D, Cummings J. Expletives: neurolinguistic and neurobehavioral perspectives on swearing. Brain Research Reviews. 1999;31:83–104. doi: 10.1016/s0165-0173(99)00060-0. [DOI] [PubMed] [Google Scholar]
- Van Lancker D, Klein KK. Preserved recognition of familiar personal nouns in global aphasia. Brain & Language. 1990;39:511–529. doi: 10.1016/0093-934x(90)90159-e. [DOI] [PubMed] [Google Scholar]
- Van Lancker D, Sidtis JJ. The identification of affective-prosodic stimuli by left- and right- hemisphere-damaged subjects: all errors are not created equal. Journal of Speech and Hearing Research. 1992;35:963–970. doi: 10.1044/jshr.3505.963. [DOI] [PubMed] [Google Scholar]
- Van Lancker D, Bogen JE, Canter GJ. A case report of pathological rule-governed syllable intrusion. Brain and Language. 1983;20:12–20. doi: 10.1016/0093-934x(83)90028-7. [DOI] [PubMed] [Google Scholar]
- Van Lancker Sidtis D. When novel sentences spoken or heard for the first time in the history of the universe are not enough: toward a dual-process model of language. International Journal of Language and Communication Disorders. 2004;39:1–44. doi: 10.1080/13682820310001601080. [DOI] [PubMed] [Google Scholar]
- Van Lancker Sidtis D. Has neuroimaging solved the problems of neurolinguistics? Brain and Language. 2006a;98:276–290. doi: 10.1016/j.bandl.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Van Lancker Sidtis D. Where in the brain is nonliteral language? Metaphor and Symbol. 2006b;21:213–244. [Google Scholar]
- Van Lancker Sidtis D. Formulaic and novel language in a ‘dual process’ model of language competence: evidence from surveys, speech samples, and schemata. In: Corrigan RL, Moravcsik EA, Ouali H, Wheatley KM, editors. Formulaic Language: Volume 2 Acquisition, loss, psychological reality, functional applications. Amsterdam: Benjamins Publishing Co; 2008. pp. 151–176. [Google Scholar]
- Van Lancker Sidtis D, Postman WA. Formulaic expression in spontaneous speech of left- and right- hemisphere-damaged subjects. Aphasiology. 2006;20:411–426. [Google Scholar]
- Van Lancker Sidtis D, Rallon G. Tracking the incidence of formulaic expressions in everyday speech: methods for classification and verification. Language and Communication. 2004;24:207–240. [Google Scholar]
- Van Lancker Sidtis D, McIntosh R, Grafton R. PET activation studies comparing two speech tasks widely used in surgical mapping. Brain and Language. 2003;85:245–261. doi: 10.1016/s0093-934x(02)00596-5. [DOI] [PubMed] [Google Scholar]
- Van Lancker Sidtis D, Pachana N, Cummings J, Sidtis J. Dysprosodic speech following basal ganglia insult: toward a conceptual framework for the study of the cerebral representation of prosody. Brain and Language. 2006;97:135–153. doi: 10.1016/j.bandl.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Wechsler D. WAIS-R: Manual: Wechsler Adult Intelligence Scale—Revised. San Antonio, TX: The Psychological Corporation; 1981. [Google Scholar]
- Wray A. Formulaic language and the lexicon. Cambridge: Cambridge University Press; 2002. [Google Scholar]
- Wray A, Perkins M. The functions of formulaic language: an integrated model. Language and Communication. 2000;20:1–28. [Google Scholar]


