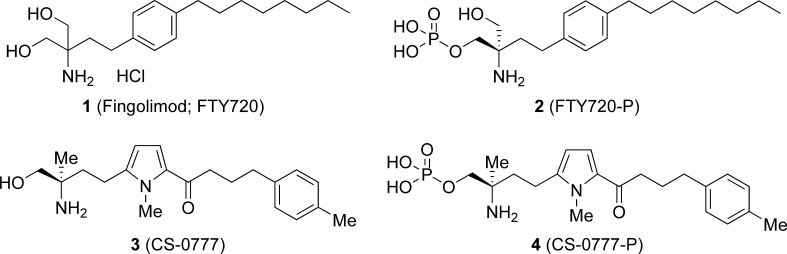
Abstract

CS-0777 (3) is phosphorylated in vivo, and the phosphate of CS-0777 (CS-0777-P) (4) acts as a selective S1P receptor-1 (S1P1) modulator. We report herein the synthesis of CS-0777 and CS-0777-P, pharmacological effects such as S1P1 and S1P3 agonist activity in vitro, peripheral blood lymphocyte lowering effects and the suppressive effect on experimental autoimmune encephalomyelitis (EAE), and also the pharmacokinetics in rats. CS-0777-P had ∼320-fold greater agonist activity for human S1P1 (EC50; 1.1 nM) relative to S1P3 (EC50; 350 nM). Following administration of single oral doses of 0.1 and 1 mg/kg of CS-0777 in rats, lymphocyte counts decreased significantly, with a nadir at 12 h postdose and recovery to vehicle control levels by 5 days postdose. In the EAE model compared to the vehicle-treated group, significant decreases in the cumulative EAE scores were observed for the 0.1 and 1 mg/kg CS-0777 groups in rats. CS-0777 is currently in clinical trials for the treatment of multiple sclerosis (MS).
Keywords: S1P1, agonist, CS-0777, lymphocyte, EAE, MS
Sphingosine 1-phosphate (S1P) receptor-1 (S1P1) is one of five G-protein-coupled receptors which bind S1P, a bioactive lipid mediator involved in cell differentiation, morphogenesis, angiogenesis, motility, and multiple other processes.1,2 Modulation of S1P receptors may have important clinical applications. In particular, targeting S1P1 with synthetic modulators appears to have clinical utility in the suppression of autoimmunity by affecting lymphocyte trafficking.1−4 Knowledge of the physiology and pharmacology of S1P1 has evolved rapidly through studies involving fingolimod (FTY720) (1), a S1P-like derivative of the fungal metabolite myriocin that modulates S1P1 signaling on lymphocytes and other cells (see Figure 1).5−7 The systemic administration of fingolimod induces a dose-responsive decrease in circulating lymphocytes, which contributes to its efficacy in animal models of autoimmune and inflammatory diseases. According to recent information, the active phosphorylated form of fingolimod (FTY720-P) (2),8,9 which is generated in vivo via a sphingosine kinase, acts as an S1P1 agonist or functional antagonist. Binding of FTY720-P to S1P1 induces receptor internalization and inhibits receptor recycling, depriving T and B cells of their ability to sense an S1P gradient and, thus, preventing their exit from lymphoid organs.3−7 Recently, the clinical efficacy of fingolimod has been demonstrated in phase 3 studies in patients with relapsing remitting multiple sclerosis (MS).10,11 However, FTY720-P lacks specificity due to its affinity for S1P3, S1P4, and S1P5.12,13 While the beneficial effects of fingolimod are thought to be mediated via S1P1, these other S1P receptor subtypes may contribute to off-target effects such as bradycardia, which is reported to be mediated via S1P3 in rodents.14,15 As a result, selective S1P1 modulators have been proposed as a means to avoid potential side effects, such as bradycardia, that have been identified in clinical trials of fingolimod.16,17 We therefore focused our attention on synthesizing compounds with greater S1P1 selectivity. Comprehensive evaluation of various properties of these synthesized compounds, such as the affinity for each S1P receptor subtype, immunosuppressive efficacy in vivo, and toxicity and pharmacokinetic (PK) aspects, led to the identification of a promising clinical candidate, CS-0777 (3), conclusively. We report herein the synthesis of CS-0777 (3) and the phosphate of CS-0777 (CS-0777-P) (4). In addition, we evaluated pharmacological effects such as S1P1 and S1P3 agonist activity in vitro, peripheral blood lymphocyte lowering effects, and the suppressive effect on the experimental autoimmune encephalomyelitis (EAE) model and also the PK profile in rats.
Figure 1.
The synthetic route of CS-0777 (3) is shown in Scheme 1. Treatment of 5(18) with Ac2O and NEt3 resulted in providing diacetate 6 in quantitative yield. Then, acylation at the 5-position on the pyrrole ring was carried out in the presence of an excess amount of 4-(4-methylphenyl)butanoyl chloride (7) and 4-(dimethylamino)pyridine (DMAP) in toluene at 110 °C to afford enol ester 8. The two acetyl groups and the enol ester moiety were removed by saponification with aqueous LiOH in THF-MeOH to afford CS-0777 (3) in good yield.
Scheme 1.
The chemical synthesis of CS-0777-P (4) was carried out as shown in Scheme 2. After protection of the amino group of 3 with allyl chloroformate (AllocCl) and KHCO3 in aqueous AcOEt, phosphorylation using (AllylO)2PN(i-Pr)2 was performed in the presence of tetrazole, and subsequent treatment with 3-chloroperoxybenzoic acid (mCPBA) in CH2Cl2 cleanly provided 9 in moderate yield. Both the allyl and Alloc groups of 9 were deprotected with Pd(PPh3)4 and pyrrolidine in CH3CN to give the desired CS-0777-P (4).
Scheme 2.
However, this synthetic route was still unsatisfactory because of the low yield and multistep reactions. In order to circumvent these issues, we already reported one-step biotransformation of α,α-disubstituted α-amino alcohol derivatives.19 Among our microbial library, strains belonging to Circinella muscae, which was reported to have microbial phosphorylation activity of ML-236B,20 were found to convert CS-0777 (3) to CS-0777-P (4) in 73% yield, as shown in Scheme 3. Thus, the biotransformation method is more practical and efficient than the previous route shown in Scheme 2.
Scheme 3.

Next, agonist-specificity CS-0777-P (4) was determined by measuring agonist-evoked [35S]GTPγ-S binding activity to rat and human S1P1 and S1P3 expressed in transfected CHO-K1 cells as shown in Table 1.21 The active FTY720-P (2) was included as a comparator. CS-0777-P (4) demonstrated potent agonist activity for rat and human S1P1 (EC50 = 1.8 and 1.1 nM, respectively) and at least 100-fold greater activity for S1P3 (EC50 = 200 and 350 nM, respectively). In comparison, the agonist activity of FTY720-P (2) for rat and human S1P1 (EC50 = 0.29 and 0.37 nM, respectively) was approximately 5- to 10-fold greater than that for rat and human S1P3 (EC50 = 1.3 and 3.3 nM, respectively). Relative to S1P3 agonist activity, CS-0777-P (4) appears to have greater selectivity for S1P1 compared to FTY720-P (2). As a result, CS-0777 is expected to avoid potential side effects corresponding to S1P3. In addition, the agonist activity of CS-0777-P (4) for human S1P5 (EC50 = 21 nM) was approximately 60-fold weaker than that of FTY720-P (EC50 = 0.34 nM), and both compounds had no ability to bind human S1P2 (data not shown).
Table 1. In Vitro Agonist-Evoked GTPγ-S Binding to Rat and Human S1P1 and S1P3a.
| EC50(nM) |
|||||
|---|---|---|---|---|---|
| rat |
human |
||||
| compd | S1P1 | S1P3 | S1P1 | S1P3 | selectivity (human S1P1 vs S1P3) |
| 4 (CS-0777-P) | 1.8 | 200 | 1.1 | 350 | 320 |
| 2 (FTY720-P) | 0.29 | 1.3 | 0.37 | 3.3 | 9 |
Note: EC50 is defined as the midpoint between the binding ratio of the vehicle and the maximum response of the test compound.
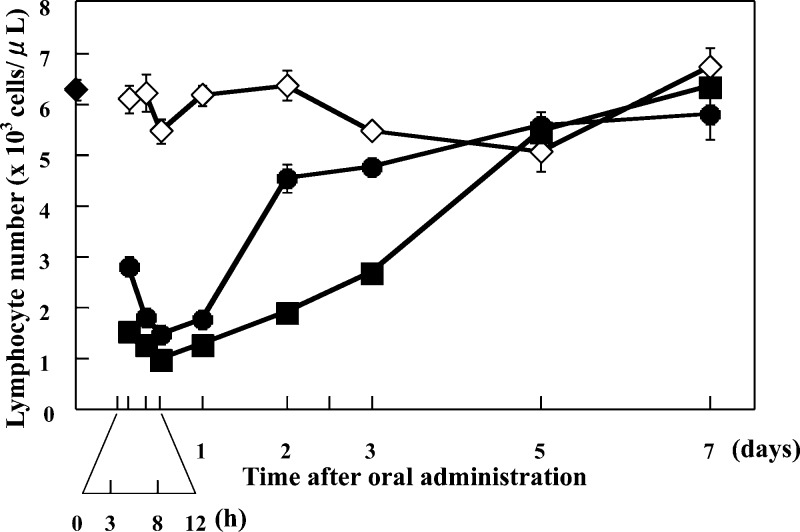
In a second series of studies, the effects of CS-0777 (3) on peripheral blood lymphocyte counts and the suppression of the development of EAE were investigated in rats. In Lewis rats administered a single oral dose of 0.1 or 1 mg/kg of CS-0777, peripheral blood lymphocyte counts decreased to 27.4% and 18.4% of vehicle-treated control values for the 0.1 and 1 mg/kg dose levels, respectively, 12 h after CS-0777 administration. By 48 h postdose, lymphocyte counts had recovered to 71.5% and 30.1% of control for 0.1 and 1 mg/kg doses, respectively, and by 5 days postdose, lymphocyte counts of both CS-0777 administered groups were similar to those of vehicle-treated rats (Figure 2).21
Figure 2.
Effects of a single oral dose of CS-0777 on lymphocyte counts in rats. Vehicle (1% MC solution, ◇), 0.1 mg/kg of CS-0777 (●), or 1 mg/kg of CS-0777 (◼) was orally administered to rats. The rats were anesthetized and abdominally dissected 3 h, 8 h, 12 h, 1 day, 2 days, 3 days, 5 days, and 7 days after oral administration. The blood was drawn from the inferior vena cava of administered rats, and lymphocyte number was counted by an automatic hemacytometer. The blood of untreated rats was also drawn, and lymphocyte number was counted (◆). Five rats were used for each group. Lymphocyte numbers are shown as mean ± SE.
Next, we evaluated the suppressive effect of CS-0777 on EAE in rats. EAE has been used as a preclinical animal model for proof of concept studies for multiple sclerosis therapy. EAE was induced to Lewis rats (6-week old females) by injection of myelin basic protein guinea pig fragment 68−82 with adjuvant into both hind footpads of rats on day 0. Vehicle or 0.01, 0.1, or 1 mg/kg of CS-0777 was orally administered every day from day 0 to day 20. The EAE score was evaluated daily from day 8 to day 21 in accordance with the following criteria: 0, no abnormality; 1, flaccid tail; 2, mild paralysis of limb and/or ataxia; 3, unilateral hind limb paralysis; 4, bilateral hind limb paralysis; 5, tetraplegia; 6, dead.21 Cumulative EAE scores were calculated by summing up the daily scores. Vehicle-administered rats began to show clinical signs of EAE from day 12, and thereafter, an acute increase of the EAE score was observed, reaching a maximal level on day 15. Mean cumulative EAE scores, calculated by summing the daily scores, were 14.0, 9.6, 0, and 0 for the vehicle and 0.01, 0.1, and 1 mg/kg of CS-0777 groups, respectively. Compared to the vehicle-treated group, statistically significant decreases in the cumulative EAE scores were observed for the 0.1 and 1 mg/kg of CS-0777 groups (nonparametric Dunnett test [joint ranking], p < 0.01) (Figure 3).21 Thus, CS-0777 has a potent suppressive effect on EAE in rats, and from the above data, CS-0777 is expected to be a promising agent for the treatment of MS.
Figure 3.
Suppressive effect of CS-0777 on rat EAE.
The pharmacokinetics of CS-0777 and CS-0777-P were evaluated in male Sprague−Dawley rats. The PK parameters of CS-0777 and CS-0777-P in rats after single oral administration of CS-0777 at doses of 0.1 and 1 mg/kg are presented in Table 2, and the blood concentration versus time profiles of CS-0777 and CS-0777-P are shown in Figure 4. After oral administration, the predominant form in blood was CS-0777-P. The blood CS-0777 concentration reached maximum blood concentration (Cmax) from 3.25 to 4.75 h postdose. Meanwhile, blood CS-0777-P reached Cmax at 9.0−9.5 h postdose and declined with a similar T1/2 to that of CS-0777. The Cmax and AUC of CS-0777-P were ca. 10-fold or larger than those of CS-0777. The oral bioavailability of CS-0777-P, calculated as the AUC ratio of CS-0777-P after oral and intravenous administration of CS-0777, was 80.6−90.8%.22
Table 2. Pharmacokinetic Parameters and Bioavailability of CS-0777 and CS-0777-P to Ratsa.
| compd | dose (mg/kg) | Cmax (ng/mL) | Tmax (h) | T1/2 (h) | AUC0-inf (ng·h/mL) | Fb (%) | CLc (mL/(min·kg)) | Vssc (L/kg) |
|---|---|---|---|---|---|---|---|---|
| CS-0777 | 0.1 | 1.89 ± 0.20 | 4.75 ± 3.50 | 5.40 (n = 1) | 31.7 (n = 1) | 72.2 (n = 1) | 38.0 ± 2.3 | 17.1 ± 0.7 |
| 1 | 18.2 ± 2.0 | 3.25 ± 2.06 | 10.4 ± 1.2 | 346 ± 35 | 69.5 ± 6.9 | 33.7 ± 2.7 | 18.1 ± 1.0 | |
| CS-0777-P | 0.1 | 26.4 ± 3.4 | 9.50 ± 1.00 | 10.9 ± 1.0 | 686 ± 136 | 80.6 ± 15.9 | nad | nad |
| 1 | 220 ± 19 | 9.00 ± 2.00 | 11.6 ± 0.6 | 6280 ± 540 | 90.8 ± 7.7 | nad | nad |
Note: Each value is the mean ± standard deviation (SD) of four animals in the rat study.
The F value (%) of CS-0777-P was calculated by dividing the CS-0777-P area under the curve (AUC) after oral administration of CS-0777 by that after intravenous administration of CS-0777 at the same dosage.
The total body clearance (CL) and distribution volume at steady state (Vss) were calculated based on the data after intravenous administration of CS-0777.
Not applicable.
Figure 4.
Blood concentration versus time profiles of CS-0777 and CS-0777-P to rats.
In conclusion, we discovered CS-0777 (3), a structurally novel S1P1 selective modulator showing potent and selective S1P1 agonist activity in vitro, peripheral blood lymphocyte lowering and suppression of the development of EAE in a rat model, and an excellent PK profile. CS-0777 is currently undergoing human clinical trials. The initial clinical results for single dose administration of CS-0777 in healthy subjects and a 12-week open-label study in patients with multiple sclerosis have recently been completed and will be reported separately elsewhere.23
Acknowledgments
We thank Dr. Tsuyoshi Nakamura, Dr. Takuya Ikeda, Dr. Takaaki Jojima, Dr. Takahiro Yamane, Taiji Goto, Takeshi Fukuda, Takashi Tsuji, and Yumiko Mizuno for preparing the key intermediates and their helpful discussions; Dr. Masakazu Tamura, Rie Ohya, and Kiyoaki Yonesu for performing the S1P1-3 agonist selectivity assays and their helpful discussions; and Taku Moriguchi, Yasunori Ono, and Emi Kurosawa for performing screenings to provide the biotransformation.
Author Contributions
T. Nishi, S. Miyazaki, T. Takemoto, K. Suzuki, Y. Iio contributed to the Design, Synthesis of CS-0777; K. Nakajima and T. Ohnuki contributed to the Synthesis of CS-0777-P and FTY720-P; Y. Kawase and F. Nara contributed to the In vitro study of CS-0777-P and FTY-720-P; S. Inaba and T. Izumi contributed to the PK study of CS-0777 and CS-0777-P; and H. Yuita, K. Oshima, H. Doi, R. Inoue, W. Tomisato, T. Kagari, and T. Shimozato contributed to the In vivo study of CS-0777.
The author contribution for T. Shimozato was erroneously reported in the version of this paper published on March 2, 2011. The correct version was reposted on March 8, 2011.
References
- Cyster J. G. Annu. Rev. Immunol. 2005, 23, 127. [DOI] [PubMed] [Google Scholar]
- Kihara A.; Igarashi Y. Biochim. Biophys. Acta 2008, 1781, 496. [DOI] [PubMed] [Google Scholar]
- Mandala S.; Hajdu R.; Bergstrom J.; Quackenbush E.; Xie J.; Milligan J.; Thornton R.; Shei G.-J.; Card D.; Keohane C.; Rosenbach M.; Hale J.; Lynch C. L.; Rupprecht K.; Parsons W.; Rosen H. Science 2002, 296, 346. [DOI] [PubMed] [Google Scholar]
- Rivera J; Proia R. L.; Olivera A. Nat. Rev. Immunol. 2008, 8, 753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann V.; Davis M. D.; Heise C. E.; Albert R.; Cottens S.; Hof R.; Bruns C.; Prieschl E.; Baumruker T.; Hiestand P.; Foster C. A.; Zollinger M.; Lynch K. R. J. Biol. Chem. 2002, 277, 21453. [DOI] [PubMed] [Google Scholar]
- Chiba K. Pharmacol. Ther. 2005, 108, 308. [DOI] [PubMed] [Google Scholar]
- Brinkmann V. Pharmacol. Ther. 2007, 115, 84. [DOI] [PubMed] [Google Scholar]
- Hale J. J.; Yan L.; Neway W. E.; Hajdu R.; Bergstrom J. D.; Milligan J. A.; Shei G.-J.; Chrebet G. L.; Thornton R. A.; Card D.; Rosenbach M.; Rosen H.; Mandala S. Bioorg. Med. Chem. 2004, 12, 4803. [DOI] [PubMed] [Google Scholar]
- Kiuchi M.; Adachi K.; Tomatsu A.; Chino M.; Takeda S.; Tanaka Y.; Maeda Y.; Sato N.; Mitsutomi N.; Sugahara K.; Chiba K. Bioorg. Med. Chem. 2005, 13, 425. [DOI] [PubMed] [Google Scholar]
- Kappos L.; Radue E.-W.; O’Conner P.; Polman C.; Hohlfeld R.; Calabresi P.; Selmaj K.; Agoropoulou C.; Leyk M.; Zhang-Auberson L.; Burtin P. N. Engl. J. Med. 2010, 362, 387. [DOI] [PubMed] [Google Scholar]
- Cohen J. A.; Barkhof F.; Comi G.; Hartung H.-P.; Khatri B. O.; Montalban X.; Pelletier J.; Capra R.; Gallo P.; Izquierdo G.; Tiel-Wilck K.; de Vera A.; Jin J.; Stites T.; Wu S.; Aradhye S.; Kappos L. N. Engl. J. Med. 2010, 362, 402. [DOI] [PubMed] [Google Scholar]
- Brinkmann V.; Cyster J. G.; Hla T. Am. J. Transplant. 2004, 4, 1019. [DOI] [PubMed] [Google Scholar]
- Matloubian M.; Lo C. G.; Cinamon G.; Lesneski M. J.; Xu Y.; Brinkmann V.; Allende M. L.; Proia R. L.; Cyster J. G. Nature 2004, 427, 355. [DOI] [PubMed] [Google Scholar]
- Forrest M.; Sun S.-Y.; Hajdu R.; Bergstrom J.; Card D.; Doherty G.; Hale J.; Keohane C.; Meyers C.; Milligan J.; Mills S.; Nomura N.; Rosen H.; Rosenbach M.; Shei G.-J.; Singer I. I.; Tian M.; West S.; White V.; Xie J.; Proia R. L.; Mandala S. J. Phamacol. Exp. Ther. 2004, 309, 758. [DOI] [PubMed] [Google Scholar]
- Sanna M. G.; Liao J.; Jo E.; Alfonso C.; Ahn M.-Y.; Peterson M. S.; Webb B.; Lefebvre S.; Chun J.; Gray N.; Rosen H. J. Biol. Chem. 2004, 279, 13839. [DOI] [PubMed] [Google Scholar]
- Kappos L.; Antel J.; Comi G.; Montalban X.; O’Conner P.; Polman C. H.; Haas T.; Korn A. A.; Karlsson G.; Radue E. W. N. Engl. J. Med. 2006, 355, 1124. [DOI] [PubMed] [Google Scholar]
- Brinkmann V. Pharmacol. Ther. 2007, 115, 84. [DOI] [PubMed] [Google Scholar]
- Nakamura T.; Tsuji T.; Iio Y.; Miyazaki S.; Takemoto T.; Nishi T. Tetrahedron: Asymmetry 2006, 17, 2781. [Google Scholar]
- Ohnuki T.; Tsuji T.; Miyazaki S.; Moriguchi T.; Nishi T. Synlett 2009, 910. [Google Scholar]
- Endo A.; Yamashita H.; Naoki H.; Iwashita T.; Mizukawa Y. J. Antibiot. 1985, 38, 328. [DOI] [PubMed] [Google Scholar]
- Shimozato T.; Tomisato W.; Doi H.; Kagari T.; Inoue R.; Yuita H.; Oshima K.; Makino T.; Suzuki K.; Sato S.; Tamura M.; Kawase Y.; Yonesu K.; Nara F.. Eur. J. Pharmacol. 2011, in preparation
- Inaba S.; Goto M.; Yabe Y.; Tanaka H.; Takahashi M.; Ikeda T.; Iwabuchi H.; Izumi T.. Drug Metab. Dispos. 2011, , in preparation
- Moberly J.; Rohatagi S.; Zahir H.; Hsu C.; Noveck R.; Truitt K.. J. Clin. Pharmacol. 2011, , in press [DOI] [PubMed]