Abstract
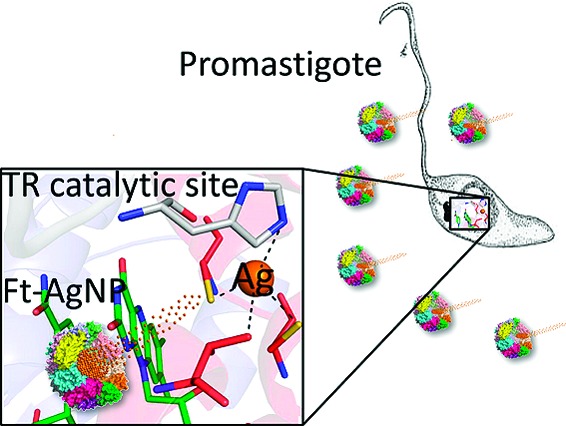
In Leishmania the glutathione/glutathione reductase eukaryotic redox sys-tem is replaced by the unique trypanothione/trypanothione reductase (TR) system. In vitro, silver is a more effective TR inhibitor than antimony, the first line drug against leishmaniasis in most endemic countries, and its mechanism of inhibition is similar to that of Sb(III). In particular, silver binds with high affinity to the catalytic triad Cys52, Cys57, and His461′, thereby inhibiting TR. Here, Ag(0) activity was tested on the promastigote and amastigote stages of Leishmania infantum using a drug-delivery system consisting in Ag(0) nanoparticles encapsulated by ferritin molecules (PfFt−AgNPs). These were able to induce an antiproliferative effect on the parasites at metal concentrations lower than those used with antimony.
Keywords: Trypanothione reductase, Leishmania, silver, nanoparticles, drug delivery
Leishmaniasis affects at present 12 million people worldwide.1 Because effective vaccines are not yet available, the chemotherapy remains the only treatment option for controlling the infection. Except for the recent encouraging advances in chemotherapy obtained with amphotericin B and its new liposomal formulations, miltefosine and paromomycin, the treatment modalities for leishmaniasis infections mostly rely on antimony-based drugs, which date back over 60 years and suffer from poor efficacy, high toxicity, and increasing resistance.2 The Leishmania life cycle involves two principal morphological stages, promastigote and amastigote. The first one develops within an insect vector, a phlebotomine sand-fly; the second one infects the macrophages of vertebrate hosts, which produce considerable amounts of H2O2 to eliminate intruding parasites. The search for urgently needed drugs against Leishmania parasites is focusing on metabolic pathways vital for the amastigote parasite and distinct from the analogous metabolism in the mammalian host. In this respect, the trypanothione metabolism enzymes are considered as promising targets of new drugs against leishmaniasis.
The trypanothione (T(SH)2) is synthesized from glutathione and spermidine by the trypanothione synthetase (TryS) and is kept reduced by the trypanothione reductase (TR). Trypanothione participates in crucial thiol−disulfide exchange reactions and serves as electron donor in several metabolic pathways, from synthesis of DNA precursors to oxidant detoxification. The T(SH)2/TR system replaces many of the antioxidant and metabolic functions of the glutathione/glutathione reductase (GR) and thioredoxin/thioredoxin reductase (TrxR) systems present in other organisms and therefore is necessary for the parasite survival.3−5 Sb(V), reduced inside the amastigote to Sb(III),6 interferes in vivo with the T(SH)2 metabolism by inducing rapid efflux of intracellular T(SH)2 and inhibiting TR in intact cells.7 We disclosed the molecular mechanism of antimonial inhibition of TR by structural analysis of reduced TR in complex with NADPH and Sb(III).8 This structure revealed that all residues of the catalytic triad, i.e. Cys52, Cys57, and His461′, are engaged in complex formation with Sb(III).
Several metal complexes have been tested on a variety of trypanosomatids and resulted to be active on different molecular targets.9−11 For example, (2,2′:6′,2′′ terpyridine)platinum(II) complexes have been found to be effective on Leishmania donovani, Trypanosoma cruzi, and Trypanosoma brucei and to have a dual action: the terpyridine moiety interacts with DNA, whereas the metal binds to the thiol containing enzymes.9 Gold(I) triphenylphosphine complex has potent antiproliferative effects on Leishmania spp. and Trypanosoma cruzi, due to the NADH fumarate reductase inhibition.10 Silver polypyridyl complexes are biologically active against Leishmania mexicana, where they interact with DNA.11 Silver has also been shown to have antibacterial action at very low concentrations, since it reacts very easily with protein carboxylates, hydroxyls, and thiols. Thus, silver compounds are used as antibacterial agents.12
Recently, our group demonstrated the ability of Pyrococcus furiosus ferritin (PfFt) to act as a protein template for the production of Ag(0) nanoparticles encapsulated by ferritin molecules (AgNPs).13 The AgNPs are characterized by a narrow size distribution (2.1 ± 0.4 nm), high stability in water solution at millimolar particle concentrations, and high thermal stability. The high solubility and stability in water are two essential properties of the silver nanoparticles (NPs), to make them suitable for biomedical applications.
In the present work, we investigated the inhibitory effect of silver on TR activity. Moreover, we determined and analyzed the X-ray structure of the complex between Ag(0) and TR in order to understand the molecular basis of protein inhibition. Finally, we measured the inhibitory effect of silver NPs in in vitro models of parasite growth and infectivity. The high solubility of PfFt-AgNPs and the fact that ferritins are known to be phagocitized by the macrophages, where the Leishmania parasites multiply,14,15 were exploited to assess the potential application of PfFt-AgNPs as antileishmania agent.
Experiments for calculation of the Ki of Ag(I) and Ag(0) were carried out by adding different concentrations of Ag(I) to a solution containing oxidized TR and 100 μM NADPH. After starting the reaction by addition of oxidized trypanothione (TS2), the absorbance at 340 nm decreases, indicating NADPH oxidation. The curve displaying trypanothione reduction by TR in the presence of Ag(I) is biphasic, since Ag(I) (E0 = 0.80 V) is reduced to Ag(0) within a few minutes by NADPH (E0 = 0.32 V). In order to separate the contribution of Ag(I) and Ag(0) to TR inhibition, TR and TS2 were added to the reaction mixture after 3 min, the time necessary for a complete reduction of Ag(I) by NADPH. The experimental data were fitted using the Michaelis−Menten equation. These experiments show that TR is strongly inhibited in vitro by silver in both oxidized and reduced form. In fact, the inhibition constant measured for Sb(III) is 1.5 μM,8 whereas the inhibition constants calculated for Ag(0) and Ag(I) are 500 ± 200 nM and 50 ± 10 nM, respectively (see Fig. S2 of the Supporting Information).
The ability of silver to inhibit TR is explained at the molecular level by the crystal structure of reduced L. infantum TR in complex with NADPH and silver solved at 3.3 Å resolution (see Supporting Information) (Figure 1). Indeed, the difference electron density map Fo − Fc shows the presence of a strong peak in both monomers, corresponding to the Sb(III) binding site, that has been assigned to a silver atom with an occupancy of 80% in both subunits (Figure 2). Ag(0) binds, as Sb(III), to the two catalytic cysteine residues (Cys52(SG)−Ag = 2.0 Å; Cys57(SG)−Ag = 2.3 Å) and to the His461′ of the 2-fold symmetry related subunit (His461′(ND1)−Ag = 2.7 Å) (Figure 2). The fourth coordination ligand is Thr335 (Thr335(OG1)−Ag = 3.3 Å). Thus, the structural analysis clearly shows that silver binds in the catalytic cleft as Sb(III) and inhibits TR activity by coordinating the same residues. Similarly to what observed for Sb(III), silver binding is only possible upon enzyme reduction, because in the oxidized enzyme the two cysteine residues form a disulfide bridge.
Figure 1.
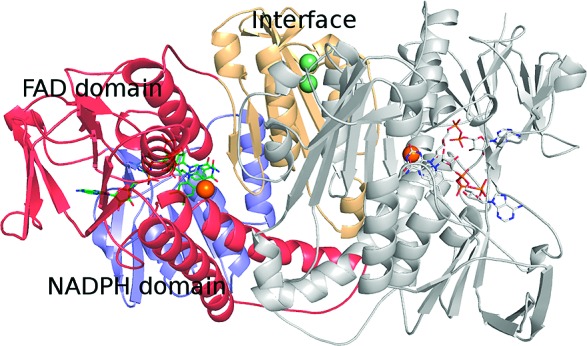
Structure of reduced TR in complex with NADPH and silver (PDB code 2X50). Silver atoms in the catalytic sites are represented as orange spheres whereas silver atoms at the dimeric interface are represented as light green spheres. NADPH and FAD molecules are represented as sticks. Figure generated by PyMol.19
Figure 2.
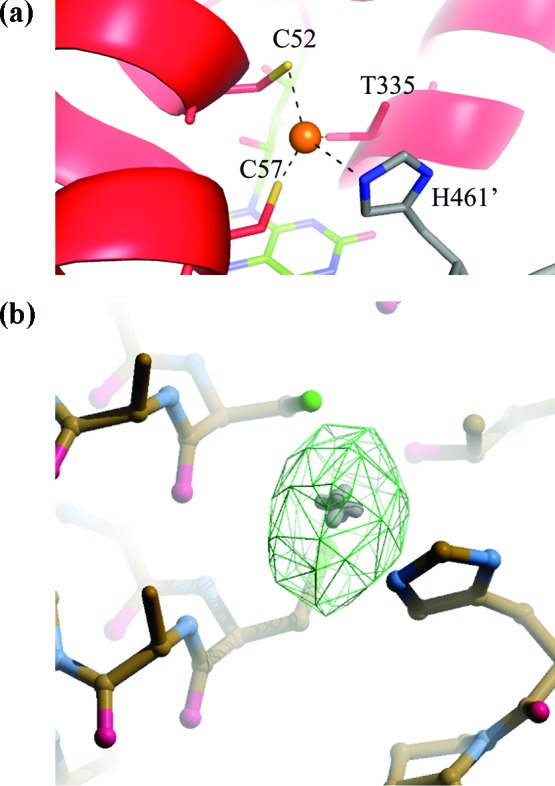
(a) Close view of the catalytic site of TR, with bound silver represented as a orange sphere. The two subunits in the asymmetric unit are colored in red and in gray respectively; the FAD molecule is colored in green. C52, C57, T335, and H461′ are represented as sticks. (b) Stick representation of the catalytic site and the Fourier difference map Fo − Fc (in green) contoured at 6σ. Figure generated by PyMol19 and Coot.20
Interestingly, inspection of the structure allowed us to identify a new dinuclear metal binding site placed at the interface between the 2-fold symmetry related subunits. The silver atoms are placed at a distance of 3.3 Å from each other, bound in a cavity formed by the two 433−437 α-helices of both subunits and coordinated by residues furnished by both subunits. The first Ag(0), displaying an occupancy of 100%, is linearly coordinated by the Cys444 residues of both subunits (S−Ag distances: 1.8 Å and 1.9 Å). The second Ag(0), displaying an occupancy of 50%, is linearly coordinated by Met447 and Met447′ (S−Ag distances: 2.2 Å and 2.4 Å) (Figure 3). It cannot be excluded that these two silver ions may contribute to enzyme inhibition by increasing the rigidity of the protein scaffold.
Figure 3.
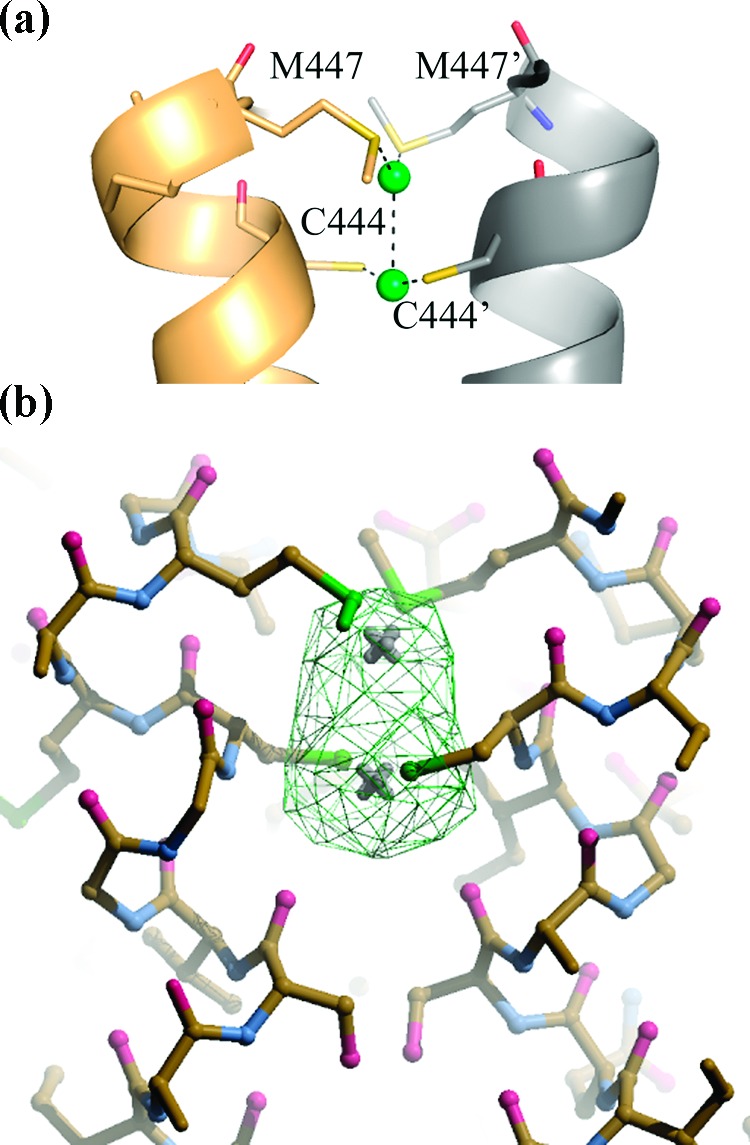
(a) Interface binding site of silver atoms to TR with bound silver represented as green spheres. The two interface domains in the asymmetric unit are colored in yellow-orange and in gray, respectively; C444, C444′, M447, and M447′ are represented as sticks. Each Ag atom is coordinated to two residues of each subunit: C444 (SG−Ag = 1.9 Å) and M447 (SD−Ag = 2.2 Å), and C444′ (SG−Ag = 1.8 Å) and M447′ (SD−Ag = 2.4 Å), respectively. (b) Stick representation of the interface silver binding site and the Fourier difference map Fo − Fc (in green) contoured at 6σ. Figure generated by PyMol19 and Coot.20
To test the silver antiparasitic activity on Leishmania cells in both amastigote and promastigote stages, we used AgNPs as silver-delivery agents (for experimental details, see Supporting Information). Nanosized particles have elicited much attention in recent years for their chemical and physical properties with respect to ionic and bulk material. In fact, recent studies demonstrated that AgNPs have a strong microbicidal effect and that this effect depends on the surface area as well as the shape of the nanoparticles.16 In addition, the use of the cagelike protein PfFt as bioreactor allows AgNP to be produced with a narrow size distribution and high stability in water solution at millimolar particle concentrations. Conversely, other AgNPs with adequate solution stability could be obtained only at submicromolar levels.13
Further, PfFt is a suitable carrier for antileishmanial drugs, since ferritins are known to be phagocitized by macrophages, where the amastigotes proliferate, thereby guaranteeing a high concentration of the encapsulated cargo inside the target cell.15
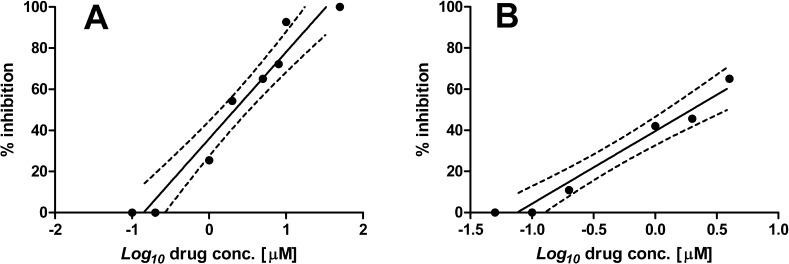
The AgNPs induced a potent dose-dependent antiproliferative effect on L. infantum promastigotes, the extracellular Leishmania stage. A dose of 50 μM Ag induced 100% promastigote mortality, whereas concentrations ranging from 1 to 10 μM determined a leishmanistatic activity. At these sublethal concentrations, parasites showed motility inhibition and body swelling, not present in control cells. Lower concentrations, ≤0.2 μM, did not affect either promastigote growth or morphology. The IC50 deduced from the dose−response curve was 2.18 ± 0.33 μM (Figure 4A).
Figure 4.
% Inhibition calculated on L. infantum stages after treatment with various concentrations of AgNPs. The data are expressed as mean ± error of two independent experiments. A: promastigotes inhibition. B: amastigotes inhibition.
The efficacy of AgNPs on the amastigote stage of L. infantum was evaluated in murine macrophages from Balb/c mice.17 Drug concentrations were selected according to the observed activity against promastigotes. The samples of AgNPs containing 8−10 μM Ag(0) were found to be highly toxic for the macrophage cells, provoking cell lysis. However, the toxic effect of AgNPs on macrophages was shown to be dose-dependent and associated with a leishmanicidal activity in the concentration range 0.2−4 μM. At these concentrations, AgNPs produced a reduction in intracellular amastigote number ranging from 10.8 to 65.0%. The AgNPs IC50, deduced from the dose−response curve, was 1.76 ± 0.24 μM (Figure 4B). At concentrations ≤0.1 μM, the PtFt−AgNPs complex exhibited neither macrophage toxicity nor efficacy against the parasites.
The IC50 values measured for both promastigote and amastigote stages are lower than the IC50 of antimonial drugs in free and encapsulated forms, which ranges from 30 to 900 μM.18 AgNPs showed leishmanicidal activity on the amastigote stage, in the concentration range 0.2−4 μM. However, a dose-dependent cytotoxicity phenomenon toward the host cells was observed at concentrations ≥4 μM. A safe range of AgNPs concentrations for the treatment of Leishmania infections can only be defined after appropriate in vivo studies.
In conclusion, the biochemical analysis here reported on reduced TR from L. infantum in complex with silver shows that this metal can inhibit TR at nanomolar concentrations and, therefore, is more effective than antimony in vitro. The structural analysis clearly demonstrates that the mechanism of inhibition is very similar to that of Sb(III). Upon TR reduction by NADPH, silver binds to the catalytic triad (Cys52, Cys57, and His461′) of the enzyme, thereby disallowing the hydride transfer from the protein to the trypanothione. These results are confirmed by in vitro experiments on both promastigote and amastigote stages, which showed that the PfFt-AgNPs are able to kill the parasite on both stages at micromolar concentrations. Although silver, as other metals (including antimony), binds to the thiols of many proteins, and selectivity issues could be raised, the authors strongly believe that the use of a novel approach, based on protein-encapsulated metals, may represent a promising strategy for the treatment of cutaneous leishmaniasis.
Acknowledgments
We acknowledge the Helmholtz−Zentrum Berlin-Electron storage ring BESSY II for provision of synchrotron radiation at beamline BL 14-1. The research leading to these results has received funding from the European Community’s Seventh Framework Programme (FP7/2007-2013) under grant agreement no. 226716. We thank Dr. Anna Rosa Sannella for her support in parasitology data analysis, and Dr. Veronica Morea for useful comments.
Glossary
Abbreviation List
- TR
trypanothione reductase
- AgNPs
silver nanoparticles
- PfFt
Pyrococcus furiosus ferritin
- T(SH)2
reduced trypanothione
- TryS
trypanothione synthetase
- TrxR
thioredoxin reductase
- NPs
nanoparticles
- NADPH
reduced nicotinamide adenine dinucleotide phosphate
- FAD
, flavin adenine dinucleotide
- TS2
oxidized trypanothione
- Tris
tris(hydroxymethyl)aminomethane/HCl
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
Supporting Information Available
Information on structure solution and overall fold, experimental procedures for protein crystallization and structure solution, TR inhibition assays, preparation of Ag(0) nanoparticles, promastigote and amastigote-macrophage assays, a figure showing the mechanism of trypanothione reduction by TR (Figure S1), and a figure showing experimental traces, Lineweaver−Burk plots, and Dixon plots (Figure S2). This information is available free of charge via the Internet at http://pubs.acs.org.
Accession Codes
Atomic coordinates and structure factors have been deposited in the Protein Data Bank (www.ebi.ac.uk/msd) with PDB accession code 2X50.
Author Contributions
§ These authors contributed equally to this work.
Supplementary Material
References
- Desjeux P. Leishmaniasis. Public health aspects and control. Clin. Dermatol. 1996, 14, 417–423. [DOI] [PubMed] [Google Scholar]
- Croft S. L.; Sundar S.; Fairlamb A. H. Drug resistance in leishmaniasis. Clin. Microbiol. Rev. 2006, 19, 111–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irigoin F.; Cibils L.; Comini M. A.; Wilkinson S. R.; Flohé L.; Radi R. Insights into the redox biology of Trypanosoma cruzi: Trypanothione metabolism and oxidant detoxification. Free Radical Biol. Med. 2008, 45, 733–742. [DOI] [PubMed] [Google Scholar]
- Krauth-Siegel R. L.; Comini M. A. Redox control in trypanosomatids, parasitic protozoa with trypanothione-based thiol metabolism. Biochim. Biophys. Acta 2008, 1780, 1236–1248. [DOI] [PubMed] [Google Scholar]
- Fairlamb A. H.; Blackburn P.; Ulrich P.; Chait B. T.; Cerami A. Trypanothione: a novel bis(glutathionyl)spermidine cofactor for glutathione reductase in trypanosomatids. Science 1985, 227, 1485–1487. [DOI] [PubMed] [Google Scholar]
- Denton H.; McGregor J. C.; Coombs G. H. Reduction of anti-leishmanial pentavalent antimonial drugs by a parasite-specific thiol-dependent reductase, TDR1. Biochem. J. 2004, 381, 405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham M. L.; Fairlamb A. H. Trypanothione reductase from Leishmania donovani. Purification, characterisation and inhibition by trivalent antimonials. Eur. J. Biochem. 1995, 230, 460–468. [DOI] [PubMed] [Google Scholar]
- Baiocco P.; Colotti G.; Franceschini S.; Ilari A. Molecular basis of antimony treatment in leishmaniasis. J. Med. Chem. 2009, 52, 2603–2612. [DOI] [PubMed] [Google Scholar]
- Lowe G.; Droz A. S.; Vilaivan T.; Weaver G. W.; Tweedale L.; Pratt J. M.; Rock P.; Yardley V.; Croft S. L. Cytotoxicity of (2,2′:6′,2′′-terpyridine)platinum(II) complexes to Leishmania donovani, Trypanosoma cruzi, and Trypanosoma brucei. J. Med. Chem. 1999, 42, 999–1006. [DOI] [PubMed] [Google Scholar]
- Vieites M.; Smircich P.; Buggeri L.; Marchán E.; Gómez-Barrio A.; Navarro M.; Garat B.; Gambino D. Synthesis and characterization of a pyridine-2-thiol N-oxide gold(I) complex with potent antiproliferative effect against Trypanosoma cruzi and Leishmania sp. insight into its mechanism of action. J. Inorg. Biochem. 2009, 103, 1300–1306. [DOI] [PubMed] [Google Scholar]
- Navarro M.; Cisneros-Fajardo E. J.; Marchan E. New silver polypyridyl complexes: synthesis, characterization and biological activity on Leishmania mexicana. Arzneimittelforschung 2006, 56, 600–604. [DOI] [PubMed] [Google Scholar]
- Liau S. Y.; Read D. C.; Pugh W. J.; Furr J. R.; Russell A. D. Interaction of silver nitrate with readily identifiable groups: relationship to the antibacterial action of silver ions. Lett. Appl. Microbiol. 1997, 25, 279–283. [DOI] [PubMed] [Google Scholar]
- Kasyutich O.; Ilari A.; Fiorillo A.; Tatchev D.; Hoell A.; Ceci P. Silver ion incorporation and nanoparticle formation inside the cavity of Pyrococcus furiosus ferritin: structural and size-distribution analyses. J. Am. Chem. Soc. 2010, 132, 3621–3627. [DOI] [PubMed] [Google Scholar]
- Kosuge H.; Terashima M.; Uchida M.; Sherlock S.; Tsao P. S.; Young M. J.; Douglas T.; Dai H.; McConnell M. V. 7 T MRI of macrophages in mouse carotid atherosclerosis using novel nanoparticle platforms. J. Cardiovasc. Magn. Reson. 2009, 11Suppl. 1151. [Google Scholar]
- Uchida M.; Terashima M.; Cunningham C. H.; Suzuki Y.; Willits D. A.; Willis A. F.; Yang P. C.; Tsao P. S.; McConnell M. V.; Young M. J.; Douglas T. A human ferritin iron oxide nano-composite magnetic resonance contrast agent. Magn. Reson. Med. 2008, 60, 1073–1081. [DOI] [PubMed] [Google Scholar]
- Rai M.; Yadav A.; Gade A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [DOI] [PubMed] [Google Scholar]
- Sereno D.; Lemesre J. L. Use of an enzymatic micromethod to quantify amastigote stage of Leishmania amazonensis in vitro. Parasitol. Res. 1997, 83, 401–403. [DOI] [PubMed] [Google Scholar]
- Pujals G.; Suñé-Negre J. M.; Pérez P.; García E.; Portus M.; Tico J. R.; Miñarro M.; Carrió J. In vitro evaluation of the effectiveness and cytotoxicity of meglumine antimoniate microspheres produced by spray drying against Leishmania infantum. Parasitology Res. 2008, 102, 1243–1247. [DOI] [PubMed] [Google Scholar]
- DeLano W. L.The PyMOL Molecular Graphics System; DeLano Scientific: San Carlos, CA, 2002. [Google Scholar]
- Emsley P.; Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr., D: Biol. Crystallogr. 2004, 60, 2126–2132. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.