Abstract
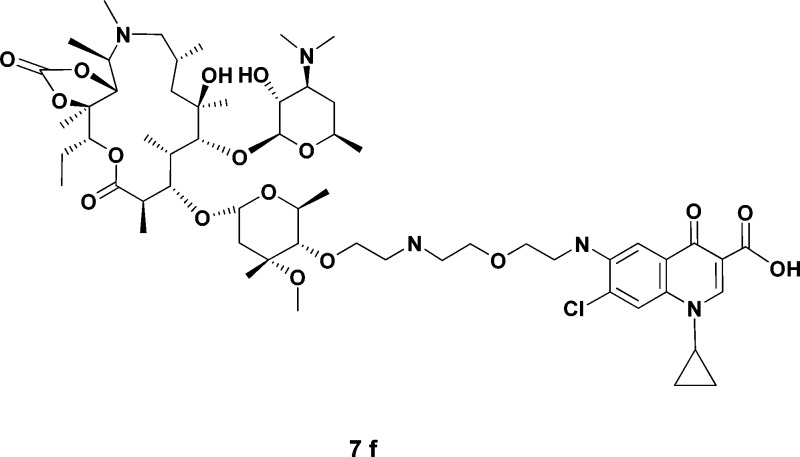
A series of novel C-4′′-substituted azithromycins was synthesized and evaluated for in vitro antibacterial activity against a panel of representative erythromycin-susceptible and macrolide-lincosamide-streptogramin (MLS) resistant pathogens. In summary, azithromycin and quinolone substructures merged in a mutually SAR-compatible design gave rise to a new class of antimicrobials with an improved spectrum and potency over azithromycin. Prototypical analogues 7f and 8f display an improved potency versus azithromycin against Gram-positive and fastidious Gram-negative pathogens. In particular, these new leads maintain activity against MLS-resistant strains of Streptococcus pneumoniae and Streptococcus pyogenes. In addition, they represent an improvement over telithromycin (1) and cethromycin (2) against the fastidious Gram-negative pathogen Haemophilus influenzae.
Keywords: Macrolide antibiotics, C-4′′-substituted azithromycins, quinolones, macrolide resistance
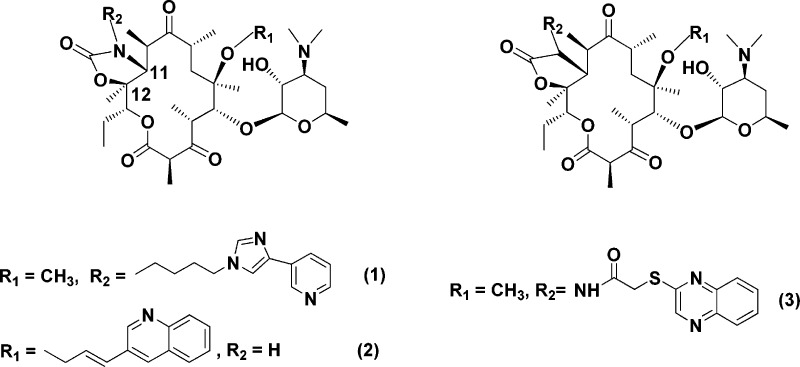
The development of bacterial resistance to currently available antibacterial agents is a growing global health problem.1 A number of solutions to the problem of bacterial resistance are possible. The most common approach is to continue modifying existing classes of antibacterial agents to provide new analogues with improved attributes. As a result of this approach, numerous macrolide analogues have been synthesized over the years.2,3 Most recently, a few major classes of 14-membered macrolides stand out with regard to their proven activity against resistant bacteria. One of the most prominent members of a relatively new family of macrolide antibacterial agents, the ketolides, is telithromycin (1, Figure 1).4
Figure 1.
Representative examples of ketolides derived from 14-membered macrolides.
Compound 1 is the only commercialized macrolide antibiotic active against resistant pathogens. It has also been proven that the C-11,C-12 cyclic carbamate present in telithromycin and cethromycin (2, ABT-773)5 can be replaced by a properly functionalized C-11,C-12 α-amino lactone ring. This type of modification led to a novel lead series exemplified by the prototype ketolide antibiotic GW773546X (3) (Figure 1).6 The ketolide series is not necessarily the only class of macrolides that can effectively address the resistance problems. Numerous other design concepts have come forward in response to the growing need for new macrolide antibiotics with improved antibacterial activity against resistant pathogens. In an effort to change the status of ketolides as the “future of macrolides”,7 a rather extensive line of studies were directed toward tethering of quinolone subunits onto the macrolide scaffold.8,9 These workers synthesized numerous C-4′′-substituted macrolides tethered to a quinolone-3-carboxylic acid substructure by a wide variety of different linkers. However, most of these derivatives were connected to C-4′′ position of the cladinose sugar through an ester bond which is known to be easily hydrolyzed by a variety of bacterial enzymes. In addition, very little effort on C-4′′ ether linked macrolides has yet been reported.10
At present, besides cethromycin11 and a few other ketolides,12−14 C-4′′ substituted azithromycins are probably one of the most advanced series of macrolides with respect to their potent and broad-spectrum antibacterial activity against all key respiratory pathogens.15
The 15-membered ring macrolide antibiotics—for example, azithromycin16—are an important series within the macrolide class of antibiotics, since they offer some advantages over 14-membered macrolides derived from erythromycin A. These advantages include better gastrointestinal tolerance, safety profile, and pharmacokinetics coupled with activity against Gram-negative pathogens. However, in contrast to 14-membered macrolides, the structure−activity relationships of their corresponding 15-membered analogues have been much less explored. In this study, we report the synthesis of numerous C-4′′ side chain modified analogues of azithromycin and azithromycin 11,12-cyclic carbonate and their antibacterial activity against some key macrolide-lincosamide-streptogramin (MLS) resistant pathogens.
We have been involved in a program dedicated to the preparation of a large number of macrolide compounds using solution-phase chemistry. The driving force for all these synthetic endeavors lay primarily in the biological profile of the macrolide analogues and their potential as antiinfective agents. In our previous work,17 we have synthesized a range of 15-membered 8a- and 9a-azahomoerythromycin analogues, which has provided us with the information toward an understanding of the essential structural features needed for antibacterial activity. In particular, within the 8a-azahomoerythromycin series, some compounds incorporating a ketolide combined with quinolone pharmacophore substructures showed potent activity against a variety of erythromycin-susceptible and MLS-resistant Gram-positive pathogens as well as fastidious Gram-negative pathogens.18 These studies have highlighted the importance of a quinolone substructure to the overall antibacterial activity and demonstrated that the quinolone moiety attached to the 8a-azaketolide core is a basic requirement for potent antibacterial activity.
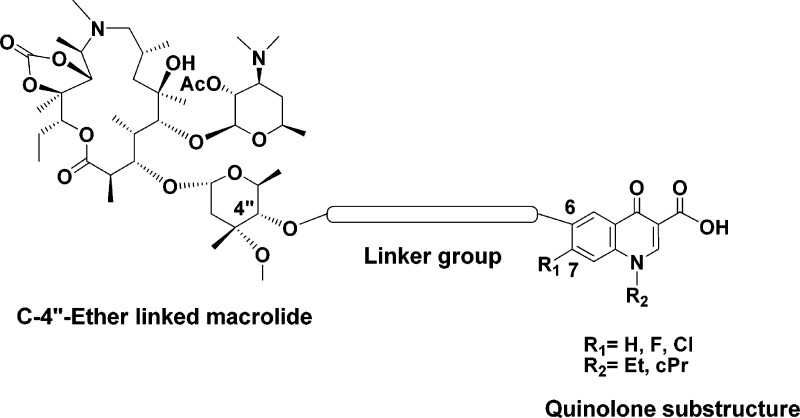
Mindful of previous results, we felt that further investigation of structure−activity relationships within the macrolide-quinolone class of compounds could lead to substantial improvements of antibacterial activity. This led us to consider the azithromycin scaffold as a suitable platform for further derivatization because of its excellent pharmacokinetic profile and ease of chemical transformation of key moieties on the scaffold. Our strategy was to attach the quinolone pharmacophore to a cladinose moiety that is remote from the macrocyclic ring and, therefore, more susceptible to such transformation than the ring itself. Basically, it was anticipated that the regioselectively protected azithromycin could be used as starting material to produce a number of analogues substituted at the C-4′′ position of the L-cladinose sugar as shown in Figure 2.
Figure 2.
Design of novel C-4′′-ether linked macrolides tethered to the quinolone subunit by different linker groups.
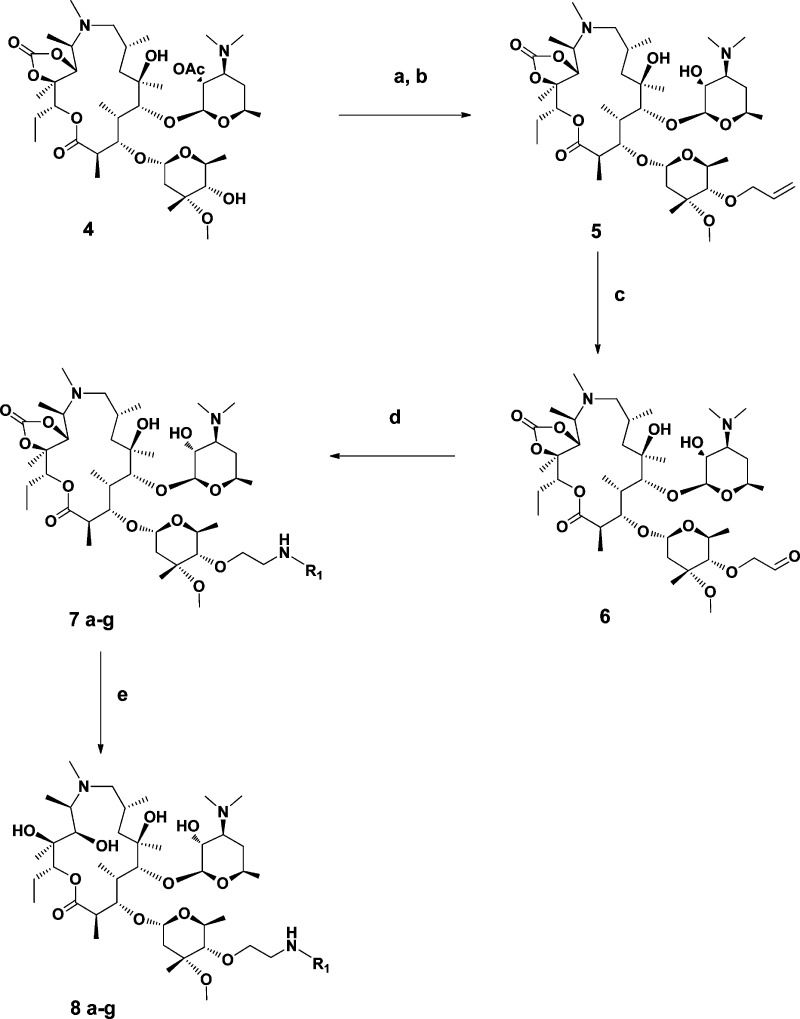
We were particularly interested in C-4′′-ether linked macrolides, since it is known that they are more stable than the corresponding ester compounds toward a range of enzymes that cause ester bond cleavage. In order to selectively alkylate the hydroxyl group at the C-4′′-position, a regioselective protection strategy had to be employed. This approach involved the selective alkylation of the C-4′′ hydroxyl group using an activated alkylating agent, such as allyl-tert-butyl carbonate. Thus, azithromycin was first protected as its 2′-O-acetyl-11,12-cyclic carbonate derivative 4 according to a modified literature procedure (Scheme 1).19
Scheme 1. Synthesis of C-4′′-Ether Linked Macrolides by Reductive Amination of the Key Intermediate 6 with Selected Amines (Chart 1).
Reagents and conditions: (a) allyl-tert-butylcarbonate, Pd(Ph3P)4, THF, reflux, 3 h; (b) MeOH, rt, 20 h; (c) OsO4, NaIO4, THF, rt, 3 h; (d) R1NH2, NaBH3CN or NaBH(OAc)3, AcOH, MeOH, rt, 3 h; or HCO2H, EtOAc, reflux, 5 h; (e) LiOH, THF/H2O, rt, 12 h.
Selective allylation with allyl-tert-butyl carbonate in the presence of a catalytic amount of Pd(Ph3P)4 followed by deprotection of the C-2′ acetyl group gave the expected 4′′-O-allyl azithromycin 11,12-cyclic carbonate 5 in 70% isolated yield. The 4′′-O-allyl azithromycin was further converted to the corresponding aldehyde 6 by one-pot oxidative cleavage of in situ formed cis-diol with OsO4/sodium periodate reagent.20
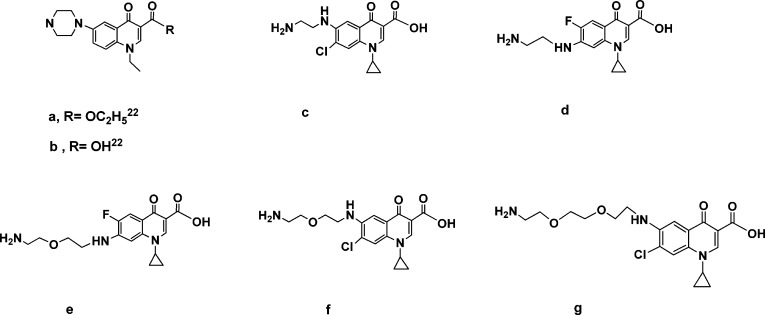
This compound served as the key intermediate for the preparation of other analogues (Scheme 1). Reductive amination21 of 6 with C-6 and C-7 substituted quinolones (Chart 1) using sodium cyanoborohydride, sodium triacetoxyborohydride, or formic acid afforded the desired products 7a−g in good yields. Deprotection of cyclic carbonates 7a−g with LiOH in aqueous THF finally gave azithromycin derivatives 8a−g, which were purified by column chromatography. The quinolones a and b were prepared by modified literature methods22 as designated. The synthesis of amines c−g was effected by heating of a mixture of fluoro- and chloro-quinolone-3-carboxylic acid derivatives at the positions C-6 and C-7 with commercially available diamines, followed by column chromatography of the formed regioisomers.
Chart 1. Quinolones Used as the R1 Substituents in Reductive Amination of 6 and HBTU Coupling of 11.
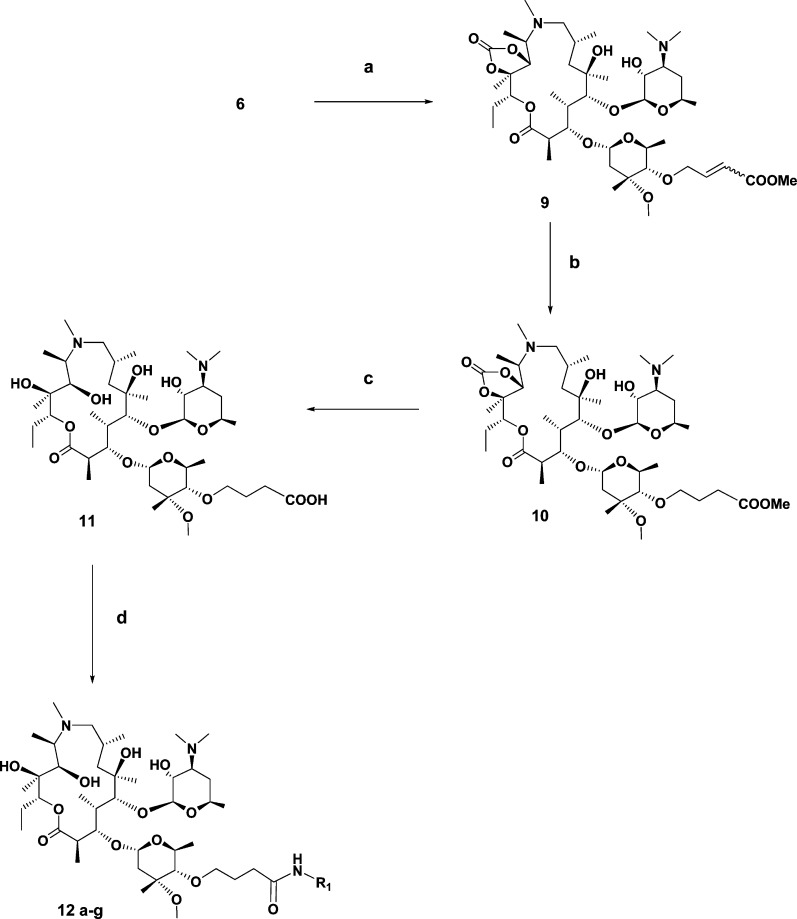
Additionally, we focused on the effects of modification of the linker-quinolone group connection moiety and linker length on antibacterial activity. From 4′′-O-(2-oxo-ethyl)azithromycin 11,12-cyclic carbonate (6), another series of azithromycin analogues was prepared through the carboxylic acid intermediate 11, as outlined in Scheme 2.
Scheme 2. Synthesis of C-4′′-Ether Linked Macrolides by HBTU-Mediated Coupling of Carboxylic Acid 11 with Selected Amines (Chart 1).
Reagents and conditions: (a) allyl-tert-butylcarbonate, Pd(Ph3P)4, THF, reflux, 3 h; (b) MeOH, rt, 20 h; (c) OsO4, NaIO4, THF, rt, 3 h; (d) R1NH2, NaBH3CN or NaBH(OAc)3, AcOH, MeOH, rt, 3 h; or HCO2H, EtOAc, reflux, 5 h; (e) LiOH, THF/H2O, rt, 12 h.
Wittig reaction of aldehyde 6 with (carbomethoxymethylene)triphenylphosphorane in refluxing benzene generated a mixture of α,β-unsaturated esters 9 highly enriched in the E isomer (E/Z = 95/5). Catalytic hydrogenation of 9 with 10 mol % Pd on carbon in methanol at room temperature afforded aliphatic ester 10, which was used in the next step without further purification. LiOH-induced hydrolysis of the ester group in 10 resulted at the same time in deprotection of C-11,12 cyclic carbonate and the formation of 4′′-O-azithromycin carboxylic acid 11, a key intermediate for further transformation. HBTU coupling of 11 with C-6-amino- and C-7-amino-substituted quinolone-3-carboxylic acids (Chart 1) was thus employed. The reaction proceeded smoothly to afford the corresponding azithromycin analogues in 60−90% isolated yield.
The antibacterial activity of the C-4′′substituted azithromycins was tested against a panel of representative pathogens selected from the Pliva Research Institute culture collection. The in vitro antibacterial activities are reported as minimum inhibitory concentrations (MICs) that were determined by the agar microdilution method according to NCCLS standards.23 Table 1 shows the in vitro activity of the azithromycin analogues and the reference compounds, azithromycin, telithromycin (1), cethromycin (2), and ciprofloxacin.
Table 1. In Vitro Antibacterial Activity of C-4′′-Ether Linked Macrolides against Selected Pathogensa,b.
|
S. aureus |
S. pneumoniae |
S. pyogenes |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| compd | Ery-S | iMLS | cMLS | M | Ery-S | cMLS | M | Ery-S | iMLS | cMLS | M | M. cat.c | H. inf.d |
| 7a | 0.5 | 64 | >64 | 8 | ≤0.125 | 64 | ≤0.125 | ≤0.125 | 1 | 64 | 16 | 0.25 | 1 |
| 7b | 1 | 16 | 32 | 2 | ≤0.125 | 32 | ≤0.125 | 0.25 | 4 | 32 | 4 | 4 | 2 |
| 7c | 2 | 64 | >64 | 4 | ≤0.125 | 64 | ≤0.125 | ≤0.125 | 0.5 | 2 | 0.5 | 0.25 | 2 |
| 7d | 4 | 64 | >64 | 4 | ≤0.125 | 64 | 0.25 | ≤0.125 | 0.25 | 8 | 4 | 0.5 | 4 |
| 7e | 8 | 64 | >64 | 64 | ≤0.125 | 16 | ≤0.125 | ≤0.125 | 1 | 32 | 4 | 0.5 | 4 |
| 7f | ≤0.125 | 0.25 | 32 | 0.25 | ≤0.125 | ≤0.125 | ≤0.125 | ≤0.125 | ≤0.125 | 0.5 | ≤0.125 | ≤0.125 | 0.5 |
| 7g | 2 | 64 | 32 | 2 | ≤0.125 | 64 | ≤0.125 | ≤0.125 | ≤0.125 | 1 | 0.5 | 0.5 | 1 |
| 8b | 2 | 64 | 64 | 4 | ≤0.125 | 64 | ≤0.125 | ≤0.125 | 0.25 | 64 | 0.5 | 4 | 8 |
| 8c | 4 | 64 | >64 | 8 | ≤0.125 | 64 | 0.25 | ≤0.125 | 1 | 16 | 8 | 0.5 | 2 |
| 8d | 2 | 64 | 64 | 2 | ≤0.125 | 32 | ≤0.125 | ≤0.125 | ≤0.125 | 1 | 1 | 1 | 4 |
| 8e | 8 | 64 | >64 | 8 | ≤0.125 | 2 | ≤0.125 | ≤0.125 | ≤0.125 | 2 | 1 | 8 | 8 |
| 8f | ≤0.125 | 0.25 | 64 | 0.5 | ≤0.125 | 0.25 | ≤0.125 | ≤0.125 | ≤0.125 | 1 | 0.125 | 0.125 | 0.5 |
| 8g | 4 | 64 | 32 | 1 | ≤0.125 | 64 | ≤0.125 | ≤0.125 | ≤0.125 | 2 | 2 | 1 | 4 |
| 12c | 4 | 32 | 64 | 4 | 0.5 | 0.5 | ≤0.125 | ≤0.125 | 0.25 | 2 | 2 | 4 | 8 |
| 12d | 8 | 8 | 64 | 16 | 1 | 1 | 4 | 1 | 1 | 16 | 16 | 2 | 8 |
| 12e | 16 | 64 | 64 | 32 | 0.5 | 8 | 0.25 | 0.25 | 0.25 | 4 | 2 | 8 | 16 |
| 12f | 2 | 64 | 64 | 8 | ≤0.125 | 4 | 0.25 | ≤0.125 | ≤0.125 | 2 | 1 | 4 | 4 |
| 12g | 16 | 64 | 64 | 8 | ≤0.125 | 64 | 0.25 | 0.25 | 0.5 | 4 | 1 | 2 | 8 |
| 1 | ≤0.125 | 0.5 | >64 | 0.25 | ≤0.125 | ≤0.125 | 0.5 | ≤0.125 | ≤0.125 | 4 | 0.25 | ≤0.125 | 1 |
| 2 | ≤0.125 | 0.25 | >64 | 0.25 | ≤0.125 | ≤0.125 | ≤0.125 | ≤0.125 | ≤0.125 | 1 | ≤0.125 | ≤0.125 | 1 |
| Azi | 1 | >64 | >64 | >64 | ≤0.125 | >64 | 4 | ≤0.125 | 8 | >64 | 1 | 0.25 | 1 |
| Cipro | 0.125 | ≤0.125 | 16 | ≤0.125 | ≤0.125 | 0.25 | 0.25 | 2 | 0.25 | 0.5 | ≤0.125 | 0.125 | ≤0.125 |
Minimum inhibitory concentration (MIC) values are given in μg/mL.
All compounds were also tested against Cipro-resistant S. aureus strain. MICs were ≥64 for all except 7f: 32 μg/mL.
M. catarrhalis.
H. influenzae. Ery-S, erythromycin-susceptible strains; iMLS, inducible resistance to MLS antibiotics; cMLS, constitutive MLS resistance; M, efflux mediated macrolide resistance; Azi, azithromycin; Cipro, ciprofloxacin.
In general, the azalides were poorly active against a constitutively MLS-resistant strain of Staphylococcus aureus (MICs 32 to >64 μg/mL). In contrast to ketolides,17 most of these compounds were also poorly active against inducibly resistant S. aureus strains. The most interesting feature of these new compounds was their effectiveness against efflux resistant staphylococci and pneumococci as well as the activity of selected analogues against constitutively MLS-resistant pneumococci.
The compounds generally maintained good activity against erythromycin-susceptible strains of S. pyogenes and S. pneumoniae as well as MLS inducibly and constitutively resistant strains of S. pyogenes. The compounds most active against constitutively resistant streptococcal strains (7f, 8f, 12c) are linked through the C-6 position of the quinolone nucleus and the C-4′′-position of azithromycin or azithromycin 11,12-cyclic carbonate by a spacer of eight or nine atoms in length. In a similar fashion, antibacterial results show a linker-length-dependent activity which peaked with compound 7f, a macrolide analogue having a nine carbon-heteroatom linker separating the quinolone ring from the C-4′′ ether oxygen on the cladinose ring. Compounds 7c and 7d, analogues having shorter carbon−heteroatom linkers than those of 7f, show a reduction in antibacterial activity with the decrease in linker length. Compound 7g, the analogue with the longest carbon−heteroatom linker in the series herein disclosed, also shows a reduction in activity in comparison to 7f. The MIC values of the C-6 linked quinolones 7c and 7f were in general 2−256-fold better than that of the C-7 linked analogues 7d and 7e, respectively, against most of the strains tested. In addition, the structure of the linker connecting the macrolide and quinolone pharmacophores appears to be critical for antibacterial activity. Amide-linked quinolone analogues 12c−g were generally not as active as macrolides 8b−g, incorporating quinolone substructures linked via an amine group.
A few compounds exhibited noteworthy levels of activity against H. influenzae and constitutively resistant S. pyogenes. In particular, 7f and 8f were more potent then azithromycin and telithromycin against H. influenzae B 0529 strain (MICs of 0.5 μg/mL for both compounds, Table 1; see also Table 2 in the Supporting Information for time-kill studies) and cMLS S. pyogenes (MICs of 0.5 and 1 μg/mL, respectively).
Among the various 4′′-ether linked azithromycin-quinolone hybrids, compound 7f exhibited the most potent and balanced activity against both susceptible and resistant bacteria. In contrast, azithromycin showed either weak or no activity against the resistant organisms. These results clearly indicated that the introduction of a quinolone substituted side chain onto the macrolide scaffold was the key factor for overcoming constitutive resistance. Design of the C-4′′ tether connecting the quinolone and azithromycin therefore appears critical for potent antibacterial activity.
In order to gain further insight into the mode of action of hybrids, we measured for 7f and 8f the inhibition of protein synthesis in an in vitro transcription/translation assay and the inhibition of the enzymes that are targeted by the quinolones, DNA gyrase, and topoisomerase IV (Table 3, Supporting Information). The activities observed in the in vitro protein assay show that the macrolones are strong inhibitors of protein synthesis with potencies comparable to that of telithromycin, demonstrating their macrolide MOA. Furthermore, the macrolones 7f and 8f were essentially devoid of DNA gyrase and Topo IV inhibitory activity, clearly indicating that the dual mode of action is not operative for these compounds. It is also important to note that all analogues were inactive against the ciprofloxacin-resistant S. aureus strain and that the quinolones a−g were inactive against all bacterial pathogens tested (Table 4, Supporting Information).
In summary, a novel series of azithromycin derivatives with potent activity against key respiratory pathogens, including those resistant to macrolide antibiotics, has been identified. These compounds are characterized by having a quinolyl moiety tethered to the C-4′′-position of the azithromycin skeleton through an ether bond. Extensive structural modification of the quinolone substituted side chain led to the discovery of several promising compounds with potent activity against both erythromycin-susceptible and MLS-constitutively resistant organisms. These studies demonstrate a great sensitivity of antibacterial activity toward structural changes of the side chain. These studies also reveal a promising potential for further systematic modification to fine-tune the biological properties of future analogues within the same C-4′′-ether linked series. As a result of extensive structural modification, macrolides 7f and 8f were identified as potential lead candidates for further evaluation.
Acknowledgments
D.P. would like to thank Slavica Milković for excellent technical assistance.
Glossary
Abbreviations
- MLS
macrolide-lincosamide-streptogramin antibiotics
- HBTU
O-(benzotriazol-1-yl)-N,N,N′,N′-tetramethyluronium hexafluorophosphate
- DIPEA
diisopropylethylamine
- SAR
structure−activity relationships
- MOA
mode of action
- Topo IV
topoisomerase IV.
Author Present Address
‡ Present address: Hercegovačka 99, 10000 Zagreb, Croatia.
Supporting Information Available
Experimental procedures, spectral data, antibacterial data for selected new compounds, and activity of 7f and 8f as inhibitors of DNA gyrase, Topo IV, and bacterial protein synthesis. This material is available free of charge via the Internet at http://pubs.acs.org.
Supplementary Material
References
- Doern G. V.; Heilmann K. P.; Huynh H. K.; Rhomberg P. R.; Coffman S. L.; Brueggemann A. B. Antimicrobial Resistance Among Clinical Isolates of Streptococcus pneumoniae in the United States During 1999−2000, Including a Comparison of Resistance Rates Since 1994−1995. Antimicrob. Agents Chemother. 2001, 45, 1721–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z.; Nemoto P. A. Discovery and Development of Ketolides as a New Generation of Macrolide Antimicrobial Agents. Curr. Med. Chem.-Anti-Infect. Agents 2002, 1, 15–34. [Google Scholar]
- Mutak S.; Maršić N.; Dominis Kramarić M.; Pavlović D. Semisynthetic Macrolide Antibacterials Derived from Tylosin. Synthesis and Structure-Activity Relationships of Novel Desmycosin Analogues. J. Med. Chem. 2004, 47, 411–431. [DOI] [PubMed] [Google Scholar]
- Agouridas C.; Denis A.; Auger J.-M.; Benedetti Y.; Bonnefoy A.; Bretin F.; Chantot J.-F.; Dussarat A.; Fromentin C.; D’Ambrieres S. G.; Lachaud S.; Laurin P.; Le Martret O.; Loyau V.; Tessot N. Synthesis and Antibacterial Activity of Ketolides (6-O-Methyl-3-oxoerythromycin Derivatives): A New Class of Antibacterials Highly Potent Against Macrolide-Resistant and Susceptible Respiratory Pathogens. J. Med. Chem. 1998, 41, 4080–4100. [DOI] [PubMed] [Google Scholar]
- Ma Z.; Clark R. F.; Brazzale A.; Wang S.; Rupp M. J.; Li L.; Griesgraber G.; Zhang S.; Yong H.; Phan L. T.; Nemoto P. A.; Chu D. T. W.; Plattner J. J.; Zhang X.; Zhong P.; Cao Z.; Nilius A. M.; Shortridge V. D.; Flamm R.; Mitten M.; Meulbroek J.; Ewing P.; Alder J.; Or Y. S. Novel Erythromycin Derivatives with Aryl Groups Tethered to the C-6 Position are Potent Protein Synthesis Inhibitors and Active against Multidrug-Resistant Respiratory Pathogens. J. Med. Chem. 2001, 44, 4137–56. [DOI] [PubMed] [Google Scholar]
- Andreotti D.; Bientinesi I.; Biondi S.; Donati D.; Erbetti I.; Lociuro S.; Marchioro C.; Pozzan A.; Ratti E.; Terreni S. A Novel Ketolide Class: Synthesis and Antibacterial Activity of a Lead Compound. Bioorg. Med. Chem. Lett. 2007, 17, 5265–5269. [DOI] [PubMed] [Google Scholar]
- Nilius A. M.; Ma Z. Ketolides: The Future of the Macrolides?. Curr. Opin. Pharmacol. 2002, 2, 493–500. [DOI] [PubMed] [Google Scholar]
- Kapić S.; Čipčić Paljetak H.; Alihodžić S.; Antolović R.; Eraković Haber V.; Jarvest R. L.; Holmes D. J.; Broskey J. P.; Hunt E. 6-Alkylquinolone-3-carboxylic acid Tethered to Macrolides Synthesis and Antimicrobial Profile. Bioorg. Med. Chem. 2010, 18, 6569–6577. [DOI] [PubMed] [Google Scholar]
- Fajdetić A.; Čipčić Paljetak H.; Lazarevski G.; Hutinec A.; Alihodžić S.; Đerek M.; Štimac V.; Andreotti D.; Šunjić V.; Berge J. M.; Mutak S.; Dumić M.; Lociuro S.; Holmes D. J.; Maršić N.; Eraković Haber V.; Spaventi R. 4′′-O-(ω-Quinolylamino-alkylamino) Propionyl Derivatives of Selected Macrolides with the Activity against the Key Erythromycin Resistant Respiratory Pathogens. Bioorg. Med. Chem. 2010, 18, 6559–6568. [DOI] [PubMed] [Google Scholar]
- Alihodžić S.; Pavlović D.; Hunt E.; Forrest A. K.; Palej I.; Kapić S.; Štimac V.. Ether Linked Macrolides Useful for the Treatment of Microbial Infections. US Patent 7,547,679 B2, 2009.
- Plata D. J.; Leanna M. R.; Rasmussen M.; McLaughlin M. A.; Condon S. L.; Kerdesky F. A. J.; King S. A.; Peterson M. J.; Stoner E. J.; Wittenberger S. J. The Synthesis of Ketolide Antibiotic ABT-773 (Cethromycin). Tetrahedron 2004, 60, 10171–10180. [Google Scholar]
- Magee T. V.; Ripp S. L.; Li B.; Buzon R. A.; Chupak L.; Dougherty T. J.; Finegan S. M.; Girard D.; Hagen A. E.; Falcone M. J.; Farley K. A.; Granskog K.; Hardink J. R.; Huband M. D.; Kamicker B. J.; Kaneko T.; Knickerbocker M. J.; Liras J. L.; Marra A.; Medina I.; Nguyen T.-T.; Noe M. C.; Obach R. S.; O’Donnell J. P.; Penzien J. B.; Reilly U. D.; Schafer J. R.; Shen Y.; Stone G. G.; Strelevitz T. J.; Sun J.; Tait-Kamradt A.; Vaz A. D. N.; Whipple D. A.; Widlicka D. W.; Wishka D. G.; Wolkowski J. P.; Flanagan M. E. Discovery of Azetidinyl Ketolides for the Treatment of Susceptible and Multidrug Resistant Community-Acquired Respiratory Tract Infections. J. Med. Chem. 2009, 52, 7446–7457. [DOI] [PubMed] [Google Scholar]
- Revill P.; Bolos J.; Serradell N. EDP-420: Ketolide Antibiotic. Drugs Fut. 2006, 31, 479–483. [Google Scholar]
- Woosley L. N.; Castanheira M.; Jones R. N. CEM-101 Activity against Gram-positive Organisms. Antimicrob. Agents Chemother. 2010, 54, 2182–2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palej Jakopović I.; Kragol G.; Forrest A. K.; Frydrych C. S. V.; Štimac V.; Kapić S.; Matanović Škugor M.; Ilijaš M.; Čipčić Paljetak H.; Jelić D.; Holmes D. J.; Hickey D. M. B.; Verbanac D.; Eraković Haber V.; Alihodžić S. Synthesis and Properties of Macrolones Characterized by Two Ether Bonds in the Linker. Bioorg. Med. Chem. 2010, 17, 6578–6588. [DOI] [PubMed] [Google Scholar]
- Đokić S.; Kobrehel G.; Lazarevski G.; Lopotar N.; Tamburašev Z. Erythromycin Series. Part 11. Ring Expansion of Erythromycin A Oxime by the Beckmann Rearrangement. J. Chem. Soc., Perkin Trans. 1 1986, 1881–1890. [Google Scholar]
- Pavlović D.; Mutak S. Synthesis and Structure-Activity Relationships of Novel 8a-Aza-8a-homoerythromycin A Ketolides. J. Med. Chem. 2010, 53, 5868–5880. [DOI] [PubMed] [Google Scholar]
- Pavlović D.; Fajdetić A.; Mutak S. Novel Hybrids of 15-Membered 8a- and 9a-Azahomoerythromycin A Ketolides and Quinolones as Potent Antibacterials. Bioorg. Med. Chem. 2010, 18, 8566–8582. [DOI] [PubMed] [Google Scholar]
- Đokić S.; Kobrehel G.; Lopotar N.; Kamenar B.; Nagl A.; Mrvoš D. Erythromycin Series. Part 13. Synthesis and Structure Elucidation of 10-Dihydro-10-deoxo-11-methyl-11-azaerythromycin A. J. Chem. Res. (S) 1988, 152–153. [Google Scholar]
- Yu W.; Mei Y.; Kang Y.; Hua Z.; Jin Z. Improved Procedure for the Oxidative Cleavage of Olefins by OsO4-NaIO4. Org. Lett. 2004, 6, 3217–3219. [DOI] [PubMed] [Google Scholar]
- Debono M.; Willard K. E.; Kirst H. A.; Wind J. A.; Crouse G. D.; Tao E. V.; Vincenzi J. T.; Counter F. T.; Ott J. L.; Ose E. E.; Omura S. Synthesis and Antimicrobial Evaluation of 20-Deoxo-20-(3,5-dimethylpiperidin-1-yl)desmycosin (Tilmicosin, EL-870) and Related Cyclic Amino Derivatives. J. Antibiot. 1989, 42, 1253–1267. [DOI] [PubMed] [Google Scholar]
- Muci A. R.; Buchwald S. L.. Practical Palladium Catalysts for C-N and C-O Bond Formation. Top. Curr. Chem. 2002, 219, 131−209. [Google Scholar]
- National Committee for Clinical Laboratory Standards. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, 5th ed.; Approved standard: NCCLS Document M7-A5; 2000. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.