Abstract
A novel series of non-ATP-competitive MK2 inhibitors based on a furan-2-carboxyamide scaffold was discovered through high-throughput screening using the affinity selection–mass spectrometry-based Automated Ligand Identification System platform. Medicinal chemistry efforts optimized the initial screening hit to leadlike compounds with significant improvements in biochemical and cellular potencies, while maintaining excellent kinase selectivity and in vitro pharmacokinetic properties. Biophysical and biochemical studies confirmed the unique non-ATP-competitive binding mode of this series and suggested that highly selective inhibitors of MK2 should be feasible by targeting the outside ATP pocket.
Keywords: Mitogen-activated protein kinase-activated protein kinase 2, non-ATP-competitive inhibitors, Automated Ligand Identification System (ALIS), saturation-transfer-difference (STD) NMR, 1H/15N-HSQC (heteronuclear single quantum coherence)
Unregulated protein kinase activity is often associated with inflammatory diseases, such as rheumatoid arthritis, which is characterized by autoimmune-mediated cartilage and bone destruction that leads to intolerable joint pain and crippling and afflicts about 1% of the world's population.1 Activation of the p38/mitogen-activated protein kinase-activated protein kinase 2 (MAPKAPK2 or MK2) pathway has been implicated in promoting pro-inflammatory cytokine production and related inflammatory diseases.2−4 However, despite numerous assessments of potent inhibitors in early stage clinical trials, no p38 MAPK inhibitors have progressed beyond phase II trials largely because of dose-dependent toxicities and unimpressive efficacies.4,5 The broad involvement of p38α MAPK and its downstream substrates in diverse cellular processes may be a major cause of related clinical trial failures.4,6,7 To minimize the apparent side effects caused by directly targeting p38α MAPK, several discovery teams have attempted inhibiting targets downstream of p38α MAPK that are responsible for its pro-inflammatory cytokine-regulating properties in macrophages.4,7 Of these MK2, a serine/threonine protein kinase that directly associates with p38α MAPK to form a heterodimer and is phosphorylated and activated by p38α MAPK has emerged as the most attractive downstream target.7−10 For example, in studies employing genetically engineered mouse models wherein MK2, MK3, and MK5 genes were knocked out, it was shown that MK2 is the primary MAPKAPK family member required for lipopolysaccharide (LPS)-induced tumor necrosis factor α (TNFα) and interleukin 6 (IL6) production.11−13 Furthermore, MK2-null mice were shown to have reduced joint damage in a collagen-induced arthritis model and no induction of asthma in a lung ovalbumin sensitization model.14,15 In contrast to the embryonic lethality associated with knocking out the gene-encoding p38α MAPK, MK2-null mice were fertile and healthy, suggesting that inhibition of MK2 in inflammatory disease states may provide efficacy comparable to direct p38α MAPK inhibition but without debilitating side effects.
Over the past few years, many small molecule MK2 inhibitors, either extracted from natural products16 or developed from various research laboratories,17−21 were reported. In particular, the ATP-competitive MK2 inhibitor from Pfizer, PF-3644022, showed efficacy in different cell and rodent models.22 From their study, Pfizer forecasted difficulties trying to use an ATP-competitive approach to inhibiting MK2 in humans due to poor biochemical efficiency (BE, ratio of binding affinity to target vs cellular activity). In light of the high cellular concentrations of ATP (ca. 2–5 mM) and affinity of ATP for P38α-activated MK2 (ATP Km = 2 μM), it was suggested that the BE increase in MK2 inhibitors could be achieved through a different binding mode. A recent report presented a class of MK2 inhibitors with a mainly uncompetitive binding mechanism.23
In general, for kinases with relatively high ATP binding affinities (Km = 1–20 μM), it has been difficult to develop desirable ATP competitive inhibitors because of problems associated with attaining sufficient general selectivity and cellular activity in addition to required novel patent space. In contrast, non-ATP-competitive inhibitors can provide distinct advantages with respect to these issues. Herein, a new series of small molecules with inhibitory activity against MK2 by virtue of a novel non-ATP-competitive binding mode are described.
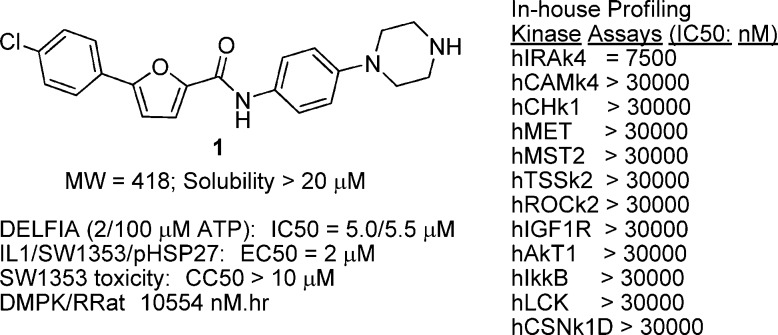
To discover novel MK2 inhibitors, we employed affinity selection–mass spectrometry (AS-MS)-based high-throughput screening using the Automated Ligand Identification System (ALIS)24 and recombinant purified MK2 protein. We employed a protein construct consisting of the minimal kinase domain of MK2 from amino acid Gln41 to Thr338. In this construct, amino and carboxy termini were truncated to both facilitate expression and slightly decrease thermal stability of the kinase domain. It was shown previously that the carboxy terminus of MK2 from residues Thr334 to Arg 364 forms an α-helical peptide that regulates MK2 nuclear localization and embeds into a substrate binding cavity on the kinase domain surface.25−27 This interaction is thought to stabilize the kinase domain;28,29 therefore, we surmised that removing this carboxy terminal peptide region by truncation at Thr338 would reduce thermal stability and in turn may increase kinase domain accessibility and in the specific context of ALIS screening may increase the probability of binding small molecule ligands with novel binding modes from the screening libraries. Compound 1 emerged as the leading hit from the ALIS screening of a mixture-based combinatorial library with the desired profile in biochemical and cell-based potency, kinase selectivity (see the Supporting Information for kinase abbreviations), DMPK (drug metabolism and pharmacokinetics), and toxicity window (Figure 1). Compound 1 showed essentially no potency shift when tested by enzyme assay at low versus high ATP concentrations (2 and 100 μM).
Figure 1.
ALIS hit 1 profiling.
To further characterize the binding mode, saturation-transfer-difference (STD) NMR studies using ATP as an active site marker were conducted to test the binding of 1 to MK2.30 In these experiments, a 2 h reference data set was first acquired using 2.5 μM MK2, 225 μM ATP, and 5 mM MgCl2. The addition of 50 μM 1 resulted in a strong set of peaks for 1 but had no effect on the intensity of ATP STD NMR peaks (Figure 2A,B). These experiments demonstrated non-ATP competitive binding of 1 to MK2. 1H/15N-heteronuclear single quantum coherence (HSQC) studies were carried out to ensure that the observed binding was site-specific. Comparison of 1H/15N-HSQC spectra collected on a sample of 50 μM MK2 with and without 1 (Figure 2C) showed that 1 binds site specifically and affects different residues than those observed for ATP binding. These NMR data provided compelling evidence that the binding of 1 to MK2 was site-specific and non-ATP competitive.
Figure 2.
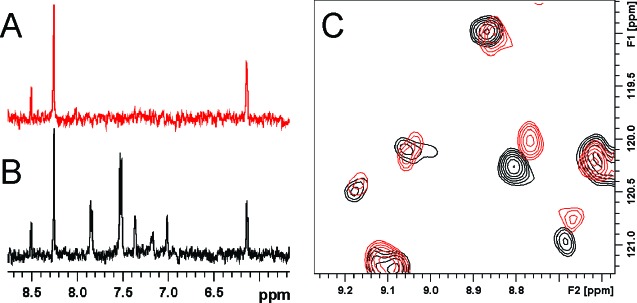
NMR characterization of binding of 1 to MK2. (A) ATP binds to the active site of MK2 and gives a large STD-NMR signal in the presence of 2.5 μM MK2. (B) The addition of 50 μM 1 results in the appearance of new STD peaks for 1 but has no effect on ATP binding. (C) A comparison of 1H/15N-HSQC spectra for apo-MK2 (black) and MK2-1 complex (red) shows that the binding of 1 affects specific MK2 residues. All NMR data were collected at 27 °C on a Bruker Avance DRX 500 spectrometer equipped with a 5 mm TXI cryoprobe.
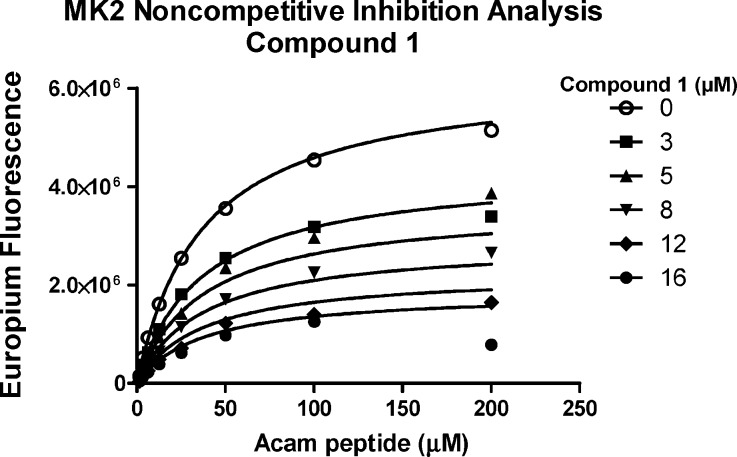
Determination of the mode of inhibition of MK2 by compound 1 with respect to peptide substrate, Acam peptide (see the Supporting Information), was made by analysis of MK2 activity in the presence of saturating ATP and varying concentrations of peptide substrate and the inhibitor (Figure 3). The data obtained were globally fit to various kinetic models of inhibition with goodness of fit evaluated by use of the Akaike information criteria (AICc), which permits direct comparison of models of inhibition of varying complexity.31 The results identified a noncompetitive inhibition model as the most likely mechanism. Mixed inhibition, wherein inhibitor 1 binds preferentially to the peptide substrate-free form of MK2, is 2-fold less likely. In contrast, competitive and uncompetitive models of inhibition were, respectively, 105 and 1011 times less likely.
Figure 3.
Enzymatic analysis of mode of inhibition by ALIS hit 1 (2.5 nM MK2, 100 μM ATP, 0–200 μM peptide substrate, Km = 35 μM).
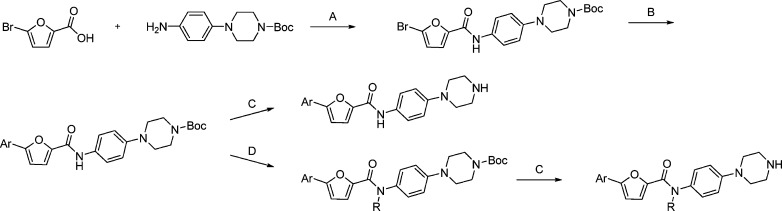
A medicinal chemistry effort was carried out to optimize this encouraging hit into a lead by improving biochemical and cell-based potency, demonstrating a favorable in vitro pharmacokinetic (PK) profile and retaining the non-ATP-competitive binding mode. A series of analogues, 2–8, with variation at the left-hand side (LHS) of the molecule were prepared following Scheme 1. 5-Bromofuran-2-carboxylic acid was reacted with aniline to give amide. 5-Aryl-furan-2-carboxamide was obtained by Suzuki cross-coupling. The removal of Boc protecting group using TFA provided the final compound.
Scheme 1. Synthesis of 5-Aryl-N-(4-(piperazin-1-yl)phenyl)furan-2-carboxamidea.
The structure–activity relationship (SAR) at LHS of this series proved to be challenging (Table 1). While an ortho-Cl substituent (2) led to a slight decrease in potency, the meta-Cl analogue (3) was inactive. Adding an ortho substitutent with either a Cl (4) or a Me group (5) to the para-Cl substitution afforded analogues with comparable affinity to compound 1. The replacement of a para-Cl group with either an electron-withdrawing CF3 group (6) or electron-donating methoxy group (7) decreased the potency by about 4-fold. The phenyl group could be replaced with a thiophene (8) with the same level of potency.
Table 1. Variation of the 5-Aryl Group of Furan on the LHS of the Molecule.
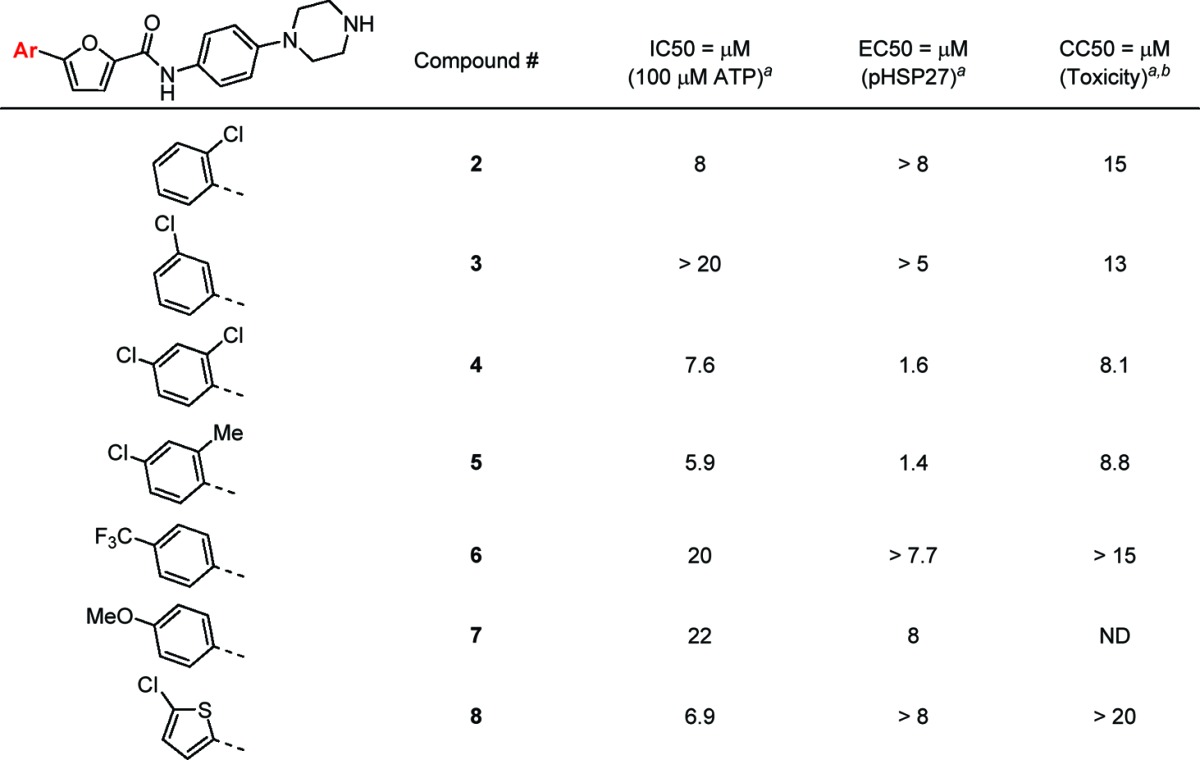
Biochemical and cell-based data represent the average values of duplicates or triplicates.
ND, not determined.
Initial efforts to improve affinity through right-hand side (RHS) changes to the series were not encouraging (Table 2). Whereas a fluorine substitution in the phenyl ring (9) reduced the affinity slightly, potency loss was observed in CF3 substitution (10). Opportunistic changes to the piperazine group at the RHS of molecule were also undertaken. Capping the distal NH group with a methyl group (11) exhibited no improvement in potency. Removal of the distal NH group and introduction of an exocyclic OH group (12) resulted in a loss of potency. The anilinic N in the piperazine ring was replaced with an sp2 carbon (13). While a potency improvement of 2-fold was observed in 13, this compound suffered from high toxicity. The change of N-arylated piperazine to N-arylated lactam (14) yielded a much less potent compound.
Table 2. Variation of N-Substitution in Furan-2-carboxamide on the RHS of the Molecule.
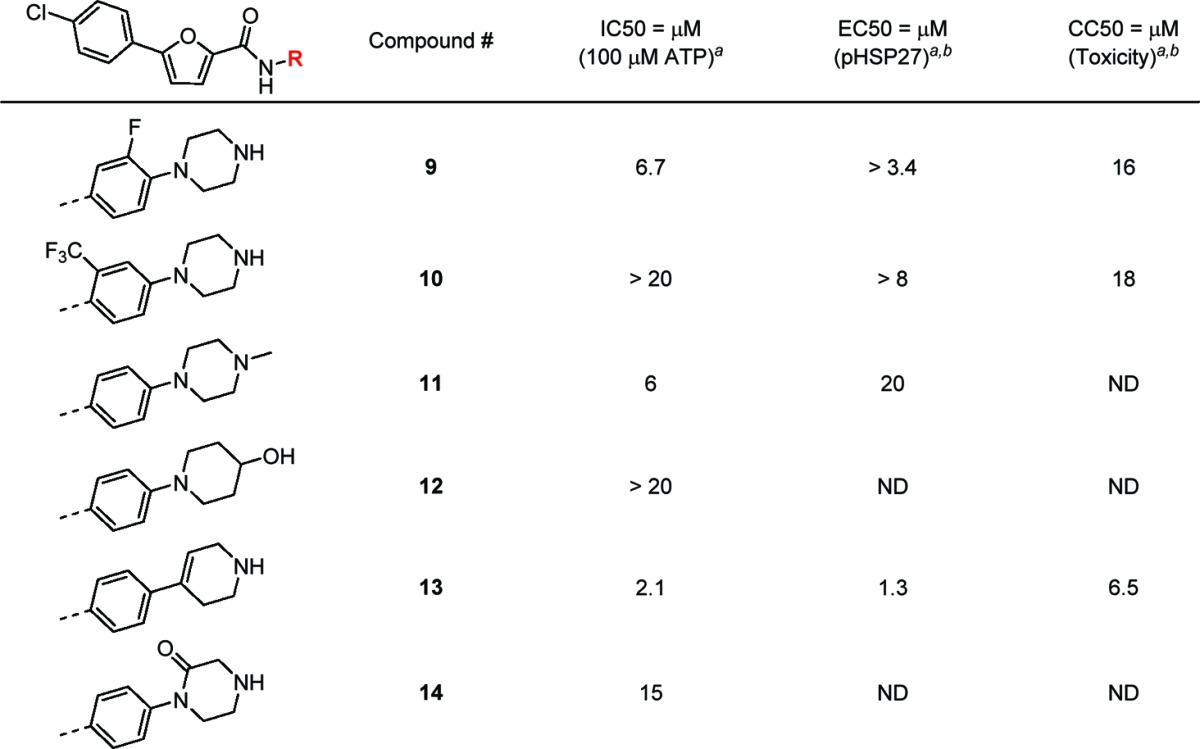
Biochemical and cell-based data represent the average values of duplicates or triplicates.
ND, not determined.
Attempts to replace the furan ring with other heterocycles such as thiophene (15), thiazole (16), isooxazole (17), and pyridine (18) were undertaken (Table 3). These compounds, 15–18, were prepared by analogy to those in Table 1 by starting with the bromo-heterocyclic carboxylic acid. Compounds generally showed a 1–2-fold reduction in affinity. In the cases of thiazole (16) and isooxazole (17) replacement, high toxicity was also observed.
Table 3. Variation of Furan with Other Heterocycles.
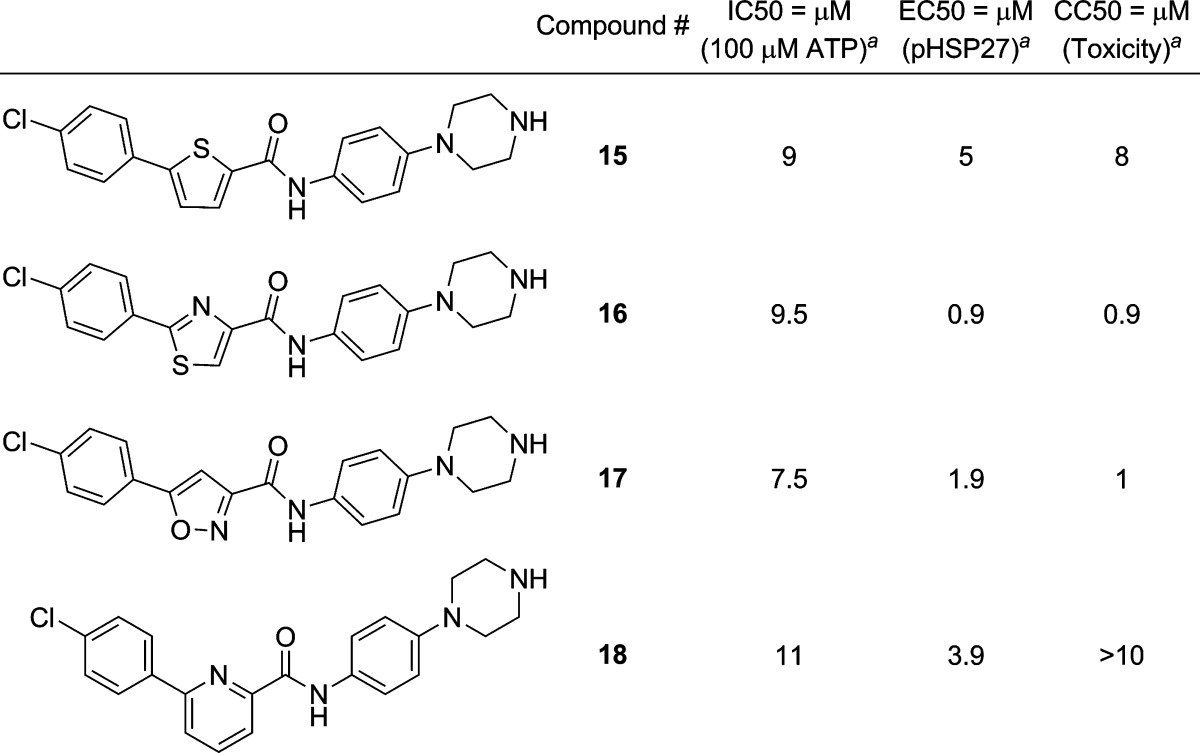
Biochemical and cell-based data represent the average values of duplicates or triplicates.
A breakthrough in potency improvement was achieved when the secondary amide was substituted with an alkyl group (Table 4). The synthesis of analogues, 19–26, follows from Scheme 1. Deprotonation of the secondary amide using sodium hydride and subsequent alkylation using alkyl halide provided the tertiary amide product, which was converted to the final compound after the removal of Boc protecting group using TFA. An 8-fold potency improvement in biochemical assay was observed when a methyl group (19) was attached to the amide. However, the cell-based potency of this compound was reduced by about 4-fold. Elongation to two carbons (20) further improved potency, not only in a biochemical assay by 40-fold but surprisingly also in cell-based activity by 2-fold. Continuing elongation to propyl (21) or iso-propyl (22) or butyl (23) provided analogues with less potency improvement than ethyl (20) but still better compounds than the starting ALIS hit 1. Notably, these alkyl-substituted analogues have shown a favorable separation between potency and cytotoxicity. Encouraged by these results, other substituted heterocycles such as imidazole (24) and pyridine (25 and 26) were explored. Biochemical potency was improved to ∼100 nM in these three analogues. Whereas the N-methylimidazole analogue (24) was much less potent in cell-based activity, N-methyl-ortho-pyridine (25) and N-methyl-para-pyridine (26) showed significant improvement in cell-based potency while retaining more than a 10-fold toxicity window.
Table 4. N-Alkylation of Furan-2-carboxamide.
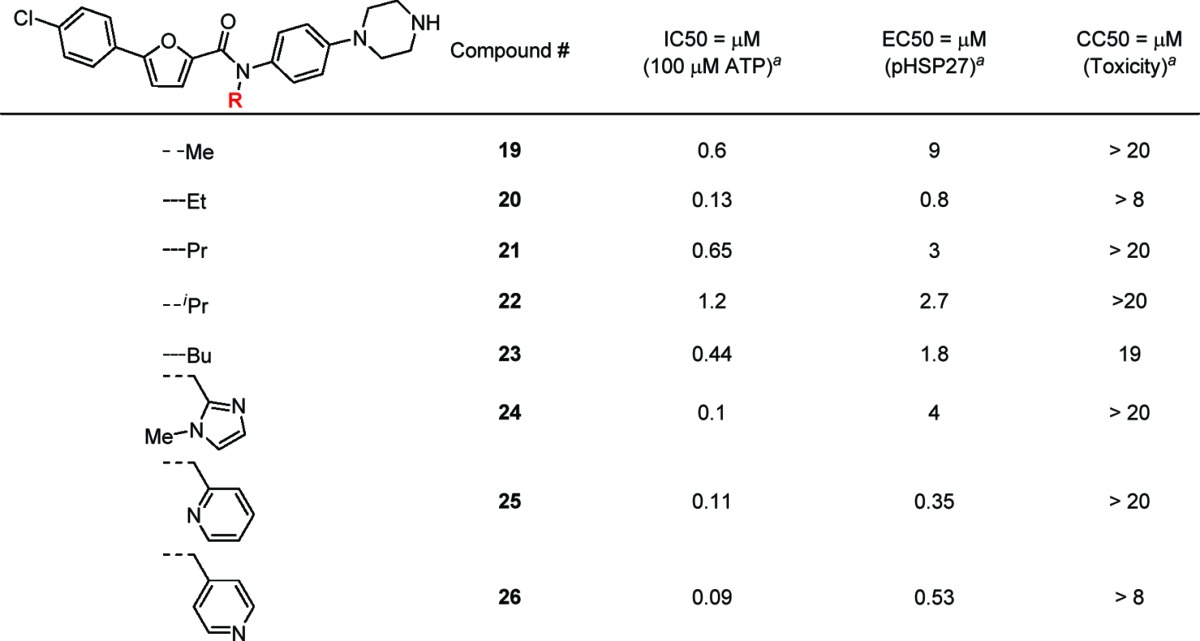
Biochemical and cell-based data represent the average values of duplicates or triplicates.
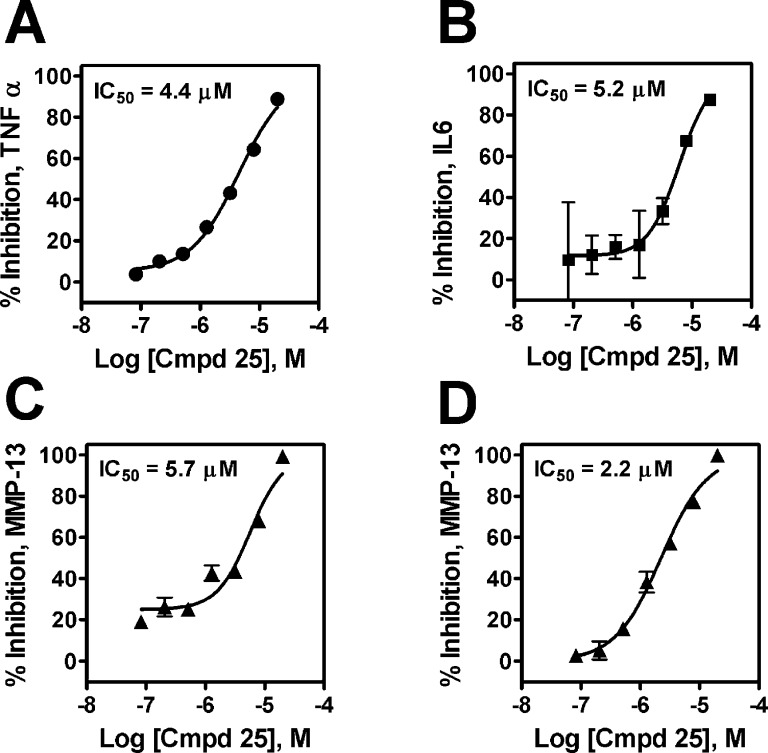
Compound 25 was profiled for kinase selectivity by screening against a broad panel of 150 protein kinases at a concentration of 10 μM, and only CK1γ3 was significantly inhibited at greater than 50%. Compound 25 bioavailability was demonstrated in an oral rat PK experiment, where an AUC of 2300 nM h was determined after a 10 mg/pk single dose. Compound 25 also demonstrated no inhibition against a panel of cytochrome P450 (CYP) enzymes up to 30 μM. Consistent with specific MK2 inhibition, compound 25 inhibited pro-inflammatory cytokine secretion from the human THP1 acute monocytic leukemia cell line, causing dose-dependent inhibition of LPS-stimulated TNFα and IL6 secretion (Figure 4A,B). Compound 25 also dose dependently inhibited IL1β-stimulated matrixmetalloprotease (MMP)13 secretion from the SW1353 chondrosarcoma cell line and human primary chondrocyte cultures (Figure 4C,D), confirming a previous report wherein MK2 activity was demonstrated to modulate secretion of MMP13 from osteoarthritis-derived chondrocytes.32
Figure 4.
Dose-dependent inhibition of inflammatory cytokine and MMP-13 production by compound 25. Treatment of LPS-stimulated THP1 cells with compound 25 dose dependently inhibits TNFα (A) and IL6 secretion (B). Treatment of IL1-β-stimulated SW1353 cells (C) and human primary osteoarthritis-derived chondrocytes (D) with compound 25 dose dependently inhibits MMP-13 secretion. For all figures, data points and error bars reflect the average and standard error of duplicate samples.
In summary, a new series of small molecules, N-alkyl-5-aryl-N-(4-(piperazin-1-yl)phenyl)furan-2-carboxamide, were developed to be potent inhibitors against MK2 with a non-ATP-competitive binding mode, enabling a high degree of MK2 selectivity. Hit-to-lead chemistry efforts resulted in significant improvements in biochemical and cellular potencies and reductions in cell toxicities, while maintaining a high degree of MK2 selectivity versus the human kinome. Of note, given its high degree of selectivity, our data suggest that compound 25 may be an excellent pharmacologic tool for specifically exploring and validating MK2 biology. We have also demonstrated another example where AS-MS screening of mixture-based combinatorial libraries combined with careful formulation of recombinant protein targets can result in the discovery of pharmacologically active compounds showing interesting and novel binding modes. The unique non-ATP-competitive binding mode, excellent kinase selectivity, and DMPK profile of this MK2 inhibitor series as exemplified by compound 25 warrant further development that may lead to a clinical candidate for inflammation or other diseases where emerging evidence implicates MK2.
Acknowledgments
Many thanks to Dr. J. Richard Miller for valuable suggestions regarding analysis of enzymatic mode of inhibition; Dr. Mingxuan Zhang and Dr. Michael Ziebell for HRMS analysis; and Michael Starks, Jason Hill, and Mark Pietrafitta for analytical support.
Author Present Address
‡ Merck Research Laboratories, 2000 Galloping Hill Road, Kenilworth, New Jersey 07033.
Supporting Information Available
Experimental procedures and spectroscopic characterization of key compounds, kinase counter screen data, CYP inhibition data, and assay protocols. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Present Address
† Merck Research Laboratories, 33 Avenue Louis Pasteur, Boston, Massachusetts 02115.
Supplementary Material
References
- Tobón G. J.; Youinou P.; Saraux A. The environment, geo-epidemiology, and autoimmune disease: Rheumatoid arthritis. Autoimmun. Rev. 2010, 9, A288. [DOI] [PubMed] [Google Scholar]
- Kumar S.; Boehm J.; Lee J. C. p38 MAP Kinases: key signalling molecules as therapeutic targets for inflammatory diseases. Nat. Rev. Drug Discovery 2003, 2, 717. [DOI] [PubMed] [Google Scholar]
- Schindler J. F.; Monahan J. B.; Smith W. G. p38 Pathway kinases as anti-inflammatory drug targets. J. Dent. Res. 2007, 86, 800. [DOI] [PubMed] [Google Scholar]
- Schett G.; Zwerina J.; Firestein G. The p38 mitogen-activated protein kinase (MAPK) pathway in rheumatoid arthritis. Ann. Rheum. Dis. 2008, 67, 909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alten R. E.; Zerbini C.; Jeka S.; Irazoque F.; Khatib F.; Emery P.; Bertasso A.; Rabbia M.; Caulfield J. P. Efficacy and safety of pamapimod in patients with active rheumatoid arthritis receiving stable methotrexate therapy. Ann. Rheum. Dis. 2010, 69, 364. [DOI] [PubMed] [Google Scholar]
- Dambach D. M. Potential adverse effects associated with inhibition of p38α/β MAP kinases. Curr. Top. Med. Chem. 2005, 5, 929. [DOI] [PubMed] [Google Scholar]
- Gaestel M; Mengel A.; Bothe U.; Asadullah K. Protein kinases as small molecule inhibitor targets in inflammation. Curr. Med. Chem. 2007, 14, 2214. [DOI] [PubMed] [Google Scholar]
- Duraisamy S.; Bajpai M.; Bughani U.; Dastidar S. G.; Ray A.; Chopra P. MK2: A novel molecular target for anti-inflammatory therapy. Expert Opin. Ther. Targets 2008, 12, 921. [DOI] [PubMed] [Google Scholar]
- White A.; Pargellis C. A.; Studts J. M.; Werneburg B. G.; Farmer B. T. II. Molecular basis of MAPK-activated protein kinase 2:p38 assembly. Proc. Natl. Acad. Sci. U.S.A. 2007, 104, 6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haar E. t.; Prabhakar P.; Liu X.; Lepre C. Crystal structure of the p38α-MAPKAP kinase 2 heterodimer. J. Biol. Chem. 2007, 282, 14684. [DOI] [PubMed] [Google Scholar]
- Kotlyarov A.; Neininger A.; Schubert C.; Eckert R.; Birchmeier C.; Volk H.-D.; Gaestel M. MAPKAP kinase 2 is essential for LPS-induced TNF-α biosynthesis. Nat. Cell Biol. 1999, 1, 94. [DOI] [PubMed] [Google Scholar]
- Shi Y.; Kotlyarov A.; Laaβ K.; Gruber A. D.; Butt E.; Marcus K.; Meyer H. E.; Friedrich A.; Volk H.-D.; Gaestel M. Elimination of protein kinase MK5/PRAK activity by targeted homologous recombination. Mol. Cell. Biol. 2003, 23, 7732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronkina N.; Kotlyarov A.; Dittrich-Breiholz O.; Kracht M.; Hitti E.; Milarski K.; Askew R.; Marusic S.; Lin L.-L.; Gaestel M.; Telliez J.-B. The mitogen-activated protein kinase (MAPK)-activated protein kinases MK2 and MK3 cooperate in stimulation of tumor necrosis factor biosynthesis and stabilization of p38 MAPK. Mol. Cell. Biol. 2007, 27, 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegen M.; Gaestel M.; Nickerson-Nutter C. L.; Lin L.-L.; Telliez J.-B. MAPKAP Kinase 2-deficient mice are resistant to collagen-induced arthritis. J. Immunol. 2006, 177, 1913. [DOI] [PubMed] [Google Scholar]
- Gorska M. M.; Liang Q.; Stafford S. J.; Goplen N.; Dharajiya N.; Guo L.; Sur S.; Gaestel M.; Alam R. MK2 controls the level of negative feedback in the NF-κB pathway and is essential for vascular permeability and airway inflammation. J. Exp. Med. 2007, 204, 1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basabe P.; Martín M.; Bodero O.; Blanco A.; Marcos I. S.; Díez D.; Urones J. G. Synthesis of (+)-makassaric acid, a protein kinase MK2 inhibitor. Tetrahedron 2010, 66, 6008. [Google Scholar]
- Argiriadi M. A.; Ericsson A. M.; Harris C. M.; Banach D. L.; Borhani D. W.; Calderwood D. J.; Demers M. D.; DiMauro J.; Dixon R. W.; Hardman J.; Kwak S.; Li B.; Mankovich J. A.; Marcotte D.; Mullen K. D.; Ni B.; Pietras M.; Sadhukhan R.; Sousa S.; Tomlinson M. J.; Wang L.; Xiang T.; Talanian R. V. 2,4-Diaminopyrimidine MK2 inhibitors. Part I: Observation of an unexpected inhibitor binding mode. Bioorg. Med. Chem. Lett. 2010, 20, 330. [DOI] [PubMed] [Google Scholar]
- Harris C. M.; Ericsson A. M.; Argiriadi M. A.; Barberis C.; Borhani D. W.; Burchat A.; Calderwood D. J.; Cunha G. A.; Dixon R. W.; Frank K. E.; Johnson E. F.; Kamens J.; Kwak S.; Li B.; Mullen K. D.; Perron D. C.; Wang L.; Wishart N.; Wu X.; Zhang X.; Zmetra T. R.; Talanian R. V. 2,4-Diaminopyrimidine MK2 inhibitors. Part II: Structure-based inhibitor optimization. Bioorg. Med. Chem. Lett. 2010, 20, 334. [DOI] [PubMed] [Google Scholar]
- Xiong Z.; Gao D. A.; Cogan D. A.; Goldberg D. R.; Hao M.-H.; Moss N.; Pack E.; Pargellis C.; Skow D.; Trieselmann T.; Werneburg B.; White A. Synthesis and SAR studies of indole-based MK2 inhibitors. Bioorg. Med. Chem. Lett. 2008, 18, 1994. [DOI] [PubMed] [Google Scholar]
- Anderson D. R.; Meyers M. J.; Vernier W. F.; Mahoney M. W.; Kurumbail R. G.; Caspers N.; Poda G. I.; Schindler J. F.; Reitz D. B.; Mourey R. J. Pyrrolopyridine inhibitors of mitogen-activated protein kinase-activated protein kinase 2 (MK-2). J. Med. Chem. 2007, 50, 2647. [DOI] [PubMed] [Google Scholar]
- Lin S.; Lombardo M.; Malkani S.; Hale J. J.; Mills S. G.; Chapman K.; Thompson J. E.; Zhang W. X.; Wang R.; Cubbon R. M.; O'Neill E. A.; Luell S.; Carballo-Jane E.; Yang L. Novel 1-(2-aminopyrazin-3-yl)methyl-2-thioureas as potent inhibitors of mitogen-activated protein kinase-activated protein kinase 2 (MK-2). Bioorg. Med. Chem. Lett. 2009, 19, 3238. [DOI] [PubMed] [Google Scholar]
- Mourey R. J.; Burnette B. L.; Brustkern S. J.; Daniels J. S.; Hirsch J. L.; Hood W. F.; Meyers M. J.; Mnich S. J.; Pierce B. S.; Saabye M. J.; Schindler J. F.; South S. A.; Webb E. G.; Zhang J.; Anderson. D. R. A benzothiophene inhibitor of mitogen-activated protein kinase-activated protein kinase 2 inhibits tumor necrosis factor α production and has oral anti-inflammatory efficacy in acute and chronic models of inflammation. J. Pharmacol. Exp. Ther. 2010, 333, 797. [DOI] [PubMed] [Google Scholar]
- Olsson H.; Sjö P.; Ersoy O.; Kristoffersson A.; Larsson J.; Nordén B. 4-Anilino-6-phenyl-quinoline inhibitors of mitogen activated protein kinase-activated protein kinase 2 (MK2). Bioorg. Med. Chem. Lett. 2010, 20, 4738. [DOI] [PubMed] [Google Scholar]
- Annis A.; Chuang C.-C.; Nazef N.. ALIS: An Affinity Selection-Mass Spectrometry System for the Discovery and Characterization of Protein-Ligand Interactions. In Methods and Principles in Medicinal Chemistry; Wanner K., Höfner G., Eds.; Mass Spectrometry in Medicinal Chemistry: Applications in Drug Discovery; Mannhold R., Kubinyi H., Folkers G., Series Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, 2007; Vol. 36, Chapter 3. [Google Scholar]
- Zu Y.-L.; Ai Y.; Huang C.-K. Characterization of an autoinhibitory domain in human mitogen-activated protein kinase-activated protein kinase 2. J. Biol. Chem. 1995, 270, 202. [DOI] [PubMed] [Google Scholar]
- Meng W.; Swenson L. L.; Fitzgibbon M. J.; Hayakawa K.; Haar E. t.; Behrens A. E.; Fulghum J. R.; Lippke J. A. Structure of mitogen-activated protein kinase-activated protein (MAPKAP) kinase 2 suggests a bifunctional switch that couples kinase activation with nuclear export. J. Biol. Chem. 2002, 277, 37401. [DOI] [PubMed] [Google Scholar]
- Engel K.; Kotlyarov A.; Gaestel M. Leptomycin B-sensitive nuclear export of MAPKAP kinase 2 is regulated by phosphorylation. EMBO J. 1998, 17, 3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kervinen J.; Ma H.; Bayoumy S.; Schubert C.; Milligan C.; Lewandowski F.; Moriarty K.; DesJarlais R. L.; Ramachandren K.; Wang H.; Harris C. A.; Grasberger B.; Todd M.; Springer B. A.; Deckman I. Effect of construct design on MAPKAP kinase-2 activity, thermodynamic stability and ligand-binding affinity. Arch. Biochem. Biophys. 2006, 449, 47. [DOI] [PubMed] [Google Scholar]
- Malawski G. A.; Hillig R. C.; Monteclaro F.; Eberspaecher U.; Schmitz A. A. P.; Crusius K.; Huber M.; Egner U.; Donner P.; Müller-Tiemann B. Identifying protein construct variants with increased crystallization propensity—A case study. Protein Sci. 2006, 15, 2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy M. A.; Senior M. M.; Wyss D. F. Screening of protein kinases by ATP-STD NMR spectroscopy. J. Am. Chem. Soc. 2005, 127, 7978. [DOI] [PubMed] [Google Scholar]
- Akaike H. An information criterion (AIC). Math. Sci. 1976, 14, 5. [Google Scholar]
- Jones S. W.; Brockbank S. M. V.; Clements K. M.; Le Good N.; Campbell D.; Read S. J.; Needham M. R. C.; Newham P. Mitogen-activated protein kinase-activated protein kinase 2 (MK2) modulates key biological pathways associated with OA disease pathology. Osteoarthritis Cartilage 2009, 17, 124. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.