Abstract
Novel colchicine derivatives possessing various substituents at the C4 position were prepared. Among them, 4-halo derivatives 3−6 were found to exhibit higher activity against cancer cell lines (A549, HT29, HCT116) as well as on mice transplanted with the HCT116 human colorectal carcinoma cell line than colchicine (1). Further, utilizing the 4-substituted colchicines, we prepared pro-drugs having a dipeptide side chain and demonstrated that these pro-drugs were activated by cathepsin B, an enzyme overexpressed in tumor cells, and exhibited selective toxicity to the tumor cells.
Keywords: Alkaloid, colchicine, structure modification, bioactivity, pro-drug, cathepsin B
Introduction
Colchicine (1) (see Figure 1) is a well-known bioactive alkaloid that is used for the therapy of acute gout. It also acts as an antimitotic agent by binding to tubulin.1 However, practical anticancer medicines derived from colchicines have not been developed so far because of their toxicity to normal cells. To solve this problem, many groups have poured much effort into the development of colchicine derivatives having high potency and reduced toxicity.2−11
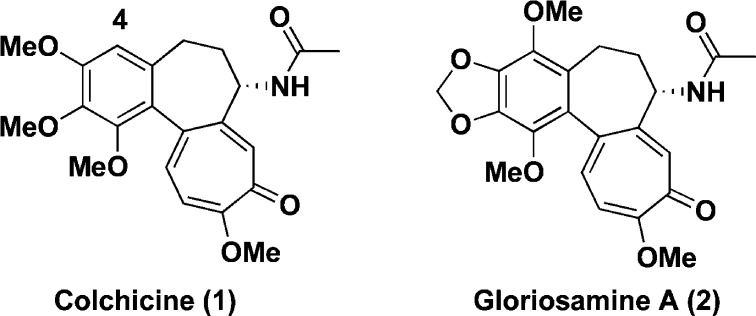
Figure 1.
Structures of colchicine (1) and gloriosamine A (2).
In our previous study of the alkaloidal constituents in the aerial parts of Gloriosa rothschildiana, we isolated a new colchicinoid, gloriosamine A (2), which is the first example of natural colchicinoid having a substituent at the C-4 position.12 Moreover, preliminary biological evaluation showed that gloriosamine A (2) demonstrated significant cytotoxicity to A549 human lung adenocarcinoma cell line. This finding prompted us to develop new colchicinoids with a substituent at the C-4 position as effective antitumor drugs.
Results and Discussion
1. 4-Substituted Colchicinoids
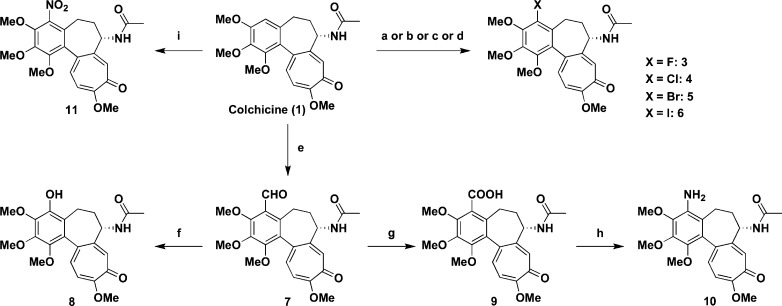
First, we prepared C-4 halogen substituted colchicine derivatives. As shown in Scheme 1, 4-fluorocolchicine (3), 4-chlorocolchicine (4), 4-bromocolchicine (5), and 4-iodocolchicine (6) were synthesized in 15%, 82%, 99%, and 99% yields from 1 by treatment with NFSi, NCS, NBS, and NIS, respectively. Structures of 4-halo derivatives were identified by careful NMR analysis. In the case of compound 3, an aromatic proton on C-4 in 1 disappeared and a quaternary carbon ascribable to C-4 appeared at 149.1 ppm with a doublet (J = 242.7 Hz) split by the installed fluorine atom.
Scheme 1. Syntheses of 3−6 from 1.
Reagents: (a) for 3, NFSi, HCOOH; (b) for 4, NCS, AcOH; (c) for 5, NBS, AcOH; (d) for 6, NIS, AcOH; (e) SnCl4, Cl2CHOMe, CH2Cl2; (f) 80% MMPP, MeOH; (g) NaClO2, NaH2PO4, 2-methyl-2-butene, t-BuOH/H2O; (h) DPPA, Et3N, THF/H2O; (i) CAN, TFAA, CH2Cl2. Abbreviations: NFSi, N-fluorodisulfoneimide; NCS, N-chlorosuccinimide; NBS, N-bromosuccinimide; NIS, N-iodosuccinimide; MMPP, magnesium bis(monoperoxyphthalate); DPPA, diphenylphosphoryl azide; CAN, cerium(IV) ammonium nitrate; TFAA, trifluoroacetic anhydride.
In addition, five 4-substituted colchicine derivatives 7−11 were prepared. According to the literature,13 4-formylcolchicine (7) was obtained by reacting with Cl2CHOMe in the presence of SnCl4 in CH2Cl2 in a quantitative yield. The Baeyer−Villiger oxidation of 7 with magnesium bis(monoperoxyphthalate) (MMPP)14 in MeOH gave 4-hydroxycolchicine (8) in 37% yield. Further, colchicine-4-carboxylic acid (9) was obtained by the Pinnick oxidation of 7 in 99% yield. The Curtius rearrangement of 9 gave 4-aminocolchicine (10) in 36% yield.
Moreover, 4-nitrocolchicine (11) was obtained in 15% yield by reacting with cerium(IV) ammonium nitrate (CAN)15 and TFAA.
The cytotoxicity of novel C-4 substituted colchicine derivatives 3−11 was evaluated using the A549 human lung adenocarcinoma cell line, the HT29 human colon adenocarcinoma grade II cell line, and the HCT116 human colorectal carcinoma cell line (Table 1). Interestingly, 4-halogenated derivatives 3−6 more strongly inhibited the examined tumor cell lines than 1, while derivative 7 with a carbon unit and 8 and 10 with an electron donor group were less active. On the other hand, nitro derivative 11 showed potent activity. To understand the different activities among the 4-derivatives of colchicines, we performed a docking study of the colchicines binding site of the tubulin protein. For example, 4-fluoro derivative 3 showed a similar binding mode to that of 1, in which interaction of the tricyclic scaffold contributes the appearance of antitumor activity, while less active compound 8 showed a different binding mode compared to that of 1 (see the Supporting Information), resulting in lowering its activity. In this binding mode, interaction of the acetylamino group on C-7 seems to be very important for exhibiting activity besides that of the tricyclic scaffold.
Table 1. IC50 Values of Colchicine Derivatives against the Tumor Cell Lines A549, HT29, and HCT116a.
| IC50 value (μM) |
|||
|---|---|---|---|
| compd | A549 | HT29 | HCT116 |
| colchicine (1) | 0.054 | 0.008 | 0.011 |
| 3 | 0.033 | 0.007 | 0.008 |
| 4 | 0.012 | 0.010 | 0.009 |
| 5 | 0.014 | 0.006 | 0.007 |
| 6 | 0.032 | 0.006 | 0.007 |
| 7 | 1.007 | 0.128 | 0.054 |
| 8 | 0.225 | 0.484 | NT |
| 10 | 2.185 | 1.550 | NT |
| 11 | 0.073 | 0.031 | 0.038 |
NT, not tested; A549, human lung adenocarcinoma; HT29, human colon adenocarcinoma; HCT116, human colorectal carcinoma.
Then, we examined the cell-growth inhibitory activities of 3 and 4 using mice transplanted with the HCT116 human colorectal carcinoma cell line by intraperitoneal injection (Table 2). Colchicine (1) at the dose of 1 mg/(kg day) caused lethality in 2 of 5 mice due to its inherent toxicity, although it showed 32.8% inhibition of tumor cell growth. Meanwhile, 4-fluorocolchicine (3) administered at the dose of 0.25 mg/(kg day) or 1 mg/(kg day) exhibited 25.0% or 55.6% inhibition of tumor cell growth, respectively, without exerting a lethal effect on the mice. 4-Chlorocolchicine (4) also showed significant efficacy (68.4% inhibition, 5 mg/(kg day), no mortality). These data demonstrated that 4-fluoro- and 4-chlorocolchicines are capable of reducing the intrinsic toxicity of colchicine (1) and show potential for use as a clinical drug.
Table 2. In Vivo Data of 3 and 4 in Mice Intraperitoneally Injected with HCT116 Human Colorectal Carcinoma Cell Line.
| compd | schedule on days | dose (mg/kg/day) | total dose (mg/kg) | tumor weight (g, mean ± S.D.) | inhibition rate (%) | mortality |
|---|---|---|---|---|---|---|
| control | 1−5 and 8−12 | 1.61 ± 0.34 | 0/5 | |||
| colchicine (1) | 1, 3, 5 | 1 | 3 | 1.08 ± 0.51 | 32.8 | 2/5 |
| 3 | 1−5 and 8−12 | 0.25 | 2.5 | 1.21 ± 0.44 | 25.0 | 0/5 |
| 3 | 1−5 and 8−12 | 1 | 10 | 0.71 ± 0.28 | 55.6 | 0/5 |
| 4 | 1−5 and 8−12 | 5 | 50 | 0.51 ± 0.08 | 68.4 | 0/5 |
2. Pro-drug Study
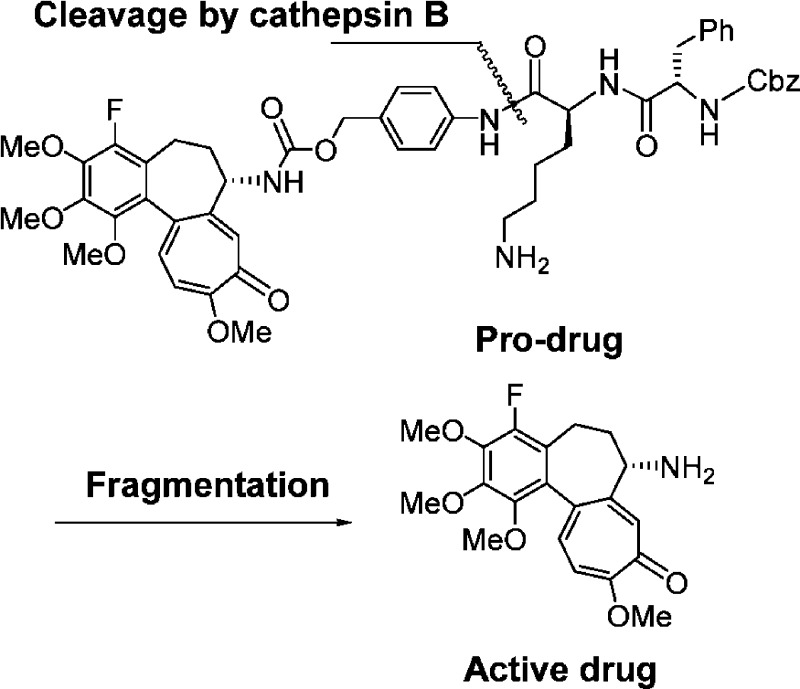
Utilizing novel 4-halocolchicinoids that were found to show potent antitumor activities in in vitro and in vivo experiments, we next designed safer and more effective antitumor agents based on a tumor-activated pro-drug strategy. Dubowchik et al. developed a novel pro-drug system16,17 that involved the release of free drug by the action of cathepsin B,18 an enzyme overexpressed in tumor cells.19 We applied the system to 4-halocolchicinoids incorporating the self-immolative p-aminobenzyloxycarbonyl (PABC) spacer20 between the active drug and the lysosomally cleavable dipeptide Phe-Lys, as shown in Figure 2.
Figure 2.
Drug release by cathepsin B.
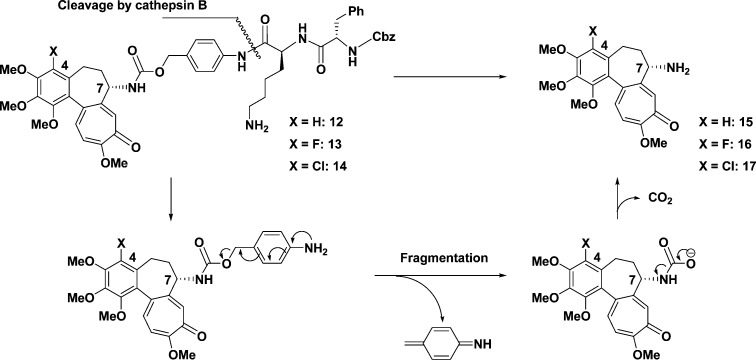
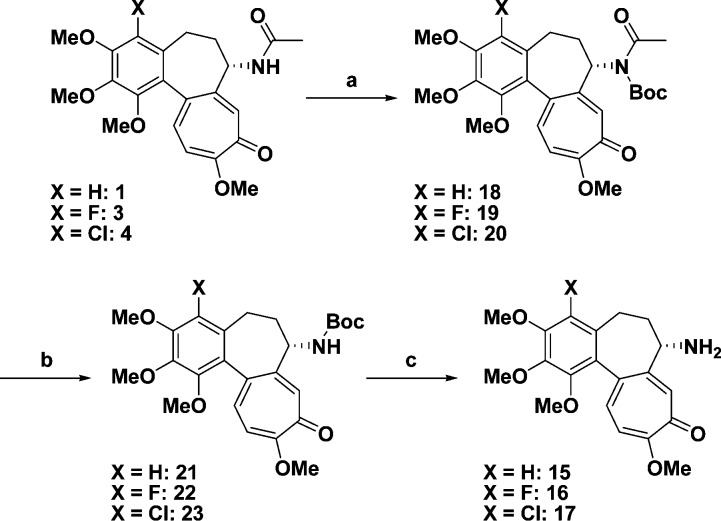
According to the idea above, we planned the synthesis of pro-drugs 12−14. For that purpose, deacetylcolchicines 15−17,10,21,22 which would be generated by the action of cathepsin B, were initially prepared (Scheme 2) and their cytotoxicity evaluated. Colchicines (1, 3, and 4) were respectively converted into N-Boc derivatives 18−20 and then sequentially deprotected to give amine derivatives 15−17.
Scheme 2. Preparation of Deacetylcolchicines 15−17.
Reagents: (a) Boc2O, DMAP, Et3N, MeCN; (b) NaOMe, MeOH; (c) TFA. Abbreviations: Boc, t-butoxycarbonyl; DMAP, dimethylamino pyridine; TFA, trifluoroacetic acid.
Among them, deacetylcolchicine (15) and 4-fluorodeacetylcolchicine (16) remained potent compared with 17, as shown in Table 3. Therefore, we planned to prepare pro-drugs 12 and 13 for further investigation.
Table 3. IC50 Values of Deacetylated Colchicine Derivatives Using Two Tumor Cell Lines, A549 and HT29. a.
| IC50 value (μM) |
||
|---|---|---|
| compd | A549 | HT29 |
| 15 | 0.036 | 0.025 |
| 16 | 0.101 | 0.080 |
| 17 | 0.301 | 0.250 |
A549, human lung adenocarcinoma; HT29, human colon adenocarcinoma.
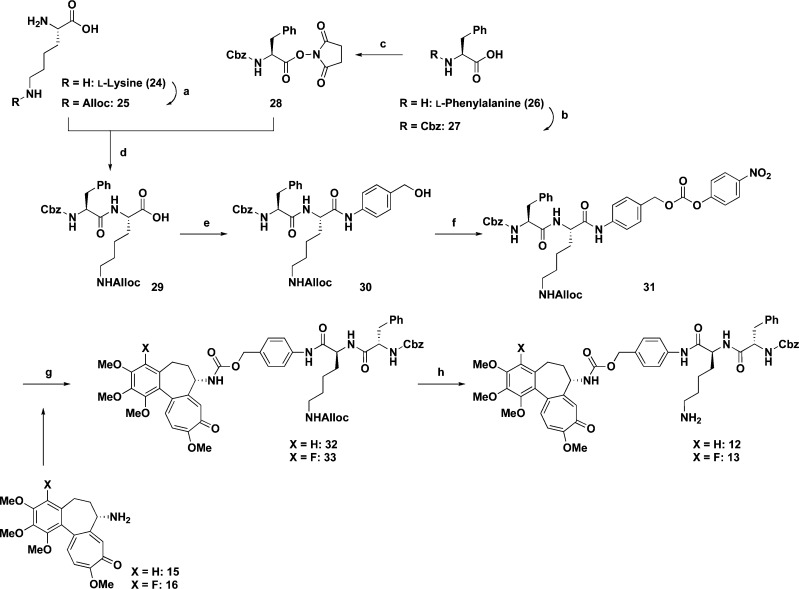
To prepare the dipeptide region, the Nε position in l-lysine (24) was protected with allylchloroformate to give 25 (61% yield). On the other hand, the amino group in phenylalanine (26) was protected by a Cbz group to afford 27 (99% yield)23,24 and then the carboxylic function was activated with N-hydroxysuccinimide to give 28 (81% yield).16,25,26 Condensation of 25 with 28 in the presence of NaHCO3 in H2O/DME afforded dipeptide 29(16) in 79% yield. The coupling reaction between 29 and p-aminobenzyl alcohol by using EDCI and HOBt in THF gave 30(16) in 96% yield. Carbonate 31(16) was obtained in 95% yield by reacting 30 with p-nitrophenylchloroformate in the presence of pyridine in THF.
The coupling of dipeptide carbonate 31 with deacetylcolchicine (15) or 4-fluorodeacetylcolchicine (16) in the presence of Et3N in NMP afforded Z-Phe-Lys(alloc)-PABC-deacetylcolchicine (32) in 80% yield or Z-Phe-Lys(alloc)-PABC-4-fluorodeacetylcolchicine (33) in a quantitative yield, respectively. Deprotection of the alloc group on the primary amine in 32 or 33 by using Pd(PPh3)4 and morpholine gave Z-Phe-Lys-PABC-deacetylcolchicine (12) in 59% yield or Z-Phe-Lys-PABC-4-fluorodeacetylcolchicine (13) in 55% yield, respectively (Scheme 3).
Scheme 3. Synthesis of 12 or 13.
Reagents: (a) AllocCl, 1 N NaOH, H2O; (b) CbzCl, 2 M NaOH; (c) HOSu, DCC, THF; (d) NaHCO3, H2O/DME (1:1.3); (e) p-aminobenzyl alcohol, HOBt, EDCI, NMM, THF; (f) p-nitrophenylchloroformate, pyridine, THF; (g) Et3N, NMP; (h) Pd(PPh3)4, morpholine, THF; (i) Boc2O, DMAP, Et3N, MeCN; (j) NaOMe, MeOH; (k) TFA. Abbreviations: Alloc, allyloxycarbonyl; Cbz, benzyloxycarbonyl; HOSu, N-hydroxysuccinimide; DCC, dicyclohexyl carbodiimide; DME, 1,2-dimethoxyethane; HOBt, 1-hydroxybenzotriazole; EDCI, 1-ethyl-3-(3-dimethyl aminopropyl)carbodiimide hydrochloride; NMM, N-methylmorpholine; THF, tetrahydrofuran; NMP, N-methyl-2-pyrrolidinone; Boc, tert-butoxycarbonyl; DMAP, dimethylamino pyridine; TFA, trifluoroacetic acid.
After obtaining pro-drugs 12 and 13, we next examined their enzymatic conversion. As the enzyme, we chose bovine spleen cathepsin B (EC 3.4.22.1, Mw ca. 40000) and prepared a stock solution by dissolving the lyophilized solid. For each assay, a 1 mM DMSO solution of pro-drug compound (4 μM) was added to the enzyme solution (96 μL), and the concentration of the substrate was adjusted to 0.04 mM. The mixture was stirred by vortexing and left to stand at 37 °C, and the reaction course was monitored by HPLC.
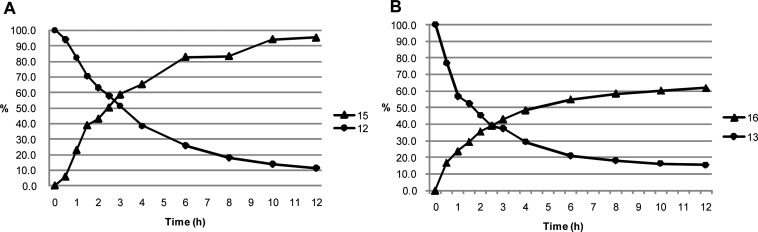
As a result, it was demonstrated that both pro-drugs 12 and 13 released free drugs 15 and 16 by the action of cathepsin B. As shown in Figure 3, the amounts of pro-drugs decreased while those of free drugs increased with time. We confirmed that pro-drugs 12 and 13 remained stable (for almost 24 h) in the absence of cathepsin B. HPLC analysis manifested the generation of the dipeptide fragment Z-Phe-Lys with the appearance of the free drugs, demonstrating a sequence of reaction involving the cleavage of the dipeptide residue by the action of cathepsin B and the fragmentation of the spacer moiety to generate the free drugs, as expected in Figure 2.
Figure 3.
Cathepsin B mediated release of free drugs from pro-drug 12 (A) and fluorinated pro-drug 13 (B) at pH 5.0, 37 °C. A 20 μL mixture was sampled at various time points and quenched with 20 μL of MeOH. The quenched reaction mixture was stored at 0 °C, and 10 μL of the mixture was injected into the HPLC. (HPLC conditions: Inertsil ODS-2 4.6 mm × 250 mm; solvent A, 0.1% (v/v) H3PO4; solvent B, CH3CN; 0 min B conc 15%; 30 min B conc 100%; column oven 40 °C; flow rate 0.5 mL/min; λ = 254 nm.)
The cytotoxicity of pro-drugs 12 and 13 and deacetyl derivatives 15 and 16 to the HT29 human colon adenocarcinoma grade II cell line and normal HUVEC was evaluated (Table 4) to estimate their selectivity for tumor cells. As a result, we found that pro-drugs 12 and 13 have approximately 2-fold higher selectivity for the HT29 tumor cell line compared with normal HUVEC. Further, the data concerning the fluoro-derivatives (13 and 16) reveals that utilization of fluoro-derivatives would be more efficient for the creation of safer antitumor medicines based on colchicines.
Table 4. Biological Evaluation of Pro-drugs 12 and 13 and Active Compounds 15 and 16 Using HT29 Human Colon Adenocarcinoma Cell Line and Normal HUVEC.
| IC50 value (μM) |
|||
|---|---|---|---|
| compd | HT29 | HUVEC | selectivity (HUVEC/HT29) |
| 12 | 0.044 | 0.075 | 1.7 |
| 13 | 0.091 | 0.151 | 1.7 |
| 15 | 0.025 | 0.034 | 1.4 |
| 16 | 0.080 | 0.075 | 0.9 |
Conclusion
A series of novel colchicinoids having a substituent at the C-4 position were synthesized from the natural alkaloid colchicine (1) and evaluated for their cytotoxicity to tumor cells, A549, HT29, and HCT116. As a result, some 4-halogenated derivatives exhibited more potent cytotoxicity to the tumor cells than 1. On evaluation of cell-growth inhibitory activity using mice transplanted with the HCT116 human colorectal carcinoma cell line, 4-fluorocolchicine (3) and 4-chlorocolchicine (4) exhibited less toxicity to mice and more potent cell-growth inhibitory activity than 1. Further, we synthesized pro-drugs 12 and 13 having a dipeptide side chain cleavable by cathepsin B, an enzyme overexpressed in solid tumors, and we confirmed that 12 and 13 were cleaved by cathepsin B to release the active drugs, resulting in an approximately 2-fold higher selectivity for tumor cells than normal cells.
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research from Japan Society for the Promotion of Science and Adaptable and Seamless Technology Transfer Program through Target-Driven R&D (A-STEP, Exploratory Research) from Japan Science and Technology Agency.
Supporting Information Available
Procedures for the preparation and spectral data of compounds 3−14, 16, 17, 19 and 20, and 22−33, procedures for in vitro and in vivo assays, and the data of the docking study. This material is available free of charge via the Internet at http://pubs.acs.org.
Supplementary Material
References
- Graening T.; Schmalz H.-G. Angew. Chem., Int. Ed. 2004, 43, 3230–3256. [DOI] [PubMed] [Google Scholar]
- Fitzgerald J. T. Biochem. Pharmacol. 1976, 25, 1383–1387. [DOI] [PubMed] [Google Scholar]
- Kerekes P.; Sharma N. P.; Brossi A.; Chignell F. C.; Quinn R. F. J. Med. Chem. 1985, 28, 1204–1208. [DOI] [PubMed] [Google Scholar]
- Rosner M.; Capraro H.-G.; Jacobson E. A.; Atwell L.; Brossi A. J. Med. Chem. 1981, 24, 257–261. [DOI] [PubMed] [Google Scholar]
- Lee S.-H.; Park S.-K.; Kim J.-M.; Kim M.-H.; Kim K.-H.; Chun K.-W.; Cho K.-H.; Youn J.-Y.; Namgoong S. K. Arch. Pharm. Chem. Life Sci. 2005, 338, 582–589. [DOI] [PubMed] [Google Scholar]
- Andreu M. J.; Timasheff N. S. Biochemistry 1982, 21, 534–543. [DOI] [PubMed] [Google Scholar]
- Dumortier C.; Yan Q.; Bane S.; Engelborghs Y. Biochem. J. 1997, 327, 685–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa-Goto K.; Jung M. K.; Hamel E.; Wu C.-C.; Bastow F. K.; Brossi A.; Ohta S.; Lee K.-H. Heterocycles 2005, 65, 541–551. [Google Scholar]
- Nakagawa-Goto K.; Chen X. C.; Hamel E.; Wu C.-C.; Bastow F. K.; Brossi A.; Lee K.-H. Bioorg. Med. Chem. 2005, 15, 235–238. [DOI] [PubMed] [Google Scholar]
- Bagnato D. J.; Eilers L. A.; Horton A. R.; Grissom B. C. J. Org. Chem. 2004, 69, 8987–8996. [DOI] [PubMed] [Google Scholar]
- Das L.; Datta B. A.; Gupta S.; Poddar A.; Sengupta S.; Janik E. M.; Bhattacharyya B. Biochemistry 2005, 44, 3249–3258. [DOI] [PubMed] [Google Scholar]
- Kitajima M.; Tanaka A.; Kogure N.; Takayama H. Tetrahedron Lett. 2008, 49, 257. [Google Scholar]
- Eidrup B. A.; Prhavc M.; Brooks J.; Bhat B.; Prakash P. T.; Song Q.; Bera S.; Bhat N.; Dande P.; Cook P. D.; Bennett C. F.; Carroll S. S.; Ball G. R.; Bosserman M.; Burlein C.; Colwell F. L.; Fay F. J.; Flores A. O.; Getty K.; LaFemina L. R.; Leone J.; MacCoss M.; McMasters R. D.; Tomassini E. J.; Langen V. D.; Wolanski B.; Olsen B. D. J. Med. Chem. 2004, 47, 5284–5297. [DOI] [PubMed] [Google Scholar]
- Heaney H.; Newbold A. J. Tetrahedron Lett. 2001, 42, 6607–6609. [Google Scholar]
- John M. M.; Stifun M.; Rachel P.; Ross W. M. Tetrahedron 2000, 56, 8019–8024. [Google Scholar]
- Dubowchik G. M.; Firestone R. A.; Padilla L.; Williner D.; Hofstead S. J.; Mosure K.; Knipe J. O.; Lasch S. J.; Trail P. A. Bioconjugate Chem. 2002, 13, 855. [DOI] [PubMed] [Google Scholar]
- Dubowchik G. M.; Firestone R. A. Bioorg. Med. Chem. Lett. 1998, 8, 3341–3346. [DOI] [PubMed] [Google Scholar]
- Otto H. H.; Schirmeister T. Chem. Rev. 1997, 97, 133–172. [DOI] [PubMed] [Google Scholar]
- Schmid B.; Chung D. E.; Warnecke A.; Fichtner I.; Kratz F. Bioconjugate Chem. 2007, 18, 702–716. [DOI] [PubMed] [Google Scholar]
- Carl P. L.; Chakravarty P. K.; Katzenellenbogen J. A. J. Med. Chem. 1981, 24, 479–480. [DOI] [PubMed] [Google Scholar]
- Ducray P.; Lebeau L.; Mioskowski C. Helv. Chim. Acta 1996, 79, 2346–2352. [Google Scholar]
- Lagnoux D.; Darbre T.; Schmitz M. L.; Reymond J.-L. Chem. Eur. J. 2005, 11, 3941–3950. [DOI] [PubMed] [Google Scholar]
- Rahman M. M.; Czaun M.; Takafuji M.; Ihara H. Chem. Eur. J. 2008, 14, 1312–1321. [DOI] [PubMed] [Google Scholar]
- Singh O. V.; Han H. Org. Lett. 2007, 9, 4801–4804. [DOI] [PubMed] [Google Scholar]
- Ogura H.; Kobayashi T.; Shimizu K.; Kawabe K.; Takeda K. Tetrahedron Lett. 1979, 4, 4745–4746. [Google Scholar]
- Becerril J.; Bolte M.; Burguete M. I.; Galindo F.; Garcia-Espana E.; Luis V. S.; Miravet F. J. J. Am. Chem. Soc. 2003, 125, 6677–6685. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.