Abstract
Hybrid liposomes are nanosized liposomal particles and can be prepared by sonication of vesicular and micellar molecules in a buffer solution. In this study, we obtained the first successful experiment resulting in a good correlation between inhibitory effects of hybrid liposomes on the growth of various tumor cells and the membrane fluidity of tumor cells (plasma membranes). The results indicated that hybrid liposomes could provide the possibility of novel membrane-targeted nanotherapy for intractable cancers.
Keywords: Hybrid liposome, nanotherapy, cancer cell, apoptosis, cell membrane, membrane fluidity
The dynamics of cell membranes (plasma membranes) are one of the cell biological characteristics of cancer cells. Tumor cell membranes show changes in the composition and construction, and the cell membranes are more fluid than those of normal cells. For example, the cell membranes of leukemia and lymphoma, which have less cholesterol and more unsaturated lipid contents in the cell membranes, showed higher fluidity as compared with those of normal lymphocytes.1−5 Similar findings were observed for other cancer cell membranes.6−10 The membrane dynamics with higher fluidity should be a fundamental property of cancer cells and closely related to the biological features of the proliferative potential, invasive potential, and metastatic ability.11−15 On the other hand, conventional chemotherapy for cancers often has severe side effects that limit their efficacy, as most current anticancer drugs are directed against a nonspecific target DNA or designed to interfere with nucleic acid biosynthesis. In recent years, molecular targeted therapeutics have attracted much attention as an efficient therapy for cancers on the basis of molecular level studies on human cells. Targeted molecules include specific proteins expressed in cancer cell membranes, such as epidermal growth factor receptor (EGFR), human EGFR-related 2 (HER2), and so on.16,17 However, there is no report on the tumor chemotherapy targeting the membrane dynamics from the viewpoint of biophysical characteristics of cancer cells.
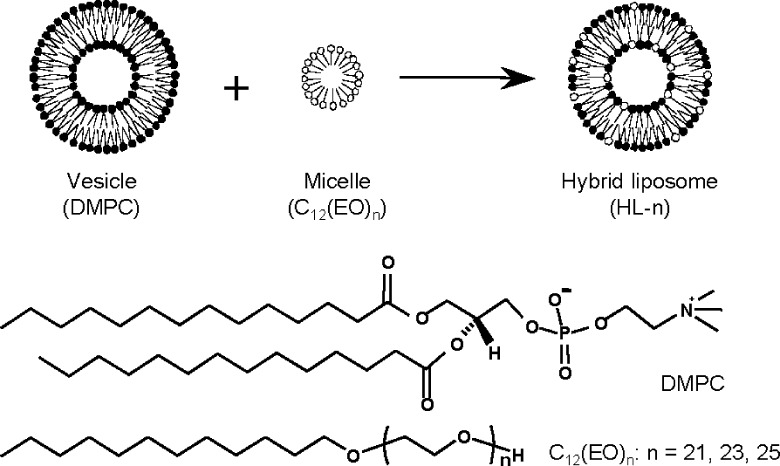
Hybrid liposomes are nanosized liposomal particles and can be prepared by sonication of vesicular and micellar molecules in a buffer solution (Scheme 1).18 The physical properties of hybrid liposomes, such as shape, size, membrane fluidity, and the temperature of the phase transition, can be controlled by changing the constituents and compositional ratios.19 In the drug delivery system (DDS) study, the therapeutic effects of an anti-tumor drug, 1,3-bis(2-chloroethyl)-1-nitrosourea (BCNU), encapsulated into hybrid liposomes composed of l-α-dimyristoylphosphatidylcholine (DMPC) and polyoxyethylene(20) sorbitan monolaurate (Tween 20) have been observed for the meningeal gliomatosis model rats in vivo.20 On the other hand, we have reported that hybrid liposomes (HL-n) composed of DMPC and polyoxyethylene(n) dodecyl ethers (C12(EO)n) without any drugs inhibited the proliferation of various cancer cells along with apoptosis in vitro(21−23) and in vivo.24,25 Furthermore, successful clinical chemotherapy with drug-free HL-n to patients with lymphoma has been reported.26,27 Significantly, we have also revealed a good correlation between the membrane fluidity of HL-n and their growth-inhibitions for colorectal cancer cells in vitro.28 HL-n having larger membrane fluidity showed higher inhibitory effects on the growth of colorectal cancer cells. In addition, HL-n distinguished lung cancer cells and normal lung cells, which have higher and lower membrane fluidities, respectively, and fused and accumulated preferentially into the lung cancer cells.10 These results suggested that the anticancer effects of HL-n could be related to the membrane dynamics of cancer cells. In this study, we investigated the inhibitory effects of HL-n on the growth of various cancer cells in vitro and obtained the first successful experiment resulting in a good correlation between growth-inhibition effects of HL-n and membrane fluidity of cancer cells.
Scheme 1. Schematic Representation of Hybrid Liposomes (HL-n) Composed of DMPC and C12(EO)n (n = 21, 23, 25).
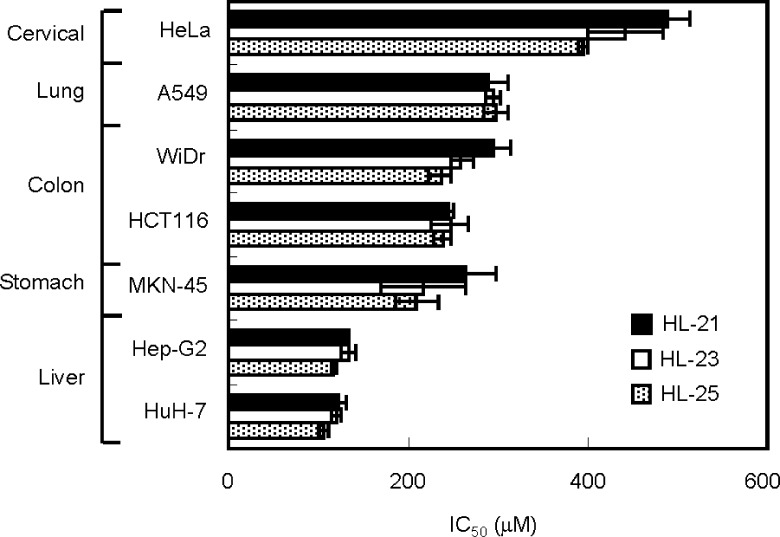
The hybrid liposomes (HL-n, n = 21, 23, 25) used in this study were prepared by sonication of a mixture containing 90 mol % DMPC and 10 mol % C12(EO)n (n = 21, 23, 25) in 5% glucose solution, as described previously.24−27 The particle size of HL-n was measured by dynamic light scattering measurements.22 HL-n had hydrodynamic mean diameters of between 60 and 90 nm with a single and narrow range of size distribution (Figure S1, Supporting Information). It is worthy to note that HL-n having less than 100 nm in diameter could avoid the clearance by the reticular endothelial system (RES) in vivo.29 Here, we examined the inhibitory effects of HL-n on the growth of various human cancer cells (liver cancer HuH-7 and Hep-G2 cells, colon cancer HCT116 and WiDr cells, stomach cancer MKN-45 cells, lung cancer A549 cells, and cervical cancer HeLa cells) in vitro on the basis of a WST-1 assay.23 The fifty percent inhibitory concentrations (IC50) are shown in Figure 1. IC50 is the concentration of HL-n necessary to inhibit the growth of cancer cells by half. HL-n potently inhibited the growth of these cancer cells at IC50 ranging from 95 to 442 μM for DMPC concentration. The IC50 values decreased in the following sequence, HeLa cells > A549 cells > HCT116 and WiDr cells > MKN-45 cells > HuH-7 and Hep-G2 cells, and HL-n could inhibit the growth of cancer cells in this order.
Figure 1.
50% inhibitory concentration (IC50) of HL-n on the growth of various cancer cells. IC50 values for cancer (HuH-7, Hep-G2, HCT116, MKN-45, WiDr, A549, HeLa) cells were estimated on the basis of a WST-1 assay after 48 h of incubation in the presence of HL-n. Mean ± S. E. from three independent experiments.
How do HL-n inhibit the growth of cancer cells? We have already reported that hybrid liposomes composed of DMPC and polyoxyethylene(n) alkyl ethers induced apoptosis in cancer cells such as liver cancer,30 lung cancer,22 breast cancer,25 colon cancer,28 lymphoma,27 and primary effusion lymphoma.31 In the present study, we confirmed the induction of apoptosis by HL-n in cancer cells on the basis of the TUNEL method using confocal laser microscopy.25 The fluorescence micrographs of cancer (A549, MKN-45, HCT116, WiDr, Hep-G2, HuH-7, and HeLa) cells are shown in Figure 2. All tumor cells employed were dyed in green or yellow after the treatment with HL-23, indicating that apoptosis could be induced by HL-23. These observations show that the inhibitory effects of HL-n were attained through the induction of apoptosis in cancer cells.
Figure 2.
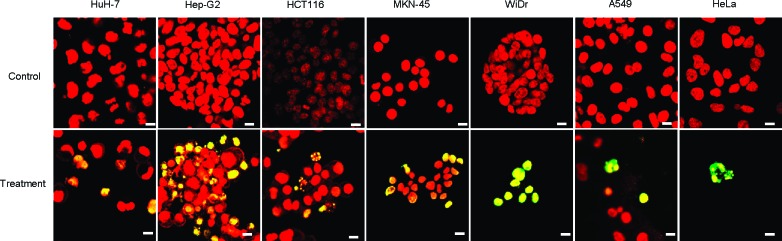
Microscopic imaging of DNA fragmentation in cancer cells treated with HL-23. After 48 h of incubation in the presence of HL-23 at the IC50, the cancer (HuH-7, Hep-G2, HCT116, MKN-45, WiDr, A549, HeLa) cells stained with TUNEL (green or yellow fluorescence) and TO-PRO-3 (red fluorescence) were observed with a confocal laser microscope. Excitation/Detection = TUNEL: 488 nm/510−570 nm, TO-PRO-3: 633 nm/640−700 nm. Scale bar, 10 μm.
To investigate the accumulation of HL-23 into the plasma membranes of tumor (A549, HCT116, Hep-G2, HuH-7, and HeLa) cells, we used fluorescent lipids NBDPC (HL-23/NBDPC) and observed the process using a TIRF microscope.28,32 The results are shown in Figure 3a. The fluorescence of HL-23/NBDPC was observed around the plasma membranes of HuH-7, Hep-G2, and HCT116 cells. Also, the mean-fluorescence intensity of NBDPC in the plasma membranes of cancer cells was plotted with time in Figure 3b. The fluorescence intensity reflects the amount of HL-23/NBDPC accumulated into the cell membranes. The fluorescence intensities for HuH-7, Hep-G2, and HCT116 cells drastically increased during 8 min after the treatment with HL-23/NBDPC, though those for A549 and HeLa cells were low and almost constant. These results indicate that HL-23/NBDPC could accumulate more in the plasma membranes of HuH-7, Hep-G2, and HCT116 cells as compared with those of A549 and HeLa cells. Importantly, the accumulations of HL-23/NBDPC into the plasma membrane of cancer cells were in good agreement with the inhibitory effects of HL-23 on the growth of cancer cells (Figure S2a, Supporting Information). This means that the cancer cells accumulating more HL-23/NBDPC could be further inhibited by HL-23.
Figure 3.
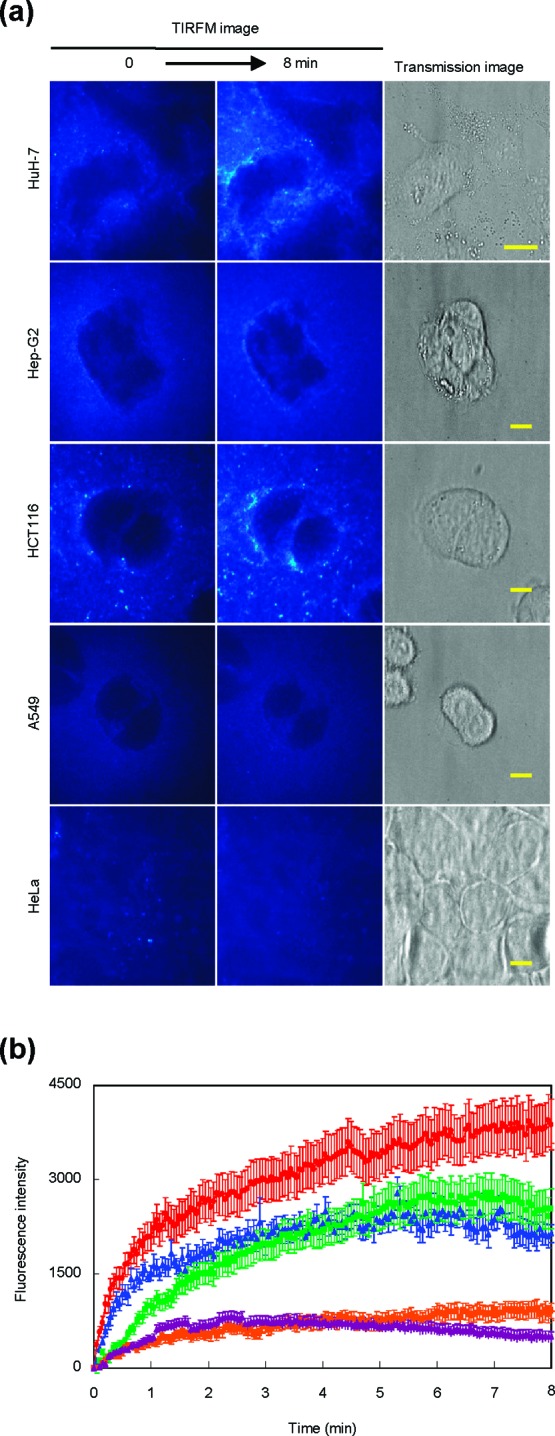
Accumulation of HL-23 including NBDPC (HL-23/NBDPC) in the plasma membranes of cancer cells. (a) TIRF images of cancer (HuH-7, Hep-G2, HCT116, A549, HeLa) cells treated with HL-23/NBDPC. Scale bar, 10 μm. (b) Time course of mean-fluorescence intensity of NBDPC embedded into cancer cell membranes (HuH-7, red; Hep-G2, green; HCT116, blue; A549, yellow; HeLa, purple). Mean ± S.E. After addition of HL-23/NBDPC into the culture solutions, the cancer cells were observed using a total internal reflection fluorescence microscope system equipped with an air-cooled CCD camera. Excitation/detection = 488 nm/510−570 nm.
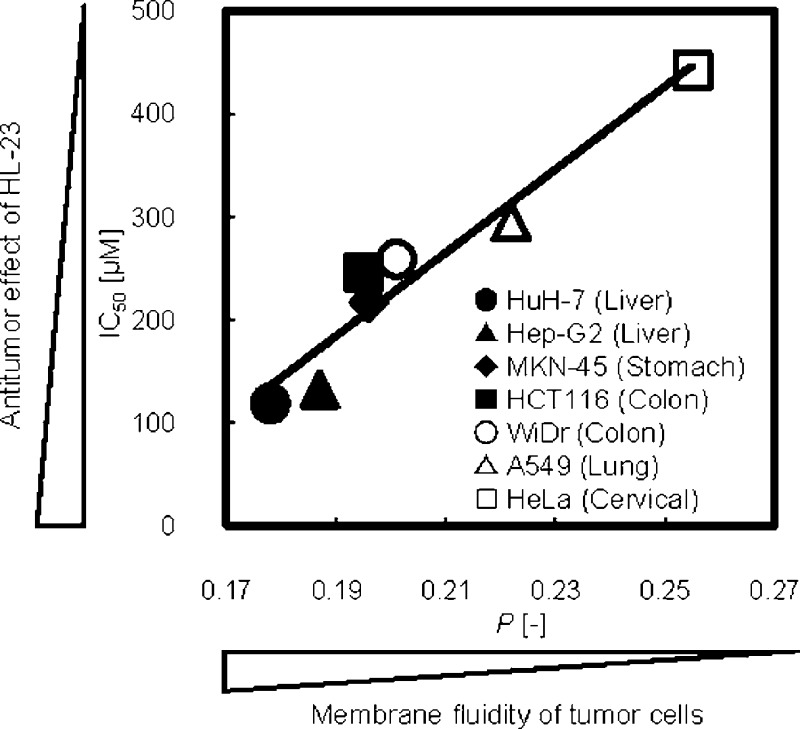
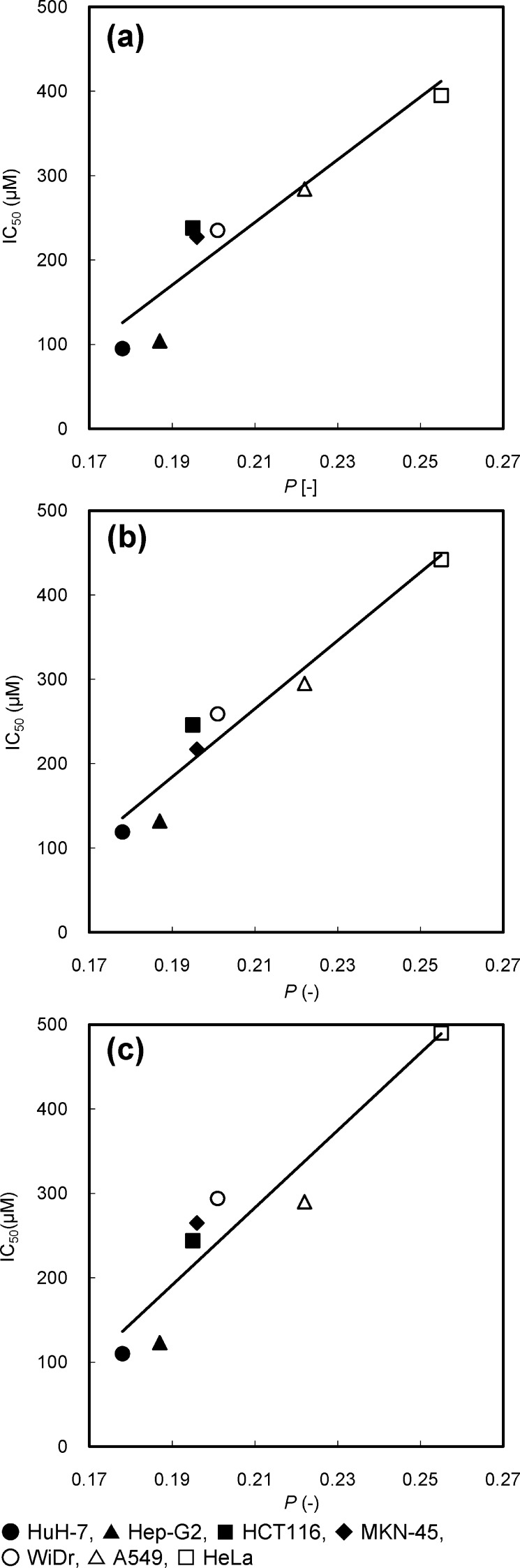
From the findings described above, it was suggested that HL-n had a broad-spectrum anticancer activity and the inhibitory effects of HL-n might be related to the affinity with the plasma membrane of cancer cells. On the other hand, a good correlation between the membrane fluidities of HL-n and their growth-inhibition effects for colorectal cancer cells was observed.28 To gain further insight into the anticancer activity of HL-n, we focused on the membrane dynamics of cancer cells and attempted to investigate the relationship between membrane fluidity of cancer cells and inhibitory effects of HL-n on the growth of cancer cells. The fluidity of cancer (A549, MKN-45, HCT116, WiDr, Hep-G2, HuH-7, and HeLa) cell membranes was evaluated from the fluorescence polarization (P) of fluorescent probe DPH embedded in the plasma membranes.24 The fluorescence depolarization is caused by the molecular motion of the fluorescent probe, which reflects the microviscosity of the surrounding region.33 As the P value of DPH in the cell membranes becomes smaller, the cancer cell membranes have larger fluidity. P values are plotted against the IC50 values of HL-n as shown in Figure 4 (The P values were summarized in Table S1 of the Supporting Information). Interestingly, good correlations between the P values of DPH in the cell membranes and the IC50 values of HL-n for the tumor cells were obtained. This result suggests that the tumor cells having larger membrane fluidity could be further inhibited by HL-n. Similarly, the P values for the cancer cells correlated with the accumulation of HL-n (Figure S2b of the Supporting Information). These results suggest that the growth-inhibition effects of HL-n should depend on the membrane fluidity of cancer cells, probably due to the membrane fusion of HL-n with cancer cells. It is plausible that HL-n could selectively fuse and accumulate into the cancer cell membranes having high fluidity and inhibit the growth of cancer cells, leading to apoptosis.
Figure 4.
Correlation between the IC50 of HL-n ((a) HL-21, (b) HL-23, (c) HL-25) on the growth of cancer cells and the fluorescence polarization (P) of DPH in the cancer cell membranes. The IC50 values of HL-n for cancer (HuH-7, Hep-G2, HCT116, MKN-45, WiDr, A549, HeLa) cells were those in Figure 1. The P values of DPH in the cancer cell membranes were evaluated on the basis of a florescence depolarization assay. Excitation/detection = 360 nm/430 nm. Correlation between the anticancer activity (IC50) of HL-n and the membrane fluidity (P) of cancer cells was obtained (HL-21: r = 0.95, p < 0.001. HL-23: r = 0.96, p < 0.001. HL-25: r = 0.94, p < 0.01).
In conclusion, we clarified that hybrid liposomes (HL-n, n = 21, 23, 25) composed of 90 mol % DMPC and 10 mol % C12(EO)n (n = 21, 23, 25) could induce apoptosis for cancer cells through the specific accumulation of HL-n into the plasma membranes. Significantly, a good correlation between the inhibitory effects of HL-n on the growth of the cancer cells and the membrane fluidity of cancer cells was observed for the first time. It was reported that the membrane fluidity increased with increasing the malignancy of cancer cells.11,12,34,35 The results in this study indicate that HL-n could provide the possibility of a novel nanotherapy for intractable cancers by targeting the cell membrane from the biophysical characteristics of cancer cells and that we should consider the relationship between biological functions and biophysical fluctuation of cancer cell membranes for future clinical applications.
Acknowledgments
This work was supported in part by a Grant-in-Aid for Science Research from the Ministry of Education, Science, and Culture of Japan (Nos. 20107007, 20360377, 20560732, and 21560813).
Glossary
Abbreviations
- BCNU
3-bis(2-chloroethyl)-1-nitrosourea
- C12(EO)n
polyoxyethylene(n) dodecyl ether
- DDS
drug delivery system
- DMPC
l-α-dimyristoylphosphatidylcholine
- DPH
1,6-diphenyl-1,3,5-hexatriene
- EGFR
epidermal growth factor receptor
- HL-n
hybrid liposomes composed of DMPC and C12(EO)n
- HER2
human EGFR-related 2
- IC50
fifty percent inhibitory concentration
- NBDPC
1-palmitoyl-2-[12-[(7-nitro-2-1,3-benzoxadiazol-4-yl)amino]dodecanoyl]-sn-glycero-3-phosphocholine
- P
fluorescence depolarization
- RES
reticular endothelial system
- TIRF
total internal reflection fluorescence
- TUNEL
terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling
- WST-1
2-methoxy-4-nitrophenyl-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium, monosodium salt.
Supporting Information Available
Experimental procedures and supporting data. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
Experimental works were mainly performed by S.N. and Y.K. Experimental design and data analysis were mainly performed by Y.K., K.G., Y.M., and R.U. All authors discussed the results and contributed to writing the paper.
Supplementary Material
References
- Shinitzky M. Membrane Fluidity in Malignancy. Adversative and Recuperative. Biochim. Biophys. Acta 1984, 738, 251–261. [DOI] [PubMed] [Google Scholar]
- Petitou M.; Tuy F.; Rosenfeld C.; Mishal Z.; Paintrand M.; Jasnin C.; Mathe G.; Inbar M. Decreased Microviscosity of Membrane Lipids in Leukemic Cells: Two Possible Mechanisms. Proc. Natl. Acad. Sci. U.S.A. 1978, 75, 2306–2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Blitterswijk W. J.; De Veer G.; Krol J. H.; Emmelot P. Comparative Lipid Analysis of Purified Plasma Membranes and Shed Extracellular Membrane Vesicles from Normal Murine Thymocytes and Leukemic GRSL Cells. Biochim. Biophys. Acta 1982, 688, 495–504. [DOI] [PubMed] [Google Scholar]
- Inbar M.; Goldman R.; Inbar L.; Bursuker I.; Goldman B.; Akstein E.; Segal P.; Ipp E.; Ben-Bassat I. Fluidity Difference of Membrane Lipids in Human Normal and Leukemic Lymphocytes as Controlled by Serum Components. Cancer Res. 1977, 37, 3037–3041. [PubMed] [Google Scholar]
- Inbar M.; Shinitzky M.; Sachs L. Microviscosity in the Surface Membrane Lipid Layer of Intact Normal Lymphocytes and Leukemic Cells. FEBS Lett. 1974, 38, 268–270. [DOI] [PubMed] [Google Scholar]
- Deliconstantions G. Physiological Aspects of Membrane Lipid Fluidity in Malignancy. Anticancer Res. 1987, 7, 1011–1021. [PubMed] [Google Scholar]
- Porat N.; Gill D.; Parola A. H. Adenosine Deaminase in Cell Transformation. J. Biol. Chem. 1988, 263, 14608–14611. [PubMed] [Google Scholar]
- Hattori T.; Andoh T.; Sakai N.; Yamada H.; Kameyama Y.; Ohki K.; Nozawa Y. Membrane Phospholipid Composition and Membrane Fluidity of Human Brain Tumour: A Spin Label Study. Neurol. Res. 1987, 9, 38–43. [DOI] [PubMed] [Google Scholar]
- Sok M.; Šentjurc M.; Schara M. Membrane Fluidity Characteristics of Human Lung Cancer. Cancer Lett. 1999, 139, 215–220. [DOI] [PubMed] [Google Scholar]
- Yukihara M.; Komizu Y.; Tanoue O.; Matsushita T.; Matsumoto Y.; Ueoka R. Specific Accumulation and Antitumor Effects of Hybrid Liposomes on the Growth of Lung Tumor Cells. Yakugaku Zasshi 2010, 130, 1581–1587. [DOI] [PubMed] [Google Scholar]
- Gonda K.; Watanabe T. M.; Ohuchi N.; Higuchi H. In Vivo Nano-Imaging of Membrane Dynamics in Metastatic Tumor Cells Using Quantum Dots. J. Biol. Chem. 2010, 285, 2750–2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisig R.; Koklič T.; Wiesner B.; Fichtner I.; Sentjurč M. Increase in Fluidity in the Membrane of MT3 Breast Cancer Cells Correlates with Enhanced Cell Adhesion in Vitro and Increased Lung Metastasis in NOD/SCID Mice. Arch. Biochem. Biophys. 2007, 459, 98–106. [DOI] [PubMed] [Google Scholar]
- Funaki N. O.; Tanaka J.; Kohmoto M.; Sugiyama T.; Ohshio G.; Nonaka A.; Yotsumoto F.; Takeda Y.; Imamura M. Membrane Fluidity Correlates with Liver Cancer Cell Proliferation and Infiltration Potential. Oncol. Rep. 2001, 8, 527–532. [DOI] [PubMed] [Google Scholar]
- Kier A. B. Membrane Properties of Metastatic and Non-Metastatic Cells Cultured from C3H Mice Injected with LM Fibroblasts. Biochim. Biophys. Acta 1990, 1022, 365–372. [DOI] [PubMed] [Google Scholar]
- Taraboletti G.; Perin L.; Bottazzi B.; Mantovani A.; Giavazzi R.; Salmona M. Membrane Fluidity Affects Tumor-Cell Motility, Invasion and Lung-Colonizing Potential. Int. J. Cancer 1989, 44, 707–713. [DOI] [PubMed] [Google Scholar]
- Baselga J. Targeting Tyrosine Kinases in Cancer: The Second Wave. Science 2006, 312, 1175–1178. [DOI] [PubMed] [Google Scholar]
- Noble M. E. M.; Endicott J. A.; Johnson L. N. Protein Kinase Inhibitors: Insights into Drug Design from Structure. Science 2004, 303, 1800–1805. [DOI] [PubMed] [Google Scholar]
- Ueoka R.; Moss R. A.; Swarup S.; Matsumoto Y.; Strauss G.; Murakami Y. Extraordinary Micellar Enantioselectivity Coupled to Altered Aggregate Structure. J. Am. Chem. Soc. 1985, 107, 2185–2186. [Google Scholar]
- Ueoka R.; Matsumoto Y.; Moss R. A.; Swarup S.; Sugii A.; Harada K.; Kikuchi J.; Murakami Y. Membrane Matrix for the Hydrolysis of Amino Acid Esters with Marked Enantioselectivity. J. Am. Chem. Soc. 1988, 110, 1588–1595. [Google Scholar]
- Kitamura I.; Kochi M.; Matsumoto Y.; Ueoka R.; Kuratsu J.; Ushio Y. Intrathecal Chemotherapy with 1,3-Bis(2-chloroethyl)-1-nitrosourea Encapsulated into Hybrid Liposomes for Meningeal Gliomatosis: An Experimental Study. Cancer Res. 1996, 56, 3986–3992. [PubMed] [Google Scholar]
- Matsumoto Y.; Kato T.; Iseki S.; Suzuki H.; Nakano K.; Iwahara M.; Ueoka R. Remarkably Enhanced Inhibitory Effects of Hybrid Liposomes on the Growth of Specific Tumor Cells. Bioorg. Med. Chem. Lett. 1999, 9, 1937–1940. [DOI] [PubMed] [Google Scholar]
- Iwamoto Y.; Matsumoto Y.; Ueoka R. Induction of Apoptosis of Human Lung Carcinoma Cells by Hybrid Liposomes Containing Polyoxyethylenedodecyl Ether. Int. J. Pharm. 2005, 292, 231–239. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y.; Iwamoto Y.; Matsushita T.; Ueoka R. Novel Mechanism of Hybrid Liposomes-Induced Apoptosis in Human Tumor Cells. Int. J. Cancer 2005, 115, 377–382. [DOI] [PubMed] [Google Scholar]
- Nagami H.; Matsumoto Y.; Ueoka R. Chemotherapy with Hybrid Liposomes for Lymphoma without Drugs in Vivo. Int. J. Pharm. 2006, 315, 167–172. [DOI] [PubMed] [Google Scholar]
- Shimoda S.; Ichihara H.; Matsumoto Y.; Ueoka R. Chemotherapy with Hybrid Liposomes for Human Breast Tumors along with Apoptosis in Vivo. Int. J. Pharm. 2009, 372, 162–178. [DOI] [PubMed] [Google Scholar]
- Ueoka R.; Matsumoto Y.; Ichihara H.; Kiyokawa T.. Chemotherapy with Hybrid Liposomes Composed of Dimyristoylphosphatidylcholine and Polyoxyethylenealkyl Ether without Drugs. In ACS Symposium Series 830. Biological Systems Engineering, 3rd ed.; Marten M. R., Park T. H., Nagamune T., Eds.; American Chemical Society: Washington, DC, 2002; pp 177−189. [Google Scholar]
- Ichihara H.; Nagami H.; Kiyokawa T.; Matsumoto Y.; Ueoka R. Chemotherapy Using Hybrid Liposomes along with Induction of Apoptosis. Anticancer Res. 2008, 28, 1187–1196. [PubMed] [Google Scholar]
- Komizu Y.; Matsumoto Y.; Ueoka R. Membrane Targeted Chemotherapy with Hybrid Liposomes for Colon Tumor Cells Leading to Apoptosis. Bioorg. Med. Chem. Lett. 2006, 16, 6131–6134. [DOI] [PubMed] [Google Scholar]
- Huang S. K.; Lee K. D.; Hong K.; Friend D. S.; Papahadjopoulos D. Microscopic Localization of Sterically Stabilized Liposomes in Colon Carcinoma-Bearing Mice. Cancer Res. 1992, 52, 5135–5143. [PubMed] [Google Scholar]
- Nakano K.; Iwamoto Y.; Takata W.; Matsumoto Y.; Ueoka R. Specific Accumulation and Growth Inhibitory Effects of Hybrid Liposomes to Hepatoma Cells in Vitro. Bioorg. Med. Chem. Lett. 2002, 12, 3251–3254. [DOI] [PubMed] [Google Scholar]
- Towata T.; Komizu Y.; Suzu S.; Matsumoto Y.; Ueoka R.; Okada S. Hybrid Liposomes Inhibit the Growth of Primary Effusion Lymphoma in Vitro and in Vivo. Leuk. Res. 2010, 34, 906–911. [DOI] [PubMed] [Google Scholar]
- Axelrod D. Total Internal Reflection Fluorescence Microscopy. Methods Cell. Biol. 1989, 30, 245–270. [DOI] [PubMed] [Google Scholar]
- Azumi T.; McGlynn S. P. Polarization of the Luminescence of Phenanthrene. J. Chem. Phys. 1962, 37, 2413–2420. [Google Scholar]
- Sok M.; Šentjurc M.; Schara M.; Stare J.; Rott T. Cell Membrane Fluidity and Prognosis of Lung Cancer. Ann. Thorac. Surg. 2002, 73, 1567–1571. [DOI] [PubMed] [Google Scholar]
- Funaki N.; Tanaka J.; Kono Y.; Nonaka A.; Yotsumoto F.; Lee J.-U.; Imamura M. Combination of α-Fetoprotein mRNA-Based Detection of Hematogenously Disseminating Hepatocellular Carcinoma Cells and Analysis of Cancer Cell Membrane Fluidity is More Accurate in Screening Patients at Risk of Postoperative Recurrence. Oncol. Rep. 2004, 11, 637–639. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.