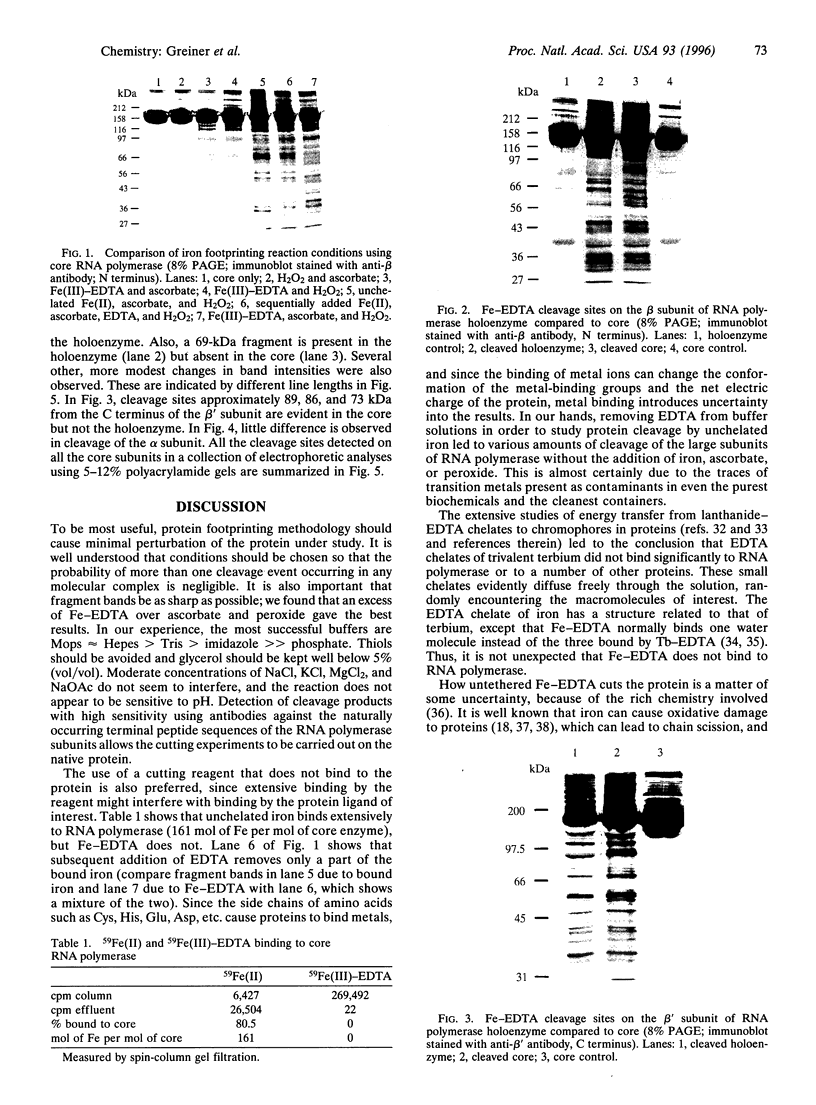
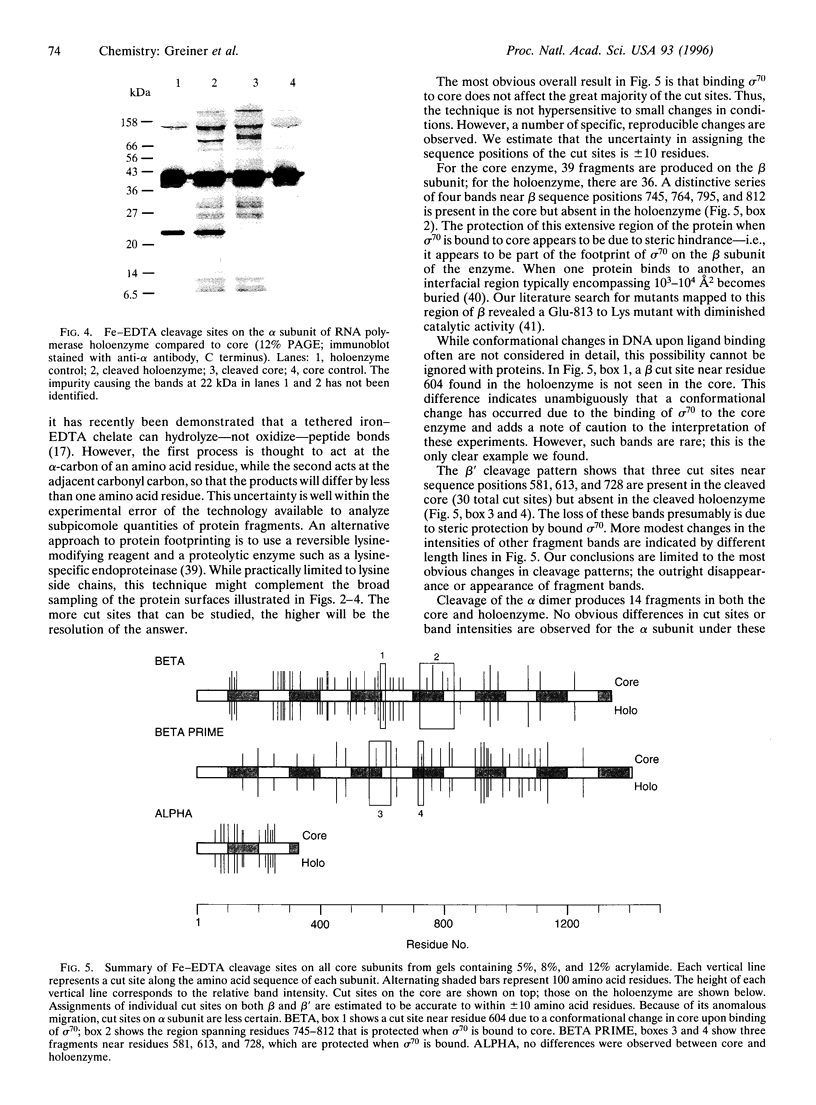
Abstract
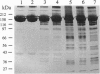
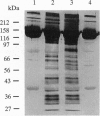
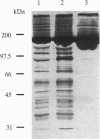
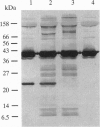
We have used a nonspecific protein cleaving reagent to map the interactions between subunits of the multisubunit enzyme RNA polymerase (Escherichia coli). We developed suitable conditions for using an untethered Fe-EDTA reagent, which does not bind significantly to proteins. Comparison of the cleaved fragments of the subunits from the core enzyme (alpha 2 beta beta') and the holoenzyme (core+sigma 70) shows that absence of the sigma 70 subunit is associated with the appearance of several cleavage sites on the subunits beta (within 10 residues of sequence positions 745, 764, 795, and 812) and beta' (within 10 residues of sequence positions 581, 613, and 728). A cleavage site near beta residue 604 is present in the holoenzyme but absent in the core, demonstrating that a conformational change occurs when sigma 70 binds. No differences are observed for the alpha subunit.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger D. K., Narberhaus F., Kustu S. The isolated catalytic domain of NIFA, a bacterial enhancer-binding protein, activates transcription in vitro: activation is inhibited by NIFL. Proc Natl Acad Sci U S A. 1994 Jan 4;91(1):103–107. doi: 10.1073/pnas.91.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess R. R., Travers A. A., Dunn J. J., Bautz E. K. Factor stimulating transcription by RNA polymerase. Nature. 1969 Jan 4;221(5175):43–46. doi: 10.1038/221043a0. [DOI] [PubMed] [Google Scholar]
- Chamberlin M. J. New models for the mechanism of transcription elongation and its regulation. Harvey Lect. 1992 1993;88:1–21. [PubMed] [Google Scholar]
- Coggins J. R., Lumsden J., Malcolm A. D. A study of the quaternary structure of Escherichia coli RNA polymerase using bis(imido esters). Biochemistry. 1977 Mar 22;16(6):1111–1116. doi: 10.1021/bi00625a013. [DOI] [PubMed] [Google Scholar]
- Dunn J. J., Bautz E. K. DNA-dependent RNA polymerase from E. coli: studies on the role of sigma in chain initiation. Biochem Biophys Res Commun. 1969 Sep 10;36(6):925–930. doi: 10.1016/0006-291x(69)90292-7. [DOI] [PubMed] [Google Scholar]
- Ermácora M. R., Ledman D. W., Hellinga H. W., Hsu G. W., Fox R. O. Mapping staphylococcal nuclease conformation using an EDTA-Fe derivative attached to genetically engineered cysteine residues. Biochemistry. 1994 Nov 22;33(46):13625–13641. doi: 10.1021/bi00250a013. [DOI] [PubMed] [Google Scholar]
- Gerlach V. L., Whitehall S. K., Geiduschek E. P., Brow D. A. TFIIIB placement on a yeast U6 RNA gene in vivo is directed primarily by TFIIIC rather than by sequence-specific DNA contacts. Mol Cell Biol. 1995 Mar;15(3):1455–1466. doi: 10.1128/mcb.15.3.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaim J. B., Greiner D. P., Meares C. F., Gennis R. B. Proximity mapping the surface of a membrane protein using an artificial protease: demonstration that the quinone-binding domain of subunit I is near the N-terminal region of subunit II of cytochrome bd. Biochemistry. 1995 Sep 12;34(36):11311–11315. doi: 10.1021/bi00036a002. [DOI] [PubMed] [Google Scholar]
- Glass R. E., Honda A., Ishihama A. Genetic studies on the beta subunit of Escherichia coli RNA polymerase. IX. The role of the carboxy-terminus in enzyme assembly. Mol Gen Genet. 1986 Jun;203(3):492–495. doi: 10.1007/BF00422075. [DOI] [PubMed] [Google Scholar]
- Goodwin D. A., Meares C. F., Watanabe N., McTigue M., Chaovapong W., Ransone C. M., Renn O., Greiner D. P., Kukis D. L., Kronenberger S. I. Pharmacokinetics of pretargeted monoclonal antibody 2D12.5 and 88Y-Janus-2-(p-nitrobenzyl)-1,4,7,10-tetraazacyclododecanetetraacetic acid (DOTA) in BALB/c mice with KHJJ mouse adenocarcinoma: a model for 90Y radioimmunotherapy. Cancer Res. 1994 Nov 15;54(22):5937–5946. [PubMed] [Google Scholar]
- Hager D. A., Jin D. J., Burgess R. R. Use of Mono Q high-resolution ion-exchange chromatography to obtain highly pure and active Escherichia coli RNA polymerase. Biochemistry. 1990 Aug 28;29(34):7890–7894. doi: 10.1021/bi00486a016. [DOI] [PubMed] [Google Scholar]
- Hanai R., Wang J. C. Protein footprinting by the combined use of reversible and irreversible lysine modifications. Proc Natl Acad Sci U S A. 1994 Dec 6;91(25):11904–11908. doi: 10.1073/pnas.91.25.11904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyduk E., Heyduk T. Mapping protein domains involved in macromolecular interactions: a novel protein footprinting approach. Biochemistry. 1994 Aug 16;33(32):9643–9650. doi: 10.1021/bi00198a033. [DOI] [PubMed] [Google Scholar]
- Hillel Z., Wu C. W. Subunit topography of RNA polymerase from Escherichia coli. A cross-linking study with bifunctional reagents. Biochemistry. 1977 Jul 26;16(15):3334–3342. doi: 10.1021/bi00634a008. [DOI] [PubMed] [Google Scholar]
- Janin J., Miller S., Chothia C. Surface, subunit interfaces and interior of oligomeric proteins. J Mol Biol. 1988 Nov 5;204(1):155–164. doi: 10.1016/0022-2836(88)90606-7. [DOI] [PubMed] [Google Scholar]
- Kim K., Rhee S. G., Stadtman E. R. Nonenzymatic cleavage of proteins by reactive oxygen species generated by dithiothreitol and iron. J Biol Chem. 1985 Dec 15;260(29):15394–15397. [PubMed] [Google Scholar]
- Kim T. K., Zhao Y., Ge H., Bernstein R., Roeder R. G. TATA-binding protein residues implicated in a functional interplay between negative cofactor NC2 (Dr1) and general factors TFIIA and TFIIB. J Biol Chem. 1995 May 5;270(18):10976–10981. doi: 10.1074/jbc.270.18.10976. [DOI] [PubMed] [Google Scholar]
- Lee J., Kashlev M., Borukhov S., Goldfarb A. A beta subunit mutation disrupting the catalytic function of Escherichia coli RNA polymerase. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6018–6022. doi: 10.1073/pnas.88.14.6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe P. A., Hager D. A., Burgess R. R. Purification and properties of the sigma subunit of Escherichia coli DNA-dependent RNA polymerase. Biochemistry. 1979 Apr 3;18(7):1344–1352. doi: 10.1021/bi00574a034. [DOI] [PubMed] [Google Scholar]
- McMahan S. A., Burgess R. R. Use of aryl azide cross-linkers to investigate protein-protein interactions: an optimization of important conditions as applied to Escherichia coli RNA polymerase and localization of a sigma 70-alpha cross-link to the C-terminal region of alpha. Biochemistry. 1994 Oct 11;33(40):12092–12099. doi: 10.1021/bi00206a012. [DOI] [PubMed] [Google Scholar]
- Meares C. F., Rice L. S. Diffusion-enhanced energy transfer shows accessibility of ribonucleic acid polymerase inhibitor binding sites. Biochemistry. 1981 Feb 3;20(3):610–617. doi: 10.1021/bi00506a025. [DOI] [PubMed] [Google Scholar]
- Ovchinnikov YuA, Monastyrskaya G. S., Gubanov V. V., Guryev S. O., Salomatina I. S., Shuvaeva T. M., Lipkin V. M., Sverdlov E. D. The primary structure of E. coli RNA polymerase, Nucleotide sequence of the rpoC gene and amino acid sequence of the beta'-subunit. Nucleic Acids Res. 1982 Jul 10;10(13):4035–4044. doi: 10.1093/nar/10.13.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovchinnikov Y. A., Lipkin V. M., Modyanov N. N., Chertov O. Y., Smirnov Y. V. Primary structure of alpha-subunit of DNA-dependent RNA polymerase from Escherichia coli. FEBS Lett. 1977 Apr 1;76(1):108–111. doi: 10.1016/0014-5793(77)80131-2. [DOI] [PubMed] [Google Scholar]
- Ovchinnikov Y. A., Monastyrskaya G. S., Gubanov V. V., Guryev S. O., Chertov OYu, Modyanov N. N., Grinkevich V. A., Makarova I. A., Marchenko T. V., Polovnikova I. N. The primary structure of Escherichia coli RNA polymerase. Nucleotide sequence of the rpoB gene and amino-acid sequence of the beta-subunit. Eur J Biochem. 1981 Jun 1;116(3):621–629. doi: 10.1111/j.1432-1033.1981.tb05381.x. [DOI] [PubMed] [Google Scholar]
- Penefsky H. S. A centrifuged-column procedure for the measurement of ligand binding by beef heart F1. Methods Enzymol. 1979;56:527–530. doi: 10.1016/0076-6879(79)56050-9. [DOI] [PubMed] [Google Scholar]
- Rana T. M., Meares C. F. Transfer of oxygen from an artificial protease to peptide carbon during proteolysis. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10578–10582. doi: 10.1073/pnas.88.23.10578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouvière P. E., De Las Peñas A., Mecsas J., Lu C. Z., Rudd K. E., Gross C. A. rpoE, the gene encoding the second heat-shock sigma factor, sigma E, in Escherichia coli. EMBO J. 1995 Mar 1;14(5):1032–1042. doi: 10.1002/j.1460-2075.1995.tb07084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THIERS R. E. Contamination in trace element analysis and its control. Methods Biochem Anal. 1957;5:273–335. doi: 10.1002/9780470110218.ch6. [DOI] [PubMed] [Google Scholar]
- Taborsky G. Oxidative modification of proteins in the presence of ferrous ion and air. Effect of ionic constituents of the reaction medium on the nature of the oxidation products. Biochemistry. 1973 Mar 27;12(7):1341–1348. doi: 10.1021/bi00731a014. [DOI] [PubMed] [Google Scholar]
- Tam J. P. Synthetic peptide vaccine design: synthesis and properties of a high-density multiple antigenic peptide system. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5409–5413. doi: 10.1073/pnas.85.15.5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K., Kusano S., Fujita N., Ishihama A., Takahashi H. Promoter determinants for Escherichia coli RNA polymerase holoenzyme containing sigma 38 (the rpoS gene product). Nucleic Acids Res. 1995 Mar 11;23(5):827–834. doi: 10.1093/nar/23.5.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers A. A., Burgessrr Cyclic re-use of the RNA polymerase sigma factor. Nature. 1969 May 10;222(5193):537–540. doi: 10.1038/222537a0. [DOI] [PubMed] [Google Scholar]
- Zomerdijk J. C., Beckmann H., Comai L., Tjian R. Assembly of transcriptionally active RNA polymerase I initiation factor SL1 from recombinant subunits. Science. 1994 Dec 23;266(5193):2015–2018. doi: 10.1126/science.7801130. [DOI] [PubMed] [Google Scholar]