Abstract
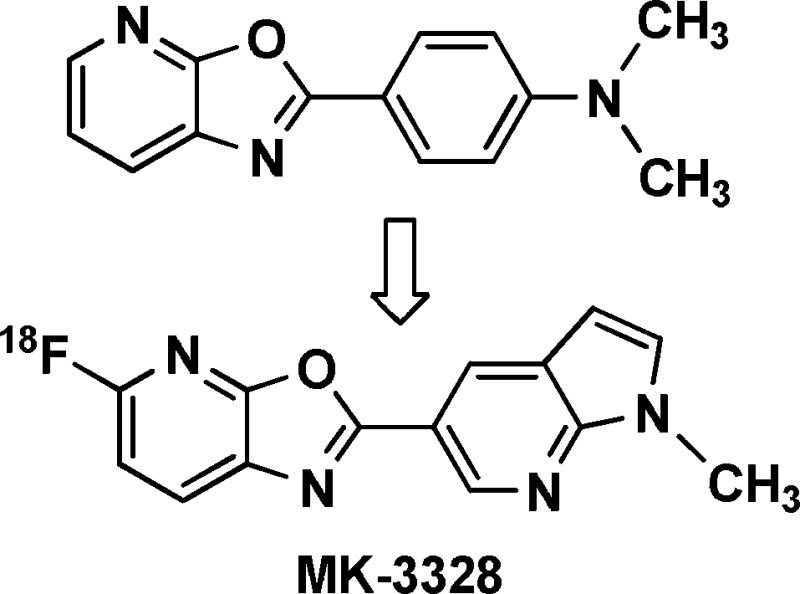
5-Fluoro-2-aryloxazolo[5,4-b]pyridines were synthesized and investigated as potential 18F containing β-amyloid PET ligands. In competition binding assays using human AD brain homogenates, compounds 14b, 16b, and 17b were identified as having favorable potency versus human β-amyloid plaque and were radiolabeled for further evaluation in in vitro binding and in vivo PET imaging experiments. These studies led to the identification of 17b (MK-3328) as a candidate PET ligand for the clinical assessment of β-amyloid plaque load.
Keywords: Alzheimer’s disease; β-amyloid plaque; fluorine-18; positron emission tomography; PET; oxazolo[5,4-b]pyridine
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder which was estimated to afflict 26.6 million people worldwide as of 2006,1 with many more being affected as caregivers of those with this debilitating disease. Although marketed treatments for AD temporarily improve cognitive symptoms in patients, they fail to significantly slow disease progression. Several novel therapeutics are under development which have the potential to intervene in the underlying mechanisms of AD, and biomarkers for detecting affected patient populations and for measuring response to these therapies are needed.2 Many in the Alzheimer’s research community believe that successful alteration of the disease’s trajectory may require therapeutic intervention at the mild cognitive impairment (MCI) symptomatic stage, before the onset of potentially irreversible neurodegeneration.3 Whereas clinical diagnosis of AD via tests of cognition is sufficient at late stages, early diagnosis of prodromal or predementia AD is less straightforward and requires evidence of both episodic memory loss and biomarker changes supportive of pathological processes.4
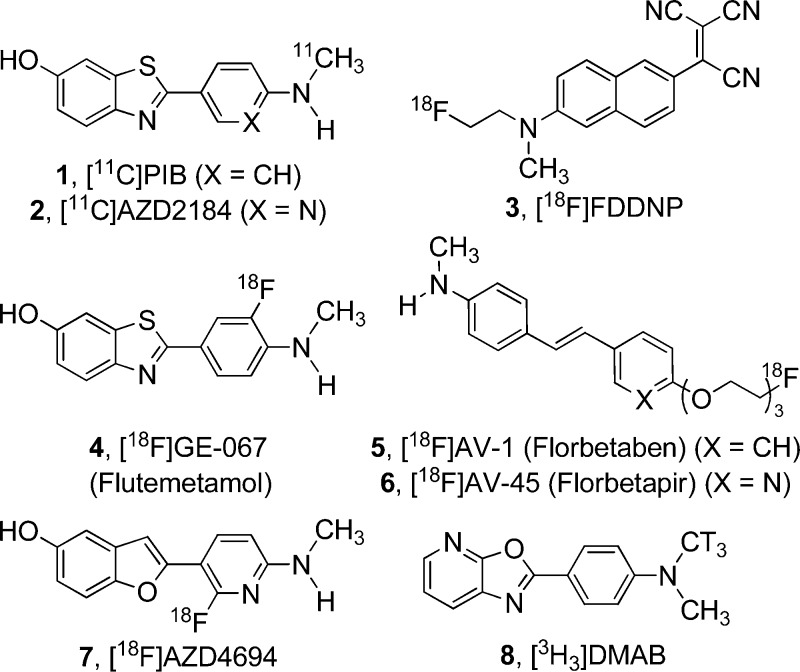
Several noteworthy efforts are underway to identify early clinical markers of AD.5 While promising, most predictive markers are indirect measures of underlying disease pathology or are medically invasive. A more direct and noninvasive approach, in particular, the assessment of β-amyloid (Aβ) plaque deposits in the brain via positron emission tomography (PET) imaging, has demonstrated success in clinical settings. To date, several Aβ-binding PET ligands have effectively differentiated AD from non-AD patients. [11C]PIB (1, Figure 1)6,7 is the most thoroughly studied amyloid PET ligand and recently has been correlated with other clinical AD biomarkers.8 Although not as well characterized as PIB, Astra-Zeneca’s [11C]AZD2184 (2) shows promise as a human amyloid imaging agent, as it has demonstrated lower levels of nonspecific binding than PIB in preclinical studies.9−11
Figure 1.
Structures of selected amyloid PET ligands and our reference radioligand 8, [3H3]DMAB.
While these tracers are effective, the short half-life of 11C (∼20 min) limits the use of [11C]PIB and [11C]AZD2184 to PET imaging centers with cyclotrons in their immediate vicinity. Because 18F has a much longer half-life (110 min), 18F-based tracers may be used at PET imaging sites much further away from radionuclide production sites, enabling increased access for patients. Several research programs have engaged in the design and discovery of 18F amyloid PET ligands, culminating in the discovery of [18F]FDDNP12 (3), GE-067 (Flutemetamol, 4),13 [18F]AV-1 (Florbetaben, 5),14,15 [18F]AV-45 (Florbetapir, 6),16,17 and AZD4694 (7).18
Based on the preclinical and clinical data available,12−18 we estimated that a successful amyloid imaging agent must have high affinity for β-amyloid plaque (Kd < 20 nM) with minimal nonspecific binding in order to provide a large specific signal for plaque detection. Also important is the behavior of the tracer in in vivo imaging studies in animals lacking plaque, where the tracer should exhibit high brain uptake and rapid washout kinetics. Published data on amyloid ligand candidates reveals that highly lipophilic tracers often exhibit elevated retention in white matter which, in imaging studies, can affect the signal-to-noise ratio and interfere with the differentiation of AD and non-AD patients.15 Decreasing lipophilicity (e.g., cLogP) is one method that may be employed to reduce nonspecific binding to brain tissue.19 Pairwise comparisons of closely related amyloid binders suggests that this approach is valid, as observed in the improved nonspecific binding profile of AZD2184 (cLogP20 = 2.84) versus PIB (cLogP = 3.45) as well as AV-45 (cLogP = 3.13) versus AV-1 (cLogP = 3.75).
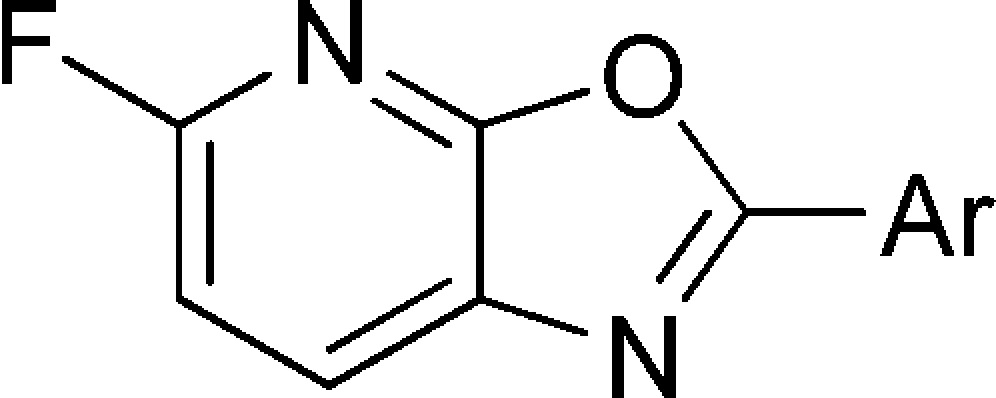
Herein we report our efforts in this area, involving the synthesis and evaluation of oxazolo[5,4-b]pyridines that can be labeled with 18F as potential imaging agents for β-amyloid plaque. To characterize binding of newly synthesized compounds, a competition binding assay in human AD cortex homogenates was employed using [3H3]DMAB (8) as a reference radioligand. This compound was identified via radiolabeling of selected structures from the Merck compound collection with high structural similarity to literature amyloid plaque ligands. [3H3]DMAB (8) has a Kd of 25 nM in human AD brain homogenates with low levels of nonspecific binding, making it a suitable reference radioligand for competition binding assays. From a design perspective, 8 also served as a lead structure in the design of the 5-fluoro-oxazolo[5,4-b]pyridines presented in this work. Potent compounds possessing low calculated logP and lacking P-glycoprotein (P-gp) susceptibility were radiolabeled with tritium for examination in more detailed in vitro binding assays and with 18F for evaluation in rhesus monkey PET studies. Because [11C]PIB does not bind with high affinity to plaque in rhesus monkey brain,21 we did not use animal models of AD in in vivo imaging experiments to predict the behavior of tracer candidates in patients.
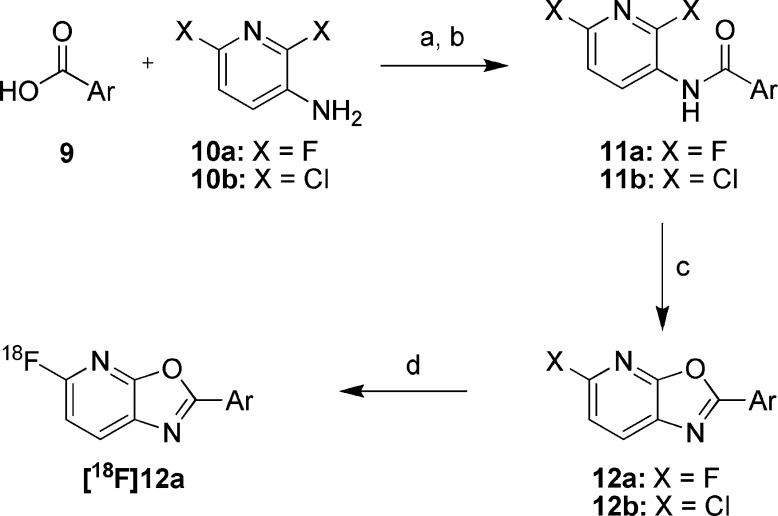
Unlabeled oxazolo[5,4-b]pyridine analogs were synthesized starting from 3-amino-2,6-difluoropyridine 10a(22) (Scheme 1) and a variety of commercially available and prepared carboxylic acids 9. Activation of the carboxylic acids 9 with Ghosez’ reagent,23 followed by reaction with 10a in the presence of excess pyridine afforded amides of type 11a. Focused libraries were prepared by parallel concentration of the crude reaction mixtures and direct passage into the next step; however, conversions were generally higher if the amides were first isolated and purified, using either chromatography or precipitation following the addition of water. Previously described acidic methods for the conversion of amides of type 11a into oxazolo[5,4-b]pyridines were poor yielding;24 however, microwave heating of the amides 11a with K2CO3 or Cs2CO3 in DMF afforded the targeted oxazolo[5,4-b]pyridines 12a, typically with high conversion. In instances where the starting carboxylate 9 contained a free N−H, the base mediated cyclizations failed to proceed, necessitating protection with a Boc or p-methoxybenzyl group, which either spontaneously deprotected during heating or was subsequently removed with trifluoroacetic acid. Synthesis of 18F labeled precursors was accomplished via the same route, substituting commercially available 3-amino-2,6-dichloropyridine 10b in the first step, leading to 5-chloro-oxazolo[5,4-b]pyridines of type 12b. Microwave heating with [18F]KF and Kryptofix 2.2.2 in DMSO then afforded [18F]12a.
Scheme 1.
Previously reported SAR in the 2-arylbenzazole classes of Aβ plaque ligands suggested that 4- and 3,4-substitutions on the 2-aryl ring are necessary for high amyloid affinity,25 while 2- and 6-substitutions are deleterious, so our own efforts primarily focused on the 3- and 4-positions (Table 1). While 4-methoxyphenyl (13) and 4-methylaminophenyl (14a) analogs demonstrated moderate potency with moderate to high clogP values, the dimethylamino analog 14b exhibited potent binding, with only a slightly higher clogP relative to 14a. Attempts to moderate lipophilicity by incorporation of a ring nitrogen, as in pyridine analogs 15a and 15b, resulted in a loss of binding affinity. Constraining the 4-substituent into a ring led to indole analogs 16a and 16b, which were potent at a cost of slightly elevated lipophilicity. Whereas aza-substitution of 4-amino phenyl compounds was deleterious for binding potency, 7-aza-substitution of the indoles 16a−b, affording the azaindoles 17a and 17b, caused only a 2-fold loss in binding potency while lowering clogP by a full unit. N-Methyl analogs (14b−17b) tended to exhibit improved binding potency relative to their respective des-methyl analogs (14a−17a). Many closely related and isomeric forms of the active indoles (16a,b) and azaindoles (17a,b) were investigated; however, the best of these compounds were 2- to 5-fold less potent (18−22) and, in most instances, were completely inactive (e.g., 23−24). All of the compounds evaluated for human P-gp susceptibility in this series were not substrates (P-gp transport ratio < 3) and exhibited high passive permeability (Papp > 20 × 10−6 cm/s), including the lead compounds 14b, 16b, and 17b.26
Table 1. Affinity of 5-Fluoro-2-aryloxazolo[5,4-b]pyridines for Human β-Amyloid Plaque Deposits.
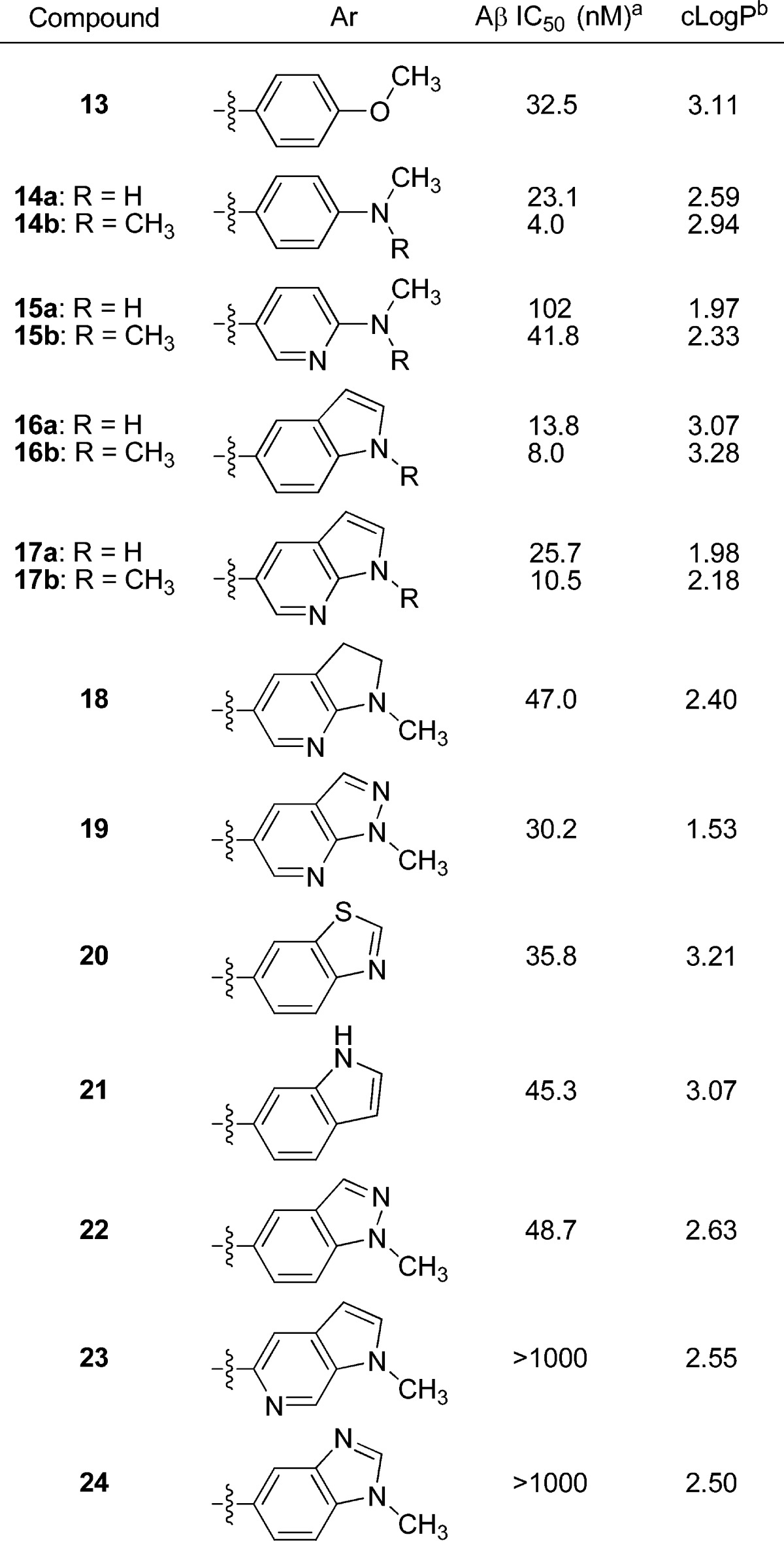 |
Values are means of at least two experiments.
Calculated using AlogP98.20
To fully characterize the binding characteristics of 14b, 16b, and 17b, their corresponding [3H3]methyl analogs were prepared using sodium hydride mediated deprotonation of 14a, 16a, and 17a, respectively, followed by reaction with [3H3]methyl iodide. Saturation binding studies were conducted using AD cortex homogenates, to which all three compounds exhibited potent and saturable binding (Table 2). While 14b and 16b exhibited more potent binding and higher Bmax/Kd ratios relative to 17b, the latter compound exhibited lower levels of nondisplacable binding in AD brain homogenates, estimated as the percent of total binding at the Kd in the presence of 2 μM unlabeled compound. The decreased level of nonspecific binding associated with 17b is consistent with the hypothesis that nonspecific binding to brain tissue correlates with lipophilicity.
Table 2. Binding Affinity of Tritium-Labeled 14b, 16b, and 17b versus Human AD Brain Homogenates from Three Different Donors.
| compd | Kd (nM)a | Bmax (nM) | Bmax/Kd | NDBb |
|---|---|---|---|---|
| [3H3]14b | 4.5 ± 0.6 | 2088 ± 231 | 464 ± 80 | 50% |
| [3H3]16b | 6.4 ± 0.9 | 1454 ± 140 | 232 ± 40 | 50% |
| [3H3]17b | 9.6 ± 0.3 | 1100 ± 290 | 117 ± 34 | 35% |
Values are means of three experiments using AD brain homogenates from different donors.
Nondisplaceable binding, estimated as the amount of binding observed at the Kd in the presence of 2 μM unlabeled compound
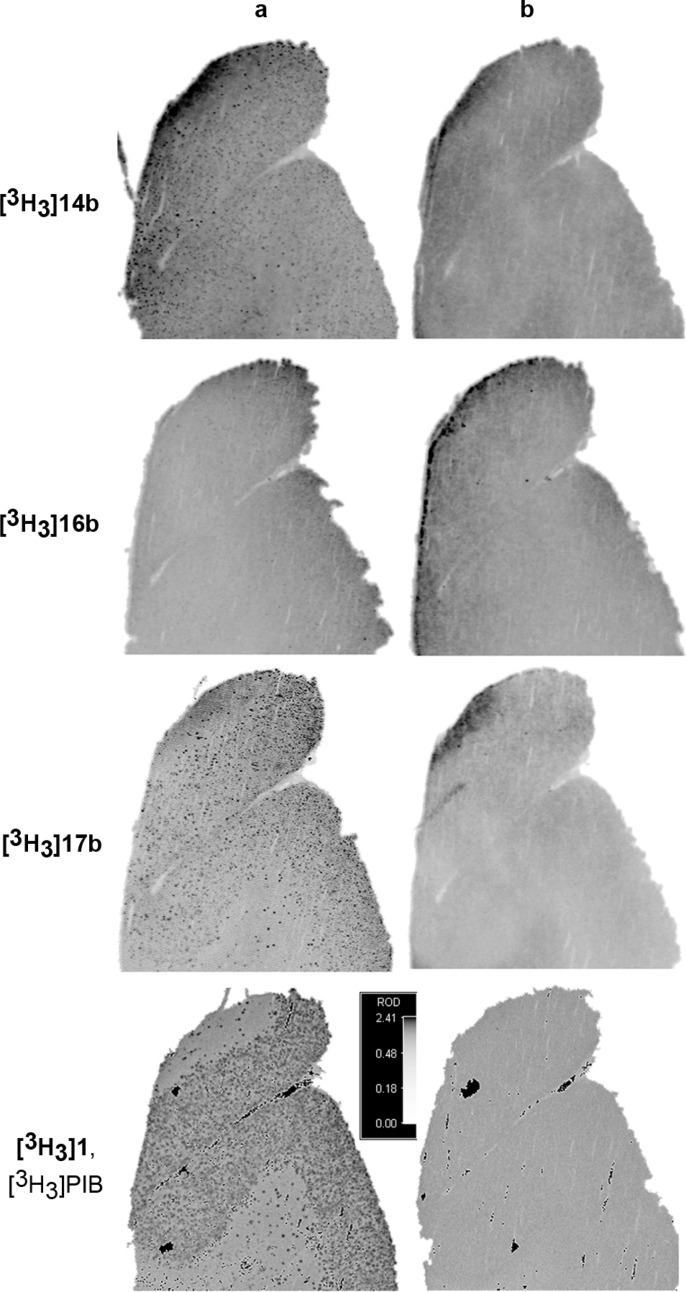
Autoradiographic imaging experiments with human AD brain slices were used to further differentiate the tritium-labeled lead compounds (Figure 2). Whereas [3H3]14b, [3H3]17b, and the positive control [3H3]PIB ([3H3]1)9 exhibited a punctate binding pattern, [3H3]16b repeatedly failed to do so, presumably due to a combination of elevated nonspecific binding and a high affinity of the compound for laboratory plastics (Figure 2, column a). Self-block with 1 μM unlabeled parent compound was used to assess nonspecific binding (Figure 2, column b). In all instances, punctate binding was not observed in the presence of a saturating concentration of unlabeled parent compound; however, all compounds exhibited some degree of diffuse background binding. Qualitatively, the specific to nonspecific binding ratio for [3H3]17b appeared to be better than that of [3H3]14b, suggesting that 17b may be a better amyloid diagnostic agent in vivo.
Figure 2.
(a) Autoradiographic images of AD human cortex after treatment with 5 nM [3H3]-labeled compound. (b) Autoradiographic images of AD human cortex after treatment with 5 nM [3H3]-labeled compound in the presence of 1 μM unlabeled compound (self-block).
Using the chemistry delineated in Scheme 1, 18F-labeled versions of 14b, 16b, and 17b were prepared for evaluation in in vivo PET imaging studies in healthy anesthetized rhesus monkeys to evaluate brain uptake and washout kinetics, and the preliminary data for [18F]17b is depicted in Figure 3.27 Here the tracer demonstrated high peak brain uptake of ca. 2.7 standardized uptake value (SUV) units in cortex and ca. 3.0 SUV in the cerebellum. As desired, [18F]17 washed out rapidly, with less than half of the peak uptake present in the cortex and cerebellum at 90 min. In humans exhibiting AD pathology, amyloid plaque deposits manifest themselves throughout the brain, including the cortex, but not in the cerebellum until terminal phases of the disease.28 The close correlation between cerebellum and cortex time activity curves in this study is desirable, as it suggests that the cerebellum will likely be a suitable baseline reference region in clinical imaging studies. In the 45−90 min summed image (Figure 3b), cortical regions show a lack of hot spots corresponding to white matter retention. Preliminarily, this may suggest that the approach of minimizing white matter binding via targeting of less lipophilic amyloid ligands is valid. Whether or not this lack of white matter retention in rhesus will translate into human clinical imaging is a matter of future study.
Figure 3.
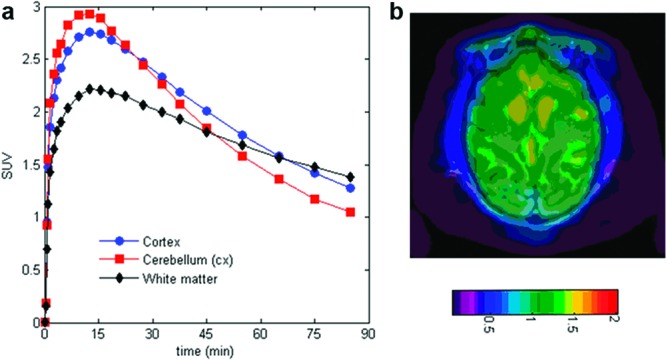
(a) Decay corrected time−activity curves for [18F]17b in cortex (red circles), cerebellum (blue squares), and white-matter (black diamonds). (b) MRI coregistered rhesus PET image at 45−90 min with [18F]17b. Cerebellum was used as a baseline reference.
In conclusion, we have investigated 5-fluoro-2-aryloxazolo[5,4-b]pyridines as potential β-amyloid PET ligands. After screening newly synthesized compounds for affinity toward AD brain homogenates, 17b (MK-3328) was identified as a promising candidate, exhibiting amyloid binding potency balanced with low levels of nonspecific binding. In vivo, [18F]17b demonstrates favorable kinetics, exhibiting high brain uptake and good washout in normal rhesus monkey PET imaging studies. Human clinical trials to evaluate [18F]MK-3328 as an 18F-based β-amyloid PET ligand are currently underway and will be reported upon in due course.
Acknowledgments
We are grateful to Ray McClain, April Cox, Anna Dudkina, and Semhal Berhane for mass-guided HPLC purification of library compounds, Joan Murphy and Charles W. Ross, III for HRMS measurements, Sandor L. Varga for NMR structure determinations, and Cheng Tang, Scott Pollack, and Ashok Chaudhary for synthesis of tritium labeled compounds.
Supporting Information Available
Representative assay and experimental procedures and characterization data for tested compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
Supplementary Material
References
- Brookmeyer R.; Johnson E.; Ziegler-Graham K.; Arrighi H. M. Forecasting the global burden of Alzheimer’s disease. Alzhimer’s Dementia 2007, 3, 186–191. [DOI] [PubMed] [Google Scholar]
- Galimberti D.; Scarpini E. Treatment of Alzheimer’s Disease: Symptomatic and Disease-Modifying Approaches. Curr. Aging Sci. 2010, 3, 46–56. [DOI] [PubMed] [Google Scholar]
- Peterson R. C.; Roberts R. O.; Knopman D. S.; Boeve B. F.; Geda Y. E.; Ivnik R. J.; Smith G. E.; Jack C. R. Jr. Mild Cognitive Impairment. Arch. Neurol. 2009, 66, 1447–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois B.; Feldman H. H.; Jacova C.; Cummings J. L.; DeKosky S. T.; Barberger-Gateau P.; Delacourte A.; Frisoni G.; Fox N. C.; Galasko D.; Gauthier S.; Hampel H.; Jicha G. A.; Meguro K.; O’Brien J.; Pasquier F.; Robert P.; Rossor M.; Salloway S.; Sarazin M.; de Souza L. C.; Stern Y.; Visser P. J.; Scheltens P. Revising the definition of Alzheimer’s disease: a new lexicon. Lancet Neurol. 2010, 9, 1118–1127. [DOI] [PubMed] [Google Scholar]
- Blennow K.; Hampel H.; Weiner M.; Zetterberg H. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nature Rev. Neurol. 2010, 6, 131–144. [DOI] [PubMed] [Google Scholar]
- Mathis C. A.; Wang Y.; Holt D. P.; Huang G.-F.; Debnath M. L.; Klunk W. E. Synthesis and Evaluation of 11C-Labeled 6-Substituted 2-Arylbenzothiazoles as Amyloid Imaging Agents. J. Med. Chem. 2003, 46, 2740–2754. [DOI] [PubMed] [Google Scholar]
- Klunk W. E.; Engler H.; Nordberg A.; Wang Y.; Blomqvist G.; Holt D. P.; Bergström M.; Savitcheva I.; Huang G.-F.; Estrada S.; Ausen B.; Debnath M. L.; Barletta J.; Price J. C.; Sandell J.; Lopresti B. J.; Wall A.; Koivisto P.; Antoni G.; Mathis C. A.; Långström B. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann. Neurol. 2004, 55, 306–319. [DOI] [PubMed] [Google Scholar]
- Degerman G. M.; Lindau M.; Wall A.; Blennow K.; Darreh-Shori T.; Basu S.; Nordberg A.; Larsson A.; Lannfelt L.; Basun H.; Kilander L. Pittsburgh compound-B and Alzheimer’s disease biomarkers in CSF, plasma and urine: An exploratory study. Dementia Geriatr. Cognit. Disord. 2010, 29, 204–212. [DOI] [PubMed] [Google Scholar]
- Swahn B.-M.; Wensbo D.; Sandell J.; Sohn D.; Slivo C.; Pyring D.; Malmström J.; Arzel E.; Vallin M.; Bergh M.; Jeppsson F.; Johnson A. E.; Juréus A.; Neelissen J.; Svensson S. Synthesis and evaluation of 2-pyridylbenzothiazole, 2-pyridylbenzoxazole and 2-pyridylbenzofuran derivatives as 11C-PET imaging agents for β-amyloid plaques. Bioorg. Med. Chem. Lett. 2010, 20, 1976–1980. [DOI] [PubMed] [Google Scholar]
- Johnson A. E.; Jeppsson F.; Sandell J.; Wensbo D.; Neelissen J. A. M.; Jureus A.; Stroem P.; Norman H.; Farde L.; Svensson S. P. S. AZD2184: a radioligand for sensitive detection of β-amyloid deposits. J. Neurochem. 2009, 108, 1177–1186. [DOI] [PubMed] [Google Scholar]
- Svante N.; Eriksdotter J. M.; Zsolt C.; Christer H.; Per J.; Hans O.; Yvonne F.-L.; Jan A.; Katarina V.; Samuel S.; Lars F. Detection of amyloid in Alzheimer’s disease with positron emission tomography using [11C]AZD2184. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 1859–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small G. W.; Kepe V.; Ercoli L.; Siddarth P.; Bookheimer S. Y.; Miller K. J.; Lavretsky H.; Burggren A. C.; Cole G. M.; Vinters H. V.; Thompson P. M.; Huang S.-C.; Satyamurthy N.; Phelps M. E.; Barrio J. R. PET of brain amyloid and tau in mild cognitive impairment. N. Engl. J. Med. 2006, 355, 2652–2663. [DOI] [PubMed] [Google Scholar]
- Vandenberghe R.; Van Laere K.; Ivanoiu A.; Salmon E.; Bastin C.; Triau E.; Hasselbalch S.; Law I.; Andersen A.; Korner A.; Minthon L.; Garraux G.; Nelissen N.; Bormans G.; Buckley C.; Owenius R.; Thurfjell L.; Farrar G.; Brooks D. J. 18F-flutemetamol amyloid imaging in Alzheimer disease and mild cognitive impairment: a phase 2 trial. Ann. Neurol. 2010, 68, 319–329. [DOI] [PubMed] [Google Scholar]
- Zhang W.; Oya S.; Kung M. P.; Hou C.; Maier D. L.; Kung H. F. F-18 PEG stilbenes as PET imaging agents targeting Aβ aggregates in the brain. Nucl. Med. Biol. 2005, 32, 799–809. [DOI] [PubMed] [Google Scholar]
- Rowe C. C.; Ackerman U.; Browne W.; Mulligan R.; Pike K. L.; O’Keefe G.; Tochon-Danguy H.; Chan G.; Berlangieri S. U.; Jones G.; Dickinson-Rowe K. L.; Kung H. P.; Zhang W.; Kung M. P.; Skovronsky D.; Dyrks T.; Holl G.; Krause S.; Friebe M.; Lehman L.; Lindemann S.; Dinkelborg L. M.; Masters C. L.; Villemagne V. L. Imaging of amyloid β in Alzheimer’s disease with 18F-BAY94-9172, a novel PET tracer: proof of mechanism. Lancet Neurol. 2008, 7, 129–135. [DOI] [PubMed] [Google Scholar]
- Zhang W.; Kung M. P.; Oya S.; Hou C.; Kung H. F. 18F-Labeled styrylpyridines as PET agents for amyloid plaque imaging. Nucl. Med. Biol. 2007, 34, 89–97. [DOI] [PubMed] [Google Scholar]
- Wong D. F.; Rosenberg P. B.; Zhou Y.; Kumar A.; Raymont V.; Ravert H. T.; Dannals R. F.; Nandi A.; Brasić J. R.; Ye W.; Hilton J.; Lyketsos C.; Kung H. F.; Joshi A. D.; Skovronsky D. M.; Pontecorvo M. J. In Vivo Imaging of Amyloid Deposition in Alzheimer Disease Using the Radioligand 18F-AV-45 (Flobetapir F 18). J. Nucl. Med. 2010, 51, 913–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juréus A.; Swahn B.-M.; Sandell J.; Jeppsson F.; Johnson A. E.; Johnström P.; Neelissen J. A. M.; Sunnemark D.; Farde L.; Svensson S. P. S. Characterization of AZD4694, a novel fluorinated Aβ plaque neuroimaging PET radioligand. J. Neurochem. 2010, 114, 784–794. [DOI] [PubMed] [Google Scholar]
- Summerfield S. G.; Read K.; Begley D. J.; Obradovic T.; Hidalgo I. J.; Coggon S.; Lewis A. V.; Porter R. A.; Jeffrey P. Central Nervous System Drug Disposition: The Relationship between in Situ Brain Permeability and Brain Free Fraction. J. Pharmacol. Exp. Ther. 2007, 322, 205–213. [DOI] [PubMed] [Google Scholar]
- AlogP98 values were calculated by Cerius2 (version 4.11) from Accelrys (San Diego, CA).Ghose A. K.; Viswanadhan V. N.; Wendolowski J. J. Prediction of Hydrophobic (Lipophilic) Properties of Small Organic Molecules Using Fragmental Methods: An Analysis of ALOGP and CLOGP Methods. J. Phys. Chem. A 1998, 102A, 3762–3772. [Google Scholar]
- Rosen R. F.; Walker L. C.; Levine H. III. PIB binding in aged primate brain: Enrichment of high-affinity sites in humans with Alzheimer’s disease. Neurobiol. Aging 2011, 32, 223–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radl S.; Hradil P. Synthesis of some 1-alkyl-1,4-dihydro-4-oxo-1,7-naphthyridine-3-carboxylic acids. Collect. Czech. Chem. Commun. 1991, 56, 2420–2429. [Google Scholar]
- Devos A.; Remion J.; Frisque-Hesbain A. M.; Colens A.; Ghosez L. Synthesis of acyl halides under very mild conditions. J. Chem. Soc., Chem. Commun. 1979, 24, 1180–1181. [Google Scholar]
- Flouzat C.; Guillaumet G. A new convenient synthesis of 2-aryl- and 2-heteroaryloxazolo[5,4-b]pyridines. Synthesis 1990, 1, 64–66. [Google Scholar]
- Cai L.; Innis R. B.; Pike V. W. Radioligand development for PET imaging of β-amyloid (Aβ)-current status. Curr. Med. Chem. 2007, 14, 19–52. [DOI] [PubMed] [Google Scholar]
- Yamazaki M.; Neway W. E.; Ohe T.; Chen I. W.; Rowe J. F.; Hochman J. H.; Chiba M.; Lin J. H. In vitro substrate identification studies for P-glycoprotein mediated transport: species difference and predictability of in vivo results. J. Pharmacol. Exp. Ther. 2001, 296, 723–735. [PubMed] [Google Scholar]
- The detailed synthesis of [18F]14b, [18F]16b, and [18F]17b and their comparison in in vivo rhesus PET imaging experiments is the subject of a separate communication: Hostetler E. D.; Sanabria-Bohórquez S.; Fan H.; Zeng Z.; Gammage L.; Miller P.; OʼMalley S.; Connolly B.; Mulhearn J.; Harrison S. T.; Wolkenberg S. E.; Barrow J. C.; Williams D. L. Jr.; Hargreaves R. J.; Sur C.; Cook J. J. [18F]Fluoroazabenzoxazoles as Potential Amyloid Plaque PET Tracers: Synthesis and In Vivo Evaluation in Rhesus Monkey. Nucl. Med. Biol., in press. [DOI] [PubMed]
- Thal D. R.; Griffin W. S. T.; Braak H. Parenchymal and vascular Aβ-deposition and its effects on the degeneration of neurons and cognition in Alzheimer’s disease. J. Cell. Mol. Med. 2008, 12, 1848–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.