Abstract

Kisspeptin is a member of the RFamide neuropeptide family that is implicated in gonadotropin secretion. Because kisspeptin-GPR54 signaling is implicated in the neuroendocrine regulation of reproduction, GPR54 ligands represent promising therapeutic agents against endocrine secretion disorders. In the present study, the selectivity profiles of GPR54 agonist peptides were investigated for several GPCRs, including RFamide receptors. Kisspeptin-10 exhibited potent binding and activation of neuropeptide FF receptors (NPFFR1 and NPFFR2). In contrast, short peptide agonists bound with much lower affinity to NPFFRs while showing relatively high selectivity toward GPR54. The possible localization of secondary kisspeptin targets was also demonstrated by variation in the levels of GnRH release from the median eminence and the type of GPR54 agonists used. Negligible affinity of the reported NPFFR ligands to GPR54 was observed and indicates the unidirectional cross-reactivity between both ligands.
Keywords: Neuropeptide FF receptors; kisspeptin; GPR54; NPFFR1, NPFFR2
Gonadotropin secretion is regulated by several upstream neuropeptides and peptide hormones at the hypothalamo−pituitary−gonadal (HPG) axis. Kisspeptins, members of the RFamide neuropeptides, are peptides that regulate hormonal levels in peripheral circulation,1 whereas in earlier studies, they were identified as antimetastatic peptides (named metastins).2 Stimulation of the receptor GPR54 by kisspeptins promotes the release of the gonadotropin-releasing hormone (GnRH) from GnRH neurons,3 which is required for increasing the plasma level of gonadotropins4 and the onset of puberty.5 It has also been disclosed that a GPR54 mutation led to idiopathic hypogonadotropic hypogonadism (IHH).6−8 Thus, the kisspeptin−GPR54 system represents a potential therapeutic target for disorders associated with gonadotropin secretion.
A number of peptide or nonpeptide GPR54 ligands have been reported. Kisspeptin-10 (Kp-10) is the minimal sequence for GPR54 activation, which corresponds to the C-terminal 10 residues of the full-length kisspeptin (Kp-54). Intraventricular or peripheral administration of Kp-10 into rats induces an increase in the plasma level of the luteinizing hormone (LH).9,10 The 3-(indazol-3-yl)maleimide derivative also stimulates the GPR54 receptor.11 In contrast, Roseweir et al. reported the peptide antagonists for GPR54, in which Leu8 in Kp-10 was substituted with d-Trp.12 More recently, small molecule GPR54 antagonists with a 2-acylamino-4,6-diphenylpyridine scaffold were reported.13 Intravenous administration of these antagonists to castrated male rats suppressed the plasma LH level.
We also previously identified two GPR54 agonist peptides through a down-sizing study of Kp-10 and the subsequent structure−activity study using a series of peptidomimetics (Figure 1).14,15 FTM080 1 is a pentapeptide agonist, which exhibits equipotent GPR54 activation to Kp-10. The 4-fluorobenzoyl group of 1 can serve as a functional surrogate of the N-terminal five residues of Kp-10. The analogue FTM145 2 was designed to offer stability under physiological conditions,16 in which the (E)-alkene dipeptide isostere at the Gly-Leu site prevents proteinase-mediated cleavage of the peptide bond17 with maintenance of potent GPR54 agonistic activity. Both peripheral administration of peptides 1 and 2 effectively induced ovulation of musk shrew (Suncus murinus) in vivo,18 suggesting that these short peptides could be useful modulating agents of hormonal levels in the circulatory system.
Figure 1.
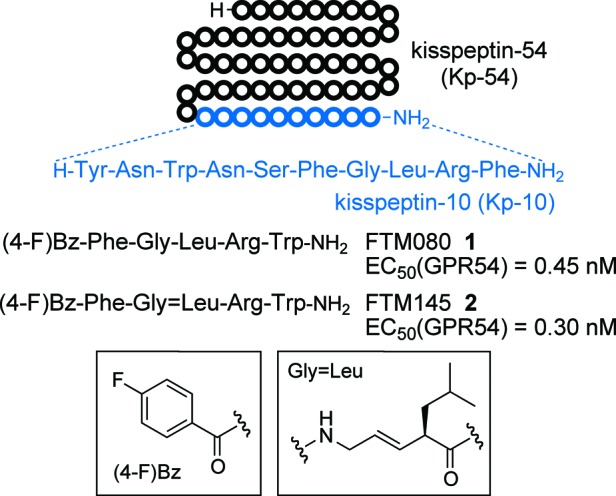
Sequences and bioactivity of GPR54 agonists. EC50 values represent the concentration required for 50% of the full agonistic activity induced by Kp-10 (1 μM).16
The aim of this study was to investigate the receptor selectivity of these peptides to avoid the potential off-target interactions, which cause adverse effects. Because the overall characteristics of the peptides resemble several other members of the neuropeptide family, these short peptides may be recognized by the other neuropeptide receptors. One recent example reported by Lyubimov et al. is the moderate activation of neuropeptide FF receptor 2 (NPFFR2/GPR74) by endogenous kisspeptin-13 (Kp-13) and kisspeptin-14 (Kp-14).19 In the report herein, the evaluation of the bioactivity of GPR54 agonist peptides against several G protein-coupled receptor (GPCRs) and the functional analysis are described.
Initially, to demonstrate the receptor selectivity of GPR54 ligands 1 and 2, the binding affinity to several GPCRs was evaluated using binding inhibition assays (Table 1). Because GPR54 shares significant homology with galanin receptors (GALRs),20 the inhibitory effects of galanin binding to three subtypes of the galanin receptors (GALR1, GALR2, and GALR3) were evaluated. As reported previously for kisspeptins, no inhibitory effect by peptides 1 and 2 to these receptors was observed at 10 μM. In contrast, GPR54 agonist peptides exhibited moderate to potent binding affinity toward three receptors of RFamide peptides. Binding of prolactin-releasing peptide to the receptor (PrRPR/GPR10) was inhibited by 10 μM peptides 1 and 2 by 21 and 16%, respectively. Significantly high inhibition was exerted by these peptides against neuropeptide SF binding to neuropeptide FF receptors (NPFFR1/GPR147 and NPFFR2/GPR74), suggesting that these peptides bind to NPFF receptors with high affinity.
Table 1. Inhibitory Effects of GPR54 Agonists on Radioligand Binding to Various Receptors.
| inhibitory rate (%)a |
||
|---|---|---|
| receptor | 1 | 2 |
| GALR1b | −e | −e |
| GALR2b | −e | −e |
| GALR3b | −e | −e |
| PrRPR/GPR10c | 21.1 | 16.1 |
| NPFFR1/GPR147d | 99.4 | 88.3 |
| NPFFR2/GPR74d | 90.7 | 95.7 |
Determined at a peptide concentration of 10 μM. The data are expressed as the mean value of duplicate analysis.
Inhibitory rate of [125I]Tyr9-galanin binding to the membrane preparation of GALR.
Inhibitory rate of [125I]-prolactin-releasing peptide-20 binding to the membrane preparation of PrRPR.
Inhibitory rate of (d-[125I]Tyr1, MePhe3)-NPFF binding to the membrane preparation of NPFFR.
No inhibition.
Dose responses of the binding affinity of GPR54 agonists with two NPFF receptors were assessed by the binding inhibition assay (Table 2). Peptides 1 and 2 showed approximately 80- and 330-fold less inhibitory activity against NPFFR1 as compared with the control neuropeptide SF (NPSF); a known endogenous ligand for NPFFR1 [IC50(1) = 0.16 μM and IC50(2) = 0.64 μM]. Of note, the full length Kp-54 and Kp-10 also bound to NPFFR1 with slightly less binding affinity than NPSF [IC50(Kp-54) = 15 nM and IC50(Kp-10) = 4.7 nM]. In the same experiment for NPFFR2, moderate binding of Kp-54 and Kp-10 to the receptor was exhibited [IC50(Kp-54) = 0.40 μM and IC50(Kp-10) = 76 nM], which is consistent with a recent report.19 The binding affinity of peptides 1 and 2 to NPFFR2 was less than a quarter of the Kp-54 binding affinity value [IC50(1) = 1.8 μM and IC50(2) = 2.4 μM]. The results demonstrated that GPR54-selectivity of peptides 1 and 2 was better than that of the original Kp-10.
Table 2. Inhibitory Effects of GPR54 Agonists on Radioligand Binding to Neuropeptide FF Receptors (NPFFRs).
| IC50 (M) |
|||
|---|---|---|---|
| peptide | GPR54a | NPFFR1b,c | NPFFR2b,c |
| Kp-54 | −d | 1.5 × 10−8 | 4.0 × 10−7 |
| Kp-10 | 1.2 × 10−10 | 4.7 × 10−9 (39) | 7.6 × 10−8 (630) |
| FTM080 1 | 7.1 × 10−10 | 1.6 × 10−7 (230) | 1.8 × 10−6 (2500) |
| FTM145 2 | 1.2 × 10−10 | 6.4 × 10−7 (530) | 2.4 × 10−6 (20000) |
| neuropeptide SF | >3.0 × 10−5 | 1.9 × 10−9 | 7.9 × 10−10 |
IC50 values indicate the concentration needed for 50% inhibition of receptor binding of [125I]kisspeptin-15 to GPR54.16
IC50 values indicate the concentration needed for 50% inhibition of receptor binding of (d-[125I]Tyr1, MePhe3)-NPFF to NPFFRs. The data were derived from the dose−response curves generated from triplicate data points.
Shown in parentheses are the selectivity indexes (SIs) calculated by SI = IC50(NPFFR)/IC50(GPR54).
Not tested.
GPR54 agonist-mediated receptor activation of NPFFRs was next evaluated by a Ca2+ flux assay using HEK293T cells expressing NPFFR along with Gqi5 (Figure 2a,b).21 Kisspeptins and peptides 1 and 2 induced NPFFR1- or NPFFR2-mediated intracellular calcium mobilization in a dose-dependent manner. Equipotent NPFFR1 activation by Kp-10 to the reference neuropeptide AF (NPAF) was observed (Kp-10: 86% activity at 0.24 μM), whereas peptides 1 and 2 exhibited slightly less potency (1, 49% activity; 2, 52% activity at 0.24 μM). However, these GPR54 agonists exerted less NPFFR2 stimulation than NPAF. Calcium mobilization responses by these peptides largely coincided with the binding affinity to NPFFR1 and NPFFR2, respectively.
Figure 2.
GPR54 agonist-mediated activation of NPFFRs. (a and b) Intracellular Ca2+ mobilization in HEK293T cells expressing NPFFR1 (a) or NPFFR2 (b). The intracellular Ca2+ response was calculated from the maximum fluorescence intensity after the addition of neuropeptide AF (NPAF; 6 μM for NPFFR1 and 1.2 μM for NPFFR2). (c and d) GPR54 agonist-mediated inhibition of forskolin-induced cAMP production in CHO cells expressing NPFFR1 (c) or NPFFR2 (d). The cAMP level was calculated from the maximum signal after the addition of NPSF (100 μM) using an AlphaScreen cAMP detection system. The data are expressed as the mean ± SD (N = 3).
Inhibitory effects of GPR54 agonists against forskolin-induced cAMP production were also examined using NPFFR-expressing CHO cells (Figure 2c,d). Because both native NPFFR1 and NPFFR2 couple to Gi/o,22,23 a decrease in cAMP levels indicates the receptor activation ability of the peptides. Kp-54 and Kp-10 reduced the cAMP level in NPFFR1-expressing CHO cells. The potency of Kp-10 was comparable to the reference NPSF. Conversely, a NPFF2-mediated decrease in cAMP levels by Kp-54 and Kp-10 peptides was considerably lower than observed with NPSF. Peptides 1 and 2 exhibited significantly low inhibitory activity against forskokin-induced cAMP production of both NPFFR1- and NPFFR2-expressing CHO cells.
Because GPR54 ligands exhibit cross-reactivity for NPFFRs, the bioactivity of NPFFR ligands for GPR54 was investigated as an opposite cross-reactivity (Figure 3 and the Supporting Information). Among the reported NPFFR ligands, NPFFR antagonis, RF9 3(24) and NPFFR agonist peptide 4(25) showed significantly low affinity with GPR54 [IC50(3) = 0.83 μM, IC50(4) = 16 μM, and IC50(Kp-10) = 78 pM], whereas both compounds did not induce intracellular calcium mobilization of GPR54-expressing CHO cells at 10 μM. Of note, although the agonistic activity of 3 for GPR54 was not observed in this study, the binding to GPR54 may possibly involve the stimulation of LH secretion.26 The other NPFFR ligands including NPSF and NPFF were not recognized by GPR54.11 In contrast, a variety of RFamide sequences have been reported to bind to NPFFRs,27 indicating that the molecular recognition by GPR54 seems to be highly stringent.
Figure 3.
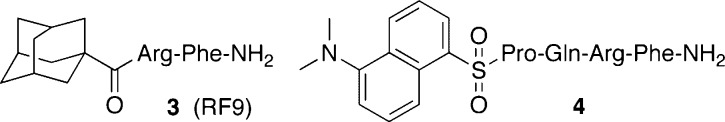
Structures of NPFF ligands with moderate binding affinity for GPR54.
Among the GPR54 agonists evaluated, peptide 2 had the best selectivity as a potent GPR54 agonist with less binding and receptor activation activities for other GPCRs, which were selected for bioevaluation, based on the receptor family or related physiological functions of RFamide ligands. The higher selectivity can be attributed to the truncation of the N terminus of endogenous kisspeptins and the substitution of the C-terminal phenylalanine with tryptophan. Alternatively, moderate to potent stimulation of NPFFRs by full-length Kp-54 and Kp-10 was observed. This is the first report on the binding of kisspeptins to NPFFR1. Of interest, Kp-10, which exhibited the most potent binding to NPFFRs among the GPR54 agonists examined, has a similar length to known NPFF receptor ligands, such as NPSF. The lower bioactivity of the full length Kp-54 and short peptides 1 and 2 may suggest that the potency of kisspeptins for NPFFRs varies during the degradation process in vivo. The recent report19 on NPFFR2 recognition by endogenous Kp-13 and Kp-14 may also support this process.
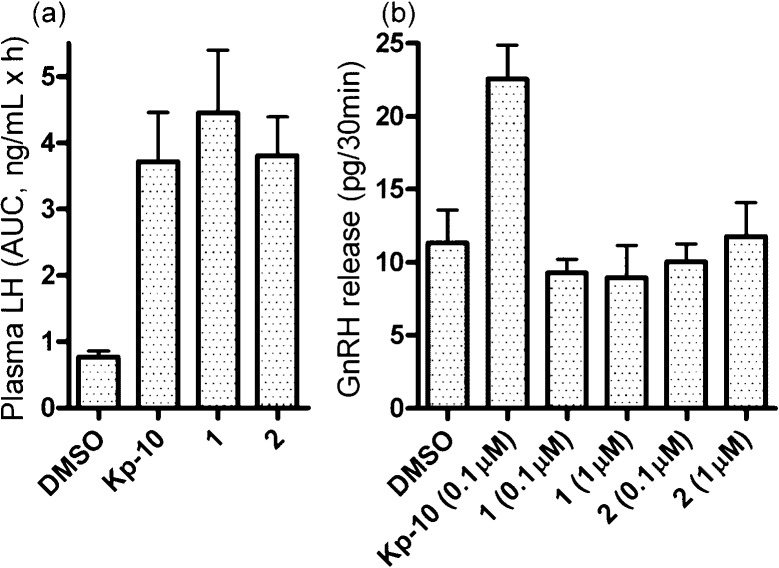
The preliminary investigation of the functional effects by peptides 1 and 2 was performed (Figure 4). Plasma LH levels were dramatically increased by in vivo administration of Kp-10, peptides 1 and 2 into the preoptic area of male rats, in which the majority of GnRH neuronal cell bodies are distributed (Figure 4a). These effects apparently coincided with the equipotent GPR54 activation of the peptides. On the other hand, in vitro experiments showed that Kp-10 (0.1 μM) stimulated GnRH release from rat median eminence, while a significantly lower release was observed by peptides 1 and 2, even at 1 μM (Figure 4b). These are likely to correspond to the potencies for NPFFR activation by these peptides. As such, the kisspeptin−NPFFR pairs, which were newly identified using a series of GPR54 agonists, may possibly represent an alternative system to activate GnRH neurons.
Figure 4.
Functional analysis of GPR54 agonists. (a) LH secretion following the administration of GPR54 agonists (0.25 nmol) into the preoptic area of male rats. The plasma LH level was determined as the area under curve (AUC) from blood samples collected every 6 min for the 3 h sampling period. The data are expressed as the mean ± SEM of five or more experiments. (b) GnRH release from the rat median eminence by treatment with GPR54 agonists in vitro. GnRH concentrations were determined by a double antibody radioimmunoassay with [125I]-labeled GnRH. The data are expressed as the mean ± SEM of triplicate or more experiments.
Kisspeptin-GPR54 and RFamide-related peptide-3 (RFRP-3)−NPFFR1 systems are well-known to regulate GnRH release in a positive and negative manner, respectively. RFRP-3, a mammalian orthologue of avian gonadotropin-inhibitory hormone (GnIH), decreases plasma LH levels in mammals in vivo.28 Our studies may suggest that kisspeptins apparently regulate two incompatible receptor signals when the receptor activation for GPR54 and NPFFRs is focused. However, it is envisaged that the LH level would be highly regulated by the expression level of these receptors or ligands, the projection pattern of neurons, and/or by some unknown mechanisms.29 Further investigation examining the kisspeptin receptor distributions should reveal the regulatory mechanism of the highly sophisticated LH secretion process as well as the molecular basis of incomplete hypogonadism in GPR54 knockout mice.30
In conclusion, the investigation of the selectivity profiles of GPR54 agonist peptides revealed unprecedented interactions between kisspeptins and NPFFRs. Among the GPR54 agonists examined, Kp-10 exhibited highly potent binding affinity and receptor activation. Five-residue peptide agonists 1 and 2 showed lower bioactivity toward NPFFRs yet equipotent bioactivity as Kp-10 toward GPR54. As compared with Kp-10, the lower GnRH release from the median eminence by peptides 1 and 2 with partial GPR54 selectivity supports the possible localization of a secondary kisspeptin receptor(s) such as NPFFRs. To further understand the regulatory systems of the HPG axis, selective GPR54 probe molecules would be beneficial. On the basis of the results using peptides 1 and 2, our ongoing efforts aim to develop potent GPR54 agonists with high selectivity.
Acknowledgments
We thank Takeda Pharmaceutical Co. Ltd. for the evaluation of biological activity for GPR54 of a series of compounds. We are grateful to the National Hormone and Peptide Program for the rat LH assay kit. The RIA was performed at the Nagoya University Radioisotope Center.
Abbreviations
GALR, galanin receptor; GnIH, gonadotropin-inhibitory hormone; GnRH, gonadotropin-releasing hormone; GPCR, G protein-coupled receptor; HPG axis, hypothalamo−pituitary−gonadal axis; IHH, idiopathic hypogonadotropic hypogonadism; Kp-10, kisspeptin-10; Kp-13, kisspeptin-13; Kp-14, kisspeptin-14; Kp-54, kisspeptin-54; LH, luteinizing hormone; NPAF, neuropeptide AF; NPFFR, neuropeptide FF receptor; NPSF, neuropeptide SF; PrRPR, prolactin-releasing peptide receptor; RFRP-3, RFamide-related peptide-3.
Supporting Information Available
Experimental procedures and characterization data. This material is available free of charge via the Internet at http://pubs.acs.org.
This work is supported by Grants-in-Aid for Scientific Research and Molecular Imaging Research Program from the Ministry of Education, Culture, Sports, Science, and Technology of Japan and by the Program for Promotion of Basic Research Activities for Innovative Biosciences (PROBRAIN) of Japan. K.T. and R.M. are grateful for Research Fellowships from the JSPS for Young Scientists.
Supplementary Material
References
- For a recent review on kisspeptins, see Oakley A. E.; Clifton D. K.; Steiner R. A. Kisspeptin Signaling in the Brain. Endocr. Rev. 2009, 30, 713–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtaki T.; Shintani Y.; Honda S.; Matsumoto H.; Hori A.; Kanehashi K.; Terao Y.; Kumano S.; Takatsu Y.; Masuda Y.; Ishibashi Y.; Watanabe T.; Asada M.; Yamada T.; Suenaga M.; Kitada C.; Usuki S.; Kurokawa T.; Onda H.; Nishimura O.; Fujino M. Metastasis Suppressor Gene KiSS-1 Encodes Peptide Ligand of a G-protein-coupled Receptor. Nature 2001, 411, 613–617. [DOI] [PubMed] [Google Scholar]
- Dhillo W. S.; Chaudhri O. B.; Patterson M.; Thompson E. L.; Murphy K. G.; Badman M. K.; McGowan B. M.; Amber V.; Patel S.; Ghatei M. A.; Bloom S. R. Kisspeptin-54 Stimulates the Hypothalamic-pituitary Gonadal Axis in Human Males. J. Clin. Endocrinol. Metab. 2005, 90, 6609–6615. [DOI] [PubMed] [Google Scholar]
- Matsui H.; Takatsu Y.; Kumano S.; Matsumoto H.; Ohtaki T. Peripheral Administration of Metastin Induces Marked Gonadotropin Release And Ovulation in the Rat. Biochem. Biophys. Res. Commun. 2004, 320, 383–388. [DOI] [PubMed] [Google Scholar]
- Shahab M.; Mastronardi C.; Seminara S. B.; Crowley W. F.; Ojeda S. R.; Plant T. M. Increased Hypothalamic GPR54 Signaling: A Potential Mechanism for Initiation of Puberty in Primates. Proc. Natl. Acad. Sci. U.S.A. 2005, 102, 2129–2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seminara S. B.; Messager S.; Chatzidaki E. E.; Thresher R. R.; Acierno J. S. Jr.; Shagoury J. K.; Bo-Abbas Y.; Kuohung W.; Schwinof K. M.; Hendrick A. G.; Zahn D.; Dixon J.; Kaiser U. B.; Slaugenhaupt S. A.; Gusella J. F.; O'Rahilly S.; Carlton M. B. L.; Crowley W. F. Jr.; Aparicio S. A. J. R.; Colledge W. H. The GPR54 Gene as a Regulator of Puberty. N. Engl. J. Med. 2003, 349, 1614–1627. [DOI] [PubMed] [Google Scholar]
- De Roux N.; Genin E.; Carel J.-C.; Matsuda F.; Chaussain J.-L.; Milgrom E. Hypogonadotropic Hypogonadism Due to Loss of Function of the KiSS1-derived Peptide Receptor GPR54. Proc. Natl. Acad. Sci. U.S.A. 2003, 100, 10972–10976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funes S.; Hedrick J. A.; Vassileva G.; Markowitz L.; Abbondanzo S.; Golovko A.; Yang S.; Monsma F. J.; Gustafson E. L. The KiSS-1 Receptor GPR54 Is Essential for the Development of the Murine Reproductive System. Biochem. Biophys. Res. Commun. 2003, 312, 1357–1363. [DOI] [PubMed] [Google Scholar]
- Gutiérrez-Pascual E.; Leprince J.; Martínez-Fuentes A. J.; Ségalas-Milazzo I.; Pineda R.; Roa J.; Duran-Prado M.; Guilhaudis L.; Desperrois E.; Lebreton A.; Pinilla L.; Tonon M. C.; Malagón M. M.; Vaudry H.; Tena-Sempere M.; Castaño J. P. In Vivo And In Vitro Structure-activity Relationships And Structural Conformation of Kisspeptin-10-related Peptides. Mol. Pharmacol. 2009, 76, 58–67. [DOI] [PubMed] [Google Scholar]
- Pheng V.; Uenoyama Y.; Homma T.; Inamoto Y.; Takase K.; Yoshizawa-Kumagaye K.; Isaka S.; Watanabe T. X.; Ohkura S.; Tomikawa J.; Maeda K.; Tsukamura H. Potencies of Centrally- or Peripherally-injected Full-length Kisspeptin Or Its C-terminal Decapeptide on LH Release in Intact Male Rats. J. Reprod. Dev. 2009, 55, 378–382. [DOI] [PubMed] [Google Scholar]
- Orsini M. J.; Klein M. A.; Beavers M. P.; Connolly P. J.; Middleton S. A.; Mayo K. H. Metastin (KiSS-1) Mimetics Identified from Peptide Structure−Activity Relationship-derived Pharmacophores And Directed Small Molecule Database Screening. J. Med. Chem. 2007, 50, 462–471. [DOI] [PubMed] [Google Scholar]
- Roseweir A. K.; Kauffman A. S.; Smith J. T.; Guerriero K. A.; Morgan K.; Pielecka-Fortuna J.; Pineda R.; Gottsch M. L.; Tena-Sempere M.; Moenter S. M.; Terasawa E.; Clarke I. J.; Steiner R. A.; Millar R. P. Discovery of Potent Kisspeptin Antagonists Delineate Physiological Mechanisms of Gonadotropin Regulation. J. Neurosci. 2009, 29, 3920–3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T.; Sasaki S.; Tomita N.; Fukui S.; Nakayama M.; Kiba A.; Kusaka M.; Matsumoto S. I.; Yamaguchi M.; Itoh F.; Baba A. 2-Acylamino-4,6-diphenylpyridine Derivatives as Novel GPR54 Antagonists with Good Brain Exposure And In Vivo Efficacy for Plasma LH Level in Male Rats. Bioorg. Med. Chem. 2010, 18, 5157–5171 and the references therein.. [DOI] [PubMed] [Google Scholar]
- Niida A.; Wang Z.; Tomita K.; Oishi S.; Tamamura H.; Otaka A.; Navenot J.-M.; Broach J. R.; Peiper S. C.; Fujii N. Design Aand Synthesis of Downsized Metastin(45−54) Analogs with Maintenance of High GPR54 Agonistic Activity. Bioorg. Med. Chem. Lett. 2006, 16, 134–137. [DOI] [PubMed] [Google Scholar]
- Tomita K.; Oishi S.; Cluzeau J.; Ohno H.; Navenoto J.-M.; Wang Z.; Peiper S. C.; Akamatsu M.; Fujii N. SAR And QSAR Studies on the N-Terminally Acylated Pentapeptide Agonists for GPR54. J. Med. Chem. 2007, 50, 3222–3228. [DOI] [PubMed] [Google Scholar]
- Tomita K.; Oishi S.; Ohno H.; Peiper S. C.; Fujii N. Development of Novel G-protein-coupled Receptor 54 Agonists with Resistance to Degradation by Matrix Metalloproteinase. J. Med. Chem. 2008, 51, 7645–7649. [DOI] [PubMed] [Google Scholar]
- Takino T.; Koshikawa N.; Miyamori H.; Tanaka M.; Takuma S.; Okada Y.; Seiki M.; Sato H. Cleavage of Metastasis Suppressor Gene Product KiSS-1 Protein/metastin by Matrix Metalloproteinases. Oncogene 2003, 22, 4617–4626. [DOI] [PubMed] [Google Scholar]
- Inoue N.; Tomikawa J.; Sasaki Y.; Uchida A.; Oishi S.; Fujii N.; Uenoyama Y.; Yamamoto N.; Maeda K. I.; Tsukamura H.. Kiss1 cDNA Cloning And Induction of Ovulation by Peripheral Administration of Various Kisspeptins in the Musk Shrew (Suncus murinus), a Reflex Ovulator. Presented at the 43rd annual meeting of the society for the study of reproduction, Milwaukee. WI, July 30−August 3, 2010; Abstract; p 128.
- Lyubimov Y.; Engstrom M.; Wurster S.; Savola J. M.; Korpi E. R.; Panula P. Human Kisspeptins Activate Neuropeptide FF2 Receptor. Neuroscience 2010, 170, 117–122. [DOI] [PubMed] [Google Scholar]
- Lee D. K.; Nguyen T.; O'Neill G. P.; Cheng R.; Liu Y.; Howard A. D.; Coulombe N.; Tan C. P.; Tang-Nguyen A.-T.; George S. R.; O'Dowd B. F. Discovery of a Receptor Related to the Galanin Receptors. FEBS Lett. 1999, 446, 103–107. [DOI] [PubMed] [Google Scholar]
- Hirasawa A.; Tsumaya K.; Awaji T.; Katsuma S.; Adachi T.; Yamada M.; Sugimoto Y.; Miyazaki S.; Tsujimoto G. Free Fatty Acids Regulate Gut Incretin Glucagon-like Peptide-1 Secretion through GPR120. Nat. Med. 2005, 11, 90–94. [DOI] [PubMed] [Google Scholar]
- Hinuma S.; Shintani Y.; Fukusumi S.; Iijima N.; Matsumoto Y.; Hosoya M.; Fujii R.; Watanabe T.; Kikuchi K.; Terao Y.; Yano T.; Yamamoto T.; Kawamata Y.; Habata Y.; Asada M.; Kitada C.; Kurokawa T.; Onda H.; Nishimura O.; Tanaka M.; Ibata Y.; Fujino M. New Neuropeptides Containing Carboxy-terminal RFamide and Their Receptor in Mammals. Nat. Cell Biol. 2000, 2, 703–708. [DOI] [PubMed] [Google Scholar]
- Elshourbagy N. A.; Ames R. S.; Fitzgerald L. R.; Foley J. J.; Chambers J. K.; Szekeres P. G.; Evans N. A.; Schmidt D. B.; Buckley P. T.; Dytko G. M.; Murdock P. R.; Milligan G.; Groarke D. A.; Tan K. B.; Shabon U.; Nuthulaganti P.; Wang D. Y.; Wilson S.; Bergsma D. J.; Sarau H. M. Receptor for the Pain Modulatory Neuropeptides FF And AF Is an Orphan G Protein-coupled Receptor. J. Biol. Chem. 2000, 275, 25965–25971. [DOI] [PubMed] [Google Scholar]
- Simonin F.; Schmitt M.; Laulin J. P.; Laboureyras E.; Jhamandas J. H.; MacTavish D.; Matifas A.; Mollereau C.; Laurent P.; Parmentier M.; Kieffer B. L.; Bourguignon J. J.; Simonnet G. RF9, a Potent And Selective Neuropeptide FF Receptor Antagonist, Prevents Opioid-induced Tolerance Associated with Hyperalgesia. Proc. Natl. Acad. Sci. U.S.A. 2006, 103, 466–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payza K.; Akar C. A.; Yang H. Y. Neuropeptide FF Receptors: Structure-activity Relationship And Effect of Morphine. J. Pharmacol. Exp. Ther. 1993, 267, 88–94. [PubMed] [Google Scholar]
- Recently, RF9-mediated LH secretion was reported. Moderate bioactivity for GPR54 may also be involved in the physiological effects: Pineda R.; Garcia-Galiano D.; Sanchez-Garrido M. A.; Romero M.; Ruiz-Pino F.; Aguilar E.; Dijcks F. A.; Blomenröhr M.; Pinilla L.; van Noort P. I.; Tena-Sempere M. Characterization of the Potent Gonadotropin-releasing Activity of RF9, a Selective Antagonist of RFamide-related Peptides and Neuropeptide FF Receptors: Physiological and Pharmacological Implications. Endocrinology 2010, 151, 1902–1913. [DOI] [PubMed] [Google Scholar]
- Mollereau C.; Mazarguil H.; Marcus D.; Quelven I.; Kotani M.; Lannoy V.; Dumont Y.; Quirion R.; Detheux M.; Parmentier M.; Zajac J. M. Pharmacological Characterization of Human NPFF1 and NPFF2 Receptors Expressed in CHO Cells by Using NPY Y1 Receptor Antagonists. Eur. J. Pharmacol. 2002, 451, 245–256. [DOI] [PubMed] [Google Scholar]
- Kriegsfeld L. J.; Gibson E. M.; Williams W. P. III; Zhao S.; Mason A. O.; Bentley G. E.; Tsutsui K. The Roles of RFamide-related Peptide-3 in Mammalian Reproductive Function and Behaviour. J. Neuroendocrinol. 2010, 22, 692–700 and the references therein.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quennell J. H.; Rizwan M. Z.; Relf H. L.; Anderson G. M. Developmental and Steroidogenic Effects on the Gene Expression of RFamide Related Peptides and Their Receptor in the Rat Brain and Pituitary Gland. J. Neuroendocrinol. 2010, 22, 309–316. [DOI] [PubMed] [Google Scholar]
- Chan Y. M.; Broder-Fingert S.; Wong K. M.; Seminara S. B. Kisspeptin/Gpr54-independent Gonadotrophin-releasing Hormone Activity in Kiss1 and Gpr54 Mutant Mice. J. Neuroendocrinol. 2009, 21, 1015–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.