Abstract
The occurrence of eight carcinogenic N-nitrosamines in biosolids from 74 wastewater treatment plants (WWTPs) in the contiguous United States was investigated. Using liquid chromatography-tandem mass spectrometry, seven nitrosamines [(N-nitrosodimethylamine (NDMA), N-nitrosomethylethylamine, N-nitrosodi-n-propylamine (NDPA), N-nitrosodibutylamine, N-nitrosopyrrolidine, N-nitrosopiperidine (NPIP), and N-nitrosodiphenylamine (NDPhA)] were detected with varying detection frequency (DF) in 88% of the biosolids samples (n = 80), with five of the seven being reported here for the first time in biosolids. While rarely detected (DF 3%), NDMA was the most abundant compound at an average concentration of 504 ± 417 ng/g dry weight of biosolids. The most frequently detected nitrosamine was NDPhA (0.7—147 ng/g) with a DF of 79%, followed by NDPA (7–505 ng/g) and NPIP (51–1185 ng/g) at 21% and 11%, respectively. The DF of nitrosamines in biosolids was positively correlated with their respective n-octanol–water partition coefficients (R2 = 0.65). The DF and sum of mean concentrations of nitrosamines in biosolids increased with the treatment capacity of WWTPs. Given their frequent occurrence in nationally representative samples and the amount of U.S. biosolids being applied on land as soil amendment, this study warrants more research into the occurrence and fate of nitrosamines in biosolids-amended soils in the context of crop and drinking water safety.
Introduction
Nitrosamines are contaminants of emerging concern frequently detected in U.S. water resources.1,2 These contaminants are known to originate as disinfectant byproducts formed during the chlorination or chloramination of drinking water and wastewater.3−5 Additionally, low yields of nitrosamines (∼0.02%) have also been shown to occur from ozonation and sunlight/UV photolysis of drinking waters containing secondary amine precursors.6,7 Though efforts have been taken to curtail the industrial applications of nitrosamines, they are formed unintentionally from various industrial processes, such as during rubber manufacturing and processing, leather tanning, metal casing, and food processing.8,9 Residential sources have also been shown to contribute to the nitrosamine load in wastewater.8,10 Currently, five nitrosamines are included in the Contaminant Candidate List 3 (CCL 3) by the U.S. Environmental Protection Agency (U.S. EPA): N-nitrosodimethylamine (NDMA), N-nitrosodiethylamine (NDEA), N-nitroso-di-n-propylamine (NDPA), N-nitrosodiphenylamine (NDPhA), and N-nitrosopyrrolidine (NPYR).11 Many nitrosamines are classified as either Group 2A (probable human carcinogen: NDMA, NDEA) or Group 2B [possibly carcinogenic to humans: N-nitrosomethylethylamine (NMEA), NDPA, N-nitrosodibutylamine (NDBA), N-nitrosomorpholine (NMOR), N-nitrosopiperidine (NPIP), NPYR, etc.] carcinogens by the International Agency for Research on Cancer (IARC).12 Much of the research conducted to date has focused on NDMA for which the U.S. EPA has established a cleanup level of 0.7 ng/L in groundwater based on a 1 in 10–6 lifetime excess cancer risk from drinking water consumption.13
Other than industrial and residential sources, nitrosamines can be additionally formed during wastewater treatment; for example, use of secondary/tertiary-amine based cationic polymers in sludge systems is known to contribute to the concentration of nitrosamine precursors14 that may undergo N-nitrosation reactions in the presence of nitrite to form nitrosamines.15 Aliphatic and alicyclic nitrosamines (NDMA, NDEA, NMOR, NPYR, NPIP, NDPA, and NDBA) with concentrations ranging from less than limit of quantification to up to 1,057 ng/L have been detected in wastewater influent.8,10,16,17 The aqueous phase removal efficiency of nitrosamines in activated sludge treatment systems was shown to be greater than 60% (except for NMOR ∼40%) and lower when the primary effluent concentrations were below 8–15 ng/L.8,10 What is more, removal efficiencies can vary significantly between wastewater treatment plants (WWTPs) (0–93%) as well as within the same plant over time (0–75%).8,10 These variations are suspected to be due to substrate competition in the cometabolic microbial degradation of nitrosamines during secondary treatment. Overall, the presence of nitrosamines in WWTP effluent is of increasing concern due to potential contamination of drinking water resources of human communities living downstream and downgradient of WWTP-effluent discharge locations. One study has demonstrated the persistence of NDMA and its precursors in surface waters impacted by wastewater, sufficiently long enough to affect the drinking water sources of communities downstream.18
Since many of the nitrosamines of interest have low partitioning coefficients and sorption to sewage sludge is believed to be negligible, very few studies have investigated the occurrence of nitrosamines in sewage sludge and biosolids (treated or processed sewage sludge). To date, the literature shows only three studies on the occurrence of select nitrosamines in U.S. sewage sludges or biosolids. Two studies reported infrequent detection of NDMA [at 0.5 to 318 ng/g dry weight (dw)], NDEA (at 1.7 to 5,520 ng/g dw), and NMOR (at 1.3 to 2.9 ng/g dw) in biosolids samples collected in 1978 and 1979.19,20 It was estimated that about 60 to 1,365 μg of nitrosamines (NDMA and NDEA) may be incorporated into 1 m2 of soil annually, as a result of land application of biosolids in the studied area.19 A more recent, third study from 2008, examining the occurrence of nitrosamines in the aqueous filtrate of untreated sewage sludge samples, reported NDMA at mean concentration of 271 to 678 ng/L and NPYR at 57 ng/L in one sludge sample among three WWTPs surveyed.15 The number of toxic chemicals detected in biosolids is steadily increasing.21−25 Monitoring of biosolids is critical in the U.S., since about 50% of the total volume produced is disposed of by application on land.26 Presence of nitrosamines in biosolids is an added concern and increases the risk of human exposure to carcinogens. The primary goal of this study was to determine the nationwide occurrence of eight carcinogenic nitrosamines in biosolids by analyzing nationally representative samples collected by the U.S. EPA during the Targeted National Sewage Sludge Survey (TNSSS), conducted in 2006/2007. The eight nitrosamines include the five from U.S. EPA’s CCL 3 list (NDMA, NDEA NDPA, NPYR, NDPhA) and additionally NMEA, NDBA, and NPIP, all being classified as possibly carcinogenic to humans (Group 2B) and all having been detected in wastewaters in past studies.8,16,17
Materials and Methods
Chemicals
Analytical standards of nitrosamines [NDMA, N-nitrosomethylethylamine (NMEA), NDEA, NDPA, N-nitrosodibutylamine (NDBA), NPYR, N-nitrosopiperidine (NPIP), N-nitrosodiphenylamine (NDPhA)], dichloromethane (DCM) (HPLC grade), acetonitrile (LC-MS grade), water (HPLC grade), ammonium acetate, and acetic acid were purchased from Sigma-Aldrich (St. Louis, MO). The deuterated isotopes NDMA-d6, NDPA-d14, and NDPhA-d6 were purchased from Cambridge Isotope Laboratories (Andover, MA). The deuterated isotope NPIP-d10 was purchased from C/D/N Isotopes Inc. (Quebec, Canada).
Biosolids Samples
Biosolids samples were collected from 74 publicly owned WWTPs that participated in the U.S. EPA’s TNSSS. The facilities selected in the survey are statistically representative of the target population consisting of WWTPs that (i) were operational during 2002 and/or 2004; (ii) had a flow rate of greater than 3.8 million liters per day (MLD) [or 1 million gallons per day (MGD)]; (iii) employed a minimum of secondary treatment; and (iv) were located in the contiguous United States. From the 3,337 WWTPs that met the above criteria, the U.S. EPA statistically selected 74 facilities using a random sampling design stratified for flow [3.8 to 38 MLD (1 to 10 MGD), 38 to 380 MLD (10 to 100 MGD), and >380 MLD (>100 MGD)] from 35 U.S. states. The combined flows of WWTPs with flow rates less than 3.8 MLD accounted for only <6% of the total flow of all WWTPs nationwide,24 and hence, their elimination from the survey is expected to introduce only minimal error to the nationwide representativeness of the samples. Detailed information on selection criteria and sampling campaign design for the survey is provided elsewhere.24
Grab samples of biosolids were collected by U.S. EPA from each facility between August 2006 and March 2007.24 Four of the facilities had two treatment systems for solids; hence, a second sample was collected to represent both treatment systems. The solids content of the biosolids samples ranged between 4% and 93%. Additionally, a duplicate grab sample was collected from six other facilities to allow for variations associated with the sampling procedure. The objective of TNSSS was to report the occurrence of selected contaminants of emerging concern (pharmaceuticals and personal care products, brominated flame retardants) as identified by U.S. EPA and the National Research Council (NRC), in biosolids. After completion of TNSSS, the samples were acquired by our laboratory and stored in amber glass jars (500 mL) at −20 °C for further analysis. From the 84 biosolids samples, four were excluded from analysis because the sample containers were either missing or broken. Additional information on the selected facilities is provided as Supporting Information (Table S1).
Nitrosamine Analysis
All glassware used in the experiments was baked at 550 °C; caps were acid washed using 10% HCl and thoroughly rinsed with ultrapure water prior to use to prevent contamination. About 6 g wet weight (ww) of biosolids was weighed in a precleaned amber glass (40 mL) vial and spiked with 250 ng each of the deuterated surrogates (NDMA-d6, NDPA-d14, NPIP-d10, and NDPhA-d6) to correct for analyte recovery and matrix interferences at the mass spectrometry (MS) interface. Nitrosamines were extracted from biosolids by adding 2 mL of DCM per g of biosolids and by placing the capped extraction vial horizontally on a rotary shaker for 2 h at 200 rpm followed by 1 h of sonication extraction at a frequency of 40 kHz. The sample was decanted, and the DCM extract was concentrated to near dryness under a gentle flow of nitrogen gas. Following addition of 2 mL of acetonitrile, each reconstituted sample was filtered using a 0.2 μm polytetrafluoroethylene syringe filter (VWR International, LLC, PA), diluted to 50% (v/v) water content, and analyzed using liquid chromatography positive electrospray ionization tandem mass spectrometry (LC-ESI-MS/MS).
Mass spectrometric analyses were carried out on an API 4000 instrument (Applied Biosystems, Framingham, MA, USA), coupled to a Shimadzu Prominence HPLC (Shimadzu Scientific Instruments, Inc., Columbia, MD, USA) and controlled by Analyst 1.5 software (Applied Biosystems, Framingham, MA, USA). Separation was carried out using XBridge BEH C8 Column, (130 Å, 3.5 μm, 4.6 × 150 mm; Waters, Milford, USA). The mobile phase consisted of solvent A (10 mM ammonium acetate and 0.01% acetic acid in water) and solvent B (100% acetonitrile) flowing at a rate of 400 μL/min with a total runtime of 15 min. The solvent gradient program consisted of 50% of solvent B for 1 min, followed by an increase from 50% to 90% over 10 min, holding at 90% for 3 min, and returning back to 50% of solvent B over 0.1 min, followed by a 2 min equilibration period prior to injection of the next sample aliquot (100 μL volume). Analytes were introduced into the mass spectrometer using an electrospray ionization probe in positive mode. Multiple reaction monitoring (MRM) was used for qualitative analysis. Optimized conditions for the ionization and fragmentation of the analytes are specified in Table S2, Supporting Information. Wet weight concentrations obtained from the analysis were converted to dw concentrations using the solid content of the analyzed biosolids. All concentrations are reported as ng/g dw. Single MRM transitions were used for both qualitative and quantitative analysis of NDMA, NMEA, NDEA, and NPYR, whereas two different transitions were used for NDPA, NDBA, NPIP, and NDPhA for identification and quantification (Figure 1; Table S2, Supporting Information). Multiple transitions were not used for the former group of analytes because a high background from interferences impaired the reproducibility of secondary transitions.
Figure 1.
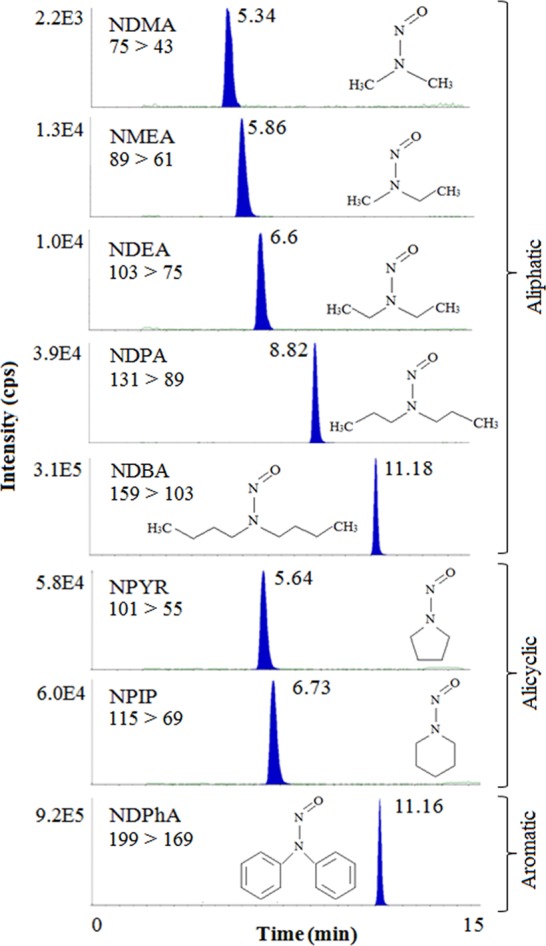
Structures, MRM transitions [parent ion m/z > product ion m/z], and LC-MS/MS chromatograms (100 μg/L concentration) of eight N-nitrosamines screened in the present study. Numbers next to the peak represent the retention times of the analytes in units of minutes.
Quality Assurance
Calibration accuracy was verified for each batch using a calibration standard solution with labeled and native analytes. Retention times of native and labeled compounds in the sample had to be within ±12 s (0.2 min) of the respective retention time established during the previous calibration. Multiple lab blanks were analyzed for each batch to check for laboratory contamination. A duplicate sample was analyzed for every five samples in a batch to evaluate analysis precision. Precision between samples and duplicates was expressed as relative percentage difference (RPD), which was calculated using the following expression:
| 1 |
where Csample and Cduplicate are the concentrations detected in the original sample and in its duplicate, respectively. Matrix spikes were performed for selected samples to confirm analyte presence in the sample and to evaluate recovery rates for analytes without deuterated labeled analogues.
Results and Discussion
Method Performance
Method detection limits (MDLs) for the various nitrosamines ranged from 0.06 to 5.7 ng/g dw (Table 1). In general, detection limits of nitrosamines in the LC-MS/MS system improved with increasing mass, the lowest observed for NDPhA (m/z 199) and the highest observed for NDMA (m/z 75). Process control samples and blanks showed no laboratory contamination. Analyte detection was further confirmed by performing matrix spike experiments in selected samples showing positive detects of nitrosamines (Figure S1, Supporting Information). Two water-immiscible solvents were tested for extraction efficiency: hexane (with poor recoveries of <20% for lower molecular weight nitrosamines) and DCM (with recoveries >32%); the latter was selected, as it yielded better recoveries (Table 1). The recoveries were improved by up to 20% by sonicating the biosolids/DCM suspension for 60 min after mixing on a rotary shaker.
Table 1. Method Performance and Concentrations of N-Nitrosamines in U.S. Biosolids.
| recovery (%)b |
biosolids
concentration (ng/g) avg. (min, max)c |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| compound | CAS# | molecular weight (g/mol) | absolute | relative | method detection limit (ng/g dw) | wet weight | dry weight | RPDd (%) | detection frequency (%) |
| NDMA | 62-75-9 | 74 | 32 ± 12 | 110 ± 11 | 5.7 | 150 (54, 248) | 504 (87, 920) | 34 | 3 |
| NMEAa | 10595-95-6 | 88 | 32 ± 7 | 1.1 | 11.4 (5.5, 14) | 121 (20, 393) | 12 | 5 | |
| NDPA | 621-64-7 | 130 | 41 ± 12 | 100 ± 5 | 0.6 | 44 (2.4, 314) | 134 (7, 505) | 46 ± 20 | 21 |
| NDBAa | 924-16-3 | 158 | 38 ± 14 | 0.1 | 0.7 (0.2, 1.9) | 1.8 (0.2, 3.3) | 21 ± 23 | 9 | |
| NPYRa | 930-55-2 | 100 | 51 ± 8 | 2.3 | 3.8 | 7.6 | 15 | 1 | |
| NPIP | 100-75-4 | 114 | 52 ± 7 | 100 ± 4 | 1.1 | 78 (12, 224) | 332 (51, 1185) | 15 | 11 |
| NDPhA | 86-30-6 | 198 | 68 ± 18 | 90 ± 10 | 0.06 | 4.5 (0.1, 91) | 10 (0.7, 147) | 23 ± 29 | 79 |
Concentrations of analytes lacking stable-isotope labeled analogues are not recovery corrected.
Relative recoveries were determined using area ratios of analyte to (stable-isotope labeled) surrogate standard. Absolute recoveries were obtained using absolute areas instead of area ratios. Absolute recoveries were determined from matrix spike studies.
Dry weight concentrations were calculated from wet weight concentrations using the solids content of the biosolids samples.
RPD: relative percentage difference.
Relative recoveries for nitrosamines computed with labeled isotopes were estimated by spiking native analytes and their respective isotopes (250 ng) in biosolids prior to extraction and subsequently analyzed. The obtained concentration ratios of analyte-to-surrogate were used to calculate relative recoveries. Relative recoveries ranged between 90% and 126% for NDMA, NDPA, NPIP, and NDPhA. Absolute recoveries were obtained using absolute concentrations instead of concentration ratios. The concentration obtained in spiked biosolids (250 ng prior extraction) minus the concentration obtained in the respective nonspiked samples were compared to the concentration obtained from an external standard with the same concentration as the spike. Mean absolute recoveries ranged from 32 ± 7% to 68 ± 18%, with recovery generally increasing with molecular weight of the target analyte (Table 1). However, NDBA showed lower recoveries from matrix spike tests (Table 1) compared to the lower molecular weight compounds NDPA and NPYR. Low absolute recoveries observed for nitrosamines could be due to the strong matrix interference from biosolids samples causing signal suppression. The observed anomaly in NDBA recoveries from matrix spike tests may be due to matrix-borne interferences in the selected biosolids samples. A similar loss in absolute recoveries has been observed in previous studies of nitrosamines due to matrix interference in wastewater samples.8,27 Higher recoveries in general are achieved in less complex matrices (e.g., drinking water) by employing solid-phase extraction (SPE) techniques;28,29 however, this technique is not applicable for concentrating analytes directly from the complex biosolids matrix. The reported concentrations of NMEA, NPYR, and NDBA in biosolids should be considered to represent conservative estimates with respect to both detection frequencies and concentrations because the concentrations were determined without labeled isotopes and because only moderate absolute recovery rates were obtained. Analysis precision expressed as RPD was good at less than 23% (average) for five nitrosamines, slightly less favorable for NDMA (34%), and marginal for NDPA (46%). The nonhomogeneity of biosolids samples (liquid/solid mixtures) was known and expected to impact analysis precision. High RPDs (average of 42%) have been observed previously for organics in biosolids samples from the U.S. EPA’s TNSSS associated with the complexity of biosolids matrix.24 Out of the six facilities with field duplicate samples available, NDPA, NDBA, and NPIP were detected in one facility each, and NDPhA was detected in three facilities. The RPDs between samples and field duplicates for most of the facilities were less than 25%, except for one, where NDPhA had an RPD of 41% (Figure S2, Supporting Information). However, this RPD is well within the default RPD limit of 50% for field duplicates that the U.S. EPA uses, a cutoff metric reflecting the sum of analytical variability and the variability in the sample collection process.24
Occurrence of Nitrosamines in U.S. Biosolids
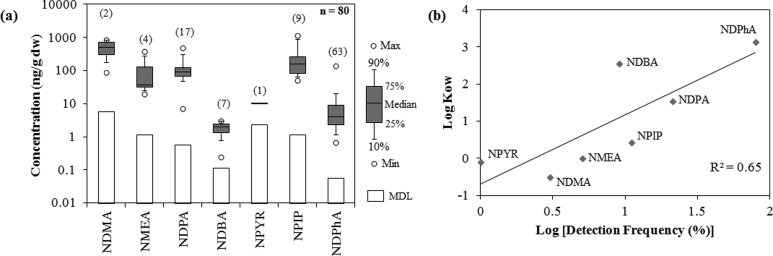
Out of the 80 biosolids samples analyzed, 70 samples (88%) tested positive for at least one nitrosamine. Seven nitrosamines were detected with varying detection frequency in the analyzed samples, of which five of them (NMEA, NDPA, NDBA, NPIP, and NDPhA) are reported here for the first time in biosolids (Figure 2a; Table 1). The most abundant nitrosamine was NDMA, detected at an average concentration of 504 (range: 87–920) ng/g dw, followed by NPIP and NDPA at 332 (range: 51–1,185) ng/g dw and 134 (range: 7–505) ng/g dw, respectively. This is in accordance with previous studies, where NDMA was shown to be the most abundant nitrosamine in wastewater and untreated sewage sludge filtrate samples.8,15 The concentration range for NDMA [54–248 ng/g wet weight (ww)] in the present study is similar to what was reported for samples collected in 1978 (215 to 374 ng/g ww)19 but higher than what was reported for samples collected in 1979 (0.5 to 93 ng/g dw).20 The latter study analyzed for nitrosamines in dried biosolids samples that could have facilitated volatilization of some NDMA, hence potentially resulting in the lower observed concentration range in the solids.20 However, in contrast to previous studies, NDMA was detected only in 3% of the biosolids samples analyzed. NDMA tested positive in 38% and 100% of the biosolids samples collected in 1978 (n = 16) and 1979 (n = 11), respectively.19,20 The only analyte not detected throughout the study was NDEA. However, NDEA was detected at a range of 0.9 to 12 ng/g dw (with 19% detection frequency)20 and 269 to 5,520 ng/g ww (with 55% detection frequency)19 in biosolids samples analyzed in past studies. The lower frequency of detection of NDMA and nondetection of NDEA in biosolids from the present study could be the result of (i) their inability to partition onto sewage sludge during secondary treatment of wastewater (due to low partitioning coefficient; log KOW of −0.5 for NDMA and 0.51 for NDEA); (ii) degradation of NDMA and NDEA in sludge processing or treatment systems (aerobic/anaerobic digesters) and/or during storage; (iii) high method detection limits for NDMA (at 5.7 ng/g dw) and NDEA (at 3.2 ng/g dw) in the present method; or (iv) a combination of the above. It is interesting to note that the lowest detection frequency was observed for NDMA (3%) and NPYR (1%) in the present study in contrast to the previous study by Padhye et al. (2009),15 where the authors reported NDMA and NPYR as the only nitrosamines present in untreated sewage sludge filtrates. This suggests that the majority of these hydrophilic nitrosamines are associated with the aqueous phase flow of wastewater treatment systems. This hypothesis was further supported by a log–log plot of n-octanol–water partition coefficient (KOW) versus the detection frequency of the various nitrosamines in biosolids, revealing a coefficient of determination (R2) value of 0.65. The latter observation shows that nitrosamines featuring elevated partition coefficients are more readily detectable in biosolids samples, presumably due to their enhanced partitioning behavior (Figure 2b). However, no significant correlation was detectable between the water content of individual biosolids samples and their content of nitrosamines (R2 < 0.1). This could be due to the significant temporal variability of nitrosamines observed between WWTPs as reported in past studies.8,10
Figure 2.
(a) Box-and-whisker plot of N-nitrosamines concentrations in biosolids samples from the U.S. EPA’s Targeted National Sewage Sludge Survey (TNSSS). Numbers within parentheses represent the number of detects out of a total of 80 samples analyzed. (b) A log–log plot of partition coefficient vs detection frequency of N-nitrosamines in biosolids showed a linear trend.
The most frequently detected nitrosamine in biosolids was NDPhA (79%) with an average concentration of 10 (range: 0.7–147) ng/g dw. NDPhA is one of five nitrosamines included in the EPA’s CCL3. With the development of new LC-MS/MS methods, only recently NDPhA has been detected as a disinfection byproduct in drinking water systems.5,30,31 Previous studies have suggested that the likely precursor for NDPhA was diphenylamine, which is widely used as an insecticide, preservative, and a solid propellant in rocket fuels, that can eventually reach WWTPs.5,32 The number of studies focusing on the occurrence and fate of NDPhA is scarce. This could be due to the fact that the frequency of NDPhA detection in water resources and drinking water systems is lower compared to the widely studied NDMA. Additionally, NDPhA has a high partition coefficient (log KOW of 3.13) compared to other nitrosamines, thus favoring its detection in solid matrices (like soil, biosolids, sediments) compared to aqueous matrices (like surface and drinking water) in which the analyte would be depleted by partitioning. NDPA and NPIP are the other two nitrosamines that were detected in more than 10% of biosolids samples analyzed. The precursors and the source for these nitrosamines in WWTPs are yet to be characterized. Piperidine, n-propylamine, and other aliphatic amines are common intermediate products in pharmaceutical industries,33 and thus industrial sources may contribute to their occurrence in wastewaters. Additionally, these amines can be formed from degradation of organic matter like proteins, amino acids, and other nitrogen-containing organic compounds.34 Aliphatic, alicyclic, and aromatic amines have been detected in wastewater34,35 and may serve as potential precursors to the respective nitrosamines in biosolids.
Variability of Nitrosamines in U.S. Biosolids
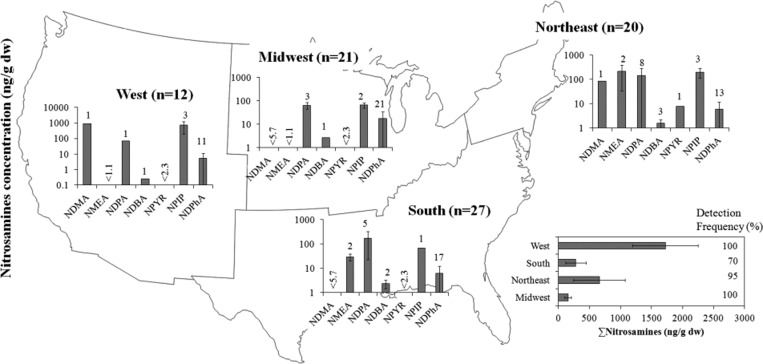
The variance in nitrosamines occurrence and concentration in biosolids is high between the studied WWTPs (Figure 2a). The concentrations of nitrosamines when plotted according to geographic location of the WWTP did not show a trend (Figure 3). However, the mean concentration of total nitrosamines was higher in WWTPs located in the West compared to other locations. When analysis data were plotted relative to the treatment capacity (flow stratum) of the WWTPs studied, the sum of mean concentration of detected nitrosamines showed an increase with increasing flow (Figure 4). This trend was particularly significant for NDMA, NPIP, and NDPhA (see bottom panel of Figure 4). Unfortunately, as a condition for participation in the study, the U.S. EPA kept undisclosed the identity of the treatment plant and additional key information (including sludge treatment systems) related to the biosolids samples. This information would have been beneficial in comparing nitrosamine concentration with respect to treatment systems. In general, nitrosamines in wastewater show large temporal variations in WWTPs (by over an order of magnitude).8,10 These large variations in the past have been associated with temporal variation in industrial discharges that contributed to the WWTP flows.8,10 The observed high concentrations of nitrosamines in large WWTPs (treating >380 ML/day) thus may be linked to industrial processes; but lacking additional data, this interpretation is purely speculative. It is a common practice in most WWTPs to use cationic polymers to aid in the process of dewatering of sewage sludge. Previous studies have shown that such polymers typically contain secondary or tertiary amine groups that may serve as precursors for nitrosamines in wastewater treatment systems.10,14 Thus, we speculate that the addition of polymers in sludge systems might have contributed to the observed variances.
Figure 3.
Spatial variation of N-nitrosamines concentrations in U.S. biosolids. The entry “n” is the total number of samples analyzed in the respective region. Numbers next to the bar depict the number of detects in the respective region. Error bars represent minima and maxima.
Figure 4.
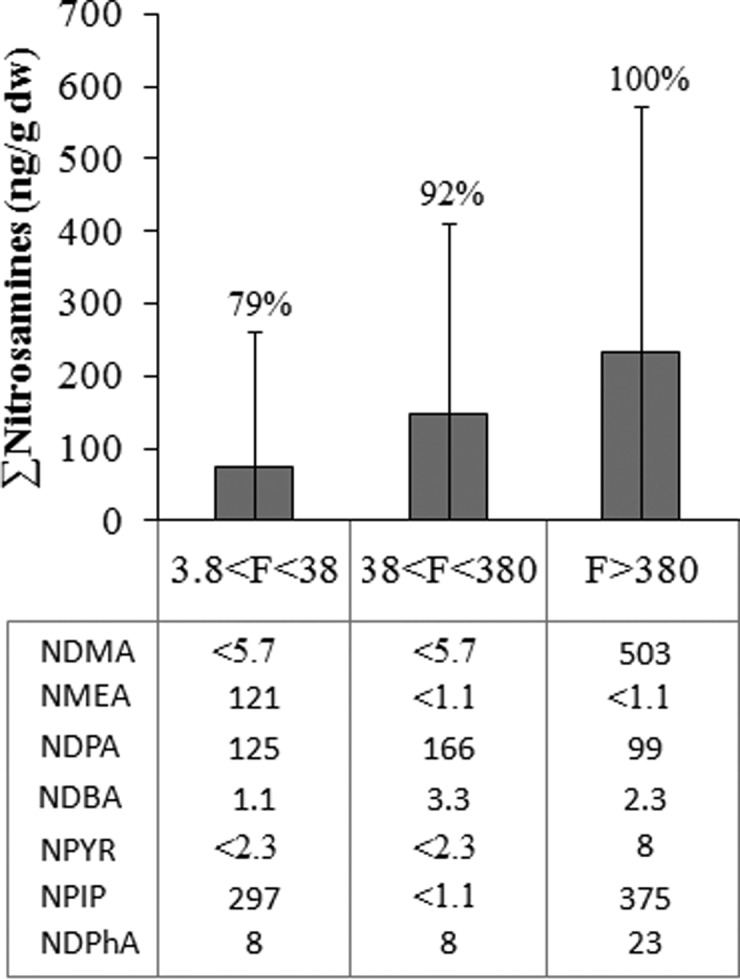
Concentrations of N-nitrosamines reported in units of ng/g dw with respect to treatment capacity of the wastewater treatment plants sampled. “F” is the flow volume in millions of liters per day. Error bars represent ± one standard deviation. Percentages provided next to the bars represent the detection frequency of nitrosamines. The lower panel summarizes concentrations of specific nitrosamines observed in individual samples.
Study Limitations, Data Gaps, and Research Needs
The archived biosolids samples from the present study were analyzed more than five years after their collection by U.S. EPA contractors in 2006–2007. Nitrosamines are volatile and have been shown to degrade in both oxic and anoxic conditions.36,37 This information would suggest that the concentrations and detection frequencies reported in the present work likely are lower than what would have been found if samples had been analyzed right away. On the other hand, storage of biosolids also could have enabled the formation of some of the nitrosamines postcollection, as a result of the conversion of precursor compounds such as secondary and tertiary amines present in biosolids. Nitrosamine formation reactions typically are temperature dependent and have been shown to increase in rate with increasing temperature.2,38 This latter fact suggests that nitrosamine formation from precursors during biosolids storage at −20 °C likely did not occur or was negligible and represents only a minor error source. To test the variation of nitrosamine concentration during storage, biosolids samples from a municipal WWTP in Mesa, Arizona were collected, analyzed once immediately, and stored at −20 °C. NDPhA was the only nitrosamine detected in the sample at a concentration of 5.7 ± 1.3 ng/g dw, which was within the range reported in the present study for the nationally representative TNSSS samples [10 (0.7 to 147) ng/g dw]. Aliquots of the biosolids sample were analyzed again for nitrosamines after 5 weeks of storage. The concentration of NDPhA showed no significant difference between initial and poststorage analysis [5.7 ± 1.3 ng/g dw (t = 0) and 4.5 ± 1.1 ng/g dw (t = 5 weeks)]. Future work is required to investigate the fate of other nitrosamines in biosolids during long storage periods. This is particularly important for facilities that store biosolids on-site for long periods of time prior to land application. If storage is found to have a significant impact on the nitrosamine content of biosolids, this information could be used to formulate best-practice protocols to limit their generation and persistence in biosolids.
Irrespective of the fact that archived samples were analyzed, the present study features some novel information regarding the environmental occurrence of these carcinogenic emerging contaminants. It furnishes the first nationwide occurrence data of seven nitrosamines in biosolids. Many past studies have focused on the “formation potential” of nitrosamines in various matrices,38−40 which investigated the potential for nitrosamines to form from a multitude of precursor compounds in water and wastewater treatment processes. Along these lines, even if some of the nitrosamines are artifacts of various reaction mechanisms during the storage period, the present study suggests the presence or accumulation of a range of nitrosamine precursor compounds that could result in the formation of nitrosamines in biosolids and in soil after land application of biosolids.
The persistence of nitrosamines in biosolids is currently unknown. Very few studies have shown the presence and formation of nitrosamines (NDMA and NDEA) in biosolids-amended soils.41,42 NDMA was shown to leach and be taken up by plants from contaminated soil.43 Provided that a significant percentage of biosolids produced in the U.S. is applied on land, research is needed to study the fate of nitrosamines in biosolids and biosolids-amended soils. It is also important to study and characterize potential nitrosamine precursors in biosolids and sludge systems for an in-depth understanding of the occurrence of nitrosamines in biosolids. Also, much of the research is primarily focused on NDMA, whereas other nitrosamines (particularly NDPhA, NDPA, and NPIP) may occur much more frequently (as shown in the present study) but may currently be overlooked. The present work shows that environmental monitoring for nitrosamines in sludge should not be restricted only to the aqueous phase of sludge samples or the filtrate thereof, as this approach may impede the successful detection of nitrosamines that reside with the solids content of the samples examined.
Acknowledgments
We thank Rick Stevens, Harry B. McCarty, and the U.S. EPA for providing the biosolids samples from the Targeted National Sewage Sludge Survey. We thank Paul Westerhoff from Arizona State University for his review and valuable feedback. This study was supported in part by the Johns Hopkins Center for a Livable Future and by National Institute of Environmental Health Sciences grants 1R01ES015445 and 1R01ES020889 and their respective supplements. The content of this work is solely the responsibility of the authors and does not necessarily represent the official views of the NIEHS or the National Institutes of Health (NIH).
Supporting Information Available
LC-MS/MS parameters, chromatograms from matrix spike experiments, nitrosamines concentration in field duplicates, and the list of facilities sampled by U.S. EPA. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Mitch W. A.; Sharp J. O.; Trussell R. R.; Valentine R. L.; Alvarez-Cohen L.; Sedlak D. L. N-Nitrosodimethylamine (NDMA) as a drinking water contaminant: A review. Environ. Eng. Sci. 2003, 20, 389–404. [Google Scholar]
- Krasner S. W.; Mitch W. A.; McCurry D. L.; Hanigan D.; Westerhoff P. Formation, Precursors, Control, and Occurrence of Nitrosamines in Drinking Water: A Review. Water Res. 2013, 47, 4433–4450. [DOI] [PubMed] [Google Scholar]
- Mitch W. A.; Sedlak D. L. Formation of N-nitrosodimethylamine (NDMA) from dimethylamine during chlorination. Environ. Sci. Technol. 2002, 36, 588–595. [DOI] [PubMed] [Google Scholar]
- Pehlivanoglu-Mantas E.; Hawley E. L.; Deeb R. A.; Sedlak D. L. Formation of nitrosodimethylamine (NDMA) during chlorine disinfection of wastewater effluents prior to use in irrigation systems. Water Res. 2006, 40, 341–347. [DOI] [PubMed] [Google Scholar]
- Zhao Y.; Boyd J. M.; Woodbeck M.; Andrews R. C.; Qin F.; Hrudey S. E.; Li X. Formation of N-nitrosamines from eleven disinfection treatments of seven different surface waters. Environ. Sci. Technol. 2008, 42, 4857–4862. [DOI] [PubMed] [Google Scholar]
- Yang L.; Chen Z.; Shen J.; Xu Z.; Liang H.; Tian J.; Ben Y.; Zhai X.; Shi W.; Li G. Reinvestigation of the nitrosamine-formation mechanism during ozonation. Environ. Sci. Technol. 2009, 43, 5481–5487. [DOI] [PubMed] [Google Scholar]
- Lee C.; Schmidt C.; Yoon J.; Von Gunten U. Oxidation of N-nitrosodimethylamine (NDMA) precursors with ozone and chlorine dioxide: Kinetics and effect on NDMA formation potential. Environ. Sci. Technol. 2007, 41, 2056–2063. [DOI] [PubMed] [Google Scholar]
- Krauss M.; Longrée P.; Dorusch F.; Ort C.; Hollender J. Occurrence and removal of N-nitrosamines in wastewater treatment plants. Water Res. 2009, 43, 4381–4391. [DOI] [PubMed] [Google Scholar]
- Ducos P.; Gaudin R.; Maire C.; Mavelle T.; Bouchikhi B.; Debry G. Occupational exposure to volatile nitrosamines in foundries using the “Ashland” core-making process. Environ. Res. 1988, 47, 72–78. [DOI] [PubMed] [Google Scholar]
- Sedlak D. L.; Deeb R. A.; Hawley E. L.; Mitch W. A.; Durbin T. D.; Mowbray S.; Carr S. Sources and fate of nitrosodimethylamine and its precursors in municipal wastewater treatment plants. Water Environ. Res. 2005, 32–39. [DOI] [PubMed] [Google Scholar]
- U.S. EPA. Contaminant Candidate List 3-CCL; 2012, http://water.epa.gov/scitech/drinkingwater/dws/ccl/ccl3.cfm.
- International Agency for Research on Cancer (IARC). Agents Classified by the IARC Monograph, Volumes 1–109; 2014, http://monographs.iarc.fr/ENG/Classification/ClassificationsGroupOrder.pdf.
- U.S. EPA. Technical Fact Sheet-N-Nitroso-dimethylamine (NDMA); 2012, http://www.epa.gov/fedfac/pdf/technical_fact_sheet_ndma.pdf.
- Mitch W. A.; Sedlak D. L. Characterization and fate of N-nitrosodimethylamine precursors in municipal wastewater treatment plants. Environ. Sci. Technol. 2004, 38, 1445–1454. [DOI] [PubMed] [Google Scholar]
- Padhye L.; Tezel U.; Mitch W. A.; Pavlostathis S. G.; Huang C. Occurrence and fate of nitrosamines and their precursors in municipal sludge and anaerobic digestion systems. Environ. Sci. Technol. 2009, 43, 3087–3093. [DOI] [PubMed] [Google Scholar]
- Yoon S.; Nakada N.; Tanaka H. Occurrence and removal of NDMA and NDMA formation potential in wastewater treatment plants. J. Hazard. Mater. 2011, 190, 897–902. [DOI] [PubMed] [Google Scholar]
- Yoon S.; Nakada N.; Tanaka H. A new method for quantifying N-nitrosamines in wastewater samples by gas chromatography—triple quadrupole mass spectrometry. Talanta 2012, 97, 256–261. [DOI] [PubMed] [Google Scholar]
- Schreiber I. M.; Mitch W. A. Occurrence and fate of nitrosamines and nitrosamine precursors in wastewater-impacted surface waters using boron as a conservative tracer. Environ. Sci. Technol. 2006, 40, 3203–3210. [DOI] [PubMed] [Google Scholar]
- Brewer W. S.; Draper A. C.; Wey S. S. The detection of dimethylnitrosamine and diethylnitrosamine in municipal sewage sludge applied to agricultural soils. Environ. Pollut. B Chem. Phys. 1980, 1, 37–43. [Google Scholar]
- Mumma R. O.; Raupach D. R.; Waldman J. P.; Hotchkiss J. H.; Gutenmann W. H.; Bache C. A.; Lisk D. J. Analytical survey of elements and other constituents in central New York State sewage sludges. Arch. Environ. Contam. Toxicol. 1983, 12, 581–587. [Google Scholar]
- Venkatesan A. K.; Halden R. U. Brominated flame retardants in US biosolids from the EPA national sewage sludge survey and chemical persistence in outdoor soil mesocosms. Water Res. 2014, 55, 133–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesan A. K.; Halden R. U. National inventory of alkylphenol ethoxylate compounds in US sewage sludges and chemical fate in outdoor soil mesocosms. Environ. Pollut. 2013, 174, 189–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesan A. K.; Halden R. U. National inventory of perfluoroalkyl substances in archived U.S. biosolids from the 2001 EPA National Sewage Sludge Survey. J. Hazard. Mater. 2013, 252–253, 413–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. EPA. Targeted National Sewage Sludge Survey Overview Report; 2009, http://water.epa.gov/scitech/wastetech/MSS/upload/2009_01_15_MSS_tnsss-tech.pdf.
- U.S. EPA. 2001 National Sewage Sludge Survey Report; 2007, http://water.epa.gov/scitech/wastetech/MSS/upload/sludgesurvey9-2007.pdf.
- U.S. EPA. Sewage sludge (biosolids): frequently asked questions; 2012, http://water.epa.gov/polwaste/wastewater/treatment/biosolids/genqa.cfm.
- Krauss M.; Hollender J. Analysis of nitrosamines in wastewater: Exploring the trace level quantification capabilities of a hybrid linear ion trap/orbitrap mass spectrometer. Anal. Chem. 2008, 80, 834–842. [DOI] [PubMed] [Google Scholar]
- Planas C.; Palacios Ó.; Ventura F.; Rivera J.; Caixach J. Analysis of nitrosamines in water by automated SPE and isotope dilution GC/HRMS: Occurrence in the different steps of a drinking water treatment plant, and in chlorinated samples from a reservoir and a sewage treatment plant effluent. Talanta 2008, 76, 906–913. [DOI] [PubMed] [Google Scholar]
- Charrois J. W.; Arend M. W.; Froese K. L.; Hrudey S. E. Detecting N-nitrosamines in drinking water at nanogram per liter levels using ammonia positive chemical ionization. Environ. Sci. Technol. 2004, 38, 4835–4841. [DOI] [PubMed] [Google Scholar]
- Zhou W.; Boyd J. M.; Qin F.; Hrudey S. E.; Li X. Formation of N-nitrosodiphenylamine and two new N-containing disinfection byproducts from chloramination of water containing diphenylamine. Environ. Sci. Technol. 2009, 43, 8443–8448. [DOI] [PubMed] [Google Scholar]
- Zhao Y.; Boyd J.; Hrudey S. E.; Li X. Characterization of new nitrosamines in drinking water using liquid chromatography tandem mass spectrometry. Environ. Sci. Technol. 2006, 40, 7636–7641. [DOI] [PubMed] [Google Scholar]
- Zhou W.; Lou L.; Zhu L.; Li Z.; Zhu L. Formation and cytotoxicity of a new disinfection by-product (DBP) phenazine by chloramination of water containing diphenylamine. J. Environ. Sci. 2012, 24, 1217–1224. [DOI] [PubMed] [Google Scholar]
- Sacher F.; Lenz S.; Brauch H. Analysis of primary and secondary aliphatic amines in waste water and surface water by gas chromatography-mass spectrometry after derivatization with 2,4-dinitrofluorobenzene or benzenesulfonyl chloride. J. Chromatogr., A 1997, 764, 85–93. [Google Scholar]
- Ábalos M.; Bayona J. M.; Ventura F. Development of a solid-phase microextraction GC-NPD procedure for the determination of free volatile amines in wastewater and sewage-polluted waters. Anal. Chem. 1999, 71, 3531–3537. [DOI] [PubMed] [Google Scholar]
- Scully F. E.; Howell G. D.; Penn H. H.; Mazina K.; Johnson J. D. Small molecular weight organic amino nitrogen compounds in treated municipal wastewater. Environ. Sci. Technol. 1988, 22, 1186–1190. [DOI] [PubMed] [Google Scholar]
- Sharp J. O.; Wood T. K.; Alvarez-Cohen L. Aerobic biodegradation of N-nitrosodimethylamine (NDMA) by axenic bacterial strains. Biotechnol. Bioeng. 2005, 89, 608–618. [DOI] [PubMed] [Google Scholar]
- Rowland I.; Grasso P. Degradation of N-nitrosamines by intestinal bacteria. Appl. Microbiol. 1975, 29, 7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W.; Chen C.; Lou L.; Yang Q.; Zhu L. Formation potential of nine nitrosamines from corresponding secondary amines by chloramination. Chemosphere 2014, 95, 81–87. [DOI] [PubMed] [Google Scholar]
- Yoon S.; Nakada N.; Tanaka H. Occurrence and fate of N-nitrosamines and their formation potential in three wastewater treatment plants in Japan. Water Sci. Technol. 2013, 68, 2118–2126. [DOI] [PubMed] [Google Scholar]
- Garcia-Rodríguez A.; Fontàs C.; Matamoros V. Formation potential of N-nitrosamines during the disinfection of treated wastewaters with sodium hypochlorite. Desalin. Water Treat. 2013, 1–8. [Google Scholar]
- Yoneyama T. Detection of N-nitrosodimethylamine in soils amended with sludges. Soil Sci. Plant Nutr. 1981, 27, 249–253. [Google Scholar]
- Mallik M.; Tesfai K.; Pancholy S. Formation of carcinogenic nitrosamines in soil treated with pesticides, and in sewage amended with nitrogen compounds. Proc. Okla. Acad. Sci. 1981, 61, 31. [Google Scholar]
- Dean-Raymond D.; Alexander M. Plant uptake and leaching of dimethylnitrosamine. Nature 1976, 262, 394–396. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.