Abstract
We report the novel combination of a selective beta adrenoceptor modulator and a norepinephrine-serotonin uptake inhibitor (sibutramine) with potential for the treatment of obesity. The synthesis and characterization of 6-[4-[2-[[(2S)-3-(9H-carbazol-4-yloxy)-2-hydroxypropyl]amino]-2-methylpropyl]phenoxy]pyridine-3-carboxamide (LY377604), a human β3-adrenergic receptor agonist and β1- and β2-adrenergic receptor antagonist with no sympathomimetic activity at the β1- and β2-adrenergic receptors, is reported. Some in vivo data in both rats and humans is presented.
Keywords: Selective beta adrenoceptor modulator, β3-adrenergic receptor agonist, norepinephrine-serotonin uptake inhibitor, sibutramine, LY377604, obesity
Obesity has increasingly been cited as a major health issue in recent decades.1 While many industrialized countries have experienced similar increases, obesity rates in the United States are among the highest in the world, with thirty-three states having a prevalence equal to or greater than 25% and nine of these have a prevalence equal to or greater than 30% in 2009.2
Three drugs have been marketed for the long-term treatment of obesity: orlistat, which inhibits pancreatic lipase and consequently fat digestion; sibutramine, which acts centrally to inhibit norepinephrine and serotonin reuptake; and rimonabant, which antagonizes the cannabinoid receptor-1 in both the brain and peripheral tissues. Rimonabant and sibutramine have recently been withdrawn, owing to concerns about psychiatric side effects and cardiovascular safety concerns, respectively. Orlistat has had modest sales, in part because of its side effects and limited efficacy.
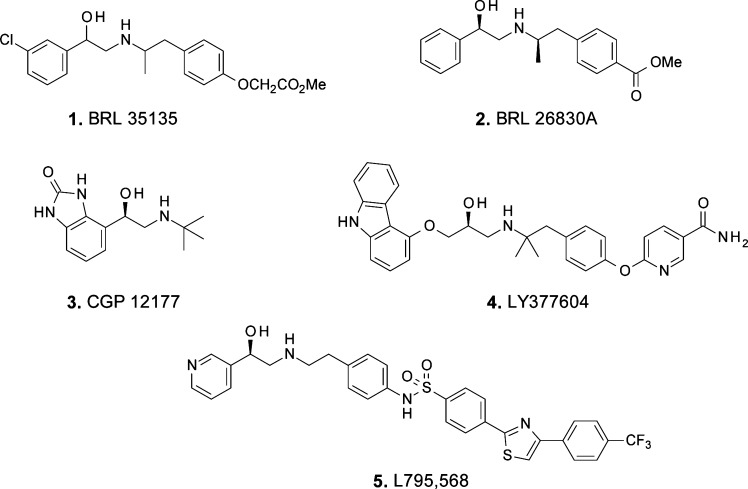
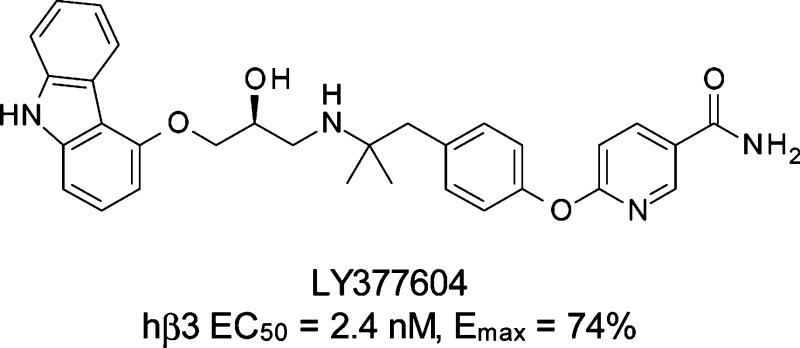
The discovery of a third β-adrenergic receptor in the early 1980s and the finding that stimulation of this receptor by selective agonists leads to marked weight loss and glycemic improvements in rodent models of obesity and diabetes, respectively, have led to intensive research efforts to develop β3 adrenergic agonists for the treatment of obesity and Type 2 diabetes in humans.3−5 Activation of the β3-adrenergic receptor markedly increases thermogenesis in rodents, and in fat tissue it stimulates oxidation of free fatty acids and lipolysis.6,7 Early clinical studies on BRL 35135 and BRL 26830A (1 and 2, Figure 1) support the potential antihyperglycemic and antiobesity effects of β3-adrenergic receptor agonists in humans.8−10 The role of brown adipose tissue (BAT) metabolism in humans is still debated, and there is disagreement, for example, on the amount of BAT that is present in adults.11 It is clear, however, that, within brown adipocytes, significant levels of uncoupling protein mRNA have been detected, along with β3-receptor mRNA.12 Furthermore, the effects of β3-receptor stimulation within BAT have been demonstrated in human subjects or tissues isolated from humans using the nonselective β-receptor agonist isoproterenol in the presence of β1- and β2-adrenergic receptor blockade or by more selective “atypical” partial β3-receptor agonists, such as CGP 12177 (3, Figure 1).13−15 In addition, treatment with β3-receptor agonists results in differentiation of preadipocytes to BAT in both rats and humans.16 These data suggest that the amount and the metabolic activity of BAT may be increased with chronic β3-receptor agonist therapy.17,18 Clinical studies with our human β3-adrenergic receptor agonist LY377604 (4) have shown an increase in energy expenditure in healthy male subjects,19 and this is further corroborated by single dose data from L-796,568 (5) in obese subjects.20,21
Figure 1.
Selected β3 adrenergic receptor agonists reported in the literature.
One dose-related side effect of sibutramine (6, Figure 2) treatment is an increase in blood pressure and heart rate due to activation of the sympathetic nervous system. We postulated that dosing in combination with LY377604 would ameliorate this by taking advantage of the β1 and β2 blocking properties of this compound, in addition to perhaps giving a synergistic increase in body weight loss in humans.
Figure 2.
Structure of sibutramine.
The synthesis of LY377604 (4) is outlined in Scheme 1.22 A mixture of 4-hydroxybenzyl alcohol (7) and 2-nitropropane was heated with potassium tert-butoxide using a Dean–Stark trap to provide 4-(2-methyl-2-nitropropyl)phenol (8), which was hydrogenated to yield the corresponding amine (9). The next step in the synthesis involved a based-promoted SNAr, whereby 4-(2-methyl-2-aminopropyl)phenol (9) was treated with powdered potassium carbonate and 6-chloronicotinamide to provide the coupled product (10) in 86% yield. In the final step, (S)-4-(oxiranylmethoxy)-9H-carbazole23 is treated with 2 equiv of the amine (10) to provide LY377604 (4) as a free base which is subsequently converted to the HCl salt or hemisuccinate.24
Scheme 1.
LY377604 was initially examined for its ability to stimulate the human β3 adrenergic receptor expressed in Chinese hamster ovary (CHO) cells. The compound was tested and found to be a potent agonist (EC50 = 2.4 nM, n = 14) and to cause a maximal increase in cyclic adenosine monophosphate (cAMP) levels that was approximately 74% of the maximal response induced by isoproterenol. LY377604 did not stimulate cAMP accumulation in CHO cells transfected with either the human β1 adrenergic receptor or the human β2 adrenergic receptor. Interestingly, this lack of efficacy to stimulate cAMP production was not a result of poor binding to the human β1 and β2 adrenergic receptors. LY377604 bound with high affinity to membranes expressing the human β1 adrenergic receptor and the human β2 adrenergic receptor. The in vitro data for LY377604 is summarized in Table 1.
Table 1. Beta Adrenergic Binding and Functional Activities of LY377604·HCl or LY377604·Hemisuccinatea.
| human β3 functional cAMP accumulation |
rat β3 functional cAMP accumulation |
human β2 functional cAMP accumulation |
human β1 functional cAMP accumulation |
human β2 radioligand binding | human β1 radioligand binding | ||||
|---|---|---|---|---|---|---|---|---|---|
| EC50 (nM) | Emax (%) | EC50 (nM) | Emax (%) | EC50 (nM) | Emax (%) | EC50 (nM) | Emax (%) | Ki (nM)b | Ki (nM)b |
| 2.4 ± 0.2 (n = 14) | 73.8 ± 1.0 (n = 14) | 5.0 ± 1.2 (n = 12) | 55.5 ± 2.0 (n = 12) | >3160 (n = 4) | <10 (n = 4) | >3160 (n = 4) | <10 (n = 4) | 0.012 ± 0.002 (n = 4) | 0.023 ± 0.005 (n = 4) |
Standard deviations are indicated by ± of the geometric mean, and the number of runs is indicated by (n).
Data obtained on LY377604·hemisuccinate.
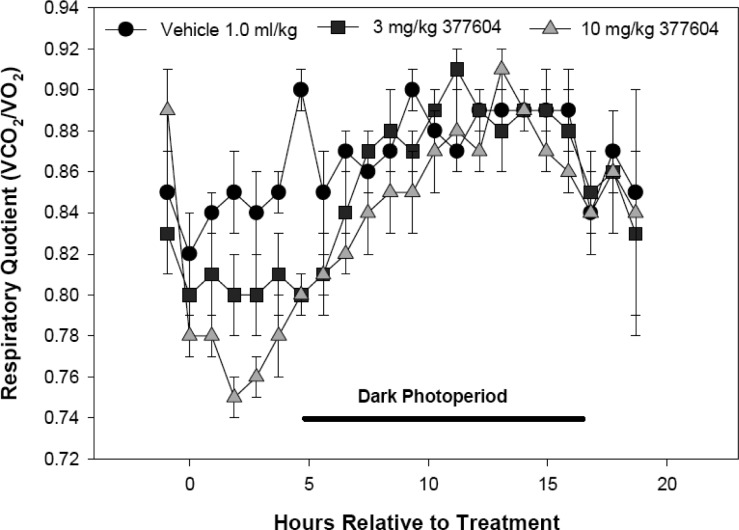
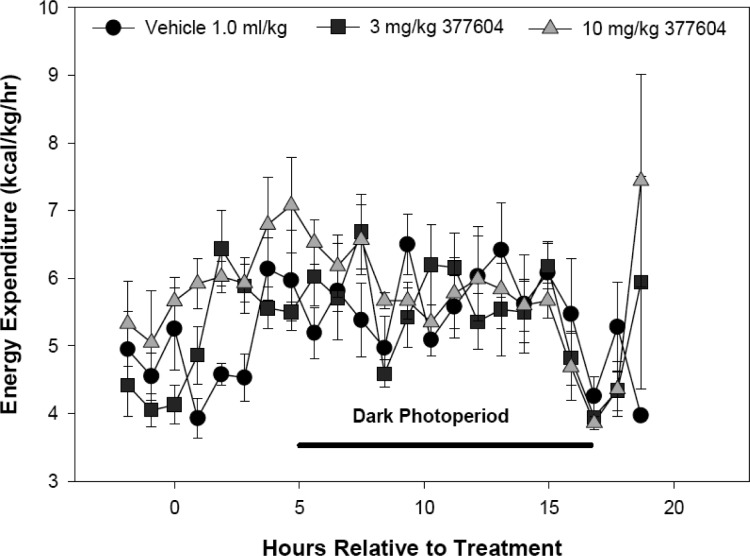
Administration of LY377604 to male Long–Evans rats fed a caloric dense diet resulted in stimulation of lipid utilization. This was observed as a decrease in respiratory quotient and persisted for about 4 h before returning to that measured from vehicle-treated rats (Figure 3).25 The increase in lipid utilization was dependent on dose. This was accompanied by a concomitant dose-dependent increase in energy expenditure (Figure 4). The increased fuel consumption was also maintained for about 4 h. We have previously reported the clinical confirmation of this increase in energy expenditure and reduction in respiratory quotient in a placebo controlled phase I dose escalation study of LY377604.19 Single doses of LY377604 also resulted in the expected β2 adrenergic antagonist activity as assessed by venous tonometry during localized isoproterenol administration in a dorsal hand vein.19
Figure 3.
LY377604 decreases the respiratory quotient in diet-induced obese male Long–Evans rats for 4 h at both 3 and 10 mg/kg orally (p < 0.05 ANOVA, SNK). The respiratory quotient was measured by indirect calorimetry.
Figure 4.
LY377604 increases caloric expenditure in diet-induced obese male Long–Evans rats for 4 h at 10 mg/kg orally (p < 0.05 ANOVA, SNK). Energy expenditure was measured by indirect calorimetry.
In healthy volunteers, LY377604 was rapidly absorbed, with maximum plasma concentrations achieved at approximately 2 h. LY377604 showed a rapid initial decline from plasma, followed by a slower terminal elimination phase; the estimated half-life was 9 h after a 60-mg dose.
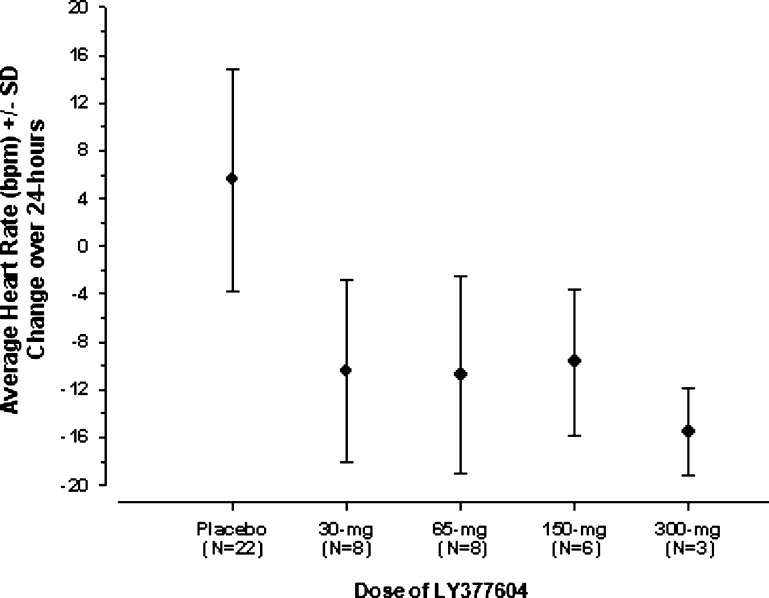
In preparation for a phase II obesity study of LY377604 in combination with sibutramine, it was determined that LY377604 did not alter the pharmacokinetics of sibutramine or its active metabolites over the dosing range studied (45–300 mg) in human volunteers. All dose levels of LY377604 blocked the cardiovascular effects of a 30 mg dose of sibutramine to a similar extent and demonstrated net decreases from baseline for heart rate (−10.5 bpm, Figure 5), systolic blood pressure (−5.5 mmHg), and diastolic blood pressure (−4.2 mmHg). Results from the phase II obesity study for this combination will be reported in due course.
Figure 5.
LY377604 blocks the sibutramine-induced increase in heart rate produced by a 30 mg dose of sibutramine at all dose levels in healthy volunteers.
Acknowledgments
We thank Robert D. Boyer, Lawrence A. Goodwin, and Douglas F. Schmidt for analytical support, Don Bennett for assay support, and Marlene L. Cohen for advice and mentorship.
Glossary
Abbreviations
- β1
beta 1
- β2
beta 2
- β3
beta 2
- LY377604
6-[4-[2-[[(2S)-3-(9H-carbazol-4-yloxy)-2-hydroxypropyl]amino]-2-methylpropyl]phenoxy]pyridine-3-carboxamide
- cAMP
cyclic adenosine monophosphate
- ANOVA
analysis of variance
- SNK
Student–Newman–Keuls
Supporting Information Available
Synthetic procedures and characterization data for all compounds, protocols for the human β adrenergic functional and binding assays, and DIO rat obesity studies. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Status
† In memoriam of Anthony J. Shuker, who passed away on March 26, 2009.
Supplementary Material
References
- Kelly T.; Wang W.; Chen C.-S.; Reynolds K.; He J. Global burden of obesity in 2005 and projections to 2030. Int. J. Obes. 2008, 32, 1431–1437. [DOI] [PubMed] [Google Scholar]
- http://www.cdc.gov/obesity/data/trends.html.
- de Souza C. J.; Burkey B. F. Beta 3-Adrenoceptor Agonists as Anti-diabetic and Anti-obesity Drugs in Humans. Curr. Pharm. Des. 2001, 7, 1433–1449. [DOI] [PubMed] [Google Scholar]
- Weyer C.; de Souza C. J. Development of β3-Adrenoceptor Agonists as Antiobesity and Antidiabetes Drugs in Humans: Current Status and Future Prospects. Drug Dev. Res. 2000, 51, 80–93. [Google Scholar]
- Arch J. R. S. The discovery of drugs for obesity, the metabolic effects of leptin and variable receptor pharmacology: perspectives from β3 adrenoceptor agonists. Naunyn-Schmiedeberg's Arch. Pharmacol. 2008, 378, 225–240. [DOI] [PubMed] [Google Scholar]
- Arch J. R. S.; Ainsworth A. T.; Cawthorne M. A.; Piercy V.; Sennitt M. V.; Thody V. E.; Wilson C.; Wilson S. Atypical β-adrenoceptor on brown adipocytes as target for anti-obesity drugs. Nature 1984, 309, 163–165. [DOI] [PubMed] [Google Scholar]
- Arch J. R. S.; Ainsworth A, T.; Ellis R. D. M.; Piercy V.; Thody V. E.; Thurlby P. L.; Wilson C.; Wilson S.; Young P. Treatment of obesity with thermogenic β-adrenoceptor agonists: studies on BRL 26830A in rodents. Int. J. Obes. 1984, 8Suppl 11–11. [PubMed] [Google Scholar]
- Connacher A. A.; Bennet W. M.; Jung R. T.; Rennie M. J. Metabolic effects of three weeks administration of the beta-adrenoceptor agonist BRL 26830A. Int. J. Obes. Relat. Metab. Disord. 1992, 16, 685–694. [PubMed] [Google Scholar]
- Mitchell T. H.; Ellis R. D.; Smith S. A.; Robb G.; Cawthorne M. A. Effects of BRL 35135, a beta-adrenoceptor agonist with novel selectivity, on glucose tolerance and insulin sensitivity in obese subjects. Int. J. Obes. 1989, 13, 757–766. [PubMed] [Google Scholar]
- Cawthorne M. A.; Sennitt M. V.; Arch J. R.; Smith S. A. BRL 35135, a potent and selective atypical beta-adrenoceptor agonist. Am. J. Clin. Nutr. 1992, 551 Suppl252S–257. [DOI] [PubMed] [Google Scholar]
- Nedergaard J.; Bengtsson T.; Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E444–E452. [DOI] [PubMed] [Google Scholar]
- Champigny O.; Ricquier D. Evidence from in vitro differentiating cells that adrenoceptor agonists can increase uncoupling protein mRNA level in adipocytes of adult humans: an RT-PCR study. J. Lipid Res. 1996, 37, 1907–1914. [PubMed] [Google Scholar]
- Lonnqvist F.; Krief S.; Strosberg A. D.; Nyberg S.; Emorine L. J.; Arner P. Evidence for a functional beta 3-adrenoceptor in man. Br. J. Pharmacol. 1993, 110, 929–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeldon N. M.; McDevitt D. G.; Lipworth B. J. Do beta 3-adrenoceptors mediate metabolic responses to isoprenaline?. Q. J. Med. 1993, 86, 595–600. [PubMed] [Google Scholar]
- Enocksson S.; Shimizu M.; Lonnqvist F.; Nordenstrom J.; Arner P. Demonstration of an in vivo functional beta 3-adrenoceptor in man. J. Clin. Invest. 1995, 95, 2239–2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himms-Hagen J.; Ricquier D.. Brown adipose tissue. In Handbook of obesity; Bray G. A., Bouchard C., James W. P. T., Eds.; Marcel Dekker: New York, pp 415–441. [Google Scholar]
- Holloway B. R. Reactivation of brown adipose tissue. Proc. Nutr. Soc. 1989, 48, 225–230. [DOI] [PubMed] [Google Scholar]
- Kawashita N. H.; Moura M. A.; Brito M. N.; Brito S. M.; Garofalo M. A.; Kettelhut I. C.; Migliorini R. H. Relative importance of sympathetic outflow and insulin in the reactivation of brown adipose tissue lipogenesis in rats adapted to a high-protein diet. Metabolism 2002, 51, 343–349. [DOI] [PubMed] [Google Scholar]
- Miller J. W.; Farid N. A.; Johnson R. D.; Smith B. P.; Dananberg J. Stimulation of energy expenditure by LY377604, a β3 adrenergic receptor agonist with β1/2 antagonist properties in healthy male subjects. Obes. Res. 1999, 7Suppl. 1121S. [Google Scholar]
- van Baak M. A.; Hul G. B. J.; Toubro S.; Astrup A.; Gottesdiener K. M.; DeSmet M.; Saris W. H. M. Acute effect of L-796568, a novel β3-adrenergic receptor agonist, on energy expenditure in obese men. Clin. Pharmacol. Ther. 2002, 71, 272–279. [DOI] [PubMed] [Google Scholar]
- Larsen T. M.; Toubro S.; van Baak M. A.; Gottesdiener K. M.; Larson P.; Saris W. H.; Astrup A. Effect of a 28-d treatment with L-796568, a novel β3-adrenergic receptor agonist, on energy expenditure and body composition in obese men. Am. J. Clin. Nutr. 2002, 76, 780–8. [DOI] [PubMed] [Google Scholar]
- Czeskis B. A.; Wheeler W. J.; Clodfelter D. K. Convergent synthesis of two 14C-labeled β3-adrenergic receptor agonists. J. Labelled Compd. Radiopharm. 2006, 49, 663–673. [Google Scholar]
- Czeskis B. A.; Wheeler W. J. Synthesis of β3 adrenergic receptor agonist LY377604 and its metabolite 4-hydroxycarbazole, labeled with carbon-14 and deuterium. J. Labelled Compd. Radiopharm. 2005, 48, 407–419. [Google Scholar]
- Crowell T. A.; Evrard D. A.; Jones C. D.; Muehl B. S.; Rito C. J.; Shuker A. J.; Thorpe A. J.; Thrasher K. J.; Czeskis B. A.; Wheeler W. J. EP Patent 0 827 746, 2002.
- Procedure based on method reported in: Chen Y.; Heiman M. L. Chronic leptin administration promotes lipid utilization until fat mass is greatly reduced and preserves lean mass of normal female rats. Regul. Pept. 2000, 92, 113–119. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.