Abstract
Previously we discovered a tricyclic indoline, N-[2-(6-bromo-4-methylidene-2,3,4,4a,9,9a-hexahydro-1H-carbazol-4a-yl)ethyl]-4-chlorobenzene-1-sulfonamide (1, Of1), from bioinspired synthesis of a highly diverse polycyclic indoline alkaloid library, that selectively resensitizes methicillin-resistant Staphylococcus aureus strains to β-lactam antibiotics. Herein, we report a thorough structure–activity relationship investigation of 1, which identified regions of 1 that tolerate modifications without compromising activity and afforded the discovery of a more potent analogue with reduced mammalian toxicity.
Introduction
Antibiotic resistance is an urgent world health concern that is aggravated by a lack of novel antibiotic discovery.1 The vast majority of antibiotic classes on the market today were discovered between 1940 and 1960, a period of time known as “the golden era” of antibiotic discovery.2 Since this time, however, only two new classes of antibiotics have been brought to the clinic. Microbial antibiotic resistance has prompted the pharmaceutical industry to develop analogues of known antibiotics; however, bacteria have been observed to develop resistant phenotypes quickly, and there are not currently enough analogues in the antibiotic pipeline to combat imminent resistance emergence.3 Methicillin-resistant Staphylococcus aureus (MRSA) is particularly concerning in this regard. Prior to the introduction of penicillin in the 1940s, the mortality of patients who developed invasive staphylococcus infections was nearly 80%, but this statistic was drastically reduced by the introduction of penicillin in the clinic.4 In just a few years, however, resistance to penicillin, mediated by β-lactamases, emerged, which led to the introduction of β-lactam antibiotics such as methicillin, which are resistant to degradation by β-lactamase. It was not long, however before S. aureus developed methicillin resistance in the form of alternative penicillin binding proteins (PBPs), such as PBP2a.5 In addition to methicillin resistance, a number of MRSA strains have developed resistant phenotypes against multiple drugs used in the clinic, thus limiting treatment options for bacterial infections and threatening the onset of a postantimicrobial era.6 Of the estimated 2 million illnesses and 23 000 deaths last year that are directly associated with antibiotic resistant bacteria in the United States, MRSA was directly responsible for 80 000 illnesses and 11 000 deaths.7 Although vancomycin as well as some antibiotic analogues are still effective for the treatment of MRSA, strains that are resistant to these last-line-of-defense treatments have already become a problem of their own.8
Resistance-modifying agents (RMAs) offer a promising solution.9 These target nonessential, resistance-conferring genes and restore antibiotic sensitivity. A notable advantage of RMAs is that they are capable of extending the market lifespan of known antibiotics that have already been optimized for large-scale production with well-studied toxicity profiles. For example, clavulanic acid is a serine β-lactamase inhibitor that is commonly used in combination with amoxicillin, under the brand name Augmentin, among others, to treat infections resulting from β-lactamase-producing bacteria.10 Clavulanic acid deactivates β-lactamase by irreversibly acylating the catalytic serine residue in the active site of the β-lactamase enzyme that would otherwise function to breakdown the β-lactam antibiotic. Although the clavulanic acid/β-lactam combination is effective for serine β-lactamases, it is ineffective against metallo-β-lactamases and does nothing to combat β-lactam resistance mediated by PBP2a.11Although efforts have been underway to discover more novel RMAs, thus far, only those that are β-lactamase inhibitors have been successfully brought to market. β-Lactam antibiotics are one of the most widely used antibiotics and have inspired numerous research efforts to overcome bacterial resistance. In addition to β-lactamase inhibitors, other classes of compounds have been reported to potentiate the activity of β-lactam antibiotics, such as compounds interfering bacterial cell wall synthesis and compounds affecting resistance-sensing pathways.12−15
Recently, our group discovered a tricyclic indoline, 1, with RMA activity against MRSA that selectively potentiates a variety of β-lactam antibiotics.16 Interestingly, in addition to β-lactam antibiotics that are susceptible to breakdown by β-lactamase, 1 also potentiates those that are β-lactamase resistant, such as methicillin, oxacillin, and meropenem. Intrigued by the unique activity of 1, we sought to conduct a structure–activity relationship (SAR) study in order to identify regions of the structure of 1 that may be modified to improve its activity and toxicity profile, as well as to allow synthesis of functional analogues to facilitate the discovery of the cellular target of 1. Herein, we report a thorough SAR study of our previously reported tricyclic indoline RMA, 1.
Results and Discussion
Modification of 1
In order to conduct our SAR investigation of 1 (Figure 1), we synthesized several series of 1 analogues, which were then evaluated for their ability to resensitize MRSA to a collection of β-lactam antibiotics. For these activity tests, we chose to use amoxicillin in combination with clavulanic acid (a.k.a., Augmentin, one of the top three most prescribed antibiotics), cefazolin (a first-generation cephalosporin), and meropenem (an ultrabroad-spectrum carbapenem). Amoxicillin/clavulanic acid and cefazolin resensitizing experiments were performed using MRSA ATCC BAA-44, for which the MICs of these two antibiotics were found to be 32/16 and 128 μg/mL, respectively. Experiments using meropenem were performed using MRSA ATCC 33592, since this strain has demonstrated greater level of resistance to meropenem, with an MIC of 16 μg/mL. To assess activity of each analogue as RMA, we employed a modified broth microdilution assay, as described previously.16 Briefly, this involves incubating MRSA with 1 or each of its analogues in 2-fold serial dilution in the presence of each individual antibiotic at its Clinical Laboratory Standards Institutes (CLSI)-defined sensitive concentrations. For amoxicillin/clavulanic acid, this concentration was 4/2 μg/mL (8-fold potentiation); for cefazolin, 8 μg/mL (16-fold potentiation); and for meropenem, 4 μg/mL (4-fold potentiation).17 Following overnight incubation, plates were examined for bacterial growth, or lack thereof. Analogues of 1were tested at concentrations ranging from 0.5 to 32 μg/mL. The minimum resensitizing concentration (MRC) was defined as the concentration of 1’s analogues at which no overnight growth was observed in the presence of a sensitive concentration of antibiotic. Compounds that displayed similar or improved RMA activity relative to 1 were further tested for their toxicity against the growth of human cervical adenocarcinoma HeLa cells by incubating a range of concentrations of each compound with cells for 24 h and assessing viability at each concentration using the CellTiter Glo mammalian viability assay (Promega). The half growth inhibitory concentration (GI50) of each analogue was determined by fitting the data using KaleidaGraph (v4.1.1, Synergy Software).
Figure 1.
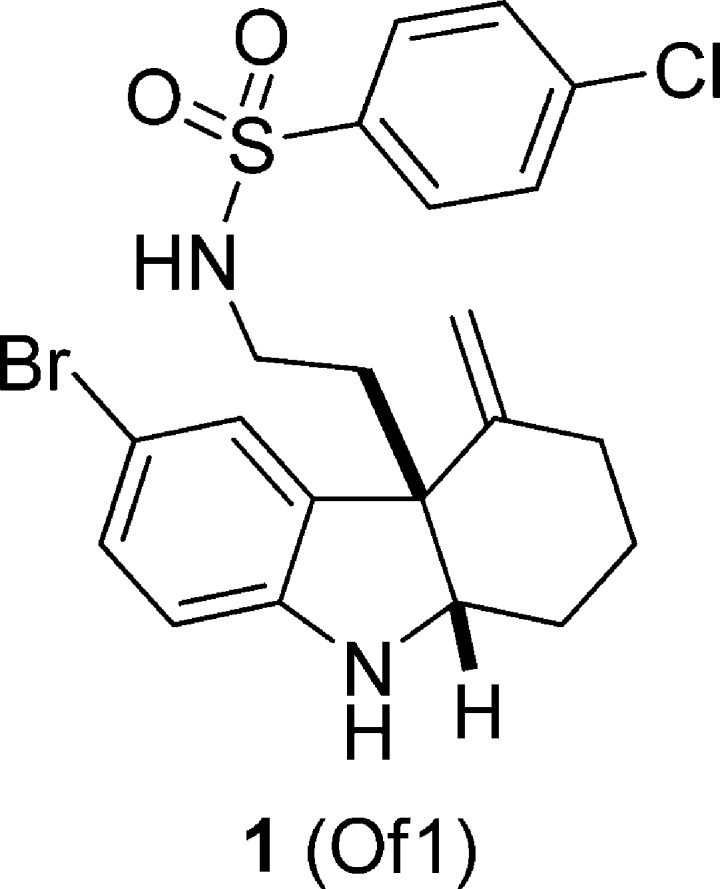
The structure of 1.
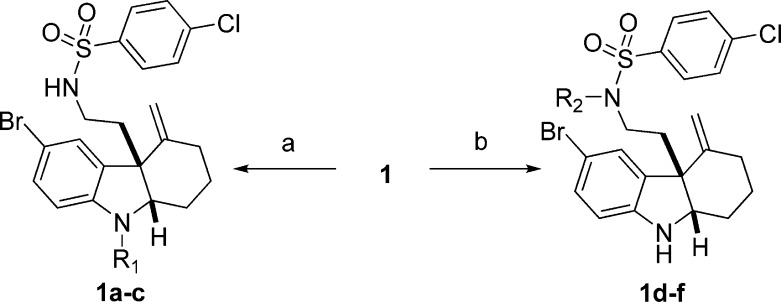
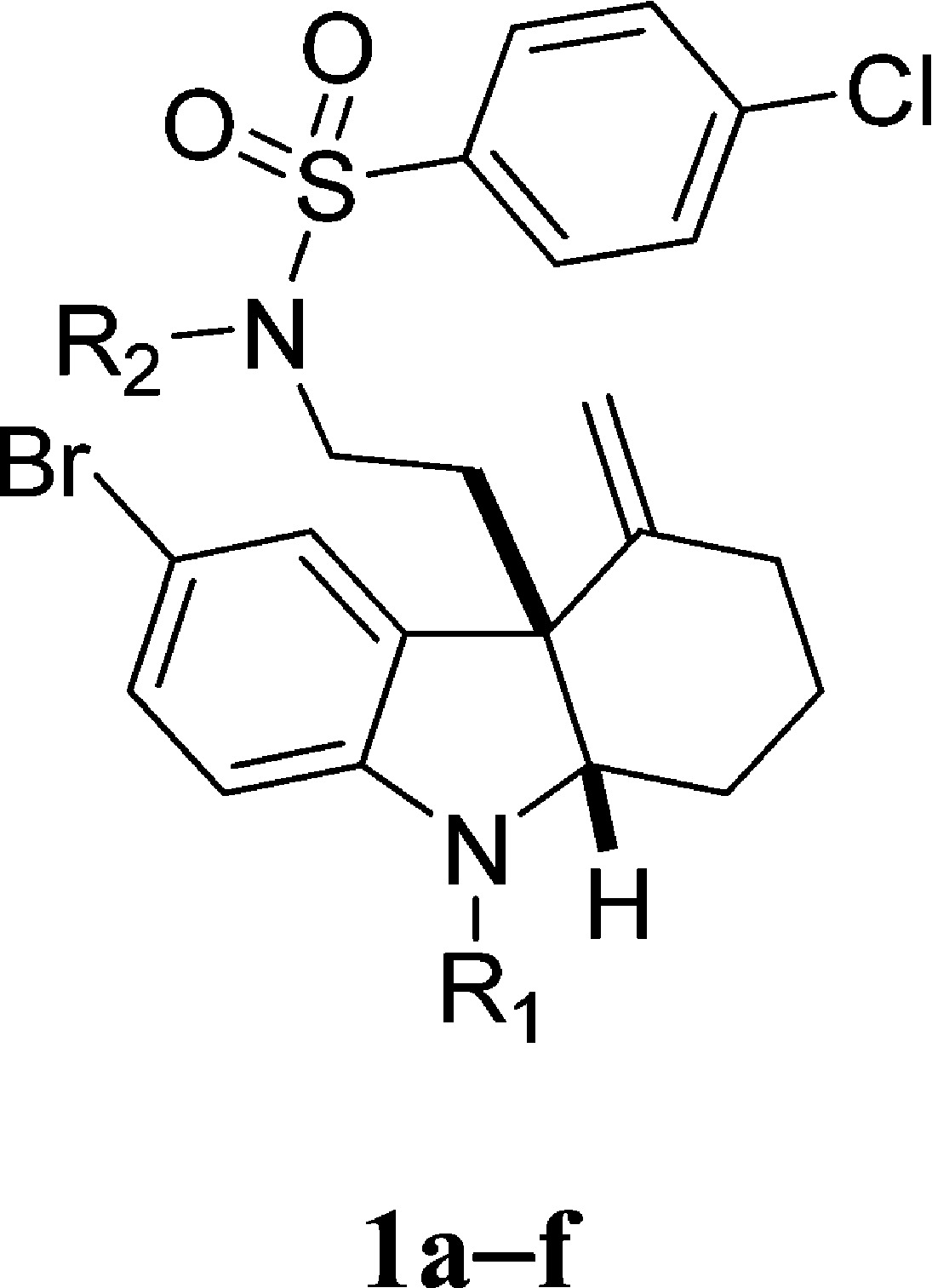
We began our SAR investigation by testing a number of functional group modifications to the indoline and sulfonamide nitrogens of 1. Due to the different pKa, different bases were used in the functionalization steps. As shown in Scheme 1, for compound 1a, selective methylation was carried out in the presence of potassium carbonate in anhydrous CH3CN; compounds 1b and 1c were prepared with corresponding electrophilic reagents under similar condition. While compound 1d was generated by using sodium hydride as a base, triethylamine was employed to activate the sulfonamide nitrogen selectively for the preparation of analogues 1e and 1f. We observed that when either or both of these moieties are modified, the RMA activity of the compound is abolished (Table1). We thus concluded that both these nitrogens must not be modified.
Scheme 1. Functionalization of the Indoline and Sulfonamide Nitrogens.
Reagents and conditions: (a) R1X, K2CO3, 0 °C, CH3CN; (b) for 1d, NaH 0 °C, then MeI, 0–60 °C, DMF; for 1e,f, R2X, Et3N, 0 °C, DCM.
Table 1. MRC Values for Indoline and Sulfonamide Nitrogen Modifications.
| entry | compd | R1 | R2 | amox/clava,b | cefazolina,b | meropenema,c |
|---|---|---|---|---|---|---|
| 1 | 1a | Me | H | >32 | >32 | >32 |
| 2 | 1b | SO2PhpCl | H | >32 | >32 | >32 |
| 3 | 1c | COPhpCl | H | >32 | >32 | >32 |
| 4 | 1d | H | Me | >32 | >32 | >32 |
| 5 | 1e | H | SO2PhpCl | >32 | >32 | >32 |
| 6 | 1f | H | COPhpCl | >32 | >32 | >32 |
All MRC values are in μg/mL.
MRSA ATCC BAA-44.
MRSA ATCC 33592.
Modifications of the Aromatic Substitution of Indoline
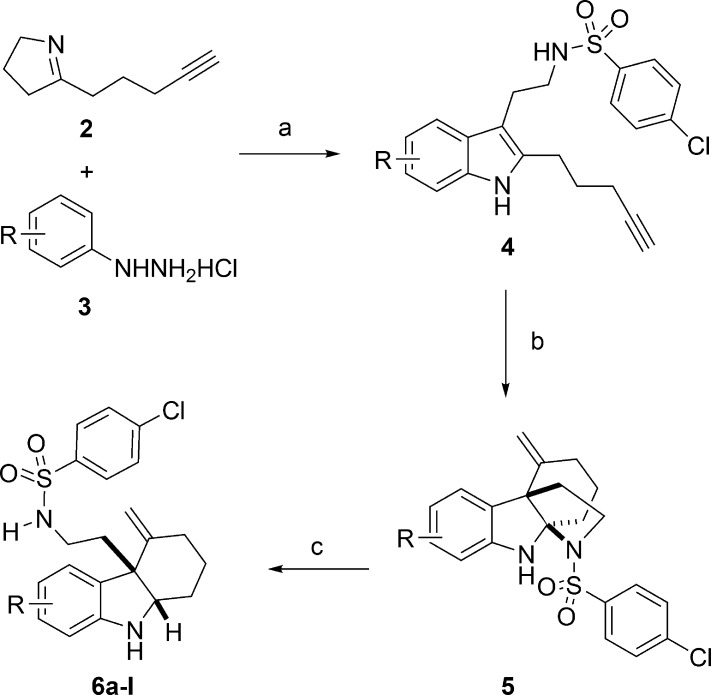
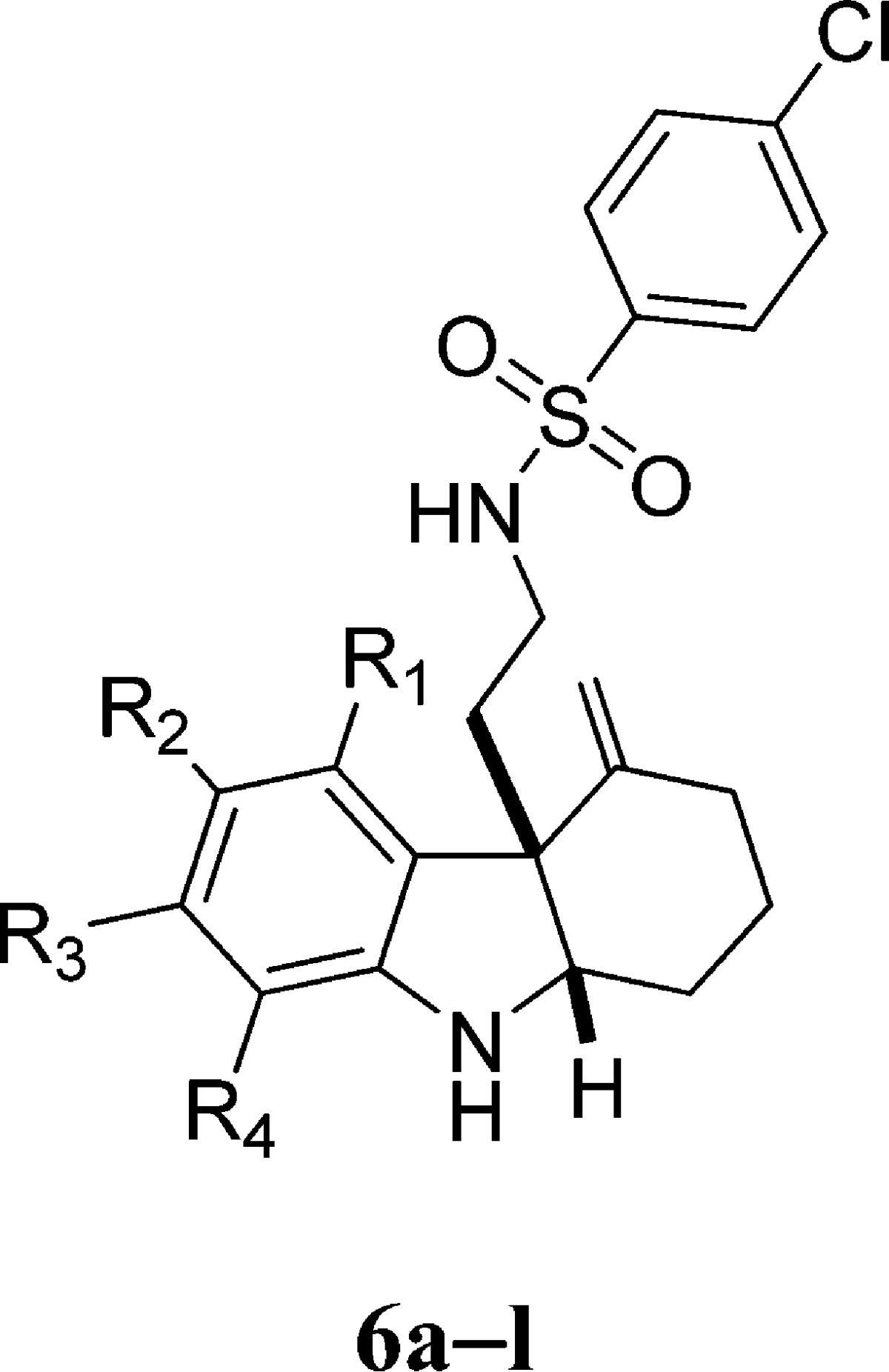
We next sought to modify the aromatic portion of the indoline ring. To prepare these analogues, we adapted the three-step synthetic route that we previously used for synthesis of the original library. As depicted in Scheme 2, cyclic imine 2 was reacted with different aryl hydrazines 3 and 4-chlorobenzenesulfonyl chloride to afford the alkynyl indole product 4 using a modified Fischer indole synthesis protocol.18 Cyclization under our standard gold catalysis conditions (5 mmol % Ph3PAuNTf2)19 followed by a reductive ring-opening reaction afforded a series of 1 analogues (6a–l). In order to test the necessity of the bromine (R2) at the 5-position of the indoline, we synthesized analogues replacing it with a methyl group (6b), a methoxy group (6c), or a hydrogen (6e). In the case of each of these modifications, RMA activity was abolished or greatly diminished for all three antibiotics tested. Moving the bromine to other positions on the indoline (e.g., 6i, 6h, and 6g) also significantly reduced its RMA activity. We thus concluded that the presence of a halogen at the R2 position is necessary for RMA activity. We further evaluated whether the identity of the halogen at the R2 position affects its activity. We found that RMA activity is optimal when bromine is maintained as the R2 halogen. However, although replacing the R2 bromine with chlorine (6a) reduced RMA activity somewhat, this analogue also showed significantly reduced toxicity against mammalian cells (Table 2). In addition, we also synthesized several 1 analogues with an additional halogen at the 7-position of indoline. We found that when the R2 halogen is chlorine, fluorine at R4 significantly reduces RMA activity (6j); however, when the identity of both R2 and R4 are chlorine (6k), the RMA activity remains similar to that of 1. Maintaining bromine at the R2 position and adding fluorine at R4 (6l), however, yield slightly improved RMA activity and reduced mammalian toxicity with respect to 1. On the basis of this series of modifications on the aromatic indoline ring, we concluded that in order to maintain RMA activity, the identity of the R2 position must be a halogen (bromine or chorine) and that the R2 position may be functionalized with a second halogen to improve or maintain activity.
Scheme 2. Synthesis of 6a–l.
Reagents and conditions: (a) pClPhSO2Cl, DMAP, 23 °C, 0.5 h, DMF; 2, 0.5 h; MsOH, then 3, 60–120 °C, 20 h; (b) Ph3PAuNTf2, 50 °C, toluene, 1–12 h; (c) AcOH, NaBH3CN, MeOH, 0 °C, 0.5 h. [Ph3PAuNTf2 = [bis(trifluoromethanesulfonyl)imidate](triphenylphosphine)gold(I).]
Table 2. MRC and GI50 Values for the Substitutions of Indoline Aromatic Ring.
| entry | compd | R1 | R2 | R3 | R4 | amox/clava,b | cefazolina,b | meropenema,c | GI50d |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | H | Br | H | H | 4 | 4 | 4 | 17.1 |
| 2 | 6a | H | Cl | H | H | 8 | 4 | 8 | 35 |
| 3 | 6b | H | Me | H | H | 16 | 16 | 32 | – |
| 4 | 6c | H | MeO | H | H | >32 | >32 | >32 | – |
| 5 | 6d | H | F | H | H | 16 | 16 | 16 | – |
| 6 | 6e | H | H | H | H | 16 | 16 | 16 | – |
| 7 | 6f | H | H | phenylene | >32 | >32 | >32 | – | |
| 8 | 6g | H | H | H | Br | >32 | 32 | 8 | – |
| 9 | 6h | H | H | Br | H | 16 | 16 | 16 | – |
| 10 | 6i | Br | H | H | H | 32 | 16 | 16 | – |
| 11 | 6j | H | Cl | H | F | 16 | 16 | 32 | – |
| 12 | 6k | H | Cl | H | Cl | 4 | 4 | 4 | 13.6 |
| 13 | 6l | H | Br | H | F | 2 | 4 | 4 | 18.1 |
MRC values are in μg/mL.
MRSA ATCC BAA-44.
MRSA ATCC 33592.
HeLa cells; GI50 values are in μg/mL.
Modifications of the Side Chain
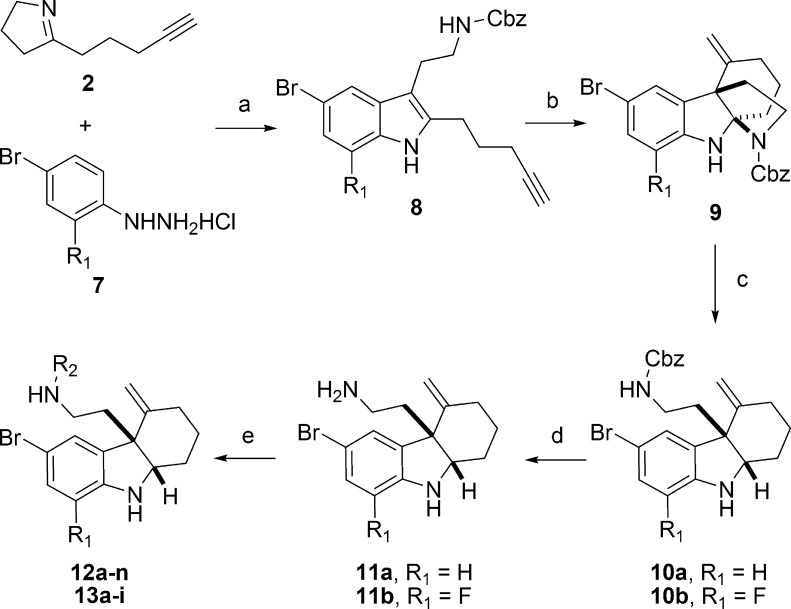
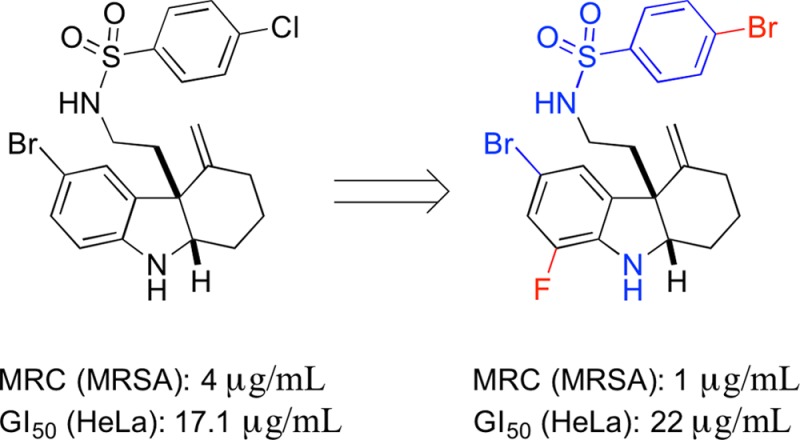
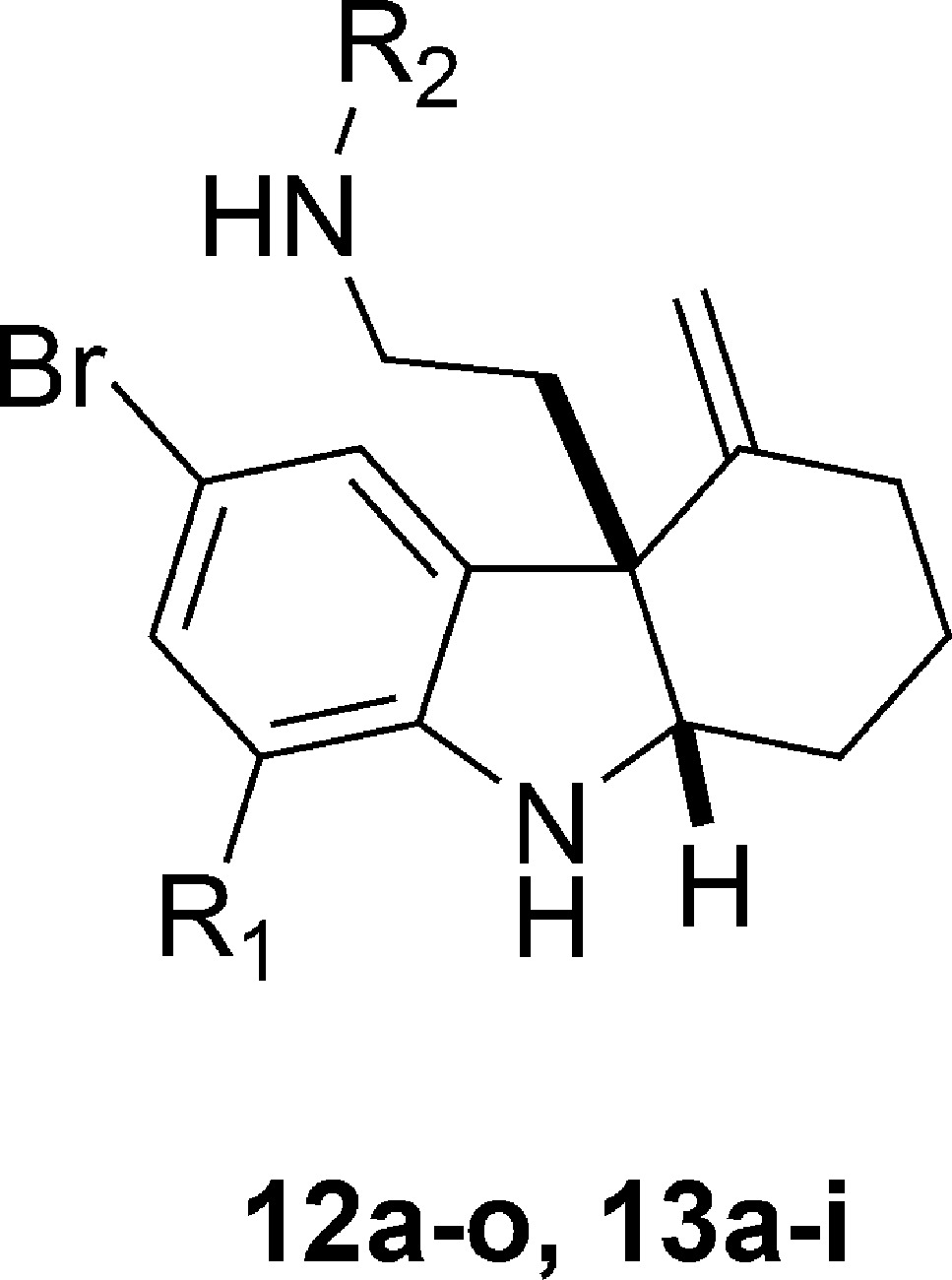
We next attempted to conduct SAR investigations on the side chain of 1. For this portion of our SAR investigation, we maintained the bromine group at the 5-position of the indoline moiety. We included several analogues in this portion of our study with fluorine at the 7-position of indoline, since 6l showed similar activity but lower mammalian cytotoxicity with respect to 1 (Table 2). The general synthetic route (Scheme 3) was similar to Scheme 2. For this series of 1 analogues, benzyl chloroformate was used as the active reagent in the indole synthesis step, which was removed successfully by treating ring-opening products 10a,b with boron trifluoride–dimethyl sulfide.20 The resulting free amine products 11a,b were further functionalized to afford compounds 12a–n and 13a–m. In addition, analogue 12o was prepared by the reduction of 12n with zinc dust in the presence of ammonium chloride.21 We first discovered that removing the side chain (R2) entirely resulted in severely reduced activity in the presence or absence of the R1 fluorine (11a,b). Likewise, replacing the sulfonamide with an amide (e.g., 12a–c) resulted in abolished RMA activity. We discovered the necessity of the phenyl ring by replacing it with a pyridine ring (12m), which resulted in a loss of RMA activity. We next attempted to modify the side chain phenyl ring. When the chlorine at the para position relative to the sulfonamide was removed from 1, the RMA activity is reduced at least 2-fold (12d). We thus attempted to modify the phenyl ring on the side chain with a series of functional groups. For these experiments, we synthesized analogues in which the chlorine was maintained and an additional chlorine was added to the meta or ortho position, respectively (12i,j). These disubstituted products did not result in improved RMA activity; however, in the presence of the fluorine at the 7-position of indoline (R1), reduced toxicity was observed for the disubstituted analogue 13f. The remainder of our modifications focused on the para position of the phenyl ring. Replacing the chlorine with a fluorine (i.e., 12f) abolished RMA activity entirely; however, this same modification in combination with the R1 as a fluorine (i.e., 13c) resulted in similar RMA activity relative to 1. A similar trend was also observed when the chlorine was replaced with an iodine in the absence (12h) or presence (13e) of the R1 fluorine. Interestingly, when the chlorine is replaced with a methyl group (12e), RMA activity is lost; however, it is regained when fluorine is added at the R1 position (13b). In addition, the mammalian toxicity of this analogue is reduced in this case. This trend was also observed when the chlorine was replaced with a cyanide (12k, 13h). Although substituting a methoxy group for the chlorine in the presence of the R1 fluorine (13a) results in slightly reduced activity, the mammalian toxicity is significantly reduced. Likewise, replacing the chlorine with a bromine (12g) resulted in slightly reduced RMA activity, and this analogue demonstrated a significant decrease in mammalian toxicity (Table 3). Intriguingly, when we added the R1 fluorine (13d) to this molecule, we observed a significant (at least 4-fold) increase in RMA activity as well as decreased toxicity relative to 1.
Scheme 3. Synthesis of 12a–n and 13a–i.
Reagents and conditions: (a) CbzCl, DMAP, 23 °C, 0.5 h, DMF; 2, 2–12 h; MsOH, then 3, 60–120 °C; (b) Ph3PAuNTf2, 50 °C, toluene, 1–12 h; (c) AcOH, NaBH3CN, MeOH, 0 °C, 0.5 h; (d) BF3·Et2O, Me2S, DCM, 23 °C, 1.5 h; (e) R2X, Et3N, DCM, 0 °C, 0.5–2 h.
Table 3. MRC and GI50 Values for 1 Analogues with Modifications on the Side Chain.
| entry | compd | R1 | R2 | amox/clava,b | cefazolina,b | meropenema,c | GI50d |
|---|---|---|---|---|---|---|---|
| 1 | 1 | H | SO2PhpCl | 4 | 4 | 4 | 17.1 |
| 2 | 11a | H | H | 32 | 32 | 32 | – |
| 3 | 11b | F | H | >32 | 32 | 16 | 16.2 |
| 4 | 12a | H | TFA | >32 | >32 | 32 | – |
| 5 | 12b | H | COBu | >32 | >32 | 32 | – |
| 6 | 12c | H | COPhpCl | >32 | >32 | >32 | – |
| 7 | 12d | H | SO2Ph | 8 | 8 | 16 | – |
| 8 | 12e | H | SO2PhpMe | >32 | >32 | >32 | – |
| 9 | 12f | H | SO2PhpF | >32 | >32 | >32 | – |
| 10 | 12g | H | SO2PhpBr | 4 | 4 | 8 | 40 |
| 11 | 12h | H | SO2PhpI | 4 | 4 | 32 | 33 |
| 12 | 12i | H | SO2Ph3,4Cl | 4 | 2 | 4 | 12.8 |
| 13 | 12j | H | SO2Ph2,4Cl | 8 | >32 | 4 | – |
| 14 | 12k | H | SO2PhpCN | 16 | 8 | 16 | – |
| 15 | 12l | H | SO2PhpNHAc | >32 | >32 | 32 | – |
| 16 | 12m | H | SO25Py | 32 | 32 | 32 | – |
| 17 | 12n | H | SO2PhpNO2 | >32 | >32 | >32 | – |
| 18 | 12o | H | SO2PhpNH2 | 16 | 16 | 16 | – |
| 20 | 13a | F | SO2PhpOMe | 8 | 4 | 8 | 49 |
| 21 | 13b | F | SO2PhpMe | 4 | 4 | 4 | 22 |
| 22 | 13c | F | SO2PhpF | 4 | 4 | 4 | 18.3 |
| 23 | 6l | F | SO2PhpCl | 2 | 4 | 4 | 18.1 |
| 24 | 13d | F | SO2PhpBr | 1 | 1 | 1 | 22 |
| 25 | 13e | F | SO2PhpI | 4 | 2 | 4 | 19.6 |
| 26 | 13f | F | SO2Ph3,4Cl | 4 | 4 | 4 | 31 |
| 27 | 13g | F | SO2PhpNHAc | 32 | 32 | 16 | 32 |
| 28 | 13h | F | SO2PhpCN | 8 | 4 | 4 | 17.0 |
| 29 | 13i | F | SO2PhpCF3 | 4 | 4 | 4 | 8.7 |
MRC values are in μg/mL.
MRSA ATCC BAA-44.
MRSA ATCC 33592.
HeLa cells; GI50 values are in μg/mL.
Evaluation of the Synergistic Activity and Hemolytic Activity of 13d
In order to assess the synergistic nature of 13d, we first investigated the antibacterial activity of 13d against both MRSA and methicillin-sensitive S. aureus (MSSA) strains. For this experiment, we employed a standard microdilution assay using MRSA ATCC BAA-44, MRSA ATCC 33592, and MSSA ATCC 25923. The assay was performed by following the procedure outlined by CLSI.22 The minimum inhibitory concentrations (MICs) of 13d against all strains was higher than 64 μg/mL, the highest concentration tested. Since 13d exhibited no antiproliferative effect on its own, we decided to confirm its synergistic activity with the three antibiotics tested in this report by performing the checkerboard (CB) test for synergy and calculating the fractional inhibitory concentration index (FICI) for each antibiotic tested in combination with 13d. CB assays and FICIs were set up and calculated as described previously.23 Briefly, 13d was diluted across 96-well microplates in MHB (8 to 0.016 μg/mL), and to each plate either amoxicillin/clavulanic acid (128 to 1 μg/mL), cefazolin (128 to 1 μg/mL), or meropenem (16 to 0.125 μg/mL) was diluted down the plate. Thusly, each well of the 96-well plate contained a unique concentration combination of 13d and antibiotic. FICI was calculated by the following formula: FICI = FICA + FICB, where FICA is the MIC of drug A in combination with B divided by the MIC of drug A on its own, and FICB is the MIC of drug B in combination divided by the MIC of drug B on its own. An FICI that is less than 0.5 indicates a synergistic drug interaction, an FICI in the range of 0.5–1 denotes an additive drug interaction, and an FICI greater than 1 indicates an antagonistic interaction. The FICI values of 13d for amoxicillin/clavulanic acid, cefazolin, and meropenem were 0.0315, 0.0156, and 0.0315, respectively. This result confirms the synergistic action of 13d in combination with all antibiotics tested in this study.
We further evaluated the toxicity of 13d by conducting a standard hemolytic assay as previously described.24,25 This assay measures the amount of hemoglobin leakage in compound-treated human red blood cells (hRBCs) and is used to measure the amount of membrane damage induced by drug treatment. The hemolytic activity assay was conducted for multiple concentrations of 13d. It caused less than 2% hemolysis of red blood cells at 64 μg/mL (64-fold above its MRC), the highest concentration tested. We thus concluded that compound 13d shows an insignificant level of toxicity against hRBCs.
Collectively, these results indicate that 13d is a more potent analogue of 1, with increased RMA activity, decreased toxicity, and potent synergy with multiple classes of β-lactam antibiotics. Furthermore, 13d shows no antiproliferative effect against MRSA or MSSA on its own.
Conclusions
We have synthesized a number of structural analogues of our tricyclic indoline RMA, 1, by taking advantage of the previously developed highly efficient synthetic approach with high functional group tolerance. These new analogues have been evaluated for their ability to potentiate three representative β-lactam antibiotics (amoxicillin/clavulanic acid, cefazolin, and meropenem) in MRSA and for their toxicity in mammalian cells. We found that neither the aniline nor the sulfonamide nitrogen can tolerate further modification. While the sulfonamide group on the side chain is crucial for the RMA activity, modifications of both aromatic systems can further fine-tune the RMA activity and the mammalian toxicity. Notably, we discovered that adding fluorine to the 7-position of the indoline increases RMA activity of the compound in multiple instances, including those in which another modification has reduced or eliminated RMA activity. Furthermore, we discovered that a number of substitutions may be added to the phenyl ring on the side chain, which will allow the development of additional 1 analogues for the discovery of its cellular target, a stepping stone to understanding the β-lactam resistome.26 In addition, we have discovered a more potent analogue of 1, compound 13d, with reduced mammalian toxicity and low hemolytic activity. We were able to confirm the synergistic activity of 13d by calculating its FICI and showed that it is highly synergistic in combination with all antibiotics tested. Further investigation of the mode of action of 1 and evaluation of its efficacy in vivo are ongoing and will be reported in due course.
Exprimental Section
Bacterial Strains
Strains ATCC BAA-44 (MRSA) and ATCC 25923 (MSSA) were gifts from the laboratories of Daniel Feldheim and Charles McHenry, respectively. Strain ATCC 33592 (MRSA) was purchased from ATCC (http://www.atcc.org).
Minimal Resensitizing Concentration (MRC) Determination
MRC screens were performed as described previously.16 Briefly, antibiotic MIC values where S. aureus is considered susceptible were determined from the CLSI handbook supplement.17 Analogues of 1 were diluted to 5 mg/mL in DMSO. Antibiotic was prepared at twice the intended final concentration in Mueller–Hinton broth (MHB). For amoxicillin/clavulanic acid, the initial concentration was 8/4 μg/mL; for meropenem, 8 μg/mL; and for cefazolin, 16 μg/mL. A 50 μL portion of the antibiotic containing media was added to each well of 96-well plates, and 100 μL was added to the top row. A 1.28 μL portion of of 5 mg/mL alkaloid solution was added to the top row of each plate to afford a concentration of 64 μg/mL in the top row of each plate, and 2-fold serial dilutions were performed down the columns. Once the plates were prepared, a day culture of MRSA was diluted to OD600 0.002, and 50 μL was added to each well. The final concentration of MRSA added was OD600 0.001, the final concentration of amoxicillin/clavulanic acid was 4/2 μg/mL, the final concentration of meropenem was 4 μg/mL, the final concentration of cefazolin was 8 μg/mL, and the highest concentration of 1 analogue tested was 32 μg/mL. Plates were incubated overnight at 37 °C with shaking. The MRC value was determined as the concentration of 1 analogue in the presence of antibiotic at which there was no observable overnight growth.
Microdilution Tests for Minimal Inhibitory Concentration (MIC) Determination
The minimal inhibitory concentrations (MICs) of active 1 analogues were determined by the broth microdilution method detailed in the CLSI handbook.22 All antimicrobial compounds were purchased from Sigma-Aldrich. The growth media used for all MIC experiments was MHB purchased from HIMEDIA through VWR (cat. 95039-356). The inoculum was prepared by diluting a bacterial day culture (OD600 0.15–0.4) to OD600 0.002. This dilution was further diluted 2-fold when added to 96-well microplates (USA Scientific CytoOne 96-well TC plate, cat. CC7682-7596) for a final inoculum concentration of OD600 0.001. All plates were incubated at 37 °C with shaking for 18 h before results were interpreted.
Microdilution Checkerboard Tests for Drug Synergy
Checkerboard assays were performed as described previously.23 Antibiotics were diluted down the columns of a 96-well microplate, while 13d was diluted across the rows. Plates were prepared that contained concentrations of antibiotics and 13d 2-fold higher than the intended final concntrations and were prepared in duplicate. All antimicrobial compounds were purchased from Sigma-Aldrich. The growth media was MHB purchased from HIMEDIA through VWR (cat. 95039-356). The inoculum was prepared by diluting a bacterial day culture (OD600 0.15–0.4) to OD600 0.002. This dilution was further diluted 2-fold when added to 96-well microplates (USA Scientific CytoOne 96-well TC plate, cat. CC7682-7596) for a final inoculum concentration of OD600 0.001. All plates were incubated at 37 °C with shaking for 18 h before results were interpreted.
Mammalian Cytotoxicity of 1 Analogues in HeLa Cells
To evaluate the cytotoxicity of 1 in mammalian cells, a cell viability assay was carried out using a CellTiter-Glo luminescent cell viability assay kit (Promega). Human cervixcal adenocarcinoma HeLa cells were seeded on white, cell-culture-treated, 96-well plates (Corning 3917) with Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal calf serum (FCS), 1% penicillin/streptomycin, at the densities of 20 000 cells/well. The medium volume for each well was 100 μL. Cells were incubated at 37 °C in 5% CO2/95% air for 16 h. The medium was removed from each well and replaced with 99 μL of warmed, fresh medium. To each well, 1 μL of 1 analogue was added in DMSO to final concentrations of 0.5–32 μg/mL. Each series was performed in triplicate. After incubation at 37 °C for another 24 h, the plates were equilibrated to room temperature for 30 min, and 100 μL of CellTiter-Glo reagent (Promega) was added to each well and mixed for 2 min on an orbital shaker. The plate was incubated at room temperature for another 10 min to stabilize the luminescent signal. The luminescence of each sample was recorded with an Envision Multilabel Plate Reader (PerkinElmer).
Hemolytic Activity Assay
Eight milliliters of freshly drawn, heparin-stabilized, human blood was centrifuged at 3500 rpm for 5 min. The supernatant was removed and the human red blood cells (hRBCs) were washed with 8 mL of D-PBS three times or until the supernatant was clear. hRBCs were then resuspended in 80 mL of D-PBS. This was further diluted in D-PBS to a final concentration of 1% of the original pellet volume. 13d was dissolved in DMSO and to 1 mL samples of the hRBC solution to final concentrations of 64, 16, and 4 μg/mL. A 1% Triton X-100 sample was used as the positive control; this was considered to produce 100% hemolysis. DMSO alone was used as the negative control. The mixtures were vortexed gently and incubated at 37 °C for 1 h with shaking. The mixtures were then centrifuged at 3000 rpm for 10 min, and 50 μL of supernatant from each sample was transferred to a well of a sterile 96-well plate containing 50 μL of water. The presence of hemoglobin was measured by absorbance at 415 nm, and the percent hemolysis was calculated using the following equation:
Each condition was assayed in triplicate. The assay was performed within 3 h of the blood draw.
Chemistry
Unless otherwise noted, reagents were obtained commercially and used without further purification. CH2Cl2 was distilled from CaH2 under a nitrogen atmosphere. THF was distilled from sodium–benzophenone under a nitrogen atmosphere. Toluene was distilled from sodium under a nitrogen atmosphere. Thin-layer chromatography (TLC) analysis of reaction mixtures was performed on Dynamicadsorbents silica gel F-254 TLC plates. Flash chromatography was carried out on Zeoprep 60 ECO silica gel. 1H and 13C NMR spectra were recorded with Varian INOVA (400, 500 MHz) and Bruker Avance-III (300 MHz) spectrometers. Mass spectral and analytical data were obtained via the PE SCIEX/ABI API QSTAR Pulsar iHybrid LC/MS/MS (Applied Biosystems) operated by the Central Analytical Laboratory, University of Colorado at Boulder. Infrared (IR) spectra were recorded on a Thermo Nicolet Avatar 370 FT-IR spectrometer. Melting point (mp) determinations were performed by using a Thomas-Hoover capillary melting point apparatus and are uncorrected. Compound purity (≥95%) was confirmed on the basis of the integration of the area under the UV absorption curve at λ = 254 or 210 nm signals using an Agilent 1260 series HPLC system coupled with a 6120 Quadrupole mass spectrometer (column: ZORBAX Narrow Bore SB-C18 RRHT, 2.1 × 50 mm, 1.8 μm, PN 827700-902). The system was eluted at 0.5 mL/min with a gradient of water/acetonitrile with 0.1% formic acid: 0–5 min, 5–95% acetonitrile; 5–7 min, 95% acetonitrile; 7–7.25 min, 95–5% acetonitrile; 7.25–8.5 min, 5% acetonitrile.
General Protocol for the One-Pot Three-Component Indole Synthesis
The activating agent 4-chlorobenzenesulfonyl chloride (1.2 equiv) was added to a solution of 4-dimethylaminopyridine (1.2 equiv) in anhydrous DMF at 0 °C. The reaction was stirred at 23 °C for 30 min. A solution of the alkynyl imine 2 (1.0 equiv) in anhydrous DMF was added and the reaction was stirred at the same temperature for 0.5 h. Then methanesulfonic acid (3.0 equiv) was next added to the above mixture at 0 °C. The reaction was then stirred at 23 °C for 2 h. Arylhydrazine 3 (1.5 equiv) was added and the mixture stirred for an addition 1 h at 23 °C. The reaction was then heated to 60–120 °C (60 °C for electron-rich arylhydrazines and 120 °C for electron-poor arylhydrazines for 20 h). The reaction was cooled down to room temperature. The residue was then dissolved in ethyl acetate and washed with brine and a saturated aqueous solution of sodium bicarbonate. The combined organic layers were dried over anhydrous Na2SO4, filtered, and concentrated in vacuo to give a crude product, which was purified by column chromatography on silica gel to give the indole product 4. 4-Chloro-N-{2-[5-chloro-2-(pent-4-yn-1-yl)-1H-indol-3-yl]ethyl}benzene-1-sulfonamide (4a): TLC (hexanes:ethyl acetate, 3:1 v/v) Rf = 0.20; light yellow oil, 32%; 1H NMR (500 MHz, CDCl3) δ 7.99 (s, 1H), 7.66 (d, J = 8.6 Hz, 2H), 7.41 (d, J = 8.6 Hz, 2H), 7.24 (d, J = 2.0 Hz, 1H), 7.21 (d, J = 8.5 Hz, 1H), 7.10 (dd, J = 8.6, 2.0 Hz, 1H), 4.40 (t, J = 6.2 Hz, 1H), 3.25 (q, J = 6.5 Hz, 2H), 2.89 (dt, J = 16.4, 7.1 Hz, 4H), 2.23 (td, J = 6.8, 2.7 Hz, 2H), 2.09 (t, J = 2.6 Hz, 1H), 1.85 (p, J = 7.1 Hz, 2H); 13C NMR (75 MHz, CDCl3) δ 139.1, 138.1, 137.4, 133.7, 129.3, 129.0, 128.3, 125.3, 121.8, 117.3, 111.6, 107.1, 83.5, 69.9, 43.0, 28.2, 24.5, 24.5, 17.7; HRMS (ESI) m/z calcd for C21H21Cl2N2O2S [M + H]+ 435.0695, found 435.0694; IR (thin film) 3377, 3297, 2924, 2854, 2359, 1575, 1475, 1325, 1160, 1094, 828 cm–1.
General Protocol for Gold(I)-Catalyzed Tandem Cyclization
To a suspension of Ph3PAuNTf2 (as the 2:1 toluene adduct) (0.05 equiv) in anhydrous toluene was added a solution of indole 4 (1.0 equiv) in anhydrous toluene. The suspension was heated to 50 °C until TLC showed that there was no starting material left (1–12 h) under argon atmosphere. The reaction mixture was then filtered through a short pad of silica gel. The filtrate was concentrated in vacuo and the residue was purified by column chromatography on silica gel to afford tetracyclic indoline product 5. 4-Chloro-16-(4-chlorobenzenesulfonyl)-13-methylidene-8,16-diazatetracyclo[7.4.3.01,9.02,7]hexadeca-2,4,6-triene (5a): TLC (hexanes:ethyl acetate, 3:1 v/v) Rf = 0.60; light yellow oil, 82%; 1H NMR (500 MHz, CDCl3) δ 7.61 (d, J = 8.8 Hz, 2H), 7.38 (d, J = 8.8 Hz, 2H), 7.05–7.03 (m, 2H), 6.51 (d, J = 8.1 Hz, 1H), 5.19 (brs, 1H), 4.92 (s, 1H), 4.77 (s, 1H), 3.48 (td, J = 8.7, 1.7 Hz, 1H), 3.03–2.98 (m, 1H), 2.72 (dt, J = 14.2, 3.8 Hz, 1H), 2.37 (ddd, J = 12.7, 10.5, 8.4 Hz, 1H), 2.24–2.12 (m, 3H), 1.93–1.86 (m, 1H), 1.75 (dt, J = 13.7, 4.5 Hz, 1H), 1.63–1.55 (m, 1H); 13C NMR (75 MHz, CDCl3) δ 147.2, 146.4, 138.9, 138.4, 133.0, 129.2, 128.4, 128.3, 124.6, 123.8, 112.8, 111.0, 93.5, 61.1, 47.4, 33.8, 32.0, 30.3, 22.8; HRMS (ESI) m/z calcd for C21H21Cl2N2O2S [M + H]+ 435.0695, found 435.0691; IR (thin film) 3054, 2986, 2926, 2850, 1478, 1423, 1335, 1265, 1156, 1090, 1001, 740 cm–1.
General Protocol for the Ring-Opening Reduction
To a solution of the tetracyclic indoline 5 (1.0 equiv) in anhydrous methanol was added acetic acid (2.0 equiv) and sodium cyanoborohydride (4.0 equiv) at 0 °C. The resulting mixture was stirred at 23 °C for 0.5 h. The solvent was removed in vacuo to give a residue, which was dissolved in ethyl acetate, and the organic layers were washed with a saturated aqueous solution of sodium bicarbonate. The combined organic layers were dried over anhydrous Na2SO4, filtered, and concentrated in vacuo to give a crude product, which was purified by column chromatography on silica gel to afford the product 6. 4-Chloro-N-[2-(6-chloro-4-methylidene-2,3,4,4a,9,9a-hexahydro-1H-carbazol-4a-yl)ethyl]benzene-1-sulfonamide (6a): TLC (hexanes:ethyl acetate, 3:1 v/v) Rf = 0.20; light yellow oil, 92%; 1H NMR (500 MHz, CDCl3) δ 7.77 (d, J = 8.6 Hz, 2H), 7.49 (d, J = 8.6 Hz, 2H), 7.01 (dd, J = 8.3, 2.2 Hz, 1H), 6.87 (s, 1H), 6.58 (d, J = 8.3 Hz, 1H), 5.13 (d, J = 5.9 Hz, 1H), 4.94 (s, 1H), 4.67 (s, 1H), 3.63 (t, J = 4.9 Hz, 1H), 3.00–2.89 (m, 1H), 2.20–2.11 (m, 2H), 2.07–2.01 (m, 1H), 1.98–1.93 (m, 1H), 1.81–1.76 (m, 1H), 1.70–1.57 (m, 2H), 1.52–1.46 (m, 1H); 13C NMR (75 MHz, CDCl3) δ 148.6, 148.2, 139.2, 138.4, 135.1, 129.4, 128.5, 127.8, 124.2, 123.8, 112.1, 111.4, 77.5, 77.0, 76.6, 65.4, 52.3, 40.2, 36.1, 32.4, 28.6, 21.7; HRMS (ESI) m/z calcd for C21H23Cl2N2O2S [M + H]+ 437.0852, found 437.0857; IR (thin film) 2264, 3285, 2934, 2858, 1637, 1604, 1587, 1477, 1428, 1328, 1160, 1093, 1014,905, 827, 617 cm–1.
General Protocol for the Removal of a Cbz Group
To a solution of substrate 10 (1.0 equiv) in dry dichloromethane was added boron trifluoride diethyl etherate (5.0 equiv) and dimethyl sulfide (10.0 equiv) dropwise at 23 °C. The resulting mixture was stirred at this temperature for 2 h. After that, TLC showed that there was no starting material left; the mixture was poured into water and 10% aqueous ammonium hydroxide and extracted with chloroform three times. The combined extracts were washed with water and then brine, dried over anhydrous Na2SO4, filtered, and concentrated in vacuo to give a crude product, which was purified by column chromatography on silica gel to afford the product 11. 2-(6-Bromo-4-methylidene-2,3,4,4a,9,9a-hexahydro-1H-carbazol-4a-yl)ethan-1-amine (11a): TLC (chloroform:methanol, 10:1 v/v): Rf = 0.10; yellow oil, 72%; 1H NMR (500 MHz, CDCl3) δ 7.20 (d, J = 2.0 Hz, 1H), 7.15 (dd, J = 8.3, 2.1 Hz, 1H), 6.55 (d, J = 8.2 Hz, 1H), 4.88 (s, 1H), 4.60 (d, J = 1.5 Hz, 1H), 3.72 (d, J = 3.2 Hz, 1H), 3.64 (s, 1H), 2.86–2.80 (m, 1H), 2.73–2.67 (m, 1H), 2.25–2.16 (m, 3H), 2.01–1.98 (m, 1H), 1.94–1.88 (m, 1H), 1.85–1.79 (m, 1H), 1.77–1.67 (m, 2H), 1.63–1.58 (m, 1H); 13C NMR (75 MHz, CDCl3) δ 149.8, 149.0, 137.1, 130.2, 127.4, 111.6, 111.6, 110.2, 65.6, 52.5, 39.3, 39.0, 32.3, 29.7, 27.6, 22.0; HRMS (ESI) m/z calcd for C15H20BrN2 [M + H]+ 307.0804, found 307.0811; IR (thin film) 3367, 2960, 2924, 2853, 1729, 1661, 1600, 1464, 1261, 1092, 870, 802 cm–1.
General Protocol for the Modification of Free Amine
To a solution of substrate 11 (1.0 equiv) in dry dichloromethane was added triethyl amine (3.0 equiv) and the corresponding sulfonyl chloride or acetyl chloride (1.2 equiv) dropwise at 0 °C. The resulting mixture was stirred at this temperature for 15 min. The reaction was quenched by aqueous sodium bicarbonate and extracted with dichloromethane three times. The combined extracts were washed with water and then brine, dried over anhydrous Na2SO4, filtered, and concentrated in vacuo to give a crude product, which was purified by column chromatography on silica gel to afford the product 12. N-[2-(6-Bromo-4-methylidene-2,3,4,4a,9,9a-hexahydro-1H-carbazol-4a-yl)ethyl]-2,2,2-trifluoroacetamide (12a): TLC (hexanes:ethyl acetate, 3:1 v/v) Rf = 0.40; yellow oil, 82%; 1H NMR (500 MHz, CDCl3) δ 7.20 (dd, J = 8.2, 2.0 Hz, 1H), 7.14 (d, J = 2.0 Hz, 1H), 6.72 (brs, 1H), 6.60 (d, J = 8.2 Hz, 1H), 5.03 (s, 1H), 4.83 (s, 1H), 3.69 (t, J = 5.2 Hz, 1H), 3.46–3.34 (m, 2H), 2.27–2.21 (m, 1H), 2.19–2.14 (m, 1H), 2.13–2.00 (m, 2H), 1.90–1.84 (m, 1H), 1.72–1.60 (m, 2H), 1.53–1.47 (m, 1H); 13C NMR (75 MHz, CDCl3) δ 156.8, 148.3, 135.5, 130.9, 127.0, 112.4, 112.1, 65.4, 52.3, 36.9, 35.4, 32.5, 29.0, 21.6; HRMS (ESI) m/z calcd for C17H19BrF3N2O [M + H]+ 403.0627, found 403.0622; IR (thin film) 3322, 2957, 2928, 2861, 1663, 1599, 1530, 1463, 1378, 1008, 823 cm–1.
General Protocol for the Modification of 1’s Indoline Amine
To a solution of substrate 1 (1.0 equiv) in dry acetonitrile was added potassium carbonate (3.0 equiv) and the corresponding iodomethane or sulfonyl chloride or acetyl chloride (1.2 equiv) dropwise at 0 °C. The resulting mixture was stirred at this temperature for 15 min. The reaction was quenched by aqueous sodium bicarbonate and extracted with dichloromethane three times. The combined extracts were washed with water and then brine, dried over anhydrous Na2SO4, filtered, and concentrated in vacuo to give a crude product, which was purified by column chromatography on silica gel to afford the products 1a–c. N-{2-[6-Bromo-9-(4-chlorobenzenesulfonyl)-4-methylidene-2,3,4,4a,9,9a-hexahydro-1H-carbazol-4a-yl]ethyl}-4-chlorobenzene-1-sulfonamide (1b): TLC (hexanes:ethyl acetate, 2:1 v/v) Rf = 0.60; light yellow oil, 72%; 1H NMR (500 MHz, CDCl3) δ 7.82 (d, J = 8.6 Hz, 2H), 7.74 (d, J = 8.6 Hz, 2H), 7.57–7.45 (m, 5H), 7.38 (dd, J = 8.6, 2.1 Hz, 1H), 6.99 (d, J = 2.1 Hz, 1H), 5.13 (d, J = 2.0 Hz, 1H), 4.86 (d, J = 2.0 Hz, 1H), 4.11 (dd, J = 7.9, 5.7 Hz, 1H), 3.98 (d, J = 5.1 Hz, 1H), 2.83–2.77 (m, 1H), 2.74–2.64 (m, 1H), 2.29–2.24 (m, 1H), 2.05–1.98 (m, 1H), 1.96–1.89 (m, 1H), 1.69–1.61 (m, 2H), 1.21–1.15 (m, 1H); 13C NMR (75 MHz, CDCl3) δ 144.8, 140.1, 139.4, 139.2, 138.4, 138.2, 137.2, 131.9, 129.8, 129.5, 128.4, 128.3, 127.0, 117.0, 116.8, 113.7, 68.3, 52.1, 39.0, 38.3, 31.6, 29.7, 19.9; HRMS (ESI) m/z calcd for C27H25BrCl2KN2O4S2 [M + K]+ 692.9448, found 692.9451; IR (thin film) 2292, 2920, 2850, 1638, 1475, 1360, 1338, 1166, 1093, 826, 756 cm–1.
Protocol for the Synthesis of 1d
To a solution of sodium hydride (0.7 mg, 0.02 mmol) in dry N,N-dimethylformamide was added 1 (6.0 mg, 0.012 mmol) dropwise at 0 °C. After 15 min, iodomethane (3.9 μL, 0.06 mmol) was added dropwise at 0 °C. The resulting mixture was stirred at this temperature for 15 min and then warmed up to 60 °C for 30 min. The reaction was quenched by aqueous ammonium chloride and extracted with ethyl acetate three times. The combined extracts were washed with water and then brine, dried over anhydrous Na2SO4, filtered, and concentrated in vacuo to give a crude product, which was purified by column chromatography on silica gel to afford the product 1d. N-[2-(6-Bromo-4-methylidene-2,3,4,4a,9,9a-hexahydro-1H-carbazol-4a-yl)ethyl]-4-chloro-N-methylbenzene-1-sulfonamide (1d): TLC (hexanes:ethyl acetate, 2:1 v/v) Rf = 0.45; light yellow oil, 5.5 mg, 89%; 1H NMR (500 MHz, CDCl3) δ 7.70 (d, J = 8.5 Hz, 2H), 7.50 (d, J = 8.5 Hz, 2H), 7.16 (dd, J = 8.2, 2.0 Hz, 1H), 7.09 (s, 1H), 6.55 (d, J = 8.2 Hz, 1H), 4.95 (s, 1H), 4.66 (s, 1H), 3.70 (t, J = 4.5 Hz, 1H), 3.18 (ddd, J = 13.6, 11.2, 5.4 Hz, 1H), 2.97 (ddd, J = 13.6, 11.0, 5.1 Hz, 1H), 2.78 (s, 3H), 2.14–2.00 (m, 3H), 1.87–1.79 (m, 1H), 1.73–1.66 (m, 2H); 13C NMR (75 MHz, CDCl3) δ 149.4, 139.1, 135.9, 130.6, 129.5, 129.4, 129.1, 128.7, 126.7, 111.8, 110.0, 109.6, 61.7, 51.4, 47.4, 37.9, 35.2, 33.1, 32.8, 21.8; HRMS (ESI) m/z calcd for C22H24BrClKN2O2S [M + K]+ 533.0062, found 533.0068; IR (thin film) 3371, 2925, 2853, 2359, 1636, 1474, 1344, 1260, 1160, 1104, 1014, 804 cm–1.
General Protocol for the Modification of 1’s Side Chain Amine
To a solution of substrate 1 (1.0 equiv) in dry dichloromethane was added triethylamine (3.0 equiv) and the corresponding sulfonyl chloride or acetyl chloride (1.2 equiv) dropwise at 0 °C. The resulting mixture was stirred at this temperature for 15 min. The reaction was quenched by aqueous sodium bicarbonate and extracted with dichloromethane three times. The combined extracts were washed with water and then brine, dried over anhydrous Na2SO4, filtered, and concentrated in vacuo to give a crude product, which was purified by column chromatography on silica gel to afford the product 1e or 1f. 2-(6-Bromo-4-methylidene-2,3,4,4a,9,9a-hexahydro-1H-carbazol-4a-yl)-N-(4-chlorobenzenesulfonyl)-S-(4-chlorophenyl)ethane-1-sulfonamido (1e): TLC (hexanes:ethyl acetate, 2:1 v/v) Rf = 0.75; colorless oil, 52%; 1H NMR (500 MHz, CDCl3) δ 7.94 (d, J = 8.7 Hz, 4H), 7.55 (d, J = 8.7 Hz, 4H), 7.25–7.12 (m, 2H), 6.57 (d, J = 8.2 Hz, 1H), 4.97 (s, 1H), 4.71 (s, 1H), 3.83 (ddd, J = 15.4, 12.5, 5.3 Hz, 1H), 3.68 (t, J = 4.4 Hz, 1H), 3.51 (ddd, J = 15.2, 12.3, 4.6 Hz, 1H), 2.33–2.12 (m, 4H), 1.89–1.79 (m, 1H), 1.75–1.67 (m, 3H); 13C NMR (75 MHz, CDCl3) δ 148.9, 148.1, 141.0, 138.0, 135.0, 130.8, 129.8, 127.7, 124.6, 112.3, 111.9, 110.2, 65.1, 52.1, 39.4, 35.5, 32.3, 20.0; HRMS (ESI) m/z calcd for C27H26BrCl2N2O4S2 [M + H]+ 654.9889, found 654.9871; IR (thin film) 2922, 2851, 1736, 1655, 1476, 1377, 1167, 1080, 826, 756, 620 cm–1.
Protocol for the Reduction of 12o
To a solution of substrate 12n (4.0 mg, 0.008 mmol) in methanol was added a solution of ammonium chloride (8.7 mg, 0.162 mmol) in water and zinc dust (5.3 mg, 0.08 mmol) at room temperature. The resulting mixture was stirred for 6 h and then filtered. The filtrate was removed under reduce pressure and the residue was diluted with 2 N sodium hydroxide and extracted with ethyl acetate three times. The combined extracts were washed with water and then brine, dried over anhydrous Na2SO4, filtered, and concentrated in vacuo to give a crude product, which was purified by column chromatography on silica gel to afford the product 12o. 2-Amino-N-[2-(6-bromo-4-methylidene-2,3,4,4a,9,9a-hexahydro-1H-carbazol-4a-yl)ethyl]benzene-1-sulfonamide (12o): TLC (hexanes:ethyl acetate, 1:1 v/v) Rf = 0.30; colorless oil, 2.9 mg, 79%; 1H NMR (500 MHz, CDCl3) δ 7.70 (d, J = 7.9 Hz, 1H), 7.35 (t, J = 7.7 Hz, 1H), 7.19–7.12 (m, 1H), 7.01 (s, 1H), 6.84 (t, J = 7.6 Hz, 1H), 6.79 (d, J = 8.2 Hz, 1H), 6.52 (d, J = 8.3 Hz, 1H), 4.97 (d, J = 6.8 Hz, 1H), 4.89 (s, 1H), 4.58 (s, 1H), 3.60 (s, 1H), 2.98–2.88 (m, 2H), 2.13 (q, J = 4.7 Hz, 2H), 1.98 (ddd, J = 15.2, 9.2, 6.2 Hz, 1H), 1.89 (ddd, J = 14.5, 9.0, 5.9 Hz, 1H), 1.77–1.73 (m, 1H), 1.69–1.62 (m, 2H), 1.52–1.47 (m, 1H); 13C NMR (75 MHz, CDCl3) δ 148.7, 147.4, 138.0, 136.0, 135.8, 134.3, 133.1, 130.6, 129.5, 126.7, 121.7, 117.5, 112.0, 111.7, 110.5, 65.2, 52.2, 40.2, 35.4, 32.2, 28.4, 21.6; HRMS (ESI) m/z calcd for C21H25BrN3O2S [M + H]+ 462.0845, found 462.0835; IR (thin film) 3370, 2924, 2853, 1712, 1599, 1481, 1454, 1318, 1260, 901, 809, 754 cm–1.
N-{2-[6-Bromo-9-(4-chlorobenzoyl)-4-methylidene-2,3,4,4a,9,9a-hexahydro-1H-carbazol-4a-yl]ethyl}-4-chlorobenzene-1-sulfonamide (1c)
TLC (hexanes:ethyl acetate, 2:1 v/v) Rf = 0.60; light yellow oil, 92%; 1H NMR (500 MHz, CDCl3) δ 7.74 (d, J = 8.6 Hz, 2H), 7.49 (d, J = 8.6 Hz, 2H), 7.44 (q, J = 8.2 Hz, 4H), 7.10 (d, J = 2.0 Hz, 1H), 5.22 (s, 1H), 5.01 (s, 1H), 4.77–4.68 (m, 1H), 4.30 (brs, 1H), 3.11–3.04 (m, 1H), 2.99–2.87 (m, 1H), 2.29 (d, J = 14.5 Hz, H), 2.04 (d, J = 10.0 Hz, 1H), 1.97–1.88 (m, 2H), 1.54 (dt, J = 13.5, 4.3 Hz, 1H); 13C NMR (75 MHz, CDCl3) δ 173.0, 144.9, 139.6, 139.4, 138.2, 137.0, 134.1, 131.2, 129.5, 129.2, 128.7, 128.5, 126.5, 117.2, 113.8, 67.4, 51.4, 39.7, 37.7, 32.7, 21.4; HRMS (ESI) m/z calcd for C28H25BrCl2KN2O3S [M + K]+ 656.9778, found 656.9780; IR (thin film) 2916, 2849, 1631, 1587, 1469, 1422, 1380, 1331, 1163, 1089, 1014, 829, 740 cm–1.
N-[2-(6-Bromo-4-methylidene-2,3,4,4a,9,9a-hexahydro-1H-carbazol-4a-yl)ethyl]-4-chloro-N-(4-chlorobenzenesulfonyl)benzamide (1f)
TLC (hexanes:ethyl acetate, 2:1 v/v) Rf = 0.70; colorless oil, 92%; 1H NMR (500 MHz, CDCl3) δ 7.84 (d, J = 8.6 Hz, 2H), 7.51 (d, J = 8.6 Hz, 2H), 7.41 (s, 5H), 7.14 (dd, J = 8.2, 2.0 Hz, 1H), 6.88 (d, J = 2.0 Hz, 1H), 6.51 (d, J = 8.2 Hz, 1H), 4.91 (s, 1H), 4.60 (s, 1H), 3.97–3.86 (m, 1H), 3.76–3.66 (m, 1H), 3.62 (t, J = 4.6 Hz, 1H), 2.21–2.09 (m, 4H), 2.07 (t, J = 4.6 Hz, 1H), 1.82–1.76 (m, 1H), 1.70–1.64 (m, 4H); 13C NMR (75 MHz, CDCl3) δ 170.2, 144.4, 140.8, 139.7, 138.4, 136.7, 133.0, 131.2, 129.6, 129.5, 129.5, 129.1, 129.0, 128.6, 126.5, 119.1, 117.1, 114.0, 67.5, 51.4, 44.3, 36.6, 32.8, 21.6; HRMS (ESI) m/z calcd for C28H25BrCl2KN2O3S [M + K]+ 656.9778, found 656.9777; IR (thin film) 3374, 2921, 2851, 1637, 1465, 1376, 1261, 1165, 1091, 1013, 827, 758 cm–1.
4-Chloro-N-{2-[5-methyl-2-(pent-4-yn-1-yl)-1H-indol-3-yl]ethyl}benzene-1-sulfonamide (4b)
TLC (hexanes:ethyl acetate, 3:1 v/v) Rf = 0.20; light yellow oil, 41%; 1H NMR (500 MHz, CDCl3) δ 7.88 (s, 1H), 7.63 (d, J = 8.8 Hz, 2H), 7.37 (d, J = 8.7 Hz, 2H), 7.18 (d, J = 8.2 Hz, 1H), 7.09 (s, 1H), 6.98 (dd, J = 8.3, 1.7 Hz, 1H), 4.50 (t, J = 6.1 Hz, 1H), 3.25 (q, J = 6.5 Hz, 2H), 2.92 (t, J = 6.7 Hz, 2H), 2.83 (t, J = 7.4 Hz, 2H), 2.42 (s, 3H), 2.20 (td, J = 6.8, 2.7 Hz, 2H), 2.08 (t, J = 2.5 Hz, 1H), 1.85–1.80 (m, 2H); 13C NMR (75 MHz, CDCl3) δ138.9, 138.2, 135.9, 133.7, 129.2, 128.9, 128.4, 128.1, 123.2, 117.5, 110.3, 106.7, 83.7, 69.7, 43.2, 28.3, 24.5, 24.5, 21.5, 17.7; HRMS (ESI) m/z calcd for C22H24ClN2O2S [M + H]+ 415.1242, found 415.1249; IR (thin film) 3394, 3298, 2937, 2860, 1587, 1477, 1456, 1326, 1161, 1093, 828, 754, 619 cm–1.
4-Chloro-N-{2-[5-methoxy-2-(pent-4-yn-1-yl)-1H-indol-3-yl]ethyl}benzene-1-sulfonamide (4c)
TLC (hexanes:ethyl acetate, 3:1 v/v) Rf = 0.25; colorless oil, 42%; 1H NMR (500 MHz, CDCl3) δ 7.97–7.90 (brm, 1H), 7.64 (d, J = 8.7 Hz, 2H), 7.37 (d, J = 8.4 Hz, 2H), 7.17 (d, J = 8.5 Hz, 1H), 6.86–6.76 (m, 2H), 4.71–4.59 (m, 1H), 3.82 (s, 3H), 3.22 (q, J = 6.6 Hz, 2H), 2.92 (t, J = 6.8 Hz, 2H), 2.81 (t, J = 7.5 Hz, 2H), 2.19 (td, J = 6.9, 2.8 Hz, 2H), 2.07 (s, 1H), 1.81 (p, J = 7.2 Hz, 2H); 13C NMR (75 MHz, CDCl3) δ 154.1, 138.9, 138.2, 136.6, 130.5, 129.2, 128.4, 128.4, 111.3, 111.2, 107.1, 100.3, 83.7, 69.7, 55.9, 43.2, 28.3, 24.6, 24.6, 17.7; HRMS (ESI) m/z calcd for C22H24ClN2O2S [M + H]+ 431.1191, found 431.1194; IR (thin film) 3395, 3296, 2940, 2833, 1683, 1500, 1455, 1434, 1396, 1327, 1217, 1159, 1095, 1030, 1014, 829, 737 cm–1.
4-Chloro-N-{2-[5-fluoro-2-(pent-4-yn-1-yl)-1H-indol-3-yl]ethyl}benzene-1-sulfonamide (4d)
TLC (hexanes:ethyl acetate, 3:1 v/v) Rf = 0.25; light yellow oil, 22%; 1H NMR (500 MHz, CDCl3) δ 8.01 (s, 1H), 7.66 (d, J = 8.2 Hz, 2H), 7.39 (d, J = 8.2 Hz, 2H), 7.19 (dd, J = 8.7, 4.3 Hz, 1H), 6.94 (dd, J = 9.5, 2.5 Hz, 1H), 6.88 (td, J = 9.0, 2.5 Hz, 1H), 4.56 (t, J = 6.3 Hz, 1H), 3.23 (q, J = 6.6 Hz, 2H), 2.91–2.83 (m, 4H), 2.21 (dt, J = 6.9, 3.8 Hz, 2H), 2.08 (d, J = 2.6 Hz, 1H), 1.84 (t, J = 7.2 Hz, 2H); 13C NMR (75 MHz, CDCl3) δ 159.3, 156.2, 139.1, 138.2, 137.7, 131.8, 129.3, 128.3, 113.1, 111.3, 111.1, 109.9, 109.6, 107.5, 107.5, 103.0, 102.7, 83.5, 69.8, 43.1, 28.2, 24.6, 24.6, 17.7; HRMS (ESI) m/z calcd for C21H21ClFN2O2S [M + H]+ 419.0991, found 419.0995; IR (thin film) 3381, 3300, 2922, 2851, 1631, 1586, 1511, 1486, 1455, 1326, 1160, 1094, 827, 616 cm–1.
4-Chloro-N-{2-[2-(pent-4-yn-1-yl)-1H-indol-3-yl]ethyl}benzene-1-sulfonamide (4e)
TLC (hexanes:ethyl acetate, 3:1 v/v) Rf = 0.25; colorless oil, 35%; 1H NMR (500 MHz, CDCl3) δ 7.98 (brs, 1H), 7.64 (d, J = 8.5 Hz, 2H), 7.38 (d, J = 8.5 Hz, 2H), 7.34 (d, J = 7.9 Hz, 1H), 7.30 (d, J = 8.1 Hz, 1H), 7.16 (t, J = 7.6 Hz, 1H), 7.06 (t, J = 7.5 Hz, 1H), 4.50 (t, J = 6.2 Hz, 1H), 3.26 (q, J = 6.6 Hz, 2H), 2.95 (t, J = 6.8 Hz, 2H), 2.86 (t, J = 7.4 Hz, 2H), 2.22 (td, J = 6.9, 2.8 Hz, 2H), 2.08 (s, 1H), 1.84 (p, J = 7.1 Hz, 2H); 13C NMR (75 MHz, CDCl3) δ 138.9, 138.2, 135.7, 135.4, 129.2, 128.4, 127.9, 121.7, 119.6, 117.8, 110.6, 107.2, 83.6, 69.7, 43.3, 28.3, 24.6, 24.5, 17.7; HRMS (ESI) m/z calcd for C21H22ClN2O2S [M + H]+ 401.1085, found 401.1073; IR (thin film) 3395, 3299, 2924, 2853, 1678, 1586, 1477, 1396, 1327, 1160, 1094, 893, 828 cm–1.
4-Chloro-N-{2-[2-(pent-4-yn-1-yl)-1H-benzo[g]indol-3-yl]ethyl}benzene-1-sulfonamide (4f)
TLC (hexanes:ethyl acetate, 3:1 v/v) Rf = 0.22; colorless oil, 31%; 1H NMR (500 MHz, CDCl3) δ 8.71 (brs, 1H), 7.97 (d, J = 8.2 Hz, 1H), 7.93 (d, J = 8.1 Hz, 1H), 7.65–7.59 (m, 2H), 7.54 (ddd, J = 8.2, 6.8, 1.3 Hz, 1H), 7.48–7.41 (m, 3H), 7.31 (d, J = 8.6 Hz, 2H), 4.44 (t, J = 6.1 Hz, 1H), 3.29 (q, J = 6.5 Hz, 2H), 3.02 (t, J = 6.7 Hz, 2H), 2.95 (t, J = 7.4 Hz, 2H), 2.26 (td, J = 6.8, 2.7 Hz, 2H), 2.14 (t, J = 2.6 Hz, 1H), 1.90 (p, J = 7.0 Hz, 2H); 13C NMR (75 MHz, CDCl3) δ 138.2, 133.7, 130.1, 129.7, 129.2, 129.0, 128.3, 125.6, 123.8, 123.5, 121.2, 120.5, 119.1, 117.9, 109.1, 83.7, 69.9, 43.6, 31.9, 31.6, 28.6, 24.6, 24.5, 22.7, 17.7; HRMS (ESI) m/z calcd for C25H24ClN2O2S [M + H]+ 451.1242, found 451.1246; IR (thin film) 3424, 2952, 2843, 1646, 1454, 1405, 1111, 1032, 1016, 623 cm–1.
N-{2-[7-Bromo-2-(pent-4-yn-1-yl)-1H-indol-3-yl]ethyl}-4-chlorobenzene-1-sulfonamide (4g)
TLC (hexanes:ethyl acetate, 3:1 v/v) Rf = 0.25; light yellow oil, 22%; 1H NMR (500 MHz, CDCl3) δ 8.56 (brs, 1H), 7.83 (d, J = 8.3 Hz, 2H), 7.55–7.43 (m, 3H), 7.30 (d, J = 7.6 Hz, 1H), 6.98 (t, J = 7.7 Hz, 1H), 4.99 (t, J = 6.5 Hz, 1H), 3.01 (q, J = 6.3 Hz, 2H), 2.90 (t, J = 7.1 Hz, 4H), 2.47 (td, J = 7.5, 2.6 Hz, 2H), 1.96 (s, 1H), 1.88 (p, J = 6.8 Hz, 2H); 13C NMR (75 MHz, CDCl3) δ 139.4, 138.1, 135.4, 134.1, 129.6, 129.3, 129.2, 128.5, 123.7, 120.5, 117.3, 111.8, 104.3, 84.3, 69.0, 42.2, 30.3, 23.8, 22.4, 20.0; HRMS (ESI) m/z calcd for C21H21BrClN2O2S [M + H]+ 479.0190, found 479.0186; IR (thin film) 3299, 2917, 2849, 1710, 1585, 1451, 1326, 1160, 1095, 1014, 828 cm–1.
N-{2-[6-Bromo-2-(pent-4-yn-1-yl)-1H-indol-3-yl]ethyl}-4-chlorobenzene-1-sulfonamide (4h)
TLC (hexanes:ethyl acetate, 3:1 v/v) Rf = 0.26; light yellow oil, 17%; 1H NMR (500 MHz, CDCl3) δ 7.99 (brs, 1H), 7.65 (d, J = 8.4 Hz, 2H), 7.44 (s, 1H), 7.39 (d, J = 8.3 Hz, 2H), 7.19 (d, J = 8.4 Hz, 1H), 7.17–7.08 (m, 1H), 4.47 (t, J = 6.3 Hz, 1H), 3.23 (q, J = 6.6 Hz, 2H), 2.92 (t, J = 6.8 Hz, 2H), 2.84 (t, J = 7.4 Hz, 2H), 2.21 (dt, J = 6.9, 3.3 Hz, 2H), 2.08 (s, 1H), 1.83 (p, J = 7.1 Hz, 2H); 13C NMR (75 MHz, CDCl3) δ 139.1, 138.2, 136.4, 136.1, 129.2, 128.3, 126.8, 122.8, 119.0, 115.1, 113.6, 107.6, 83.5, 69.9, 43.2, 28.1, 24.6, 24.4, 17.7; HRMS (ESI) m/z calcd for C21H21BrClN2O2S [M + H]+ 479.0190, found 479.0180; IR (thin film) 3356, 3299, 2924, 2851, 2359, 2342, 1716, 1587, 1506, 1463, 1326, 1159, 1094, 1014, 828 cm–1.
N-{2-[4-Bromo-2-(pent-4-yn-1-yl)-1H-indol-3-yl]ethyl}-4-chlorobenzene-1-sulfonamide (4i)
TLC (hexanes:ethyl acetate, 3:1 v/v) Rf = 0.24; colorless oil, 11%; 1H NMR (500 MHz, CDCl3) δ 8.07 (brs, 1H), 7.67 (d, J = 8.6 Hz, 2H), 7.37 (d, J = 8.6 Hz, 2H), 7.24 (d, J = 8.0 Hz, 1H), 7.21 (d, J = 7.5 Hz, 1H), 6.97 (t, J = 8.0 Hz, 1H), 4.53 (t, J = 6.1 Hz, 1H), 3.32 (q, J = 6.7 Hz, 2H), 3.15 (t, J = 7.0 Hz, 2H), 2.94–2.87 (m, 2H), 2.32–2.20 (m, 2H), 2.12 (t, J = 2.6 Hz, 1H), 1.90–1.83 (m, 2H); 13C NMR (75 MHz, CDCl3) δ 138.8, 138.5, 137.4, 136.6, 129.6, 128.7, 125.8, 124.5, 124.2, 122.7, 122.4, 113.0, 110.0, 108.4, 83.2, 69.9, 45.1, 28.0, 24.6, 17.8; HRMS (ESI) m/z calcd for C21H21BrClN2O2S [M + H]+ 479.0190, found 479.0197; IR (thin film) 3304, 2919, 2850, 1718, 1654, 1458, 1276, 1106, 913, 750 cm–1.
4-Chloro-N-{2-[5-chloro-7-fluoro-2-(pent-4-yn-1-yl)-1H-indol-3-yl]ethyl}benzene-1-sulfonamide (4j)
TLC (hexanes:ethyl acetate, 3:1 v/v) Rf = 0.20; light yellow oil, 20%; 1H NMR (500 MHz, CDCl3) δ 8.23 (brs, 1H), 7.68 (d, J = 8.7 Hz, 2H), 7.42 (d, J = 8.7 Hz, 2H), 7.05 (d, J = 1.8 Hz, 1H), 6.88 (dd, J = 10.4, 1.7 Hz, 1H), 4.54 (t, J = 6.2 Hz, 1H), 3.23 (q, J = 6.6 Hz, 2H), 2.90–2.87 (m, 4H), 2.26–2.20 (m, 2H), 2.09 (s, 1H), 1.91–1.82 (m, 2H); 13C NMR (75 MHz, CDCl3) δ 139.1, 138.2, 138.0, 129.3, 128.3, 124.8, 124.7, 113.4, 113.3, 108.2, 108.0, 107.8, 83.3, 70.0, 43.0, 28.0, 24.7, 24.5, 17.8; HRMS (ESI) m/z calcd for C21H20Cl2FN2O2S [M + H]+ 453.0601, found 453.0616; IR (thin film) 3445, 2962, 2081, 1652, 1456, 1261, 1096, 1033, 800 cm–1.
4-Chloro-N-{2-[5,7-dichloro-2-(pent-4-yn-1-yl)-1H-indol-3-yl]ethyl}benzene-1-sulfonamide (4k)
TLC (hexanes:ethyl acetate, 3:1 v/v) Rf = 0.20; light yellow oil, 24%; 1H NMR (500 MHz, CDCl3) δ 8.16 (brs, 1H), 7.66 (d, J = 8.6 Hz, 2H), 7.41 (d, J = 8.6 Hz, 2H), 7.21–7.11 (m, 2H), 4.44 (t, J = 6.3 Hz, 1H), 3.23 (q, J = 6.6 Hz, 2H), 3.03–2.79 (m, 4H), 2.26 (td, J = 6.7, 2.7 Hz, 2H), 2.11 (s, 1H), 1.88 (p, J = 7.1 Hz, 2H); 13C NMR (75 MHz, CDCl3) δ 139.2, 138.2, 138.1, 131.2, 129.9, 129.3, 128.3, 125.3, 121.1, 116.5, 116.1, 108.5, 83.3, 70.0, 43.0, 28.0, 24.8, 24.5, 17.8; HRMS (ESI) m/z calcd for C21H20Cl3N2O2S [M + H]+ 469.0306, found 469.0319; IR (thin film) 3353, 3301, 3088, 2928, 2854, 1713, 1574, 1476, 1328, 1161, 1095, 1014, 964, 912, 828, 753 cm–1.
Benzyl {2-[5-Bromo-2-(pent-4-yn-1-yl)-1H-indol-3-yl]ethyl}carbamate (8a)
TLC (hexanes:ethyl acetate, 3:1 v/v) Rf = 0.20; light yellow oil, 30%; 1H NMR (500 MHz, CDCl3) δ 8.05 (brs, 1H), 7.65 (s, 1H), 7.40–7.31 (m, 5H), 7.23 (dd, J = 8.5, 1.9 Hz, 1H), 7.16 (d, J = 8.6 Hz, 1H), 5.12 (s, 2H), 4.82 (t, J = 6.1 Hz, 1H), 3.45 (q, J = 6.7 Hz, 2H), 2.90 (t, J = 6.9 Hz, 2H), 2.85 (t, J = 7.4 Hz, 2H), 2.21 (td, J = 6.9, 2.7 Hz, 2H), 2.07 (t, J = 2.5 Hz, 1H), 1.84 (h, J = 6.1, 5.0 Hz, 2H); 13C NMR (75 MHz, CDCl3) δ 156.3, 136.8, 136.5, 134.0, 130.2, 128.6, 128.5, 128.3, 128.1, 128.1, 124.2, 120.7, 112.7, 111.9, 108.5, 83.6, 69.7, 66.7, 41.5, 28.2, 24.6, 24.5, 17.7; HRMS (ESI) m/z calcd for C23H24BrN2O2 [M + H]+ 439.1016, found 439.1016; IR (thin film) 3295, 2920, 2850, 1698, 1518, 1454, 1251, 1135, 1075, 797, 748 cm–1.
Benzyl {2-[5-Bromo-7-fluoro-2-(pent-4-yn-1-yl)-1H-indol-3-yl]ethyl}carbamate (8b)
TLC (hexanes:ethyl acetate, 3:1 v/v) Rf = 0.20; light yellow oil, 31%; 1H NMR (500 MHz, CDCl3) δ 9.82 (brs, 1H), 7.49–7.32 (m, 6H), 7.01 (dd, J = 10.1, 1.6 Hz, 1H), 5.19 (s, 2H), 4.96 (brs, 1H), 3.33 (q, J = 6.3 Hz, 2H), 2.90 (t, J = 7.5 Hz, 2H), 2.81 (t, J = 6.7 Hz, 2H), 2.47 (td, J = 7.5, 2.7 Hz, 2H), 1.98 (s, 1H), 1.83 (p, J = 6.6 Hz, 2H); 13C NMR (75 MHz, CDCl3) δ 157.7, 137.5, 136.2, 132.7, 132.7, 128.6, 128.3, 128.2, 122.3, 116.5, 110.5, 109.8, 109.6, 84.2, 69.0, 67.2, 39.5, 31.0, 23.6, 21.9, 20.1; HRMS (ESI) m/z calcd for C23H23BrFN2O2 [M + H]+ 457.0921, found 457.0924; IR (thin film) 3298, 2923, 2851, 1701, 1638, 1523, 1477, 1454, 1308, 1255, 1217, 1131, 1005, 882, 826, 697 cm–1.
16-(4-Chlorobenzenesulfonyl)-4-methyl-13-methylidene-8,16-diazatetracyclo[7.4.3.01,9.02,7]hexadeca-2,4,6-triene (5b)
TLC (hexanes:ethyl acetate, 3:1 v/v) Rf = 0.60; light yellow oil, 90%; 1H NMR (500 MHz, CDCl3) δ 7.59 (d, J = 8.7 Hz, 2H), 7.34 (d, J = 8.7 Hz, 2H), 6.92–6.84 (m, 2H), 6.48 (d, J = 7.8 Hz, 1H), 5.06 (brs, 1H), 4.89 (s, 1H), 4.78 (s, 1H), 3.48 (td, J = 8.6, 1.7 Hz, 1H), 2.95 (ddd, J = 10.3, 8.7, 6.8 Hz, 1H), 2.73 (dt, J = 14.1, 3.8 Hz, 1H), 2.37 (ddd, J = 12.5, 10.4, 8.3 Hz, 1H), 2.27 (s, 3H), 2.26–2.19 (m, 1H), 2.15 (ddd, J = 14.2, 10.2, 5.2 Hz, 1H), 1.93 (ddd, J = 13.9, 12.5, 4.6 Hz, 1H), 1.77–1.71 (m, 1H), 1.60 (tdd, J = 18.0, 9.9, 4.8 Hz, 1H); 13C NMR (75 MHz, CDCl3) δ 148.0, 145.4, 138.6, 138.5, 131.2, 129.0, 128.8, 128.5, 128.4, 125.0, 112.4, 110.1, 93.7, 61.0, 47.4, 34.2, 32.1, 30.5, 23.1, 21.0; HRMS (ESI) m/z calcd for C22H24ClN2O2S [M + H]+ 415.1242, found 415.1245; IR (thin film) 3391, 3088, 2941, 2865, 1639, 1585, 1493, 1332, 1155, 1089, 1002, 904, 813, 623 cm–1.
16-(4-Chlorobenzenesulfonyl)-4-methoxy-13-methylidene-8,16-diazatetracyclo[7.4.3.01,9.02,7]hexadeca-2,4,6-triene (5c)
TLC (hexanes:ethyl acetate, 3:1 v/v) Rf = 0.60; light yellow oil, 92%; 1H NMR (500 MHz, CDCl3) δ 7.58 (d, J = 8.6 Hz, 2H), 7.34 (d, J = 8.6 Hz, 2H), 6.70 (d, J = 2.5 Hz, 1H), 6.62 (dd, J = 8.4, 2.5 Hz, 1H), 6.48 (d, J = 8.4 Hz, 1H), 4.95 (brs, 1H), 4.90 (s, 1H), 4.77 (s, 1H), 3.76 (s, 3H), 3.50 (td, J = 8.6, 1.5 Hz, 1H), 2.94 (ddt, J = 11.2, 6.8, 5.6 Hz, 1H), 2.73 (dtd, J = 14.1, 3.8, 1.2 Hz, 1H), 2.41–2.35 (m, 1H), 2.28–2.11 (m, 3H), 1.96–1.89 (m, 1H), 1.81–1.72 (m, 1H), 1.64–1.57 (m, 1H); 13C NMR (75 MHz, CDCl3) δ 153.5, 147.6, 141.5, 138.6, 138.4, 132.7, 129.4, 129.3, 129.0, 128.5, 128.4, 128.4, 112.7, 112.7, 111.7, 110.7, 94.1, 69.6, 61.2, 56.0, 47.4, 34.3, 31.9, 30.6, 23.2; HRMS (ESI) m/z calcd for C22H24ClN2O2S [M + H]+ 431.1191, found 431.1195; IR (thin film) 3386, 3300, 2942, 2866, 1708, 1586, 1491, 1333, 1158, 1003, 829 cm–1.
16-(4-Chlorobenzenesulfonyl)-4-fluoro-13-methylidene-8,16-diazatetracyclo[7.4.3.01,9.02,7]hexadeca-2,4,6-triene (5d)
TLC (hexanes:ethyl acetate, 3:1 v/v) Rf = 0.50; light yellow oil, 78%; 1H NMR (500 MHz, CDCl3) δ 7.60 (d, J = 8.6 Hz, 2H), 7.37 (d, J = 8.6 Hz, 2H), 6.83 (dd, J = 8.2, 2.6 Hz, 1H), 6.78 (td, J = 8.8, 2.6 Hz, 1H), 6.49 (dd, J = 8.5, 4.2 Hz, 1H), 5.08 (brs, 1H), 4.92 (s, 1H), 4.75 (s, 1H), 3.51 (td, J = 8.7, 1.6 Hz, 1H), 2.98 (ddd, J = 10.4, 8.8, 6.9 Hz, 1H), 2.73 (d, J = 14.1 Hz, 1H), 2.39 (ddd, J = 12.7, 10.4, 8.5 Hz, 1H), 2.28–2.11 (m, 3H), 1.91 (td, J = 13.5, 4.6 Hz, 1H), 1.76 (dt, J = 13.4, 4.3 Hz, 1H), 1.60 (tdd, J = 13.3, 9.9, 6.4 Hz, 1H); 13C NMR (75 MHz, CDCl3) δ 158.6, 155.4, 147.2, 143.7, 143.7, 138.8, 138.4, 134.2, 134.1, 132.9, 132.8, 132.0, 132.0, 129.3, 129.3, 129.2, 129.1, 128.4, 114.7, 114.4, 112.9, 112.1, 111.8, 110.7, 110.6, 94.1, 61.1, 61.1, 47.3, 34.1, 31.9, 30.5, 23.1; HRMS (ESI) m/z calcd for C21H21ClFN2O2S [M + H]+ 419.0991, found 419.0995; IR (thin film) 3389, 3090, 2944, 2869, 1693, 1640, 1585, 1486, 1278, 1225, 1002, 884 cm–1.
16-(4-Chlorobenzenesulfonyl)-13-methylidene-8,16-diazatetracyclo[7.4.3.01,9.02,7]hexadeca-2,4,6-triene (5e)
TLC (hexanes:ethyl acetate, 3:1 v/v) Rf = 0.60; light yellow oil, 82%; 1H NMR (500 MHz, CDCl3) δ 7.57 (d, J = 8.6 Hz, 2H), 7.34 (d, J = 8.6 Hz, 2H), 7.14–7.03 (m, 2H), 6.78 (t, J = 7.5 Hz, 1H), 6.57 (d, J = 7.8 Hz, 1H), 5.17 (brs, 1H), 4.90 (s, 1H), 4.76 (s, 1H), 3.50 (dd, J = 9.6, 8.0 Hz, 1H), 2.95 (ddd, J = 10.3, 8.7, 6.9 Hz, 1H), 2.79–2.70 (m, 1H), 2.43–2.37 (m, 1H), 2.30–2.21 (m, 2H), 2.17 (td, J = 14.1, 12.4, 5.0 Hz, 1H), 1.98–1.91 (m, 1H), 1.81–1.72 (m, 1H), 1.65–1.55 (m, 1H); 13C NMR (75 MHz, CDCl3) δ 147.8, 147.6, 138.7, 138.5, 131.0, 129.1, 128.4, 124.3, 119.2, 112.6, 110.3, 93.4, 60.9, 47.5, 34.2, 32.0, 30.5, 23.1; HRMS (ESI) m/z calcd for C21H22ClN2O2S [M + H]+ 401.1085, found 401.1095; IR (thin film) 3393, 3087, 2943, 2869, 1640, 1589, 1474, 1466, 1191, 893, 830 cm–1.
20-(4-Chlorobenzenesulfonyl)-17-methylidene-12,20-diazapentacyclo[11.4.3.01,13.02,11.05,10]icosa-2,4,6,8,10-pentaene (5f)
TLC (hexanes:ethyl acetate, 3:1 v/v) Rf = 0.55; colorless oil, 82%; 1H NMR (500 MHz, CDCl3) δ 7.80 (dd, J = 6.2, 3.3 Hz, 1H), 7.61 (dd, J = 6.2, 3.3 Hz, 1H), 7.50–7.40 (m, 4H), 7.32 (d, J = 8.2 Hz, 1H), 7.26 (d, J = 6.0 Hz, 1H), 6.99 (d, J = 8.6 Hz, 2H), 5.63 (brs, 1H), 4.90 (s, 1H), 4.79 (s, 1H), 3.57 (td, J = 8.5, 1.5 Hz, 1H), 2.95–2.80 (m, 2H), 2.48 (ddd, J = 12.8, 10.7, 8.2 Hz, 1H), 2.37 (ddd, J = 12.9, 6.8, 1.5 Hz, 1H), 2.21 (t, J = 6.4 Hz, 2H), 2.10–1.98 (m, 1H), 1.88–1.77 (m, 1H), 1.73–1.63 (m, 1H); 13C NMR (75 MHz, CDCl3) δ 148.6, 143.8, 138.4, 138.0, 134.0, 128.8, 128.5, 128.2, 128.2, 125.7, 125.1, 124.6, 122.1, 121.6, 120.6, 119.3, 112.4, 93.6, 61.9, 47.8, 34.4, 32.3, 30.3, 22.9; HRMS (ESI) m/z calcd for C25H24ClN2O2S [M + H]+ 451.1242, found 451.1252; IR (thin film) 3370, 3302, 3054, 2924, 2853, 1713, 1633, 1587, 1476, 1391, 1327, 1265, 1160, 1093, 1014, 807 cm–1.
6-Bromo-16-(4-chlorobenzenesulfonyl)-13-methylidene-8,16-diazatetracyclo[7.4.3.01,9.02,7]hexadeca-2,4,6-triene (5g)
TLC (hexanes:ethyl acetate, 3:1 v/v) Rf = 0.60; light yellow oil, 72%; 1H NMR (500 MHz, CDCl3) δ 7.62 (d, J = 8.6 Hz, 2H), 7.36 (d, J = 8.7 Hz, 2H), 7.23 (d, J = 8.0 Hz, 1H), 7.00 (d, J = 7.3 Hz, 1H), 6.65 (t, J = 7.7 Hz, 1H), 5.45 (s, 1H), 4.91 (s, 1H), 4.73 (s, 1H), 3.54 (td, J = 8.6, 1.6 Hz, 1H), 2.90–2.75 (m, 2H), 2.44 (ddd, J = 12.7, 10.6, 8.4 Hz, 1H), 2.20 (dtd, J = 19.1, 14.2, 7.6 Hz, 3H), 2.01 (td, J = 14.1, 13.6, 4.7 Hz, 1H), 1.78 (tt, J = 9.1, 4.6 Hz, 1H), 1.68–1.62 (m, 1H); 13C NMR (75 MHz, CDCl3) δ 147.2, 146.5, 138.8, 137.7, 132.5, 131.0, 129.1, 128.5, 123.3, 120.3, 112.8, 103.9, 92.6, 62.3, 47.2, 34.5, 32.2, 30.4, 29.7, 22.8; HRMS (ESI) m/z calcd for C21H21BrClN2O2S [M + H]+ 479.0190, found 479.0204; IR (thin film) 3054, 2926, 2853, 1607, 1585, 1465, 1421, 1265, 1158, 1061, 1101, 896, 740 cm–1.
5-Bromo-16-(4-chlorobenzenesulfonyl)-13-methylidene-8,16-diazatetracyclo[7.4.3.01,9.02,7]hexadeca-2,4,6-triene (5h)
TLC (hexanes:ethyl acetate, 3:1 v/v) Rf = 0.60; light yellow oil, 78%; 1H NMR (500 MHz, CDCl3) δ 7.60 (d, J = 8.6 Hz, 2H), 7.40 (d, J = 8.6 Hz, 2H), 6.92 (d, J = 7.8 Hz, 1H), 6.88 (dd, J = 7.8, 1.7 Hz, 1H), 6.66 (d, J = 1.6 Hz, 1H), 5.17 (brs, 1H), 4.90 (s, 1H), 4.72 (s, 1H), 3.50 (td, J = 8.6, 1.4 Hz, 1H), 3.04–2.96 (m, 1H), 2.76–2.67 (m, 1H), 2.40–2.34 (m, 1H), 2.27–2.19 (m, 2H), 2.18–2.10 (m, 1H), 1.92–1.86 (m, 1H), 1.78–1.71 (m, 1H); 13C NMR (75 MHz, CDCl3) δ 149.0, 147.3, 139.0, 138.3, 134.3, 134.1, 132.0, 130.2, 129.3, 129.2, 129.2, 128.3, 125.5, 121.9, 121.9, 113.4, 112.8, 93.2, 60.6, 47.6, 34.1, 31.9, 29.7, 22.9; HRMS (ESI) m/z calcd for C21H21BrClN2O2S [M + H]+ 479.0190, found 479.0185; IR (thin film) 3391, 2917, 2849, 1602, 1585, 1478, 1436, 1392, 1333, 1154, 1104, 997, 752 cm–1.
3-Bromo-16-(4-chlorobenzenesulfonyl)-13-methylidene-8,16-diazatetracyclo[7.4.3.01,9.02,7]hexadeca-2,4,6-triene (5i)
TLC (hexanes:ethyl acetate, 3:1 v/v) Rf = 0.20; colorless oil, 62%; 1H NMR (500 MHz, CDCl3) δ 7.62 (d, J = 8.6 Hz, 2H), 7.38 (d, J = 8.6 Hz, 2H), 6.99–6.84 (m, 2H), 6.51 (dd, J = 7.6, 1.2 Hz, 1H), 5.24 (brs, 1H), 5.01 (s, 1H), 4.91 (s, 1H), 3.50 (t, J = 8.2 Hz, 1H), 3.16 (dd, J = 13.2, 6.3 Hz, 1H), 3.05–3.00 (m, 1H), 2.69 (ddd, J = 14.1, 4.7, 3.0 Hz, 1H), 2.32–2.26 (m, 1H), 2.16 (t, J = 6.6 Hz, 2H), 1.88 (td, J = 13.2, 5.2 Hz, 1H), 1.76–1.73 (m, 1H), 1.66–1.61 (m, 1H); 13C NMR (75 MHz, CDCl3) δ 150.2, 146.5, 138.6, 130.8, 130.2, 129.1, 128.4, 124.0, 120.5, 114.4, 109.1, 93.4, 63.9, 47.6, 33.6, 32.8, 30.8, 29.4, 22.9; HRMS (ESI) m/z calcd for C21H21BrClN2O2S [M + H]+ 479.0190, found 479.0188; IR (thin film) 3393, 2954, 2916, 2848, 1261, 1159, 1090, 912, 764 cm–1.
4-Chloro-16-(4-chlorobenzenesulfonyl)-6-fluoro-13-methylidene-8,16-diazatetracyclo[7.4.3.01,9.02,7]hexadeca-2,4,6-triene (5j)
TLC (hexanes:ethyl acetate, 3:1 v/v) Rf = 0.60; light yellow oil, 77%; 1H NMR (500 MHz, CDCl3) δ 7.63 (d, J = 8.7 Hz, 2H), 7.40 (d, J = 8.7 Hz, 2H), 6.97–6.83 (m, 2H), 5.28 (brs, 1H), 4.94 (s, 1H), 4.73 (s, 1H), 3.55 (t, J = 8.6 Hz, 1H), 2.96–2.91 (m, 1H), 2.77 (dt, J = 14.4, 3.8 Hz, 1H), 2.44–2.38 (m, 1H), 2.27–2.09 (m, 3H), 1.94 (td, J = 13.5, 4.6 Hz, 1H), 1.79–1.75 (m, 1H); 13C NMR (75 MHz, CDCl3) δ 146.6, 139.0, 137.7, 129.2, 128.2, 123.5, 120.4, 120.4, 115.9, 115.6, 113.1, 93.5, 61.7, 47.4, 34.2, 31.9, 29.7, 22.8; HRMS (ESI) m/z calcd for C21H20Cl2FN2O2S [M + H]+ 453.0601, found 453.0609; IR (thin film) 3054, 2986, 2926, 2853, 1584, 1422, 1265, 1159, 1013, 975, 896, 740, 705 cm–1.
(4,6-Dichloro-16-(4-chlorobenzenesulfonyl)-13-methylidene-8,16-diazatetracyclo[7.4.3.01,9.02,7]hexadeca-2,4,6-triene (5k)
TLC (hexanes:ethyl acetate, 3:1 v/v) Rf = 0.60; light yellow oil, 82%; 1H NMR (500 MHz, CDCl3) δ 7.63 (d, J = 8.6 Hz, 2H), 7.39 (d, J = 8.7 Hz, 2H), 7.10 (d, J = 1.9 Hz, 1H), 6.93 (d, J = 1.9 Hz, 1H), 5.44 (brs, 1H), 4.94 (s, 1H), 4.74 (s, 1H), 3.53 (td, J = 8.6, 1.6 Hz, 1H), 2.90–2.85 (m, 1H), 2.79 (dt, J = 14.3, 3.9 Hz, 1H), 2.45–2.38 (m, 1H), 2.24–2.16 (m, 3H), 1.96 (ddd, J = 14.1, 12.5, 4.7 Hz, 1H), 1.78–1.75 (m, 1H), 1.65–1.60 (m, 1H); 13C NMR (75 MHz, CDCl3) δ 146.6, 143.9, 139.0, 137.7, 133.9, 129.2, 128.4, 127.8, 123.9, 123.2, 115.7, 113.1, 92.9, 62.2, 47.1, 34.1, 32.2, 30.2, 22.6; HRMS (ESI) m/z calcd for C21H20Cl3N2O2S [M + H]+ 469.0306, found 469.0305; IR (thin film) 3380, 2928, 2856, 2360,1700, 1636, 1583, 1464, 1336, 1156, 1092, 1010, 904, 631 cm–1.
4-Chloro-N-[2-(6-methyl-4-methylidene-2,3,4,4a,9,9a-hexahydro-1H-carbazol-4a-yl)ethyl]benzene-1-sulfonamide (6b)
TLC (hexanes:ethyl acetate, 3:1 v/v) Rf = 0.30; pink oil, 95%; 1H NMR (500 MHz, CDCl3) δ 7.74 (d, J = 8.6 Hz, 2H), 7.46 (d, J = 8.6 Hz, 2H), 6.90–6.84 (m, 1H), 6.75–6.69 (m, 1H), 6.58 (d, J = 7.8 Hz, 1H), 5.29 (s, 1H), 4.93 (s, 1H), 4.70 (d, J = 1.4 Hz, 1H), 3.57 (t, J = 5.2 Hz, 1H), 3.03–2.98 (m, 1H), 2.90–2.78 (m, 1H), 2.24 (s, 3H), 2.17–2.10 (m, 2H), 2.07–1.92 (m, 2H), 1.82–1.76 (m, 1H), 1.68–1.55 (m, 3H), 1.48–1.43 (m, 1H); 13C NMR (75 MHz, CDCl3) δ 149.3, 147.0, 138.9, 138.54, 133.3, 129.3, 128.9, 128.5, 128.4, 124.6, 111.7, 110.7, 65.3, 52.3, 40.4, 36.6, 32.7, 29.1, 21.90, 21.0; HRMS (ESI) m/z calcd for C22H26ClN2O2S [M + H]+ 417.1398, found 417.1399; IR (thin film) 2927, 2856, 1491, 1476, 1395, 1329, 1276, 1161, 1014, 827, 752 cm–1.
4-Chloro-N-[2-(6-methoxy-4-methylidene-2,3,4,4a,9,9a-hexahydro-1H-carbazol-4a-yl)ethyl]benzene-1-sulfonamide (6c)
TLC (hexanes:ethyl acetate, 3:1 v/v) Rf = 0.40; pink oil, 97%; 1H NMR (500 MHz, CDCl3) δ 7.75 (d, J = 8.8 Hz, 2H), 7.47 (d, J = 8.8 Hz, 1H), 6.81–6.75 (brm, 1H), 6.67 (dd, J = 8.5, 2.5 Hz, 1H), 6.56 (s, 1H), 5.56 (brs, 1H), 5.01 (s, 1H), 4.77 (s, 1H), 3.79 (s, 1H), 3.76 (s, 3H), 3.05–3.02 (m, 1H), 2.84–2.81 (m, 1H), 2.24–2.19 (m, 1H), 2.12–2.04 (m, 2H), 2.02–1.97 (m, 1H), 1.96–1.88 (m, 1H), 1.71–1.65 (m, 1H), 1.64–1.56 (m, 1H), 1.50–1.44 (m, 1H); 13C NMR (75 MHz, CDCl3) δ 138.9, 138.4, 129.5, 129.4, 129.3, 128.5, 112.9, 112.5, 110.7, 65.4, 55.9, 53.0, 40.3, 36.4, 32.7, 28.6, 22.0; HRMS (ESI) m/z calcd for C22H26ClN2O3S [M + H]+ 433.1347, found 433.1336; IR (thin film) 3286, 3087, 2927, 2854, 1930, 1637, 1587, 1488, 1435, 1329, 1278, 1263, 1219, 1161, 1094, 828 cm–1.
4-Chloro-N-[2-(6-fluoro-4-methylidene-2,3,4,4a,9,9a-hexahydro-1H-carbazol-4a-yl)ethyl]benzene-1-sulfonamide (6d)
TLC (hexanes:ethyl acetate, 3:1 v/v) Rf = 0.20; light yellow oil, 84%; 1H NMR (500 MHz, CDCl3) δ 7.75 (d, J = 8.6 Hz, 2H), 7.48 (d, J = 8.6 Hz, 2H), 6.76 (td, J = 8.8, 2.6 Hz, 1H), 6.64 (dd, J = 8.3, 2.6 Hz, 1H), 6.58 (dd, J = 8.4, 4.3 Hz, 1H), 5.16 (t, J = 6.1 Hz, 1H), 4.94 (s, 1H), 4.67 (s, 1H), 3.63 (t, J = 5.0 Hz, 1H), 3.04–2.96 (m, 1H), 2.91–2.83 (m, 1H), 2.23–2.09 (m, 2H), 2.09–2.01 (m, 1H), 1.96 (ddd, J = 14.1, 8.4, 5.6 Hz, 1H), 1.80 (tt, J = 9.5, 4.9 Hz, 1H), 1.70–1.57 (m, 1H), 1.52–1.45 (m, 1H); 13C NMR (75 MHz, CDCl3) δ 148.8, 139.1, 138.4, 129.4, 128.5, 114.3, 114.0, 112.1, 111.6, 111.3, 111.1, 111.0, 65.7, 52.5, 40.2, 36.2, 32.5, 28.7, 21.8; HRMS (ESI) m/z calcd for C21H23ClFN2O2S [M + H]+ 421.1147, found 421.1147; IR (thin film) 3734, 2929, 1507, 1485, 1276, 1260, 1160, 1260, 1160, 1093, 1014, 828, 751 cm–1.
4-Chloro-N-[2-(4-methylidene-2,3,4,4a,9,9a-hexahydro-1H-carbazol-4a-yl)ethyl]benzene-1-sulfonamide (6e)
TLC (hexanes:ethyl acetate, 3:1 v/v) Rf = 0.20; light yellow oil, 95%; 1H NMR (500 MHz, CDCl3) δ 7.73 (d, J = 8.6 Hz, 2H), 7.46 (d, J = 8.6 Hz, 2H), 7.06 (td, J = 7.6, 1.3 Hz, 1H), 6.91 (dd, J = 7.3, 1.3 Hz, 1H), 6.75 (td, J = 7.4, 1.0 Hz, 1H), 6.66 (d, J = 7.8 Hz, 1H), 5.08 (t, J = 6.0 Hz, 1H), 4.92 (d, J = 1.6 Hz, 1H), 4.67 (d, J = 1.3 Hz, 1H), 3.60 (t, J = 4.9 Hz, 1H), 3.06–2.96 (m, 1H), 2.93–2.87 (m, 1H), 2.15 (q, J = 5.5 Hz, 2H), 2.09–1.93 (m, 2H), 1.85–1.74 (m, 1H), 1.71–1.57 (m, 2H), 1.52–1.47 (m, 1H); 13C NMR (75 MHz, CDCl3) δ 149.5, 149.3, 139.0, 138.5, 133.0, 129.3, 128.5, 128.0, 124.1, 119.3, 111.8, 110.7, 65.2, 52.2, 40.4, 36.4, 32.5, 28.8, 21.8; HRMS (ESI) m/z calcd for C21H24ClN2O2S [M + H]+ 403.1242, found 403.1241; IR (thin film) 3287, 2930, 2856, 1606, 1478, 1464, 1396, 1328, 1276, 1261, 1160, 1094, 1014, 902, 828, 751 cm–1.
4-Chloro-N-(2-{7-methylidene-6bH,7H,8H,9H,10H,10aH,11H-benzo[a]carbazol-6b-yl}ethyl)benzene-1-sulfonamide (6f)
TLC (hexanes:ethyl acetate, 3:1 v/v) Rf = 0.40; light yellow oil, 90%; 1H NMR (500 MHz, CDCl3) δ 7.81 (dt, J = 6.9, 3.5 Hz, 1H), 7.63 (dd, J = 6.2, 3.3 Hz, 1H), 7.56 (d, J = 8.6 Hz, 1H), 7.46 (dp, J = 6.7, 3.5 Hz, 2H), 7.30 (d, J = 8.2 Hz, 1H), 7.25 (d, J = 8.5 Hz, 2H), 7.08 (d, J = 8.2 Hz, 1H), 5.39 (t, J = 6.3 Hz, 1H), 4.97 (s, 1H), 4.79 (s, 1H), 3.80 (t, J = 5.0 Hz, 1H), 2.97 (dtd, J = 13.0, 7.3, 5.6 Hz, 1H), 2.71 (dtd, J = 12.9, 7.5, 5.1 Hz, 1H), 2.22 (ddd, J = 13.7, 8.2, 5.3 Hz, 1H), 2.16–2.00 (m, 3H), 1.86 (tt, J = 10.1, 5.3 Hz, 1H), 1.65 (dtt, J = 14.9, 9.7, 4.6 Hz, 2H), 1.50 (dtd, J = 11.3, 6.4, 3.3 Hz, 1H); 13C NMR (75 MHz, CDCl3) δ 149.5, 145.4, 138.8, 138.1, 133.8, 129.2, 128.6, 128.3, 126.3, 125.6, 125.1, 122.2, 121.4, 121.0, 119.6, 111.6, 65.7, 53.1, 40.5, 37.1, 32.5, 29.7, 29.5, 21.1; HRMS (ESI) m/z calcd for C25H26ClN2O2S [M + H]+ 453.1398, found 453.1404; IR (thin film) 3355, 3289, 3056, 2929, 2856, 1718, 1638, 1586, 1519, 1445, 1265, 1161, 1094, 1014, 901, 803, 752 cm–1.
N-[2-(8-Bromo-4-methylidene-2,3,4,4a,9,9a-hexahydro-1H-carbazol-4a-yl)ethyl]-4-chlorobenzene-1-sulfonamide (6g)
TLC (hexanes:ethyl acetate, 3:1 v/v) Rf = 0.40; light yellow oil, 83%; 1H NMR (500 MHz, CDCl3) δ 7.85–7.64 (m, 2H), 7.48 (d, J = 8.8 Hz, 2H), 7.22 (d, J = 8.0 Hz, 1H), 6.86 (d, J = 7.2 Hz, 1H), 6.63 (t, J = 7.6 Hz, 1H), 4.94 (s, 1H), 4.88 (t, J = 6.2 Hz, 1H), 4.68 (s, 1H), 3.71–3.63 (m, 1H), 3.06–2.85 (m, 2H), 2.16 (q, J = 5.6 Hz, 2H), 2.07 (q, J = 9.1, 8.3 Hz, 1H), 1.99 (ddd, J = 14.2, 8.5, 5.6 Hz, 1H), 1.82 (ddt, J = 12.3, 8.3, 5.0 Hz, 1H), 1.67 (dtt, J = 16.8, 9.8, 5.0 Hz, 2H), 1.50 (ddt, J = 13.8, 10.2, 6.1 Hz, 1H); 13C NMR (75 MHz, CDCl3) δ 148.6, 139.1, 138.4, 130.6, 129.4, 128.5, 122.9, 120.2, 112.1, 64.7, 53.4, 40.3, 36.4, 32.4, 28.7, 21.5; HRMS (ESI) m/z calcd for C21H23BrClN2O2S [M + H]+ 481.0347, found 491.0350; IR (thin film) 3373, 3054, 2927, 2853, 1588, 1454, 1422, 1265, 1164, 1098, 897, 829 cm–1.
N-[2-(7-Bromo-4-methylidene-2,3,4,4a,9,9a-hexahydro-1H-carbazol-4a-yl)ethyl]-4-chlorobenzene-1-sulfonamide (6h)
TLC (hexanes:ethyl acetate, 3:1 v/v) Rf = 0.30; light yellow oil, 86%; 1H NMR (500 MHz, CDCl3) δ 7.73 (d, J = 8.5 Hz, 2H), 7.49 (d, J = 8.5 Hz, 2H), 6.89–6.82 (m, 1H), 6.78–6.64 (m, 2H), 4.93 (s, 1H), 4.76 (t, J = 6.3 Hz, 1H), 4.67 (s, 1H), 3.70 (brs, 1H), 3.62 (t, J = 4.8 Hz, 1H), 3.03–2.84 (m, 2H), 2.24–2.10 (m, 2H), 2.07–2.01 (m, 1H), 1.99–1.93 (m, 1H), 1.84–1.72 (m, 1H), 1.69–1.60 (m, 2H), 1.53–1.46 (m, 1H); 13C NMR (75 MHz, CDCl3) δ 151.2, 148.8, 139.1, 138.4, 132.0, 129.4, 128.5, 125.3, 121.7, 121.6, 113.4, 112.0, 65.3, 51.8, 40.2, 36.3, 32.3, 28.7, 21.5; HRMS (ESI) m/z calcd for C21H23BrClN2O2S [M + H]+ 481.0347, found 481.0350; IR (thin film) 3367, 3285, 2925, 2854, 1713, 1600, 1478, 1434, 1328, 1161, 1094, 1014, 901, 828, 738 cm–1.
N-(2-((4aS,9aS)-5-Bromo-4-methylene-2,3,4,4a,9,9a-hexahydro-1H-carbazol-4a-yl)ethyl)-4-chlorobenzenesulfonamide (6i)
TLC (hexanes:ethyl acetate, 3:1 v/v) Rf = 0.30; colorless oil, 73%; 1H NMR (500 MHz, CDCl3) δ 7.73 (d, J = 8.5 Hz, 2H), 7.49 (d, J = 8.5 Hz, 2H), 6.89–6.82 (m, 1H), 6.78–6.64 (m, 2H), 4.93 (s, 1H), 4.76 (t, J = 6.3 Hz, 1H), 4.67 (s, 1H), 3.70 (brs, 1H), 3.62 (t, J = 4.8 Hz, 1H), 3.03–2.84 (m, 2H), 2.24–2.10 (m, 2H), 2.07–2.01 (m, 1H), 1.99–1.93 (m, 1H), 1.84–1.72 (m, 1H), 1.69–1.60 (m, 2H), 1.53–1.46 (m, 1H); 13C NMR (75 MHz, CDCl3) δ 153.1, 150.6, 149.0, 136.1, 135.4, 131.4, 130.0, 129.5, 128.5, 123.2, 111.9, 111.3, 64.8, 50.6, 41.2, 39.9, 32.8, 29.3, 22.4; HRMS (ESI) m/z calcd for C21H22BrClN2NaO2S [M + Na]+ 503.0166, found 503.0170; IR (thin film) 2954, 2923, 2853, 1713, 1662, 1571, 1462, 1377, 1331, 1278, 1162, 1096, 1014, 906, 827 cm–1.
4-Chloro-N-[2-(6-chloro-8-fluoro-4-methylidene-2,3,4,4a,9,9a-hexahydro-1H-carbazol-4a-yl)ethyl]benzene-1-sulfonamide (6j)
TLC (hexanes:ethyl acetate, 3:1 v/v) Rf = 0.50; light yellow oil, 89%; 1H NMR (500 MHz, CDCl3) δ 7.77 (d, J = 8.6 Hz, 2H), 7.50 (d, J = 8.5 Hz, 2H), 6.89 (dd, J = 9.9, 1.9 Hz, 1H), 6.72 (d, J = 1.9 Hz, 1H), 4.96 (s, 1H), 4.83 (t, J = 6.2 Hz, 1H), 4.67 (s, 1H), 3.70 (s, 1H), 3.01–2.89 (m, 2H), 2.21–2.12 (m, 2H), 2.10–2.04 (m, 1H), 2.01–1.95 (m, 1H), 1.86–1.80 (m, 1H), 1.71–1.65 (m, 3H), 1.56–1.48 (m, 1H); 13C NMR (75 MHz, CDCl3) δ 148.1, 147.3, 139.2, 138.3, 137.7, 137.6, 135.8, 135.6, 129.5, 128.5, 120.0, 120.0, 115.5, 115.2, 112.4, 66.0, 52.9, 40.1, 36.0, 32.4, 31.9, 28.6, 21.6; HRMS (ESI) m/z calcd for C21H22Cl2FN2O2S [M + H]+ 455.0758, found 455.0760; IR (thin film) 3054, 2986, 2926, 2853, 1584, 1422, 1265, 1159, 1013, 975, 896, 740 cm–1.
4-Chloro-N-[2-(6,8-dichloro-4-methylidene-2,3,4,4a,9,9a-hexahydro-1H-carbazol-4a-yl)ethyl]benzene-1-sulfonamide (6k)
TLC (hexanes:ethyl acetate, 3:1 v/v) Rf = 0.60; light yellow oil, 88%; 1H NMR (500 MHz, CDCl3) δ 7.76 (d, J = 8.6 Hz, 2H), 7.50 (d, J = 8.6 Hz, 2H), 7.08 (d, J = 1.9 Hz, 1H), 6.79 (d, J = 1.9 Hz, 1H), 4.95 (d, J = 1.4 Hz, 1H), 4.81 (t, J = 6.3 Hz, 1H), 4.67 (s, 1H), 3.89 (brs, 1H), 3.69 (t, J = 4.8 Hz, 1H), 3.02–2.88 (m, 2H), 2.19–2.14 (m, 2H), 2.11–2.01 (m, 1H), 1.99–1.93 (m, 1H), 1.87–1.77 (m, 1H), 1.73–1.64 (m, 2H), 1.53–1.50 (m, 1H); 13C NMR (75 MHz, CDCl3) δ 147.9, 145.7, 139.3, 138.3, 135.8, 129.5, 128.5, 127.4, 123.5, 122.7, 116.0, 112.4, 65.2, 53.3, 40.1, 36.1, 32.3, 28.5, 21.4; HRMS (ESI) m/z calcd for C21H21Cl3N2NaO2S [M + Na]+ 493.0282, found 493.0292; IR (thin film) 3360, 3291, 2926, 2855, 1714, 1638, 1580, 1464, 1329, 1161, 1094, 904, 754 cm–1.
N-[2-(6-Bromo-8-fluoro-4-methylidene-2,3,4,4a,9,9a-hexahydro-1H-carbazol-4a-yl)ethyl]-4-chlorobenzene-1-sulfonamide (6l)
TLC (hexanes:ethyl acetate, 3:1 v/v) Rf = 0.20; light yellow oil, 77%; 1H NMR (500 MHz, CDCl3) δ 7.76 (d, J = 8.6 Hz, 2H), 7.50 (d, J = 8.6 Hz, 2H), 7.02 (dd, J = 9.5, 1.7 Hz, 1H), 6.84 (d, J = 1.7 Hz, 1H), 4.96 (d, J = 1.4 Hz, 1H), 4.76 (t, J = 6.3 Hz, 1H), 4.67 (s, 1H), 3.74 (brs, 1H), 3.69 (t, J = 4.9 Hz, 1H), 3.04–2.85 (m, 2H), 2.21–2.12 (m, 2H), 2.10–2.03 (m, 1H), 2.02–1.94 (m, 1H), 1.86–1.78 (m, 1H), 1.72–1.63 (m, 2H), 1.55–1.49 (m, 1H); 13C NMR (75 MHz, CDCl3) δ 150.8, 148.1, 147.5, 139.2, 138.3, 138.2, 138.2, 136.3, 136.1, 129.5, 128.5, 128.5, 128.1, 122.8, 122.8, 118.2, 117.9, 112.4, 109.7, 109.6, 66.0, 52.9, 52.9, 40.1, 36.0, 32.4, 28.5, 21.6; HRMS (ESI) m/z calcd for C21H22BrClFN2O2S [M + H]+ 499.0252, found 499.0258; IR (thin film) 3359, 3303, 2927, 2854, 1705, 1623, 1588, 1520, 1472, 1396, 1330, 1261, 1161, 1094, 1014, 903, 828, 753 cm–1.
Benzyl N-[2-(6-Bromo-4-methylidene-2,3,4,4a,9,9a-hexahydro-1H-carbazol-4a-yl)ethyl]carbamate (10a)
TLC (hexanes:ethyl acetate, 2:1 v/v) Rf = 0.30; light yellow oil, 95%; 1H NMR (500 MHz, CDCl3) δ 7.41–7.31 (m, 5H), 7.15 (d, J = 7.1 Hz, 2H), 6.55 (d, J = 8.6 Hz, 1H), 5.09 (s, 2H), 4.94 (s, 1H), 4.83 (brs, 1H), 4.71 (s, 1H), 3.74 (t, J = 4.4 Hz, 1H), 3.39–3.26 (m, 1H), 3.22–3.15 (m, 1H), 2.21–2.19 (m, 2H), 2.10–2.04 (m, 1H), 2.00–1.94 (m, 1H), 1.87–1.80 (m, 1H), 1.71–1.66 (m, 3H), 1.57–1.52 (m, 1H); 13C NMR (75 MHz, CDCl3) δ 156.3, 149.0, 148.9, 136.3, 130.5, 128.5, 128.1, 127.2, 112.0, 111.7, 110.4, 66.7, 65.3, 52.3, 38.0, 35.7, 32.3, 28.2, 21.8; HRMS (ESI) m/z calcd for C23H25BrN2NaO2 [M + Na]+ 464.0092, found 464.1006; IR (thin film) 3344, 3032, 2924, 2853, 1711, 1601, 1519, 1473, 1375, 1259, 1102, 902, 807 cm–1.
N-[2-(6-Bromo-4-methylidene-2,3,4,4a,9,9a-hexahydro-1H-carbazol-4a-yl)ethyl]pentanamide (12b)
TLC (chloroform:methanol, 10:1 v/v) Rf = 0.75; light yellow oil, 82%; 1H NMR (500 MHz, CDCl3) δ 7.16 (d, J = 6.7 Hz, 2H), 6.55 (d, J = 8.9 Hz, 1H), 5.45 (brs, 1H), 4.94 (d, J = 1.4 Hz, 1H), 4.72 (s, 1H), 3.99 (brs, 1H), 3.76 (t, J = 4.4 Hz, 1H), 3.44–3.38 (m, 1H), 3.28–3.16 (m, 1H), 2.36 (t, J = 7.5 Hz, 1H), 2.23–2.20 (m, 1H), 2.14–2.08 (m, 2H), 2.08–2.00 (m, 1H), 1.99–1.92 (m, 1H), 1.87–1.79 (m, 1H), 1.75–1.66 (m, 2H), 1.65–1.57 (m, 2H), 1.33 (dq, J = 14.6, 7.3 Hz, 2H), 0.91 (t, J = 7.3 Hz, 3H); 13C NMR (75 MHz, CDCl3) δ 173.1, 140.4, 131.5, 131.0, 114.3, 104.5, 66.4, 52.0, 38.3. 36.6, 36.2, 34.9, 32.7, 29.8, 27.7, 27.2, 22.6, 14.1; HRMS (ESI) m/z calcd for C20H28BrN2O [M + H]+ 391.1380, found 391.1368; IR (thin film) 3312, 3084, 2955, 2930, 2860, 1727, 1661, 1587, 1542, 1467, 1392, 1325, 1254, 1202, 909, 821 cm–1.
N-[2-(6-Bromo-4-methylidene-2,3,4,4a,9,9a-hexahydro-1H-carbazol-4a-yl)ethyl]-4-chlorobenzamide (12c)
TLC (hexanes:ethyl acetate, 1:1 v/v) Rf = 0.70; light yellow oil, 97%; 1H NMR (500 MHz, CDCl3) δ 7.59 (d, J = 8.5 Hz, 2H), 7.39 (d, J = 8.5 Hz, 2H), 7.20 (d, J = 2.0 Hz, 1H), 7.17 (dd, J = 8.2, 2.0 Hz, 1H), 6.57 (d, J = 8.2 Hz, 1H), 6.24 (brs, 1H), 4.98 (d, J = 1.5 Hz, 1H), 4.77 (s, 1H), 3.80 (t, J = 4.5 Hz, 1H), 3.60–3.57 (m, 1H), 3.52–3.42 (m, 1H), 2.32–2.20 (m, 2H), 2.19–2.15 (m, 1H), 2.12–2.06 (m, 1H), 1.88–1.81 (m, 1H), 1.74–1.67 (m, 2H), 1.58–1.53 (tm, 1H); 13C NMR (75 MHz, CDCl3) δ 166.2, 149.2, 149.1, 137.6, 136.2, 132.8, 130.6, 128.8, 128.8, 128.2, 127.1, 112.1, 111.8, 110.5, 65.2, 52.3, 37.1, 35.3, 32.3, 28.4, 21.6; HRMS (ESI) m/z calcd for C22H22BrClN2NaO [M + Na]+ 467.0496, found 467.0498; IR (thin film) 3309, 2927, 2855, 1636, 1597, 1567, 1544, 1485, 1316, 1262, 1094, 1013, 976 cm–1.
N-[2-(6-Bromo-4-methylidene-2,3,4,4a,9,9a-hexahydro-1H-carbazol-4a-yl)ethyl]benzenesulfonamide (12d)
TLC (hexanes:ethyl acetate, 1:1 v/v) Rf = 0.50; light yellow oil, 77%; 1H NMR (500 MHz, CDCl3) δ 7.87–7.79 (m, 2H), 7.62–7.57 (m, 1H), 7.53 (t, J = 7.6 Hz, 2H), 7.13 (dd, J = 8.2, 2.0 Hz, 1H), 6.98 (d, J = 2.0 Hz, 1H), 6.52 (d, J = 8.2 Hz, 1H), 4.91 (s, 1H), 4.89 (brs, 1H), 4.63 (s, 1H), 3.62 (t, J = 4.8 Hz, 1H), 3.04–2.91 (m, 2H), 2.15–2.13 (m, 2H), 2.05–1.97 (m, 1H), 1.97–1.90 (m, 1H), 1.79–1.74 (m, 1H), 1.66–1.61 (m, 2H), 1.53–1.45 (m, 1H); 13C NMR (75 MHz, CDCl3) δ 148.8, 148.7, 139.8, 135.6, 132.7, 130.6, 129.2, 128.8, 128.2, 127.0, 112.1, 111.8, 110.6, 65.3, 52.3, 40.2, 36.1, 32.4, 28.5, 21.7; HRMS (ESI) m/z calcd for C21H24BrN2O2S [M + H]+ 447.0736, found 448.0742; IR (thin film) 3361.8, 2926, 2855, 1638, 1598, 1475, 1324, 1159, 1092, 902, 689 cm–1.
N-[2-(6-Bromo-4-methylidene-2,3,4,4a,9,9a-hexahydro-1H-carbazol-4a-yl)ethyl]-4-methylbenzene-1-sulfonamide (12e)
TLC (hexanes:ethyl acetate, 3:1 v/v) Rf = 0.20; light yellow oil, 80%; 1H NMR (500 MHz, CDCl3) δ 7.71 (d, J = 8.3 Hz, 2H), 7.33 (d, J = 8.3 Hz, 2H), 7.30 (dd, J = 8.2, 2.1 Hz, 2H), 6.94 (d, J = 2.1 Hz, 1H), 5.09 (d, J = 2.1 Hz, 1H), 4.80 (d, J = 2.0 Hz, 1H), 3.97 (dd, J = 8.1, 5.8 Hz, 1H), 3.55 (t, J = 6.2 Hz, 1H), 2.79–2.72 (m, 1H), 2.55–2.49 (m, 1H), 2.46 (s, 3H), 2.23 (dt, J = 15.2, 5.0 Hz, 1H), 2.05–2.01 (m, 1H), 1.96–1.86 (m, 1H), 1.71–1.55 (m, 4H), 1.52–1.46 (1, 1H); 13C NMR (75 MHz, CDCl3) δ 144.7, 139.4, 136.7, 135.9, 131.8, 130.1, 127.0. 126.8, 126.7, 117.1, 113.6, 67.8, 52.1, 38.7, 38.4, 31.8, 29.7, 21.6; HRMS (ESI) m/z calcd for C22H25BrKN2O2S [M + K]+ 499.0452, found 499.0455; IR (thin film) 3292, 3064, 2924, 2854, 1724, 1683, 1597, 1465, 1356, 1334, 1184, 1162, 1094, 908, 815, 665 cm–1.
N-[2-(6-Bromo-4-methylidene-2,3,4,4a,9,9a-hexahydro-1H-carbazol-4a-yl)ethyl]-4-fluorobenzene-1-sulfonamide (12f)
TLC (hexanes:ethyl acetate, 3:1 v/v) Rf = 0.20; light yellow oil, 70%; 1H NMR (500 MHz, CDCl3) δ 7.90 (dd, J = 8.6, 5.0 Hz, 2H), 7.81 (dd, J = 8.8, 5.1 Hz, 2H), 7.50 (d, J = 8.5 Hz, 1H), 7.39–7.34 (m, 1H), 6.98 (d, J = 2.0 Hz, 1H), 5.11 (s, 1H), 4.85 (s, 1H), 4.17 (brs,, 1H), 4.12 (dd, J = 8.0, 5.7 Hz, 1H), 2.81–2.76 (m, 1H), 2.73–2.66 (m, 1H), 2.28–2.17 (m, 2H), 2.08–1.98 (m, 1H), 1.93 (q, J = 8.9, 8.4 Hz, 1H), 1.95–1.89 (m, 1H), 1.73–1.64 (m, 2H), 1.44–1.41 (m, 1H); 13C NMR (75 MHz, CDCl3) δ 144.9, 139.3, 138.4, 131.8, 129.8, 129.7, 129.6, 129.6, 127.0, 117.0, 116.9, 116.68, 116.7, 116.6, 116.3, 113.5, 68.3, 52.1, 39.0, 38.2, 31.6, 30.0, 20.0; HRMS (ESI) m/z calcd for C21H22BrFN2NaO2S [M + Na]+ 487.0462, found 487.0481; IR (thin film) 3298, 3077, 2926, 2855, 1712, 1651, 1592, 1493, 1469, 1358, 1337, 1293, 1238, 907, 839, 738 cm–1.
4-Bromo-N-[2-(6-bromo-4-methylidene-2,3,4,4a,9,9a-hexahydro-1H-carbazol-4a-yl)ethyl]benzene-1-sulfonamide (12g)
TLC (hexanes:ethyl acetate, 3:1 v/v) Rf = 0.30; light yellow oil, 81%; 1H NMR (500 MHz, CDCl3) δ 7.76–7.59 (m, 4H), 7.19 (d, J = 8.3 Hz, 1H), 7.02 (d, J = 2.0 Hz, 1H), 6.63 (s, 1H), 4.98 (s, 1H), 4.71 (s, 1H), 3.81–3.68 (m, 1H), 3.02–2.90 (m, 2H), 2.26–2.16 (m, 1H), 2.15–2.09 (m, 1H), 2.09–2.01 (m, 1H), 2.00–1.94(m, 1H), 1.70–1.64 (m, 2H), 1.50–1.44 (m, 1H); 13C NMR (75 MHz, CDCl3) δ 149.7, 144.9, 139.3, 139.0, 138.3, 132.9, 132.1, 131.9, 128.4, 127.1, 117.1, 116.7, 114.9, 68.5, 52.1, 39.3, 32.0, 31.6, 30.3, 20.0; HRMS (ESI) m/z calcd for C21H23Br2N2O2S [M + H]+ 524.9842, found 524.9839; IR (thin film) 3290, 3088, 2924, 2853, 1712, 1638, 1574, 1468, 1389, 1359, 1339, 1231, 1167, 1092, 1068, 1010, 908, 821, 738 cm–1.
N-[2-(6-Bromo-4-methylidene-2,3,4,4a,9,9a-hexahydro-1H-carbazol-4a-yl)ethyl]-4-iodobenzene-1-sulfonamide (12h)
TLC (hexanes:ethyl acetate, 3:1 v/v) Rf = 0.50; yellow oil, 92%; 1H NMR (500 MHz, CDCl3) δ 7.88 (d, J = 8.5 Hz, 2H), 7.53 (d, J = 8.5 Hz, 2H), 7.15 (dd, J = 8.3, 2.0 Hz, 1H), 6.99 (d, J = 2.0 Hz, 1H), 6.53 (d, J = 8.2 Hz, 1H), 4.94 (d, J = 1.3 Hz, 1H), 4.92 (brs, 1H), 4.68 (d, J = 1.2 Hz, 1H), 3.62 (t, J = 4.9 Hz, 1H), 3.01–2.86 (m, 2H), 2.21–2.10 (m, 2H), 2.05–2.01 (m, 1H), 1.98–1.92 (m, 1H), 1.82–1.76 (m, 1H), 1.67–1.57 (m, 2H), 1.51–1.46 (m, 1H); 13C NMR (75 MHz, CDCl3) δ 148.8, 148.6, 139.6, 138.4, 135.4, 130.7, 128.4, 127.0, 112.2, 111.9, 110.7, 100.0, 65.4, 52.3, 40.2, 36.3, 32.4, 29.7, 21.6; HRMS (ESI) m/z calcd for C21H22BrIN2NaO2S [M + Na]+ 594.9522, found 594.9523; IR (thin film) 3283, 3082, 2926, 2855, 1716, 1636, 1601, 1570, 1474, 1384, 1329, 1262, 1161, 1090, 1006, 816, 737 cm–1.
N-[2-(6-Bromo-4-methylidene-2,3,4,4a,9,9a-hexahydro-1H-carbazol-4a-yl)ethyl]-3,4-dichlorobenzene-1-sulfonamide (12i)
TLC (hexanes:ethyl acetate, 3:1 v/v) Rf = 0.50; light yellow oil, 72%; 1H NMR (500 MHz, CDCl3) δ 7.91 (d, J = 2.0 Hz, 1H), 7.63 (dd, J = 8.4, 2.1 Hz, 1H), 7.60 (s, 1H), 7.15 (dd, J = 8.3, 2.0 Hz, 1H), 7.00 (d, J = 2.0 Hz, 1H), 6.54 (d, J = 8.3 Hz, 1H), 5.12 (t, J = 6.3 Hz, 1H), 4.97 (d, J = 1.4 Hz, 1H), 4.72 (s, 1H), 3.74 (s, 1H), 3.63 (t, J = 5.1 Hz, 1H), 3.07–2.95 (m, 1H), 2.93–2.83 (m, 1H), 2.21–2.11 (m, 2H), 2.08–2.01 (m, 1H), 2.00–1.94 (m, 1H), 1.84–1.77 (m, 1H), 1.68–1.56 (m, 2H), 1.53–1.43 (m, 1H); 13C NMR (75 MHz, CDCl3) δ 148.7, 148.4, 139.7, 137.5, 135.3, 133.8, 131.2, 131.2, 130.8, 128.9, 128.6, 126.9, 126.1, 125.6, 112.2, 111.9, 110.9, 65.4, 52.4, 40.3, 36.5, 32.5, 29.0, 21.6; HRMS (ESI) m/z calcd for C21H22BrCl2N2O2S [M + H]+ 514.9957, found 514.9963; IR (thin film) 2268, 2960, 2337, 1720, 1601, 1455, 1370, 1260, 1164, 1032, 801, 700 cm–1.
N-[2-(6-Bromo-4-methylidene-2,3,4,4a,9,9a-hexahydro-1H-carbazol-4a-yl)ethyl]-2,4-dichlorobenzene-1-sulfonamide (12j)
TLC (hexanes:ethyl acetate, 2:1 v/v) Rf = 0.40; light yellow oil, 77%; 1H NMR (500 MHz, CDCl3) δ 8.16–8.03 (m, 1H), 7.87 (dd, J = 6.9, 2.4 Hz, 1H), 7.82–7.67 (m, 2H), 7.14 (dd, J = 8.3, 2.0 Hz, 1H), 7.02 (d, J = 2.1 Hz, 1H), 6.55 (d, J = 8.3 Hz, 1H), 5.82 (t, J = 6.1 Hz, 1H), 4.96 (s, 1H), 4.71 (s, 1H), 3.68 (t, J = 5.0 Hz, 1H), 3.21–2.99 (m, 3H), 2.26–2.15 (m, 2H), 2.15–2.08 (m, 1H), 2.06–2.00 (m, 1H), 1.87–1.80 (m, 1H), 1.74–1.62 (m, 2H), 1.55–1.46 (m, 1H); 13C NMR (75 MHz, CDCl3) δ 148.9, 141.7, 139.5, 135.3, 132.3, 131.3, 130.7, 127.6, 126.8, 112.1, 111.9, 111.0, 65.2, 52.3, 40.2, 36.2, 32.4, 31.1, 21.8; HRMS (ESI) m/z calcd for C21H21BrCl2N2NaO2S [M + Na]+ 536.9776, found 536.9794; IR (thin film) 3292, 3087, 2924, 2853, 1712, 1637, 1600, 1572, 1555, 1456, 1373, 1337, 1260, 1166, 1102, 903, 817, 624 cm–1.
N-[2-(6-Bromo-4-methylidene-2,3,4,4a,9,9a-hexahydro-1H-carbazol-4a-yl)ethyl]-4-cyanobenzene-1-sulfonamide (12k)
TLC (hexanes:ethyl acetate, 3:1 v/v) Rf = 0.20; colorless oil, 67%; 1H NMR (500 MHz, CDCl3) δ 7.93 (d, J = 8.4 Hz, 2H), 7.83 (d, J = 8.4 Hz, 2H), 7.16 (dd, J = 8.3, 2.0 Hz, 1H), 6.99 (d, J = 2.0 Hz, 1H), 6.54 (d, J = 8.2 Hz, 1H), 5.13 (brs, 1H), 4.97 (s, 1H), 4.71 (s, 1H), 3.62 (t, J = 5.1 Hz, 1H), 3.04–2.90 (m, 2H), 2.24–2.10 (m, 2H), 2.09–2.01 (m, 1H), 1.99–1.92 (m, 1H), 1.83–1.78 (m, 1H), 1.67–1.61 (m, 2H), 1.50–1.46 (m, 1H); 13C NMR (75 MHz, CDCl3) δ 148.8, 148.4, 144.3, 135.2, 133.0, 130.8, 127.6, 126.9, 117.3, 116.4, 112.2, 111.9, 110.8, 65.4, 52.3, 40.3, 36.6, 32.5, 29.7, 29.0, 21.6; HRMS (ESI) m/z calcd for C22H23BrN3O2S [M + H]+ 472.0689, found 472.0696; IR (thin film) 3364, 2923, 2864, 2844, 1653, 1474, 1332, 1275, 1260, 1161, 1054, 1016, 750, 634 cm–1.
N-(4-{[2-(6-Bromo-4-methylidene-2,3,4,4a,9,9a-hexahydro-1H-carbazol-4a-yl)ethyl]sulfamoyl}phenyl)acetamide (12l)
TLC (hexanes:ethyl acetate, 1:3 v/v) Rf = 0.30; colorless oil, 74%; 1H NMR (500 MHz, CDCl3) δ 7.76 (d, J = 8.8 Hz, 2H), 7.67 (d, J = 8.8 Hz, 2H), 7.13 (dd, J = 8.2, 2.0 Hz, 1H), 6.99 (d, J = 2.0 Hz, 1H), 6.52 (d, J = 8.2 Hz, 1H), 4.92 (d, J = 1.4 Hz, 1H), 4.82 (t, J = 6.3 Hz, 1H), 4.65 (s, 1H), 3.62 (t, J = 4.8 Hz, 1H), 2.94 (q, J = 7.2 Hz, 2H), 2.23 (s, 3H), 2.17–2.14 (m, 2H), 2.05–2.00 (m, 1H), 1.97–1.91 (m, 1H), 1.80–1.75 (m, 1H), 1.69–1.64 (m, 2H), 1.50 (dt, J = 11.7, 6.0 Hz, 1H); 13C NMR (75 MHz, CDCl3) δ 169.0, 148.4, 142.1, 135.9, 132.2, 130.8, 130.1, 128.3, 127.9, 127.2, 119.6, 112.3, 112.1, 65.1, 52.4, 39.9, 35.9, 32.3, 24.8, 21.5; HRMS (ESI) m/z calcd for C23H26BrKN3O3S [M + K]+ 542.0510, found 542.0515; IR (thin film) 3318, 3056, 2925, 2854, 1678, 1592, 1533, 1488, 1401, 1371, 1321, 1264, 1155, 094, 1005, 824, 725 cm–1.
N-[2-(6-Bromo-4-methylidene-2,3,4,4a,9,9a-hexahydro-1H-carbazol-4a-yl)ethyl]pyridine-2-sulfonamide (12m)
TLC (chloroform:methanol, 10:1 v/v) Rf = 0.50; colorless oil, 77%; 1H NMR (500 MHz, CDCl3) δ 8.82–8.65 (m, 1H), 8.01 (d, J = 7.9 Hz, 1H), 7.94 (td, J = 7.7, 1.7 Hz, 1H), 7.52 (ddd, J = 7.5, 4.7, 1.3 Hz, 1H), 7.13 (dd, J = 8.3, 2.0 Hz, 1H), 7.01 (d, J = 2.0 Hz, 1H), 6.54 (d, J = 8.2 Hz, 1H), 5.32 (t, J = 6.1 Hz, 1H), 4.92 (s, 1H), 4.64 (s, 1H), 3.68 (t, J = 4.7 Hz, 1H), 3.07 (dtd, J = 8.7, 6.2, 2.1 Hz, 2H), 2.16–2.15 (m, 2H), 2.11–2.04 (m, 1H), 2.02–1.96 (m, 1H), 1.88–1.76 (m, 1H), 1.70–1.65 (m, 2H), 1.56–1.49 (m, 1H); 13C NMR (75 MHz, CDCl3) δ 157.4, 150.1, 148.9, 148.8, 138.1, 135.7, 130.6, 127.0, 126.7, 122.3, 112.1, 111.8, 110.6, 110.5, 65.3, 52.3, 40.7, 36.0, 32.4, 28.4, 21.8; HRMS (ESI) m/z calcd for C20H23BrN3O2S [M + H]+ 448.0689, found 448.0695; IR (thin film) 3363, 2926, 2854, 1720, 1637, 1578, 1474, 1426, 1331, 1174, 902, 811 cm–1.
N-[2-(6-Bromo-4-methylidene-2,3,4,4a,9,9a-hexahydro-1H-carbazol-4a-yl)ethyl]-2-nitrobenzene-1-sulfonamide (12n)
TLC (hexanes:ethyl acetate, 1:1 v/v) Rf = 0.50; yellow oil, 87%; 1H NMR (500 MHz, CDCl3) δ 8.16–8.03 (m, 1H), 7.87 (dd, J = 6.9, 2.4 Hz, 1H), 7.82–7.67 (m, 2H), 7.14 (dd, J = 8.3, 2.0 Hz, 1H), 7.02 (d, J = 2.1 Hz, 1H), 6.55 (d, J = 8.3 Hz, 1H), 5.82 (t, J = 6.1 Hz, 1H), 4.96 (s, 1H), 4.71 (s, 1H), 3.68 (t, J = 5.0 Hz, 1H), 3.21–2.99 (m, 3H), 2.26–2.15 (m, 2H), 2.15–2.08 (m, 1H), 2.06–2.00 (m, 1H), 1.87–1.80 (m, 1H), 1.74–1.62 (m, 2H), 1.55–1.46 (m, 1H); 13C NMR (75 MHz, CDCl3) δ 148.9, 148.6, 135.4, 133.7, 133.5, 132.8, 131.0, 130.7, 126.9, 125.4, 112.1, 111.8, 110.6, 65.4, 52.4, 46.0, 40.7, 36.4, 32.5, 28.7, 21.7; HRMS (ESI) m/z calcd for C21H22BrKN3O4S [M + K]+ 530.0146, found 530.0151; IR (thin film) 3367, 3091, 2932, 2857, 2360, 1735, 1600, 1540, 1475, 1422, 1362, 1342, 1261, 1166, 812, 738 cm–1.
2-(6-Bromo-8-fluoro-4-methylidene-2,3,4,4a,9,9a-hexahydro-1H-carbazol-4a-yl)ethan-1-amine (11b)
TLC (choloroform:methanol, 10:1 v/v) Rf = 0.10; yellow oil, 81%; 1H NMR (500 MHz, CDCl3) δ 7.01 (d, J = 9.0 Hz, 2H), 4.88 (s, 1H), 4.58 (s, 1H), 3.76 (t, J = 4.1 Hz, 1H), 2.81 (dt, J = 11.5, 5.6 Hz, 1H), 2.68 (td, J = 12.0, 11.3, 5.2 Hz, 1H), 2.20 (dt, J = 8.9, 4.8 Hz, 2H), 2.01 (ddd, J = 13.9, 10.9, 5.4 Hz, 1H), 1.94–1.88 (m, 1H), 1.85–1.69 (m, 3H), 1.62–1.58 (m, 1H); 13C NMR (75 MHz, CDCl3) δ 150.7, 149.2, 147.5, 139.7, 139.6, 136.5, 136.3, 123.3, 123.2, 117.7, 117.4, 111.8, 109.1, 109.0, 66.2, 53.1, 53.1, 39.1, 38.8, 32.2, 27.5, 21.9; HRMS (ESI) m/z calcd for C15H19BrFN2 [M + H]+ 325.0710, found 325.0714; IR (thin film) 3369, 2931, 2857, 1636, 1591, 1471, 1315, 848 cm–1.
Benzyl N-[2-(6-Bromo-8-fluoro-4-methylidene-2,3,4,4a,9,9a-hexahydro-1H-carbazol-4a-yl)ethyl]carbamate (10b)
TLC (hexanes:ethyl acetate, 3:1 v/v) Rf = 0.40; light yellow oil, 92%; 1H NMR (500 MHz, CDCl3) δ 7.43–7.31 (m, 5H), 7.06–6.97 (m, 2H), 5.10 (s, 2H), 4.96 (s, 1H), 4.84 (t, J = 5.8 Hz, 1H), 4.70 (s, 1H), 3.79 (dd, J = 8.9, 4.9 Hz, 2H), 3.36–3.29 (m, 1H), 3.21–3.14 (m, 1H), 2.21 (t, J = 5.8 Hz, 2H), 2.11–2.05 (m, 1H), 2.01–1.97 (m, 1H), 1.85–1.83 (m, 1H), 1.78–1.69 (m, 2H), 1.60–1.54 (m, 1H); 13C NMR (75 MHz, CDCl3) δ 156.3, 150.7, 148.5, 147.5, 139.0, 139.0, 136.5, 136.5, 136.3, 128.5, 128.1, 128.1, 123.1, 123.0, 118.0, 117.7, 112.3, 109.3, 109.2, 77.5, 77.1, 76.7, 66.7, 65.9, 52.8, 52.8, 38.0, 35.4, 32.3, 28.0, 21.8; HRMS (ESI) m/z calcd for C23H25BrFN2O2 [M + H]+ 459.1078, found 459.1085; IR (thin film) 3416, 3298, 2923, 2851, 1701, 1577, 1523, 1477, 1454, 1371, 1308, 1255, 1217, 1131, 882, 826 cm–1.
N-[2-(6-Bromo-8-fluoro-4-methylidene-2,3,4,4a,9,9a-hexahydro-1H-carbazol-4a-yl)ethyl]-4-methoxybenzene-1-sulfonamide (13a)
TLC (hexanes:ethyl acetate, 2:1 v/v) Rf = 0.20; light yellow oil, 91%; 1H NMR (500 MHz, CDCl3) δ 7.77 (d, J = 8.9 Hz, 2H), 7.10–6.93 (m, 3H), 6.84 (d, J = 1.8 Hz, 1H), 4.93 (d, J = 1.5 Hz, 1H), 4.65 (t, J = 6.3 Hz, 1H), 4.62 (s, 1H), 3.89 (s, 3H), 3.75 (brs, 1H), 3.70 (t, J = 4.6 Hz, 1H), 3.00–2.89 (m, 2H), 2.16 (t, J = 6.2 Hz, 2H), 2.09–2.01 (m, 1H), 1.99–1.93 (m, 1H), 1.85–1.78 (m, 1H), 1.71–1.64 (m, 2H), 1.56–1.51 (m, 1H); 13C NMR (75 MHz, CDCl3) δ 162.9, 150.7, 148.2, 147.5, 138.4, 138.3, 136.4, 136.2, 131.2, 129.2, 122.9, 122.8, 118.1, 117.8, 114.3, 112.38, 109.5, 109.4, 65.9, 55.6, 52.9, 52.9, 40.1, 35.7, 32.3, 28.3, 21.7; HRMS (ESI) m/z calcd for C22H25BrFN2O3S [M + H]+ 495.0748, found 495.0754; IR (thin film) 3054, 2986, 2924, 2850, 1597, 1421, 1265, 1157, 1094, 896, 835, 740 cm–1.
N-[2-(6-Bromo-8-fluoro-4-methylidene-2,3,4,4a,9,9a-hexahydro-1H-carbazol-4a-yl)ethyl]-4-methylbenzene-1-sulfonamide (13b)
TLC (hexanes:ethyl acetate, 3:1 v/v) Rf = 0.50; colorless oil, 83%; 1H NMR (500 MHz, CDCl3) δ 7.72 (d, J = 8.3 Hz, 2H), 7.33 (d, J = 8.0 Hz, 2H), 7.01 (dd, J = 9.6, 1.7 Hz, 1H), 6.84 (d, J = 1.7 Hz, 1H), 4.93 (d, J = 1.5 Hz, 1H), 4.63 (d, J = 7.6 Hz, 1H), 3.73 (brs, 1H), 3.70 (t, J = 4.7 Hz, 1H), 3.02–2.90 (m, 2H), 2.45 (s, 3H), 2.16 (dd, J = 7.6, 4.7 Hz, 2H), 2.05 (dt, J = 14.0, 7.9 Hz, 1H), 1.96 (dt, J = 14.5, 7.4 Hz, 1H), 1.81 (td, J = 10.9, 5.4 Hz, 1H), 1.75–1.65 (m, 1H), 1.52 (tt, J = 10.3, 4.8 Hz, 1H); 13C NMR (75 MHz, CDCl3) δ 148.2, 143.6, 138.4, 136.7, 129.8, 129.0, 127.1, 122.9, 122.8, 118.1, 117.9, 112.4, 65.9, 52.9, 40.1, 35.8, 32.3, 28.3, 21.7, 21.6; HRMS (ESI) m/z calcd for C22H25BrFN2O2S [M + H]+ 479.0799, found 479.0793; IR (thin film) 3054, 2925, 2850, 1422, 1265, 971, 896, 741 cm–1.
N-[2-(6-Bromo-8-fluoro-4-methylidene-2,3,4,4a,9,9a-hexahydro-1H-carbazol-4a-yl)ethyl]-4-fluorobenzene-1-sulfonamide (13c)
TLC (hexanes:ethyl acetate, 3:1 v/v) Rf = 0.20; light yellow oil, 72%; 1H NMR (500 MHz, CDCl3) δ 7.95–7.80 (m, 2H), 7.21 (dd, J = 9.5, 7.6 Hz, 2H), 7.02 (dd, J = 9.6, 1.7 Hz, 1H), 6.84 (d, J = 1.8 Hz, 1H), 4.95 (s, 1H), 4.81 (t, J = 6.3 Hz, 1H), 4.66 (s, 1H), 3.76 (brs, 1H), 3.70 (t, J = 4.8 Hz, 1H), 3.00–2.90 (m, 1H), 2.21–2.12 (m, 1H), 2.11–2.02 (m, 1H), 2.00–1.94 (m, 1H), 1.89–1.78 (m, 1H), 1.71–1.64 (m, 2H), 1.57–1.47 (m, 1H); 13C NMR (101 MHz, CDCl3) δ 150.7, 148.1, 147.5, 138.2, 138.2, 136.3, 136.2, 135.8, 135.8, 129.8, 129.7, 122.8, 122.8, 118.2, 117.9, 116.6, 116.3, 112.4, 109.7, 109.6, 66.0, 52.9, 52.9, 40.1, 36.0, 32.4, 28.5, 21.6; HRMS (ESI) m/z calcd for C21H22BrF2N2NaO2S [M + Na]+ 483.0548, found 483.0553; IR (thin film) 3054, 2986, 2925, 2849, 1593, 1495, 1469, 1265, 896, 840 cm–1.
4-Bromo-N-[2-(6-bromo-8-fluoro-4-methylidene-2,3,4,4a,9,9a-hexahydro-1H-carbazol-4a-yl)ethyl]benzene-1-sulfonamide (13d)
TLC (hexanes:ethyl acetate, 3:1 v/v) Rf = 0.40; light yellow oil, 81%; 1H NMR (500 MHz, CDCl3) δ 7.75–7.60 (m, 4H), 6.99 (d, J = 9.5 Hz, 1H), 6.82 (s, 1H), 4.93 (s, 1H), 4.82 (t, J = 6.3 Hz, 1H), 4.64 (s, 1H), 3.73 (brs, 1H), 3.66 (t, J = 4.9 Hz, 1H), 2.91 (ddd, J = 10.0, 8.1, 5.1 Hz, 2H), 2.13 (q, J = 5.2 Hz, 2H), 2.02 (q, J = 7.9, 7.4 Hz, 1H), 1.94 (ddd, J = 14.4, 8.3, 6.2 Hz, 1H), 1.79 (td, J = 12.4, 11.3, 5.8 Hz, 1H), 1.64 (p, J = 6.4, 5.8 Hz, 2H), 1.49 (dq, J = 12.7, 6.3 Hz, 1H); 13C NMR (75 MHz, CDCl3) δ 150.8, 148.0, 147.5, 138.8, 138.2, 138.1, 136.3, 136.1, 132.5, 128.6, 127.7, 122.8, 122.8, 118.2, 118.0, 112.5, 109.7, 109.6, 66.0, 52.9, 52.9, 40.1, 36.0, 32.4, 28.5, 21.6; HRMS (ESI) m/z calcd for C21H22Br2N2O2S [M + H]+ 542.9747, found 542.9751; IR (thin film) 3773, 2929, 2859, 1620, 1528, 1327, 1260, 1222, 1161, 1068, 1010, 902, 822, 764 cm–1.
N-[2-(6-Bromo-8-fluoro-4-methylidene-2,3,4,4a,9,9a-hexahydro-1H-carbazol-4a-yl)ethyl]-4-iodobenzene-1-sulfonamide (13e)
TLC (hexanes:ethyl acetate, 3:1 v/v) Rf = 0.50; yellow oil, 81%; 1H NMR (500 MHz, CDCl3) δ 7.89 (d, J = 8.5 Hz, 2H), 7.54 (d, J = 8.5 Hz, 2H), 7.03 (dd, J = 9.5, 1.7 Hz, 1H), 6.85 (d, J = 1.7 Hz, 1H), 4.96 (d, J = 1.5 Hz, 1H), 4.82 (t, J = 6.4 Hz, 1H), 4.67 (s, 1H), 3.75 (brs, 1H), 3.69 (t, J = 4.9 Hz, 1H), 3.00–2.88 (m, 2H), 2.23–2.12 (m, 2H), 2.09–2.03 (m, 1H), 2.00–1.95 (m, 1H), 1.87–1.78 (m, 1H), 1.72–1.63 (m, 2H), 1.57–1.47 (m, 1H); 13C NMR (101 MHz, CDCl3) δ 150.3, 148.0, 147.9, 139.4, 138.4, 138.2, 138.1, 136.2, 136.1, 128.4, 122.8, 122.8, 118.2, 118.0, 112.5, 109.7, 109.6, 100.1, 65.9, 52.9, 52.8, 40.1, 36.0, 32.3, 28.5, 21.6; HRMS (ESI) m/z calcd for C21H21BrFIN2NaO2S [M + Na]+ 590.9609, found 590.9616; IR (thin film) 3054, 2986, 2918, 2849, 1624, 1571, 1471, 1336, 1265, 1163, 1125, 896, 819, 740 cm–1.
N-[2-(6-Bromo-8-fluoro-4-methylidene-2,3,4,4a,9,9a-hexahydro-1H-carbazol-4a-yl)ethyl]-3,4-dichlorobenzene-1-sulfonamide (13f)
TLC (hexanes:ethyl acetate, 3:1 v/v) Rf = 0.75; colorless oil, 80%; 1H NMR (500 MHz, CDCl3) δ 7.92 (d, J = 2.1 Hz, 1H), 7.65 (dd, J = 8.4, 2.1 Hz, 1H), 7.61 (d, J = 8.4 Hz, 1H), 7.03 (dd, J = 9.5, 1.7 Hz, 1H), 6.86 (d, J = 1.8 Hz, 1H), 4.98 (d, J = 1.5 Hz, 1H), 4.94 (t, J = 6.3 Hz, 1H), 4.70 (s, 1H), 3.04–2.89 (m, 2H), 2.22–2.15 (m, 2H), 2.13–2.05 (m, 1H), 2.02–1.95 (m, 1H), 1.86–1.81 (m, 1H), 1.72–1.62 (m, 3H), 1.55–1.49 (m, 1H); 13C NMR (75 MHz, CDCl3) δ 150.8, 147.9, 147.5, 139.7, 138.1, 138.0, 137.6, 136.3, 133.8, 131.2, 129.0, 126.0, 122.8, 122.7, 118.3, 118.0, 112.53, 109.8, 109.7, 66.0, 52.9, 52.9, 40.2, 36.2, 32.4, 28.7, 21.6; HRMS (ESI) m/z calcd for C21H21BrCl2FN2O2S [M + H]+ 532.9863, found 532.9869; IR (thin film) 3365, 3085, 2925, 2855, 1624, 1560, 1456, 1333, 1203, 1164, 1059, 903, 820, 613 cm–1.
N-(4-{[2-(6-Bromo-8-fluoro-4-methylidene-2,3,4,4a,9,9a-hexahydro-1H-carbazol-4a-yl)ethyl]sulfamoyl}phenyl)acetamide (13g)
TLC (hexanes:ethyl acetate, 1:3 v/v) Rf = 0.50; colorless oil, 62%; 1H NMR (500 MHz, CDCl3) δ 7.78 (d, J = 8.8 Hz, 2H), 7.67 (d, J = 8.6 Hz, 2H), 7.43 (brs, 1H), 7.01 (dd, J = 9.6, 1.7 Hz, 1H), 6.85 (d, J = 1.8 Hz, 1H), 4.94 (s, 1H), 4.70 (t, J = 6.3 Hz, 1H), 4.63 (s, 1H), 3.75 (brs, 1H), 3.68 (t, J = 4.7 Hz, 1H), 2.98–2.90 (m, 2H), 2.24 (s, 4H), 2.18–2.15 (m, 2H), 2.08–2.02 (m, 1H), 1.99–1.93 (m, 1H), 1.85–1.78 (m, 1H), 1.72–1.63 (m, 2H), 1.55–1.49 (m, 1H); 13C NMR (75 MHz, CDCl3) δ 168.6, 150.8, 148.1, 141.8, 138.3, 136.4, 136.2, 134.5, 128.4, 128.3, 122.8, 122.8, 119.4, 118.2, 117.9, 112.4, 65.9, 52.9, 40.1, 35.8, 32.3, 28.3, 24.8, 21.6; HRMS (ESI) m/z calcd for C23H26BrFN3O3S [M + H]+ 522.0857, found 522.0857; IR (thin film) 3243, 3092, 2980, 2944, 1695, 1591, 1473, 1399, 1371, 1317, 1261, 1158, 1035, 848, 763 cm–1.
N-[2-(6-Bromo-8-fluoro-4-methylidene-2,3,4,4a,9,9a-hexahydro-1H-carbazol-4a-yl)ethyl]-4-cyanobenzene-1-sulfonamide (13h)
TLC (hexanes:ethyl acetate, 3:1 v/v) Rf = 0.20; colorless oil, 75%; 1H NMR (500 MHz, CDCl3) δ 7.94 (d, J = 8.4 Hz, 2H), 7.83 (d, J = 8.4 Hz, 2H), 7.03 (dd, J = 9.5, 1.7 Hz, 1H), 6.84 (d, J = 1.8 Hz, 1H), 5.06 (brs, 1H), 4.98 (s, 1H), 4.70 (s, 1H), 3.77 (brs, 1H), 3.69 (t, J = 5.1 Hz, 1H), 3.03–2.89 (m, 2H), 2.27–2.11 (m, 2H), 2.10–2.04 (m, 1H), 2.02–1.96 (m, 1H), 1.86–1.81 (m, 1H), 1.74–1.63 (m, 2H), 1.54–1.47 (m, 1H); 13C NMR (75 MHz, CDCl3) δ 150.3, 147.9, 147.8, 144.1, 138.0, 137.9, 136.2, 136.1, 133.0, 127.6, 122.7, 122.7, 118.3, 118.1, 117.3, 116.4, 112.5, 109.8, 109.8, 66.0, 52.8, 52.8, 40.2, 36.3, 32.4, 28.8, 21.5; HRMS (ESI) m/z calcd for C22H22BrFN3O2S [M + H]+ 490.0595, found 490.0600; IR (thin film) 3054, 2924, 2849, 1570, 1462, 1421, 1265, 1019, 971, 896, 740 cm–1.
N-[2-(6-Bromo-8-fluoro-4-methylidene-2,3,4,4a,9,9a-hexahydro-1H-carbazol-4a-yl)ethyl]-4-(trifluoromethyl)benzene-1-sulfonamide (13i)
TLC (hexanes:ethyl acetate, 3:1 v/v) Rf = 0.40; light yellow oil, 71%; 1H NMR (500 MHz, CDCl3) δ 7.93 (d, J = 8.1 Hz, 2H), 7.77 (d, J = 8.2 Hz, 2H), 6.99 (dd, J = 9.4, 1.7 Hz, 1H), 6.82 (d, J = 1.7 Hz, 1H), 4.94 (s, 2H), 4.66 (s, 1H), 3.73 (s, 1H), 3.66 (t, J = 5.1 Hz, 1H), 3.06–2.81 (m, 2H), 2.13 (dt, J = 12.4, 5.8 Hz, 2H), 2.09–1.90 (m, 2H), 1.86–1.73 (m, 1H), 1.68–1.61 (m, 2H), 1.47 (dd, J = 11.0, 5.8 Hz, 1H); 13C NMR (75 MHz, CDCl3) δ 150.8, 147.9, 147.5, 143.4, 138.1, 138.0, 136.3, 136.1, 134.7, 134.2, 127.5, 126.4, 126.4, 126.3, 126.3, 125.0, 122.8, 122.7, 121.4, 118.3, 118.0, 112.5, 109.8, 109.7, 66.0, 52.9, 52.9, 40.2, 36.3, 32.4, 28.7, 21.6; HRMS (ESI) m/z calcd for C22H22BrF4N2O2S [M + H]+ 533.0516, found 533.0520; IR (thin film) 3054, 2987, 2926, 2853, 1559, 1507, 1421, 1323, 1265, 1063, 896, 740 cm–1.
Acknowledgments
This work was supported by the University of Colorado, the Colorado Bioscience Discovery Evaluation Grant, and National Institutes of Health Institutional Training Grant GM008732 (to J.D.P).
Glossary
Abbrevitions Used
- MRSA
methicillin-resistant S. aureus
- MSSA
methicillin-sensitive S. aureus
- PBP
penicillin binding protein
- RMA
resistance-modifying agent
- CLSI
Clinical Laboratory Standards Institutes
- SAR
structure–activity relationship
- DMSO
dimethyl sulfoxide
- TFA
trifluoroacetic acid
- DMEM
Dulbecco’s modified Eagle’s medium
- FCS
fetal calf serum
- MHB
Mueller–Hinton broth
- MIC
minimal inhibitory concentration
- MRC
minimum resensitizing concentration
- TLC
thin-layer chromatography
- OD
optical density
- IR
infrared
- mp
melting point.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
References
- Payne D. J. Microbiology. Desperately seeking new antibiotics. Science 2008, 321, 1644–1645. [DOI] [PubMed] [Google Scholar]
- Lewis K. Platforms for antibiotic discovery. Nat. Rev. Drug Discovery 2013, 12, 371–387. [DOI] [PubMed] [Google Scholar]
- Coates A. R. M.; Halls G.; Hu Y. Novel classes of antibiotics or more of the same?. Br. J. Pharmacol. 2011, 163, 184–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowy F. D. Antimicrobial resistance: The example of Staphylococcus aureus. J. Clin. Invest. 2003, 111, 1265–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stryjewski M. E.; Corey G. R. Methicillin-resistant Staphylococcus aureus: An evolving pathogen. Clin. Infect. Dis. 2014, 58, 10–19. [DOI] [PubMed] [Google Scholar]
- Cohen M. L. Epidemiology of drug resistance: Implications for a post-antimicrobial era. Science 1992, 257, 1050–1055. [DOI] [PubMed] [Google Scholar]
- Center for Disease Control and Prevention. Antibiotic Resistance Threats; CDC: Atlanta, GA, 2013. [Google Scholar]
- Bal A. M.; Garau J.; Gould I. M.; Liao C. H.; Mazzei T.; Nimmo G. R.; Soriano A.; Stefani S.; Tenover F. C. Vancomycin in the treatment of meticillin-resistant Staphylococcus aureus (MRSA) infection: End of an era?. J. Glob. Antimicrob. Resist. 2013, 1, 23–30. [DOI] [PubMed] [Google Scholar]
- Abreu A. C.; McBain A. J.; Simões M. Plants as sources of new antimicrobials and resistance-modifying agents. Nat. Prod. Rep. 2012, 29, 1007–1021. [DOI] [PubMed] [Google Scholar]
- Reading C.; Cole M. Clavulanic acid: A beta-lactamase-inhiting beta-lactam from Streptomyces clavuligerus. Antimicrob. Agents Chemother. 1977, 11, 852–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossolini G. M. Acquired metallo-beta-lactamases: An increasing clinical threat. Clin. Infect. Dis. 2005, 41, 1557–1558. [DOI] [PubMed] [Google Scholar]
- Worthington R. J.; Melander C. Overcoming resistance to β-lactam antibiotics. J. Org. Chem. 2013, 78, 4207–4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worthington R. J.; Richards J. J.; Melander C. Small molecule control of bacterial biofilms. Org. Biomol. Chem. 2012, 10, 7457–7474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H.; Gill C. J.; Lee S. H.; Mann P.; Zuck P.; Meredith T. C.; Murgolo; She X.; Kales S.; Liang L.; Liu J.; Wu J.; Maria J. S.; Su J.; Pan J.; Hailey J.; Mcguiness D.; Tan C. M.; Flattery A.; Walker S.; Black T.; Roemer T. Discovery of wall teichoic acid inhibitors as potential anti-MRSA β-lactam combination agents. Chem. Biol. 2013, 20, 272–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquina L. W.; Santa Maria J. P.; Walker S. Teichoic acid biosynthesis as an antibiotic target. Curr. Opin. Microbiol. 2013, 16, 531–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podoll J. D.; Liu Y.; Chang L.; Wall S.; Wang W.; Wang X. Bio-inspired synthesis yields a tricyclic indoline that selectively resensitizes methicillin-resistant Staphylococcus aureus (MRSA) to β-lactam antibiotics. Proc. Natl. Acad. Sci. U. S. A. 2013, 110, 15573–15578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing; 17th Informational Supplement; CLSI: Wayne, PA, 2007. [Google Scholar]
- Yeo S. J.; Liu Y.; Wang X. A one-pot three-component reaction for the preparation of highly functionalized tryptamines. Tetrahedron 2012, 68, 813–818. [Google Scholar]
- Liu Y.; Xu W.; Wang X. Gold(I)-catalyzed tandem cyclization approach to tetracyclic indolines. Org. Lett. 2010, 12, 1448–1451. [DOI] [PubMed] [Google Scholar]
- Sanchez I. H.; Lopez F. J.; Soria J. J.; Larraza M. I.; Flores H. J. Total synthesis of (±)-elwesine, (±)-epielwesine, and (±)-oxocrinine. J. Am. Chem. Soc. 1983, 105, 7640–7643. [Google Scholar]
- Wu C.; Decker E. R.; Blok N.; Bui H.; You T. J.; Wang J.; Bourgoyne A. R.; Knowles V.; Berens K.; Holland G. W.; Brock T. A.; Dixon R. A. F. Discovery, modeling, and human pharmacokinetics of N-(2-acetyl-4,6-dimethylphenyl)-3-(3,4-dimethylisoxazol-5-ylsulfamoyl)thiophene-2-carboxamide (TBC3711), a second generation, ETA selective, and orally bioavailable endothelin antagonist. J. Med. Chem. 2004, 47, 1969–1986. [DOI] [PubMed] [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Methods for Dilution Amntimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard; CLSI: Wayne, PA, 2009. [Google Scholar]
- Sopirala M. M.; Mangino J. E.; gebreyes W. A.; Beller B.; Bannerman T.; Balada-Llasat J. M.; Pancholi P. Synergy testing by Etest, microdilution checkerboard, and time-kill methods for pan-drug-resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 2010, 54, 4678–4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng H.; Kumar K. Antimicrobial activity and protease stability of peptides containing fluorinated amino acids. J. Am. Chem. Soc. 2007, 129, 15615–15622. [DOI] [PubMed] [Google Scholar]
- Wang F.; Qin L.; Pace C. J.; Malonis R.; Gao J. Solubilized gramicidin A as potential systemic antibiotics. ChemBioChem 2012, 13, 51–55. [DOI] [PubMed] [Google Scholar]
- Wright G. D. The antibiotic resistome: The nexus of chemical and genetic diversity. Nat. Rev. Microbiol. 2007, 5, 175–186. [DOI] [PubMed] [Google Scholar]