Abstract
Understanding the role of GABAC receptors in the central nervous system is limited due to a lack of specific ligands. Novel γ-aminobutyric acid (GABA) analogues based on 3-(aminomethyl)-1-oxo-1-hydroxy-phospholane 17 and 3-(guanido)-1-oxo-1-hydroxy-phospholane 19 were investigated to obtain selective GABAC receptor antagonists. A compound of high potency (19, KB = 10 μM) and selectivity (greater than 100 times at ρ1 GABAC receptors as compared to α1β2γ2L GABAA and GABAB(1b,2) receptors) was obtained. The cyclic phosphinic acids (17 and 19) are novel lead agents for developing into more potent and selective GABAC receptor antagonists with increased lipophilicity for future in vivo studies.
Keywords: γ-Aminobutyric acid, ligand-gated ion channels, GABA receptors, cyclic phosphinic acids, two-electrode voltage clamp, ρ1 GABAC homology model
γ-Aminobutyric acid 1 (GABA 1, Figure 1) is the major inhibitory neurotransmitter in the mammalian central nervous system (CNS) and is essential for the overall balance between neuronal excitation and inhibition.1,2 GABA influences neurons via three major classes of receptors that are grouped on the basis of their subunit composition, gating properties, and pharmacological profiles: GABAA, GABAB, and GABAC (GABA ρ) receptors. GABAA and GABAC receptors are ionotropic receptors, belonging to the Cys loop family of ligand-gated ion channels, which also incorporates nicotinic acetylcholine, strychnine-sensitive glycine, serotonin type 3, and some invertebrate anionic glutamate receptors.1,2 Both GABAA and GABAC receptors are chloride channels that mediate fast synaptic inhibition when activated by GABA. In contrast, GABAB receptors are members of the family 3 class metabotropic receptors; these receptors couple via G proteins (Gi/o) to interact with inwardly rectifying potassium (GIRK) and voltage-gated calcium channels, mediating slow, longer lasting synaptic inhibition by increasing potassium and decreasing calcium conductances.3
Figure 1.
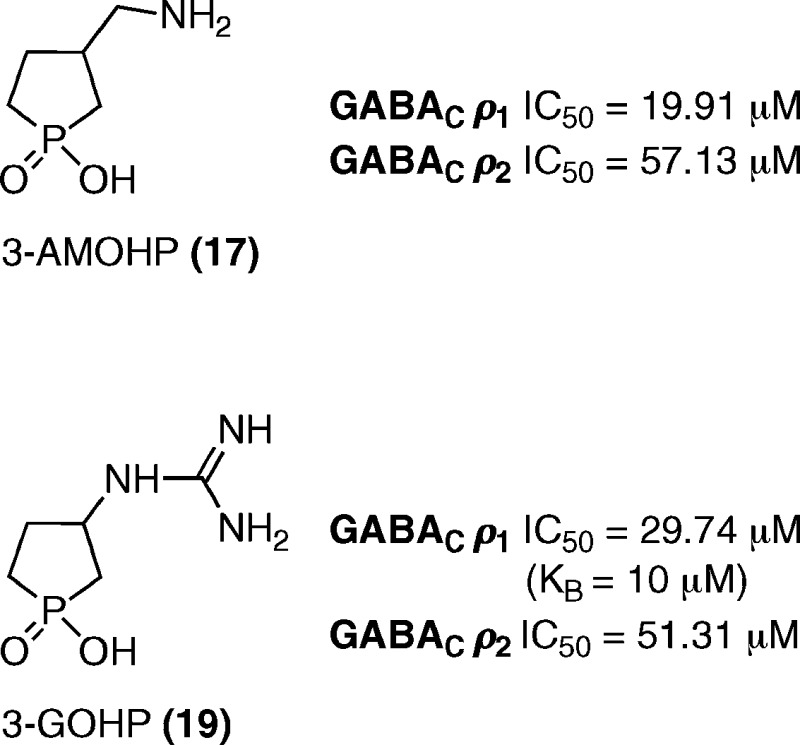
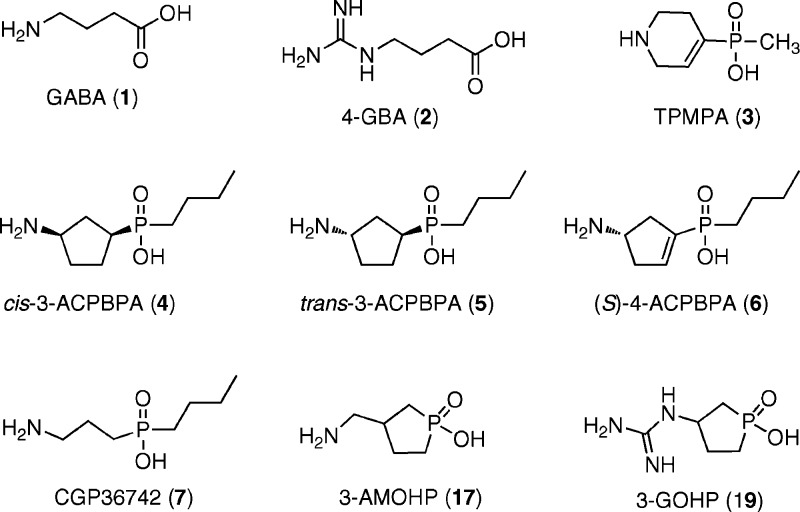
Structures of GABA (1), compounds active at GABA receptors (2−7), and synthesized novel 3-AMOHP (17) and 3-GOHP (19).
The ionotropic GABAA receptors are transmembrane protein complexes composed of five heteropentameric subunits. So far, 16 human GABAA receptor subunits have been identified, and they have been classified into α (α1−α6), β (β1−β3), γ (γ1−γ3), δ, ε, π, and θ. Although a wide range of different GABAA receptor combinations exist in vivo, the most common is the α1β2γ2 combination, constituting approximately 18% of all GABAA receptors in the human brain.4 The GABAB receptors consist of heterodimers, which are composed of two subunits, a ligand-binding subunit (GABAB1), and a signal transduction subunit (GABAB2).3
The GABAC receptor has distinct pharmacology, physiology, and subunit composition to that of GABAA and GABAB receptors.1,5,6 Consisting of homo-oligomeric or pseudohomo-oligomeric pentameric compositions of ρ subunits (ρ1, ρ2, and ρ3 subunits) in mammals,5,7 the receptors are highly expressed in many parts of the brain, including the superior colliculus,8 cerebellum,9 hippocampus (high ρ2 subunit expression),10 and, most prominently, the retina (high ρ1 subunit expression).11
The first selective GABAC receptor antagonist developed was 1,2,5,6-tetrahydropyridine-4-yl-methyl-phosphinic acid 3 (TPMPA 3, Figure 1).12 TPMPA 3 has been shown to enhance memory in chicks,13 inhibit myopia development in chicks,14 modulate the sleep-waking behavior in rats,15 and exert influence on the lateral nucleus of the amygdala (LA).16 However, TPMPA 3 probably does not cross the blood brain barrier, and there have been no reports delineating the CNS effects of TPMPA 3 upon systemic administration.
Recently, we have synthesized a series of conformationally restricted 3-aminocyclopentane/cyclopentene alkylphosphinic acids from a structure−function study of various aminocyclopentane/cyclopentene phosphinic acid analogues of GABA, and these conformationally restricted alkyl phosphinic acids are highly potent and selective for GABAC receptors.17 (S)-4-ACPBPA 6 is the most potent and selective GABAC receptor antagonist of this series. Interestingly, (S)-4-ACPBPA 6 is a conformationally restricted analogue of the orally active GABAB/C receptor antagonist 3-aminopropyl-n-butylphosphinic acid 7 (CGP36742 or SGS742) (GABAB, GABAC, and GABAA: IC50 = 38, 62, and 508 μM, respectively). CGP36742 or SGS742 reached phase II clinical trials, enhancing memory in mildly memory-impaired subjects.18 However, because CGP36742 (SGS742) is approximately equipotent on GABAB as it is on GABAC receptors, it is not clear whether the pharmacological effects of CGP36742 are due to its GABAB or GABAC activity. In continuation of our efforts to identify the role of GABAC receptors in the CNS, we discovered that the selective GABAC receptor antagonists, cis- and trans-(3-aminocyclopentanyl)-n-butylphosphinic acid (cis-3-ACPBPA 4 and trans-3-ACPBPA 5; Figure 1), inhibit myopia progression in chicks and facilitate learning and memory in the Morris Water Maze task in rats.19
The discovery of potent and selective GABAC antagonists emphasizes some important pharmacological differences between these GABA receptor families. Thus, GABAC receptors may be of clinical and pharmacological interest as potential therapeutic targets for myopia, in enhancing cognition and managing memory-related disorders, and its presence in the amygdala might be an alternative target for the development of antianxiety drugs.16
To date, the structural manipulations made in developing GABA analogues for the GABAC receptor have mainly been confined to the carboxylic acid end of the molecule or restricting the conformations of the flexible GABA backbone. As the structure−activity-relationship (SAR) profile of selective GABAC receptor ligands is limited, there is a need to develop more structurally diverse GABAC receptor ligands to understand the physiological role of these receptors. During the course of our ongoing research, we envisioned a novel template for the GABAC receptor by modifying the terminal nitrogen to incorporate a guanidino functional group, which is known to act at GABA receptors20 along with restraining the phosphinic acid moiety (bioisostere of the carboxylic acid). Therefore, in the present study, we report the design, synthesis, and pharmacological evaluation of synthetically challenging cyclic phosphinic acid analogues as GABAC receptor antagonists. The cyclic phosphinic acid nucleus has not been previously used for GABA analogues, and only one paper has described similar cyclic phosphinic acid derivatives as glutamate receptor agonists.21
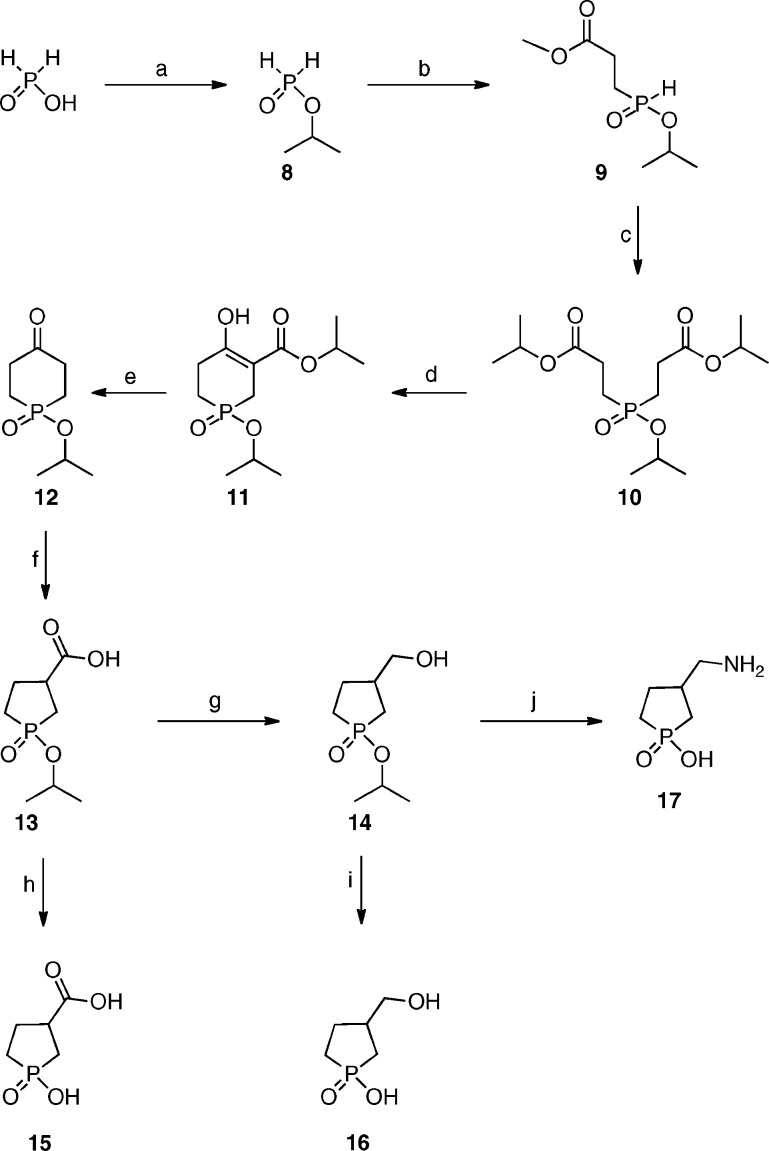
The synthesis of 3-(aminomethyl)-1-oxo-1-hydroxy-phospholane 17 (3-AMOHP) is depicted in Scheme 1. The intermediate isopropoxy-1-oxophosphorinan-4-one 12 was prepared in five steps from water-free hypophosphoric acid, with few modifications as previously described by Verkade et al.22 Hypophosphoric acid was treated with commercially available tetraisopropyl orthosilicate to afford isopropyl phosphinate 8, which was treated with methyl acrylate to provide diester 9. The diester 9 was treated with methyl acrylate in isopropanol to afford triester 10, which was cyclized in the presence of base to afford compound 11. The selective hydrolysis of compound 11 is a key step to afford intermediate isopropoxy-1-oxophosphorinan-4-one 12. The selectivity relies on the difference in reactivity of the carboxylic and phosphinic esters. We investigated several different reaction conditions (such as different concentrations of HCl in isopropanol at room/elevated temperature), and the best condition, which gave a reasonable yield of the intermediate 12, was the treatment of the compound 11 with 3 N hydrochloric acid in the presence of isopropanol.
Scheme 1. Synthesis of Compounds 15−17.
Reagents and conditions: (a) (i-PrO)4Si, CH3CN, reflux for 2 h, 86%. (b) CH2=CHCOOCH3, NaOMe, room temperature for 24 h, 89%. (c) CH2=CHCOOCH3, NaOPri, i-PrOH, room temperature, 95%. (d) n-BuLi, THF, 0 °C to reflux for 4 h, 86% or KOtBu, toluene, 0 °C for 5 h, 83%. (e) 3 N HCl, i-PrOH, reflux for 12 h, 49%. (f) Tl(NO3)3·3H2O, CH2Cl3, room temperature for 48 h, 86%. (g) ClCOOCH2CH3, Et3N, THF, −10 °C for 1 h; NaBH4 0−5 °C, 4 h, 79%. (h) 3 N HCl, reflux for 8 h, 60%. (i) 3 N HCl, reflux for 5 h, 68%. (j) DEAD, 1 M NH3 in benzene, PPh3, THF, 12 h; 3 N HCl, reflux for 3 h, 55%.
The phospholane-1-oxide carboxylic acid 13 was synthesized from keto phosphinane-1-oxide 12 via ring contraction along with oxidation reaction in one step.23 This observation may be of synthetic utility for five-membered heterocycle formation; as to the authors knowledge, it is the first demonstration of direct one-step access to heterocyclic five-membered carboxylic acid from heterocyclic six-membered ketone. The carboxylic acid 13 was reduced to primary alcohol 14 using NaBH4 through in situ mixed carbonic−carboxylic acid anhydride. Finally, the amine was generated via a one pot Mitsunobu−Staudinger reaction, followed by phosphinate ester hydrolysis using aqueous HCl and the crude product purified via ion-exchange chromatography and recrystallization to give the free amine 17 (Scheme 1). Compounds 15 and 16 were prepared by hydrolysis of corresponding phosphinate esters using aqueous HCl.
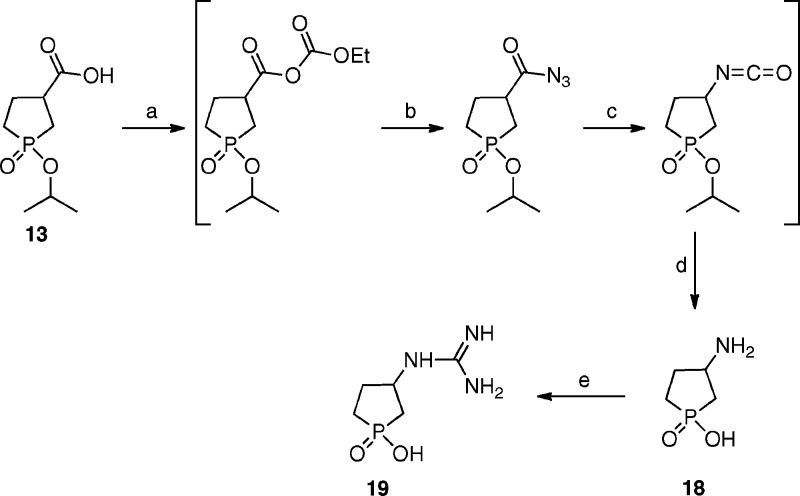
The syntheses of 3-(amino)-1-oxo-1-hydroxy-phospholane (3-AOHP) 18 and 3-(guanido)-1-oxo-1-hydroxy-phospholane (3-GOHP) 19 are depicted in Scheme 2. Modified Curtius rearrangement of the acid 13 and hydrolysis of the intermediate isocyanates with aqueous HCl afforded the amine 18. Compound 19 was prepared by guanylation of amine 18 using formamidinesulfinic acid in the presence of a base.
Scheme 2. Synthesis of Compounds 18 and 19.
Reagents and conditions: (a) ClCOOCH2CH3, Et3N, acetone, −10 °C for 1 h. (b) NaN3, H2O, 2 h. (c) Toluene, reflux for 2 h. (d) 3 N HCl, reflux for 3 h, 48%. (e) Formamidinesulfinic acid, NaOH, H2O, room temperature for 12 h, 76%.
The functional characterization of the cyclic phosphinic acids at GABA receptors was performed using two-electrode voltage clamp on recombinant human GABA receptors expressed in Xenopus oocytes.17,24 The cyclic phosphinic acids (15−19) were evaluated for activity alone and in the presence of GABA on GABAA (α1β2γ2L), GABAB (1b/2), and GABAC (ρ1 and ρ2) receptors to determine whether they behave as agonists, antagonists, or modulators. It has previously been observed that orientations of the acid, backbone, and amine group are important for antagonist activity at GABA receptors, but the distance between acid−amine counterparts and the use of alkyl phosphinic acids as a bioisostere of a carboxylic acid are also an important criterion to develop selective GABAC antagonists.17
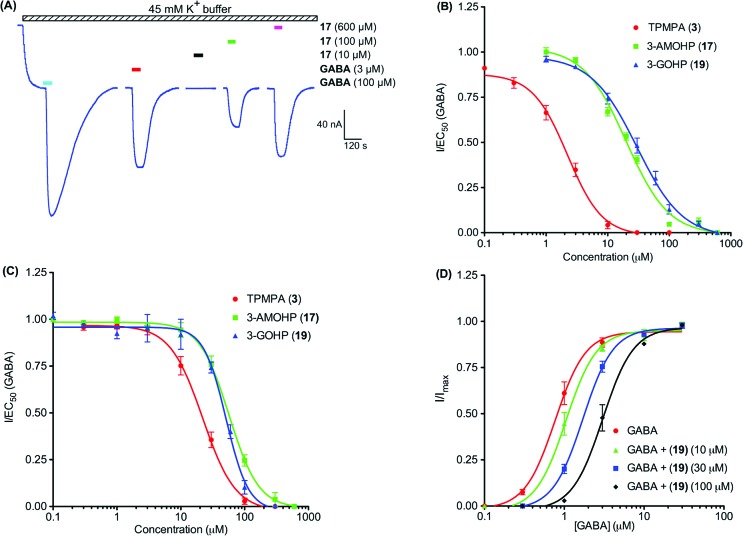
As shown in Table 1, we have examined the effects of cyclic phosphinic acids (15−19) on GABAA, GABAB, and GABAC receptors. Compounds 15 and 16, which lack the terminal nitrogen showed no effects at 100 μM on all three GABA receptors. It proved that both the acid and the amino counterparts are important for the ligand's affinity at these receptors. 3-AMOHP 17 was found to be a potent antagonist (IC50 = 19.91 μM) at ρ1 GABAC receptors, inactive (at 600 μM) at α1β2γ2L GABAA receptors, and a weak agonist at GABAB(1b/2) receptors. Figure 2A shows the effect of 3-AMOHP 17 on GABAB(1b/2) receptors expressed in Xenopus oocytes. 3-AMOHP 17 shows activation itself at 100 (33.22 ± 4.65%) and 600 μM (45.17 ± 5.23%), indicating its weak agonist nature at GABAB(1b/2) receptors. 3-GOHP 19 was developed based on the observed activities of 4-guanidinobutanoic acid 2 (4-GBA), which is an antagonist at GABAA and GABAC receptors with no effects on GABAB receptors.20 ω-Guanidino acids are known to act like GABA at GABA receptors, indicating that guanidino acids behave as though the guanidino group is equivalent to the amino functionality with a more basic nature.20 3-GOHP 19 is a potent and selective antagonist (KB = 10.0 ± 1.6 μM) at the ρ1 GABAC receptor. 3-GOHP 19 has reduced activity (29% inhibition at 600 μM) at α1β2γ2L GABAA receptors and was inactive (at 600 μM) at GABAB(1b/2) receptors, indicating that substituting the amine functionality with a guanidino moiety is well tolerated at the GABAC receptor ligand-binding site. 3-AOHP 18 was inactive at all three GABA receptors, which indicates that the distance between the acidic group and the terminal nitrogen of GABA receptor ligands appears to be important for ligand affinity at these receptors. Figure 2B shows the inhibitory concentration−response curves for the active analogues (TPMPA 3, 3-AMOHP 17, and 3-GOHP 19) against GABA (1 μM) at ρ1 GABAC receptors. The active compounds were further tested at human ρ2 GABAC receptors. Figure 2C shows the inhibitory concentration−response curves for the TPMPA 3 (IC50 = 22.09 μM), 3-AMOHP 17 (IC50 = 57.13 μM), and 3-GOHP 19 (IC50 = 51.31 μM) against GABA (1 μM) at ρ2 GABAC receptors. Both compounds (17 and 19) are moderately potent antagonists at ρ2 GABAC receptors. In addition, 3-GOHP 19 caused a parallel rightward shift of the GABA concentration−response curves in the presence of three antagonist concentrations, indicating its competitive nature (Figure 2D; KB = 10.0 ± 1.6 μM).
Table 1. Pharmacological Data.
| human GABAC receptors IC50 (95% CI)/KB (μM) |
||||
|---|---|---|---|---|
| compd | ρ1 | ρ2 | human GABAB (1b/2) receptors EC50 (μM) or % inhibition |
human α1β2γ2L GABAA receptors KB (μM) or % inhibition |
| TPMPA (3) | IC50 = 2.22 (1.32−6.09) μM | IC50 = 22.09 (18.87−25.86) μM | EC50 = ∼500 μMa | KB = 320 μMa |
| KB = 2.1 μMa | KB = 14.9 μMb | |||
| (S)-4-ACPBPA (6) | KB = 4.97 μM c | 22.5 ± 1.9%c,d | 24.2 ± 1.7%c,e | |
| CGP36742 (7) | IC50 = 62 μMf | IC50 = 38 μMf | IC50 = 508 μMf | |
| 15 | inactive at 100 μM | inactive at 100 μM | inactive at 100 μM | |
| 16 | inactive at 100 μM | inactive at 100 μM | inactive at 100 μM | |
| 17 | IC50 = 19.91 (16.79−23.60) μM | IC50 = 57.13 (54.92−63.39) μM | weak agonist g | inactive at 600 μM |
| 18 | inactive at 600 μM | 9.76 ± 4.6% h | inactive at 600 μM | |
| 19 | IC50 = 29.74 (26.64−35.42) μM | IC50 = 51.31 (47.30−55.40) μM | inactive at 600 μM | 29.21 ± 2.3% e |
| KB = 10 ± 1.6 μMi | ||||
Data from ref (12).
Data from ref (24).
Data from ref (17).
Percentage inhibition by 300 μM compound of the current produced by a submaximal does of GABA (3 μM, EC50). Data are the means ± SEMs (n = 3−5 oocytes).
Percentage inhibition by 600 μM compound of the current produced by a submaximal does of GABA (30 μM, EC50). Data are the means ± SEMs (n = 3 oocytes).
Data from ref (18).
Figure 2A showing the weak agonist effects of 3-AMOHP 17.
Percentage inhibition by 600 μM compound of the current produced by a submaximal does of GABA (3 μM, EC50). Data are the means ± SEMs (n = 3 oocytes).
The KB value is the mean ± SEM.
Figure 2.
(A) Sample current trace showing the effects of 3-AMOHP 17 on human GABAB(1b/2) receptors coexpressed with GIRK1/4 channels in Xenopus oocytes using 45 mM K+ buffer (forward hatched bar). 3-AMOHP 17 had no effect at 10 μM (black bar) but activated GABAB receptors at 100 (green bar) and 600 μM (purple bar) in a concentration-dependent manner, indicating weak agonist effects at this receptor. (B) Inhibitory concentration−response curves for TPMPA 3 (red dot, n = 4), 3-AMOHP 17 (green square, n = 3), and 3-GOHP 19 (blue triangle, n = 4) against GABA (1 μM) at human ρ1 GABA receptors expressed in Xenopus oocytes. Data are the means ± SEMs (n = 3−4 oocytes). (C) Inhibitory concentration−response curves for TPMPA 3 (red dot, n = 3), 3-AMOHP 17 (green square, n = 4), and 3-GOHP 19 (blue triangle, n = 4) against GABA (1 μM) at human ρ2 GABA receptors expressed in Xenopus oocytes. Data are the means ± SEMs (n = 3−4 oocytes). (D) Concentration−response curves of GABA alone (red dot, n = 3) and GABA in the presence of 10 (green triangle, n = 4), 30 (blue square, n = 3), and 100 μM (⧫, n = 4) 19 at human ρ1 GABAC receptors expressed in Xenopus oocytes. Data are the means ± SEMs (n = 3−4 oocytes).
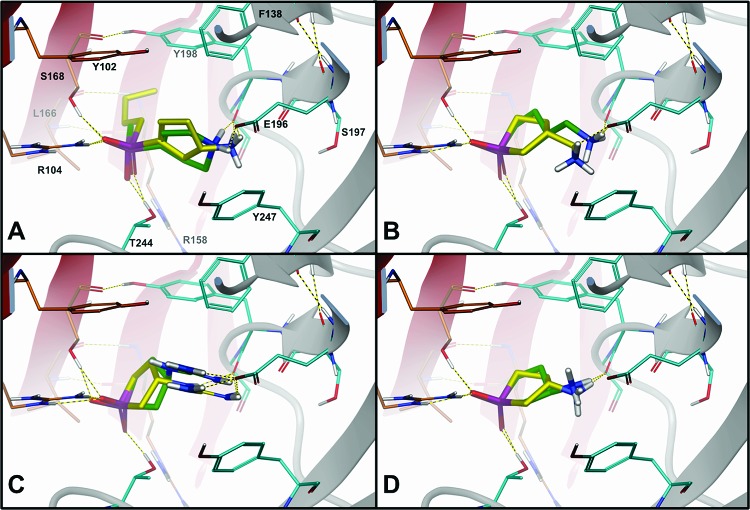
To delineate the key interactions responsible for differences in binding affinity, the structures of TPMPA 3, (S)-4-ACPBPA 6, and each stereoisomer of 3-AMOHP 17, 3-AOHP 18, and 3-GOHP 19 were flexibly docked into the ligand-binding site of a ρ1 GABAC homology model (see the Supporting Information for details). Figures 3B,C shows the S- and R-stereoisomers of both 3-AMOHP 17 and 3-GOHP 19. These compounds are predicted to bind similarly to TPMPA 3 and (S)-4-ACPBPA 6 (Figure 3A). The binding affinity is largely ascribed to various electrostatic interactions,25−28 including (i) a salt bridge interaction between the phosphinic acid and the Arg104,25 (ii) a salt bridge interaction between the basic amine/guanidino and the Glu196,25 (iii) hydrogen bond contacts to Ser168 and Thr244 and to the backbone carbonyl of Tyr198,26,27 and (iv) a cation···π attraction between the basic amine and the Tyr247, an experimentally determined interaction.28 While similar binding energies are predicted for the structures of 3-AMOHP 17 and 3-GOHP 19 (see the Supporting Information), the larger guanidino group of 3-GOHP 19 incurs a slightly higher ligand strain penalty (∼3−4 kcal/mol) than 3-AMOHP 17, which may account for it is slightly decreased activity on ρ1 GABAC activity. The complete inactivity of 3-AOHP 18 on ρ1 GABAC is most likely due to the fact that neither the S- nor the R-stereoisomer can optimally span the width of the binding site (Figure 3D); therefore, they are unable to interact with Arg104 and Glu196 simultaneously, amino acids known to be important for GABA binding. Furthermore, they cannot establish a favorable cation···π attraction with Tyr247. Among all ligands docked, the largest difference is seen with (S)-4-ACPBPA 6, which additionally inserts a butyl chain into a hydrophobic pocket enclosed by Tyr198, Leu166, and Arg158 (Cα, Cβ, Cγ, and Cδ atoms) (Figure 3A). This moiety potentially liberates thermodynamically unfavorable waters29 and highlights a region for further lead optimization. In conclusion, the synthesis of novel cyclic phosphinic acid template has been developed. The activity of these compounds has been investigated at the three major GABA receptor families, and 3-AMOHP 17 and 3-GOHP 19 (ω-guanidino acid) are discovered as potent and selective GABAC receptor antagonists. This is first demonstration of a ω-guanidino acid as a selective GABAC receptor antagonist. These results offer new knowledge of the architecture of GABAC receptor ligand's for studies regarding the binding site and receptor flexibility. In future studies, it might be useful to generate complete SARs of this novel template for developing into more potent and selective GABAC receptor antagonists.
Figure 3.
View of the ρ1 GABAC ligand-binding site with predicted binding modes. (A) TPMPA 3 (green carbons) and (S)-4-ACPBPA 6 (yellow carbons). (B) Stereoisomer of 3-AMOHP 17: (R)-3-AMOHP (green carbons) and (S)-3-AMOHP (yellow carbons). (C) Stereoisomer of 3-GOHP 19: (R)-3-GOHP (green carbons) and (S)-3-GOHP (yellow carbons). (D) Stereoisomer of 3-AOHP 18: (R)-3-AOHP (green carbons) and (S)-3-AOHP (yellow carbons).
Acknowledgments
We are thankful to Bruce Tattam and Dr. Keith Fischer for technical assistance with mass spectrometry measurements. We are also thankful to Hye-Lim Kim for technical assistance with the two-electrode voltage clamp method.
Supporting Information Available
Synthetic experimental procedures, analytical and spectral characterization data of all synthesized compounds including elemental analyses, and details of molecular modeling and pharmacology. This material is available free of charge via the Internet at http://pubs.acs.org.
N.G. acknowledges support from an Endevour International Postgraduate Research Scholarship (EIPRS) and the John Lamberton Scholarship.
Supplementary Material
References
- Chebib M.; Johnston G. A. R. GABA-activated ligand gated ion channels: Medicinal chemistry and molecular biology. J. Med. Chem. 2000, 43, 1427–1447. [DOI] [PubMed] [Google Scholar]
- Bormann J. The “ABC” of GABA receptors. Trends Pharmacol. Sci. 2000, 21, 16–19. [DOI] [PubMed] [Google Scholar]
- Marshall F. H.; Jones K. A.; Kaupmann K.; Bettler B. GABAB receptors-the first 7TM heterodimers. Trends Pharmacol. Sci. 1999, 20, 396–399. [DOI] [PubMed] [Google Scholar]
- Whiting P. J. GABA-A receptor subtypes in the brain: a paradigm for CNS drug discovery?. Drug Discovery Today 2003, 8, 445–450. [DOI] [PubMed] [Google Scholar]
- Enz R.; Cutting G. R. Molecular composition of GABAC receptors. Vision Res. 1998, 38, 1431–1441. [DOI] [PubMed] [Google Scholar]
- Zhang J.; Xue F.; Chang Y. Structural determinants for antagonist pharmacology that distinguish the ρ1 GABAC receptor from GABAA receptors. Mol. Pharmacol. 2008, 74, 941–951. [DOI] [PubMed] [Google Scholar]
- Ogurusu T.; Yanagi K.; Watanabe M.; Fukaya M.; Shingai R. Localization of GABA receptor rho 2 and rho 3 subunits in rat brain and functional expression of homooligomeric rho 3 receptors and heterooligomeric rho 2 rho 3 receptors. Recept. Channels 1999, 6, 463–475. [PubMed] [Google Scholar]
- Boue-Grabot E.; Roudbaraki M.; Bascles L.; Tramu G.; Bloch B.; Garret M. Expression of GABA receptor ρ subunits in rat brain. J. Neurochem. 1998, 70, 899–907. [DOI] [PubMed] [Google Scholar]
- Rozzo A.; Armellin M.; Franzot J.; Chiaruttini C.; Nistri A.; Tongiorgi E. Expression and dendritic mRNA localization of GABAC receptor ρ1 and ρ2 subunits in developing rat brain and spinal cord. Eur. J. Neurosci. 2002, 15, 1747–1758. [DOI] [PubMed] [Google Scholar]
- Alakuijala A.; Palgi M.; Wegelius K.; Schmidt M.; Enz R.; Paulin L.; Saarma M.; Pasternack M. GABA receptor ρ subunit expression in the developing rat brain. Dev. Brain Res. 2005, 154, 15–23. [DOI] [PubMed] [Google Scholar]
- Enz R.; Brandstaetter J. H.; Waessle H.; Bormann J. Immunocytochemical localization of the GABAC receptor ρ subunits in the mammalian retina. J. Neurosci. 1996, 16, 4479–4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata Y.; Woodward R. M.; Miledi R.; Overman L. E. The first selective antagonist for a GABAC receptor. Bioorg. Med. Chem. Lett. 1996, 6, 2073–2076. [Google Scholar]
- Gibbs M. E.; Johnston G. A. R. Opposing roles for GABAA and GABAC receptors in short-term memory formation in young chicks. Neuroscience 2005, 131, 567–576. [DOI] [PubMed] [Google Scholar]
- Stone R. A.; Liu J.; Sugimoto R.; Capehart C.; Zhu X.; Pendrak K. GABA, experimental myopia, and ocular growth in chick. Invest. Opthalmol. Vis. Sci. 2003, 44, 3933–3946. [DOI] [PubMed] [Google Scholar]
- Arnaud C.; Gauthier P.; Gottesmann C. Study of a GABAC receptor antagonist on sleep-waking behavior in rats. Psychopharmacology (Berlin) 2001, 154, 415–419. [DOI] [PubMed] [Google Scholar]
- Cunha C.; Monfils M.-H.; LeDoux J. E. GABAC receptors in the lateral amygdala: A possible novel target for the treatment of fear and anxiety disorders?. Front. Behav. Neurosci. 2010, 4, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R. J.; Chebib M.; Hibbs D. E.; Kim H.-L.; Johnston G. A. R.; Salam N. K.; Hanrahan J. R. Novel γ-aminobutyric acid ρ1 receptor antagonists; synthesis, pharmacological activity and structure-activity relationships. J. Med. Chem. 2008, 51, 3825–3840. [DOI] [PubMed] [Google Scholar]
- Froestl W.; Gallagher M.; Jenkins H.; Madrid A.; Melcher T.; Teichman S.; Mondadori C. G.; Pearlman R. SGS742: The first GABAB receptor antagonist in clinical trials. Biochem. Pharmacol. 2004, 68, 1479–1487. [DOI] [PubMed] [Google Scholar]
- Chebib M.; Hinton T.; Schmid K. L.; Brinkworth D.; Qian H.; Matos S.; Kim H.-L.; Abdel-Halim H.; Kumar R. J.; Johnston G. A. R.; Hanrahan J. R. Novel, potent, and selective GABAC antagonists inhibit myopia development and facilitate learning and memory. J. Pharmacol. Exp. Ther. 2009, 328, 448–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chebib M.; Gavande N.; Wong K. Y.; Park A.; Premoli I.; Mewett K. N.; Allan R. D.; Duke R. K.; Johnston G. A. R.; Hanrahan J. R. Guanidino acids act as ρ1 GABAC receptor antagonists. Neurochem. Res. 2009, 34, 1704–1711. [DOI] [PubMed] [Google Scholar]
- Conway S. J.; Miller J. C.; Bond A. D.; Clark B. P.; Jane D. E. Synthesis and biological evaluation of phospholane and dihydrophosphole analogues of the glutamate receptor agonist AP4. J. Chem. Soc., Perkin Trans. 1 2002, 1625–1627. [Google Scholar]
- Wroblewski A. E.; Verkade J. G. 1-Oxo-2-oxa-1-phosphabicyclo[2.2.2]octane: A new mechanistic probe for the basic hydrolysis of phosphate esters. J. Am. Chem. Soc. 1996, 118, 10168–10174. [Google Scholar]
- Ferraz H. M. C. Jr.; Silva L. F. Thallium trinitrate mediated ring contraction of monocyclic ketones: Stereochemical aspects. Tetrahedron Lett. 1997, 38, 1899–1902. [Google Scholar]
- Chebib M.; Hanrahan J. R.; Kumar R. J.; Mewett K. N.; Morriss G.; Wooller S.; Johnston G. A. R. (3-Aminocyclopentyl)methylphosphinic acids: Novel GABAC receptor antagonists. Neuropharmacology 2007, 52, 779–787. [DOI] [PubMed] [Google Scholar]
- Osolodkin D. I; Chupakhin V. I.; Palyulin V. A.; Zefirov N. S. Molecular modeling of ligand−receptor interactions in GABAC receptor. J. Mol. Graphics Modell. 2009, 27, 813–821. [DOI] [PubMed] [Google Scholar]
- Abdel-Halim H; Hanrahan J. R; Hibbs D. E.; Johnston G. A. R.; Chebib M. A molecular basis for agonist and antagonist actions at GABAC receptors. Chem. Biol. Drug Des. 2008, 71, 306–327. [DOI] [PubMed] [Google Scholar]
- Adamian L.; Gussin H. A.; Tseng Y. Y.; Muni N.; Feng F.; Qian H.; Pepperberg D. R.; Liang J. Structural model of rho1 GABAC receptor based on evolutionary analysis: Testing of predicted protein-protein interactions involved in receptor assembly and function. Protein Sci. 2009, 18, 2371–2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lummis S. C.; Beene D. L.; Harrison N. J.; Lester H. A.; Dougherty D. A. A cation-pi binding interaction with a tyrosine in the binding site of the GABAC receptor. Chem. Biol. 2005, 12, 993–997. [DOI] [PubMed] [Google Scholar]
- Abel R.; Young T.; Farid R.; Berne B. J.; Friesner R. A. Role of the active-site solvent in the thermodynamics of factor Xa ligand binding. J. Am. Chem. Soc. 2008, 130, 2817–2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.