Abstract

A series of potent and selective β1-adrenoreceptor ligands were identified (IC50 range, 0.04–0.25 nM; β1/β2 selectivity range, 65–450-fold), labeled with the PET radioisotope fluorine-18 and evaluated in normal Sprague–Dawley rats. Tissue distribution studies demonstrated uptake of each radiotracers from the blood pool into the myocardium (0.48–0.62% ID/g), lung (0.63–0.97% ID/g), and liver (1.03–1.14% ID/g). Dynamic μPET imaging confirmed the in vivo dissection studies.
Keywords: PET, β1-selective ligand, cardiac imaging, heart failure, 18F-labeling
The β1- and β2-adrenoreceptors (β-AR) play an important role in the regulation of heart function and have been extensively studied in recent decades,1,2 as changes in the number and ratio of cardiac β-ARs have been associated with several diseases of the heart including myocardial ischemia,3 congestive heart failure,4 cardiomyopathy,5 and hypertention.6 In the healthy human heart, β1-AR is the dominant subtype (4:1 ratio, β1:β2-AR) and widely distributed. However, in the failing heart, a predictable decrease in cardiac β1-AR density (3:2 ratio, β1:β2-AR density observed in heart tissue of postmortem heart failure patients), coupled with enhanced sympathetic activity, boosting cardiac-derived noradrenaline and adrenaline levels, leads to further desensitization and subsequent down-regulation of β1-ARs as well as a decrease in cardiac contractility, the hallmark of heart failure.7 In addition, it has been shown that β1-AR down-regulation precedes clinical heart failure and may indeed be an early clinical marker of left ventricular dysfunction.8
To date, the human heart has been extensively studied with positron emission tomography (PET) and single photon emission computed tomography (SPECT) to assess myocardial blood flow and substrate metabolism.9 However, these imaging techniques have yet to realize the opportunity of noninvasive assessment of cardiac AR density, distribution, and occupancy using endogenous ligands or drugs,10 where the capacity of PET to be used for repeat measurements may significantly aid in monitoring and treatment of various heart diseases.11 Identification of the population at risk for developing heart failure before clinical symptoms are noticed would greatly improve patient management as well as the stratification of a patient population that would benefit from placement of an implantable cardioverter-defibrillator (ICD).12
At present, however, no optimal radioligands for imaging cardiac β1-AR using SPECT or PET are available,13 where the most extensively studied tracer in cardiovascular disease patients is the nonselective ligand [11C]CGP-12177.14 Regrettably, its demonstrated capacity for visualization and quantification of β-AR density alone rather than the β1-AR population specifically is less valuable for assessment of cardiac diseases.15
In contrast, the β1-AR selective antagonist ICI 89,406 (1; Scheme 1) has previously been shown to produce effective blockage of β1-AR during exercise in patients with angina pectoris.16 As such, Schaefer et al. chose this compound as their lead structure in the development of new β1-AR selective ligands for PET and SPECT.17−19 Notably, the authors developed an O-methyl derivative of 1, labeled with carbon-11 ([11C]OMe-ICI 89,406; [11C]2), that exhibited high affinity and selectivity for β1-AR in vitro. Unfortunately, in vivo studies did not demonstrate high specific binding to myocardial β1-AR; rapid metabolism and high nonspecific binding in the myocardium were observed.20
Scheme 1. Synthesis of ICI 89, 406, and Analogues.

Reagents and conditions: (a) HCl, dioxane, or TFA, CH2Cl2, 55–98%. (b) MeCN or i-PrOH, i-Pr2NEt, Et2BOMe, Yb(OTf)3, 43–45%; method A, B, or C, 10–45%.
Our goal in the present study was to expand on the known structure–activity profile of 1 and evaluate multiple 18F-labeled derivatives through in vivo imaging studies.21,22 Traditionally, ICI 89,406 along with a very small set of nonfluorinated derivatives were prepared via the acid-catalyzed epoxide opening of (S)-(oxiran-2-ylmethoxy)benzonitrile with an appropriately functionalized amine (Scheme 1).19 The various fluorinated β1-ligands prepared in our study took advantage of this synthetic disconnection approach, where several functionalized amines were first prepared and then exposed to a series of substituted (S)-(oxiran-2-ylmethoxy)benzonitriles to create a diverse set of compounds with A and B regions as shown in Chart 1.
Chart 1. Diverse Moieties for the A and B Region Linked via the ICI 89,406 Backbone.
In conjunction, two complementary synthetic strategies were developed to generate the requisite functionalized amino-urea derivatives. Within each analogue, the central urea linkage was constructed through condensation of tert-butyl-2-aminoethylcarbamate with either a commercially available isocyanate or an in situ-generated p-nitrophenylcarbamate (Schemes 2 and 3 and Supporting Information).
Scheme 2.
Scheme 3. Synthesis of Carbamates 38, 40, 51–60, 63, and 65.
PG (protecting group) = Boc or Cbz. Reagents and conditions: (a) (i) 4-Nitrophenyl chloroformate, pyridine, solvent, overnight; (ii) NBoc-ethylenediamine, base, DMF or THF, 15 min −2 h, 29–94%. (b) H2, Pd/C, EtOH, 90–96%. (c) 2-Fluoroethyl tosylate, K2CO3, DMF, 50–80 °C, 2–24 h, 42–96%.
In certain cases, additional functional group manipulations were required to complete the construct prior to reaction with the substituted (S)-(oxiran-2-ylmethoxy)benzonitriles, while in others, simple deprotection of the derived urea remained to generate the requisite coupling partner.23
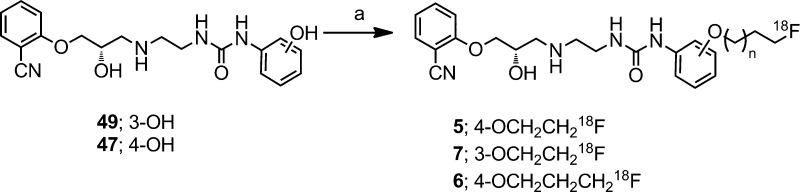
To generate multiple radioligands more efficiently, the radiosynthetic procedure that we chose for labeling compounds 5–7 utilized a well-established, two-step process that allows for labeling of multiple phenolic precursors with various 18F-labeled alkyl substituents (Scheme 4).24 A robotic radiosynthesis station, which can conduct multistep radiosynthetic procedures, was developed and evaluated with our selected precursor phenols.
Scheme 4.
For preparation of the conjugate 18F-labeled alkylfluorides, precursor materials were required that contain activated leaving groups such as sulfonate esters (e.g., tosylates, mesylate). Primary derivatives were preferred because of their ease of displacement with fluorine-18 as compared to secondary derivatives, which often eliminate under the basic fluorination conditions. With these caveats in mind, we first generated the 18F-labeled radioligands [18F]fluoroethyl-(41) and [18F]fluoropropyl tosylate (62) from their respective bis-tosylate precursors using a combination of published methods.25 Subsequent reaction of 41 and 62 with phenol precursors 47 or 49 under standard Williamson conditions afforded [18F]5–7 after high-performance liquid chromatography (HPLC) purification (11–31% conversion after 30 min; see the Supporting Information for details). Notably, for each radiotracer, ∼25 mCi of the final compound was isolated from ∼500 mCi of [18F]NaF in high radiochemical purity; the total synthesis time was 120 min. Specific activity values ranged from 750 to 2000 mCi/μmol depending upon the final radioactive concentration. Significantly, the single-step labeling process, ([18F]KF, K222, MeCN, 90 °C, 15 min), using the derivative tosylate precursors of [18F]5–7, demonstrated rather poor chemical efficiency (<2% radiochemical yield).
Initial synthetic efforts were directed toward introduction of a fluorine atom into the B region of 1 (Table 1). Importantly, the aromatic fluorides 3 and 4 both exhibited potent β1-AR binding activity (IC50 = 0.2 and 0.1 nM, respectively) and β1/β2-AR selectivity (69- and 150-fold, respectively), revealing a modest preference for locating fluorine at the para-position of the aromatic ring. Alternatively, extension of the methoxy function in 2 to fluoroethoxy derivative 5 resulted in a 3-fold increase in selectivity while maintaining β1-AR binding potency.26 Interestingly, further extension to yield a fluoropropoxy function (6) resulted in decreased β1-AR binding potency and subtype selectivity as compared to 2. Maintaining the fluoroethoxy group in 5 with transposition to the meta-position did, however, improve β1-AR potency albeit with reduced selectivity (e.g., 5 and 7, Table 1). Relocating the fluorine atom from the B region (5) to the A region as in 12 induced a marked decrease in β1-AR potency and selectivity. Lastly, when the fluoroethoxy group was replaced with a fluorinated amino derivative, β1-AR potency and selectivity significantly decreased (8). Taken together, fluorine substitution was better tolerated on the B ring with regard to both β1-AR potency and selectivity.
Table 1. Fluorinated Analogues of ICI 89,406 (1) and OMe-ICI 89,406 (2).
| compound | β1a | β2/β1 | cLogD |
|---|---|---|---|
| A1–B1 (2) | 0.11 | 145 | 0.85 |
| A1–B2 (3) | 0.2 | 69 | 0.57 |
| A1–B3 (4) | 0.1 | 150 | 1.23 |
| A1–B4 (5) | 0.08 | 448 | 1.05 |
| A1–B5 (6) | 0.25 | 65 | 1.42 |
| A1–B6(7) | 0.04 | 265 | 1.03 |
| A1–B7 (8) | 1.5 | 13 | 0.39 |
| A2–B8 (9) | 21 | 164 | –0.53 |
| A3–B8 (10) | 2 | 70 | –1.87 |
| A1–B9 (11) | 1 | 511 | –1.66 |
| A2–B10 (12) | 2 | 32 | 2.07 |
| A1–B11 (13) | 0.1 | 50 | 0.92 |
| A1–B12 (14) | 0.3 | 345 | 0.19 |
| A1–B13 (15) | 0.05 | 723 | 0.86 |
| A1–B14(16) | 0.05 | >1000 | 0.47 |
| A1–B15 (17) | 0.35 | 163 | 0.35 |
| A1–B16 (18) | 26 | 8 | 1.04 |
| A1–B17 (19) | 10 | 10 | –0.13 |
| A1–B18 (20) | >100 | 0.05 | –0.59 |
| A1–B19 (21) | 20 | 4 | –0.74 |
| A1–B20 (22) | 10 | 4 | 0.92 |
| A1–B21 (23) | 3 | 30 | 0.42 |
| A1–B22 (24) | 0.14 | 6 | 1.28 |
| A1–B23 (25) | 5 | 0.1 | 0.91 |
IC50 values are expressed in nanomolar concentrations.
Previous studies have identified potent β1-AR selective ICI 89,406 analogues, which contain a carboxylic acid residue in the para-position of the B ring.27 Hence, a set of fluorinated analogues (9–11), which contain a related substitution pattern, were prepared. Compounds 9 and 10 exhibit moderate binding potency to β1-AR (IC50 = 21 and 2 nM, respectively), while maintaining good β1/β2-AR selectivity (164- and 70-fold, respectively; Table 1). Transposing the fluorine atom from the A region to the B region (11) once again resulted in a significant increase in β1-AR binding activity and selectivity as compared to 9 and 10. Although compounds 9–11 were comparable in their selectivity profile to 3–7, the β1-AR binding potency was approximately 10-fold lower.
Despite numerous reports of β1-AR ligands that contain an aryloxy propanolamine pharmacophore, many of these compounds are in fact not selective for β1-AR.28 Considering 5–7 contain both the aryloxy propanolamine backbone and a urea-substituted aromatic system (B region), one may conclude that the enhanced β1-AR potency and selectivity is in fact attributed to these additional structural features. As such, supplemental structure–activity relationship (SAR) studies were conducted to evaluate structural requirements within the aryloxy propanolamine backbone of ligands 5–7 necessary to maintain potency and selectivity. For example, introduction of a heteroatom in the B ring of parent structures 4 and 5 afforded the fluorinated pyridine derivatives 14 and 13, respectively. Notably, 14 exhibited a 3-fold decrease in β1-AR potency and a nearly 2-fold increase in β1-AR selectivity as compared to 4, while compound 13 maintained β1-AR potency, albeit with markedly decreased selectivity as compared to 5. Replacement of the fluorine atom in 14 with iodine or bromine yielded compounds 15 and 16, which exhibited increased β1-AR potency and selectivity; 15 and 16 may prove interesting structural targets as 124I and 76Br-labeled PET radiotracers.
Introduction of an additional nitrogen atom in the B ring of 16 generated a fluorinated pyrimidine analogue, which resulted in a minor decrease in β1-AR potency and selectivity (17; IC50 = 0.35 nM; 163-fold), whereas replacement of the B ring with 5-membered heterocycles such as thiazole (18) or tetrazole (19) proved detrimental for both β1-AR potency and selectivity. Not surprisingly, substitution of the B ring with saturated heterocycles as in 20 and 21 markedly decreased potency and selectivity and confirmed the aromatic structural requirements outlined above. A small structure–activity profile of the urea functionality within the linker connecting the A and B regions in 5–7 was also generated, where removal of either nitrogen atom within the urea functionality of 4 and 14 to afford the derived amides 24 and 23, respectively, resulted in >10-fold decrease in both potency and selectivity. Replacement of the urea moiety with a sulfonamide linker (25) also proved detrimental. Overall, no improved functional groups were identified.
In summary, compounds 3–7, 13, and 14, each containing a fluorine atom, exhibited β1-AR binding (IC50 = 0.04–0.30 nM) and good receptor selectivity (50–450-fold) suitable for imaging studies. Our criteria for radioligand selection were the following: (a) β1-binding affinity, <1 nM; (b) β1/β2 selectivity >50; (c) moderate cLogD values; and (d) readily labeled with fluorine-18. Compounds 5–7 were thus selected as representative candidates for labeling with fluorine-18 as they achieved our selection criteria, provided a range of activity and selectivity values, and maintained a cLogD value near one.
Biodistribution studies of [18F]5–7 in Sprague–Dawley (SD) rats are summarized in Table 2. In general, high extraction of the radiotracers from the blood pool into tissues such as heart, lung, and liver was observed at 30 min postinjection. Heart uptake of compounds [18F]5–7 was 0.48, 0.42, and 0.62% ID/g, at 30 min, with corresponding heart:lung ratios of 1:2, 1:1.5, and 1:1.27, respectively. Similar values were also observed between the heart and the liver. In these cases, uptake of radioligands [18F]5–7 appears unaffected by the position or length of the fluoroalkyl side chain.
Table 2. Biodistribution of [18F]-ICI 89,406 Analogues in Ratsab.
| organ | [18F]5b | [18F]6a | [18F]7b |
|---|---|---|---|
| blood | 0.18 ± 0.07 | 0.21 ± 0.01 | 0.14 ± 0.05 |
| heart | 0.48 ± 0.16 | 0.42 ± 0.15 | 0.62 ± 0.03 |
| lung | 0.97 ± 0.26 | 0.63 ± 0.27 | 0.79 ± 0.11 |
| liver | 1.14 ± 0.33 | 1.03 ± 0.52 | 1.14 ± 0.12 |
| spleen | 0.37 ± 0.02 | 0.40 ± 15 | 0.52 ± 0.09 |
| kidney | 1.26 ± 0.29 | 1.38 ± 0.16 | 2.01 ± 0.21 |
| femur | 0.27 ± 0.05 | 0.63 ± 0.16 | 0.16 ± 0.01 |
| muscle | 0.08 ± 0.02 | 0.09 ± 0.01 | 0.10 ± 0.01 |
Data are expressed as the % ID/g ± SD with three animals per data point at 30 min postinjection.
Data are expressed as the % ID/g ± SEM with two animals per data point at 30 min postinjection.
Considering low expression of the β1 receptor subtype, the observed preferential uptake of these tracers in lung tissue is likely nonspecific in nature. While this detail is consistent with the published literature,20 several authors report challenges associated with the in vivo demonstration of subtype selectivity, including achieving a proper balance of specific activity and receptor occupancy as well as mediating the pharmacologic effects of preferred blocking agents (i.e., bradycardia from metaprolol). Despite achieving appropriate levels of specific activity in the present study, the observed tissue distribution will likely complicate future in vivo blocking studies.
Despite low statistical power, the abbreviated in vivo dissection studies outlined above help to establish gross differences in compound distribution and facilitate SAR development. More extensive, statistically relevant studies are however required, to distinguish subtle distribution differences and finalize lead candidate selection.
Dynamic PET imaging studies carried out on SD rats supported the biodistribution results. All three radiotracers were clearly visualized in both the myocardium and the liver at 30 min postinjection; however, at 60 min, significant wash-out of all three radiotracers was observed. Importantly, no interference from uptake in the lung was detected (Figure 1). In vivo metabolism study of [18F]7 revealed progressive loss of the parent species over time beginning at 83% and ending at 44% (2 and 30 min, respectively); a 30 min urine sample contained 76% of the parent species. Two unique metabolites were observed by HPLC analysis.
Figure 1.

Images of the rat heart at 30 min. (a) Horizontal long axis, (b) short axis, and (c) vertical long axis.
Considering the proximity of data derived from [18F]5–7, compound [18F]7 was chosen as a representative example for more detailed in vivo evaluation. Time–activity plots generated through further study of [18F]7 showed rapid uptake of the radiotracer from the blood pool into the myocardium, lung, and liver <5 min postinjection, after which a steady level of the tracer was observed in blood, heart, and lung tissues as liver levels decreased markedly over time (Figure 2). Importantly, the time-dependent imaging study demonstrated [18F]7 does in fact accumulate in cardiac tissue within minutes after injection. Target:nontarget organ ratios remained relatively stable over time (Figure 3; Supporting Information).
Figure 2.

Time–activity curves from the imaging data of [18F]7.
Figure 3.

Images of [18F]7 in the rat heart at various time points. (a) Horizontal long axis, (b) short axis, and (c) vertical long axis.
In conclusion, multiple 18F-labeled derivatives of the β1-selective ligand ICI 89,406 were developed and evaluated as potential cardiac imaging tracers. An extensive structure–activity study identified a series of fluorine-substituted β1-selective ligands, with potent β1-AR affinity and β1/β2 selectivity. In vivo evaluation of [18F]5–7 in SD rats demonstrated uptake of the radiotracers in heart (0.48–0.62% ID/g), lung (0.63–0.97% ID/g), and liver (1.03–1.14% ID/g) tissue; results were confirmed through μPET imaging study.
Despite clear visualization of the myocardium, the considerable uptake of these tracers in nontarget tissues intimates a lack of specific binding, which may limit overall application of current imaging data and general utility of existing pharmacophore design. Future studies will attempt to reconcile these issues of selectivity and be directed toward decreasing liver uptake and improving overall cardiac retention.
Supporting Information Available
Full experimental procedures and characterization data for all new compounds described in this study. This material is available free of charge via the Internet at http://pubs.acs.org.
Supplementary Material
References
- Riemann B.; Schaefers M.; Law. M. P.; Wichter T.; Schober O. Radioligands for imaging myocardial α- and β-adrenoreceptors. Nuklearmedizin 2003, 42, 4–9. [PubMed] [Google Scholar]
- Brodde O. E.; Bruck H.; Leineweber K. Cardiac Adrenoreceptors: Physiological and pathophysiological relevance. J. Pharmacol. Sci. 2006, 100, 323–337. [DOI] [PubMed] [Google Scholar]
- Corr P. B.; Crafford W. A. Enhanced alpha-adrenergic responsiveness in ischemic myocardium: Role of alpha-adreergic blockade. Am. Heart J. 1981, 102, 605–612. [DOI] [PubMed] [Google Scholar]
- Molenaar P.; Parsonage W. A. Fundamental considerations of beta-adrenoreceptor subtypes in human heart failure. TRENDS Pharmacol. Sci. 2005, 26, 368–375. [DOI] [PubMed] [Google Scholar]
- de Jong R. M.; Willemsen T. M.; Slart R. H. J.; Blanksma P. K.; van Waarde A.; Cornel J. H.; Vaalburg W.; van Veldhuisen D. J.; Elsinga P. H. Myocardial beta-adreoreceptor downregulation in idiopathic dilated cardiomyopathy measured in vivo with PET using the new radioligand (S)-[11C]CGP12388. Eur. J. Nucl. Med. Mol. Imaging 2005, 32, 443–447. [DOI] [PubMed] [Google Scholar]
- Yamada S.; Ishima T.; Tomita T.; Hatashi T.; Okada T.; Hayashi E. Alterations in cardiac alpha and beta adrenoreceptors during the development of spontaneous hypertension. J. Pharmacol. Exp. Ther. 1984, 228, 454–459. [PubMed] [Google Scholar]
- Heusch G. Alpha-adrenergic mechanisms in myocardial ischemia. Circulation 1990, 81, 1–13. [DOI] [PubMed] [Google Scholar]
- Bergmann S. R. Cardiac positron emission tomography. Semin. Nucl. Med. 1998, 38, 320–340. [DOI] [PubMed] [Google Scholar]
- Kopka K.; Schoeber O.; Wagner S. 18F-lablelled cardiac PET tracers: Selected probes for the molecular imaging of transporters, receptors and proteases. Basic Res. Cardiol. 2008, 103, 131–143. [DOI] [PubMed] [Google Scholar]
- Kopka K.; Law M. P.; Breyholz H.-J.; Faust A.; Hoeltke C.; Riemann B.; Schober O.; Schaefer M.; Wagner S. Non-invasive molecular imaging of beta-adrenoreceptors in vivo:Perspectives for PET-radioligands. Curr. Med. Chem. 2005, 12, 2057–2074. [DOI] [PubMed] [Google Scholar]
- de Jong R. M.; Blanksma P. K.; van Waarde A.; Veldhuisen D. J. Measurement of myocardial beta-adrenoreceptos density in clinical studies: A role for positron emission tomography. Eur. J. Nucl. Med. 2002, 29, 88–97. [DOI] [PubMed] [Google Scholar]
- Estes N. A. M.; Weinstock J.; Wang P. J.; Homoud M. K.; Link M. S. Use of antiarrhythmics and implantable cardioverter-defibrillators in conjestive heart failure. Am. J. Cardiol. 2003, 91, 45–52. [DOI] [PubMed] [Google Scholar]
- Momose M.; Reder S.; Raffel D. M.; Watzlowik P.; Wester H.-J.; Nguyen N.; Elsinga P. H.; Bengel F. M.; Remien J.; Schwaiger M. Evaluation of cardiac beta-adrenoreceptors in the isolated perfused rat heart using (S)-11C-CGP12388. J. Nucl. Med. 2004, 45, 471–477. [PubMed] [Google Scholar]
- Langer O.; Halldin C. PET and SPET tracers for mapping the cardiac nervous system. Eur. J. Nucl. Med. 2002, 29, 416–434. [DOI] [PubMed] [Google Scholar]
- Schaefers M.; Riemann B.; Levkau B.; Wichter T.; Schaefer K.; Kopka K.; Breithardt; Schober O. Current status and future application of cardiac receptor imaging with positron emission tomography. Nucl. Med. Commun. 2002, 23, 113–115. [DOI] [PubMed] [Google Scholar]
- Majid P. A.; Schreuder J. E.; de Feyter P. J.; Roos J. P. Clinical, electrocardiographic, and hemodynamic effects of ICI89,406, a new cardioselective beta-adrenoreceptor antagonist with intrinsic sympathomimetic activity, in patients with angina pectoris. J. Cardiovasc. Pharmacol. 1980, 2, 435–444. [DOI] [PubMed] [Google Scholar]
- Law M. P.; Wagner S.; Renner C.; Pike V. W.; Schober O.; Schaefers M. Preclinical evaluation of and 18F-labelled beta1-adrenoreceptor selective radioligand based on ICI89,406. Nucl. Med. Biol. 2010, 37, 517–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner S.; Law M. P.; Riemann B.; Pike V. W.; Breyholz H.-J.; Hoeltke C.; Faust A.; Schober O.; Schaefers M.; Kopka K. Synthesis of (R)- and (S)-[O-methyl-11C]N-[2-[3-(2-cyano-phenoxy)-2-hydroxy-propylamino]-ethyl]-N′-(4-methoxy-phenyl)-urea as candidate high affinity beta1-adrenoreceptor PET radioligands. J. Labelled Compd. Radiopharm. 2005, 48, 721–733. [Google Scholar]
- Kopka K.; Wagner S.; Riemann B.; Law M. P.; Puke C.; Luthra S. K.; Pike V. W.; Wichter T.; Schmitz W.; Schober O.; Schaefers M. Design of new beta1-selective adrenoreceptor ligands as potential radioligands for in vivo imaging. Bioorg. Med. Chem. 2003, 11, 3513–3527. [DOI] [PubMed] [Google Scholar]
- Law M. P.; Wagner S.; Kopka K.; Pike V. W.; Schober O.; Schaefers M. Are [O-methyl-11C]derivatives of ICI 89,406 beta1-adrenoreceptor selective radioligands suitable for PET?. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 174–185 and references therein. [DOI] [PubMed] [Google Scholar]
- Purohit A.; Harris T. Robinson S. P.; Yalamanchili P.; Azure M. T.; Casebier S. D.. Preparation of [18F]containing phenylether derivatives as cardiac beta1 adrenoreceptor ligands for imaging congestive heart failure. WO 2008/083054A2.
- Radeke H. S.; Purohit A.; Harris T. D.; Hansen K.; Broekema M.; Hu C.; Jones R.; Azure M.; Cdebaca M.; Yalamanchili P.. Synthesis and evaluation of fluorinated beta1-adrenoreceptor selective ligands Abstract of Papers, 240th ACS National Meeting, Boston, MA, USA, August 22–26, 2010.
- Pence M.; Mee H. T.; Chang G.. Enhanced chemoselective aminolysis of epoxides using diethylmethoxyborane, Abstracts of Papers, 230th ACS National Meeting, August 28–September 1, 2005.
- Musachio J. L. Radiosynthesis and reactivities of novel [18F]2-fluoroethyl arylsulfonates. J. Labelled Compd. Radiopharm. 2005, 48, 735–747. [Google Scholar]
- Zuhayra M. New approach for the synthesis of [18F]fluoroethyltyrosine for cancer imaging: Simple, fast, and high yielding automated synthesis. Bioorg. Med. Chem. 2009, 17, 7441–7448. [DOI] [PubMed] [Google Scholar]
- Wagner S.; Law M. P.; Riemann B.; Pike V. W.; Breyholz H.-J.; Hoeltke C.; Faust A.; Renner C.; Schober O.; Schaefers M.; Kopka K. Synthesis of and 18F-labeled high affinity beta1-adrenoreceptor PET radioligand based on ICI 89,406. J. Labelled Compd. Radiopharm. 2006, 49, 177–195. [Google Scholar]
- Wagner S.; Kopka K.; Law M. P.; Riemann B.; Pike V. W.; Schober O.; Schaefers M. Synthesis and first in vivo evaluation of new selective high affinity beta1-adrenoreceptor radioligands for SPECT based on ICI 89, 406. Bioorg. Med. Chem. 2004, 12, 4117–4132. [DOI] [PubMed] [Google Scholar]
- Large M. S.; Smith L. H. J. Med. Chem. 1982, 25, 1286–1292. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.