Abstract

A series of FC131 [cyclo(-d-Tyr-Arg-Arg-Nal-Gly-)] analogues containing amidine type peptide bond isosteres were synthesized as selective CXC chemokine receptor type 4 (CXCR4) antagonists. An isosteric amidine substructure was constructed by a macrocyclization process using nitrile oxide-mediated C−N bond formation. All of the amidine-containing FC131 analogues exhibited potent SDF-1 binding inhibition to CXCR4. The Nal-Gly-substituted analogue was characterized as one of the most potent cyclic pentapeptide-based CXCR4 antagonists reported to date. The improved activity against human immunodeficiency virus (HIV) type-1 X4 strains suggested that addition of another basic amidine group to the peptide backbone effectively increases the selective binding of the peptides to CXCR4 receptor.
Keywords: Amidine, chemokine, CXCR4 antagonist, FC131, nitrile oxide, peptidomimetics
CXC chemokine receptor type 4 (CXCR4) is a G protein-coupled receptor1 for stromal cell-derived factor 1 (SDF-1)2 that plays a critical role in the metastasis of mammary carcinoma3 and in human immunodeficiency virus (HIV) type-1 infection.4 CXCR4 is an important therapeutic target for these diseases.5 To date, several types of CXCR4 antagonists with a variety of scaffolds have been reported (Figure 1).6−11 Although the scaffolds of these antagonists have little in common, the antagonists all contain a number of basic groups. For example, the polyphemusin II-derived anti-HIV peptide, T140 1,6 has seven basic Arg and Lys residues. Another example is the small molecule antagonist AMD3100, which contains eight secondary or tertiary amino nuclei.7 Crystal structure analysis and mutation experiments of the receptor indicated that the ion-pairing interaction between the basic functional groups of the antagonists and the acidic residues in CXCR4 contributes to the potent bioactivity.12−14
Figure 1.
Structures of reported CXCR4 antagonists. Bold residues are basic residues. Nal = 3-(2-naphthyl)alanine.
FC131 [cyclo(-d-Tyr-Arg-Arg-Nal-Gly-), Nal = 3-(2-naphthyl)alanine] 2 is a highly potent CXCR4 antagonist (Figure 1).15 Using the peptide library approach, the potent anti-HIV activity of T140 1 was reproduced with the appropriate arrangement of basic and aromatic residues on the cyclic pentapeptide framework of FC131. Further systematic structure−activity studies, such as alanine-scanning or amino acid optimizations, have been conducted to identify the structural and electrostatic requirements for the bioactivity of FC131.16 Substitution of an Arg residue in 2 with the epimeric N-methyl-d-arginine led to identification of cyclic pentapeptide-based CXCR4 antagonist, FC122 3, which is the most potent CXCR4 antagonist among the FC131 derivatives reported to date.16 However, backbone modification of 2 using peptide bond isosteres did not improve the bioactivity.17−19 For example, replacement of several peptide bonds with reduced amide bonds 5 or alkene dipeptide isosteres 6 resulted in greatly reduced bioactivity (Figure 2), which suggests that these isosteric substructures are not appropriate for modifications of FC131. On the basis of these previous studies of FC131 derivatives and the common structural features of highly potent CXCR4 antagonists, we envisioned that addition of basic functional group(s) onto FC131 could improve its potency.
Figure 2.
Structures of the peptide bond and the mimetics.
Recently, we established a novel synthetic approach for amidine type peptide bond isosteres 7 using nitrile oxide-mediated C−N bond formation.20 Amidine type peptide bond isosteres were designed based on substitution of the peptide bond carbonyl (C=O) group with an imino (C=NH) group.21,22 Under physiological conditions, the positive charge of the protonated amidines 7′ is delocalized over two nitrogens. Substructure 7′ contributes both the double bond character of peptide bond 4 and the basic character of reduced amide bond isostere 5′. Therefore, the addition of this acyclic amidine group to the framework was expected to enhance the bioactivity without inducing large conformational change in the backbone structure. Accordingly, amidine-containing FC131 analogues 15a,b and 15d−f were designed, in which each peptide bond was replaced with the amidine substructure (Table 1). Compounds 15c and 15g were also designed as epimers of 15b (at the Nal position) and 15f (at the Tyr position), respectively. In this study, we investigated the contribution of amidine units to the bioactivity of amidine-containing FC131 analogues 15a−g.
Table 1. Inhibitory Activity of FC131 and the Derivatives 15a−g against [125I]-SDF-1 Binding to CXCR4.
| peptide | sequencea | IC50 (nM)b |
|---|---|---|
| FC131 (2) | cyclo(-d-Tyr-Arg-Arg-Nal-Gly-) | 126 ± 68 |
| FC122 (3) | cyclo(-d-Tyr-d-MeArg-Arg-Nal-Gly-) | 37 ± 20 |
| 15a | cyclo(-d-Tyr-Arg-Arg-Nal-Gly-Ψ-) | 9.4 ± 3.0 |
| 15b | cyclo(-d-Tyr-Arg-Arg-Nal-Ψ-Gly-) | 4.2 ± 0.31 |
| 15c | cyclo(-d-Tyr-Arg-Arg-d-Nal-Ψ-Gly-) | 4.9 ± 1.1 |
| 15d | cyclo(-d-Tyr-Arg-Arg-Ψ-Nal-Gly-) | 11 ± 2.9 |
| 15e | cyclo(-d-Tyr-Arg-Ψ-Arg-Nal-Gly-) | 16 ± 7.2 |
| 15f | cyclo(-d-Tyr-Ψ-Arg-Arg-Nal-Gly-) | 679 ± 132 |
| 15g | cyclo(-Tyr-Ψ-Arg-Arg-Nal-Gly-) | 334 ± 6.2 |
Ψ indicates the ψ[−C(=NH)−NH−] substructure. Nal, 3-(2-naphthyl)alanine.
IC50 values are the concentrations for 50% inhibition of the [125I]-SDF-1α binding to CXCR4 transfectant of HEK293 cells.
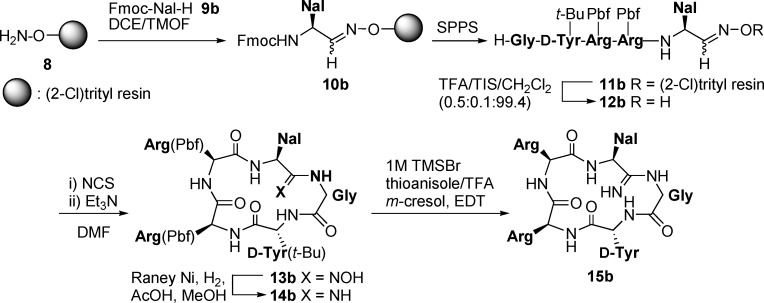
Synthesis of the l-Nal-Gly-substituted analogue 15b is shown in Scheme 1 as a representative preparation of peptides 15a−g. The first Nal residue was loaded onto aminooxy-2-chlorotrityl resin 8(20) by treatment with Fmoc-3-(2-naphthyl)alaninal 9b under acid-free conditions to give aldoxime resin 10b. To prevent possible intramolecular cyclization between side chain guanidino and aldehyde groups in the preparation of aldoxime resins 10d and 10e, di-Boc-protected arginine [Arg(Boc)2]-derived aldehyde was utilized for the preparation of Arg-Arg- and Arg-Nal-substituted analogues 15d and 15e. Peptide elongation was performed by the standard Fmoc-based solid-phase synthesis using N,N′-diisopropylcarbodiimide (DIC)/N-hydroxybenzotriazole (HOBt) in DMF. The cleavage of peptide aldoxime resin 11b provided the linear peptide aldoxime 12b, which was treated with N-chlorosuccinimide and triethylamine to afford cyclic amidoxime (N-hydroxyamidine) 13b.20 After Raney Ni-mediated reduction to the amidine 14b, deprotection with a cocktail of 1M TMSBr, thioanisole/TFA, m-cresol, and 1,2-ethanedithiol gave the desired amidine-containing FC131 analogue 15b. The analogues 15a and 15c−g were synthesized by the same procedure. During this nitrile oxide-mediated cyclization, significant epimerizations of the activated C termini of the peptides were not observed.23
Scheme 1. Synthesis of Amidine-Containing FC131 Analogue 15b.
The potency of the resulting FC131 analogues 15a−g to inhibit [125I]-SDF-1 binding to CXCR4 was evaluated (Table 1). Peptides 15a−e were more potent than the control peptides 2 and 3. This indicates that the basic amidine units had the expected effect of increasing the affinity with the receptor. By contrast, substitution of the Tyr-Arg dipeptide decreased the CXCR4 antagonistic activity (15f and 15g). These observations were consistent with our previous study, in that the d-Tyr-Arg peptide bond is an indispensable functional group that is required to maintain the peptide conformation and the interaction with the receptor. Potent bioactivity of d-MeArg-substituted peptide (3) indicated that the amide hydrogen of Arg is not critical to the bioactivity,16 while the local backbone conformation, particularly with respect to the orientation of d-Tyr carbonyl oxygen, may contribute to the receptor binding. Less potent bioactivity of 15f and 15g supports the significant contribution of d-Tyr carbonyl group in peptides 2 and 3.
Nal-Gly-modified analogues 15b and 15c were the most potent inhibitors of the compounds synthesized in this study. At this Nal-Gly dipeptide position, the amidine substructure was more appropriate than the reduced amide motif (−CH2−NH−), which exhibited slightly lower bioactivity than FC131 in our previous study.17 It is interesting that modification at the Arg-Nal dipeptide (15d) gave potent bioactivity, whereas replacement of this dipeptide with the reduced amide bond in our earlier study reduced receptor binding.17 This indicates that the high bioactivity of 15d could be caused by conformational advantage rather than the basicity. The partial double bond character of the amidine motif in 15d might favorably constrain the cyclic configuration and place the side chains in the appropriate spatial orientations of the pharmacophore.
Recent reports of the docking model of FC131-CXCR4 interactions indicated that the amino group of the Gly-d-Tyr peptide bond forms a hydrogen bond to the carbonyl group of Ala95.24,25 It was also suggested that the Arg-Arg dipeptide is surrounded by acidic residues in CXCR4 (Glu288 and Asp262).24,25 The potent bioactivities of Gly-d-Tyr- and Arg-Arg-substituted analogues 15a and 15e may support the presence of these favorable interactions, which were enhanced by the introduction of positively charged amidine motifs. In particular, the amidine in the Arg-Arg dipeptide could form salt bridge(s) with the negatively charged residues.
Interestingly, both stereoisomers of Nal-Gly- and Tyr-Arg-modified analogues (15b,c and 15f,g) showed similar antagonistic activities [15b (IC50 = 4.2 nM) and 15c (IC50 = 4.9 nM); 15f (IC50 = 679 nM) and 15g (IC50 = 334 nM)]. This is in contrast to the suggestion that the bioactivity of FC131 derivatives is sensitive to the configurations of the component residues.16,17 These results may suggest that the local conformation around the amidine motif is more flexible in cyclic pentapeptides than in the original peptide bond. Of note, none of the peptides 15a−g showed inhibition against SDF-1-CXCR7 interaction (data not shown), which is reported to be an alternative receptor of SDF-1.
Anti-HIV activity based on inhibition of human immunodeficiency virus type 1 (HIV-1) entry into target cells was examined by the MAGI assay using NL4-3, IIIB, and Ba-L strains (Table 2). NL4-3 and IIIB strains use CXCR4 for entry into cells, and the FC131 analogues 15a−e showed very potent anti-HIV activity against these strains. The two Tyr-Arg-substituted peptides 15f and 15g only moderately inhibited infection with these two strains, which was similar to their inhibitory effects against SDF-1-CXCR4 binding. The Ba-L strain uses CCR5 for entry to cells, and none of the peptides showed inhibitory activity against this strain even with the peptides at 10 μM. This result indicates that peptides 15a−g show similar target specificity to FC131 as selective CXCR4 antagonists.26 The cytotoxicity of analogues 15a−g was not observed even at 10 μM in the MAGI assay.
Table 2. Anti-HIV Activity of FC131 and the Derivatives 15a−g.
| EC50 (nM)a |
|||
|---|---|---|---|
| peptide | NL4-3 | IIIB | Ba-L |
| FC131 (2) | 21 ± 4.3 | 21 ± 5.9 | −b |
| FC122 (3) | 7.6 ± 0.34 | 7.6 ± 1.1 | −b |
| 15a | 1.3 ± 0.43 | 0.61 ± 0.10 | −b |
| 15b | 1.4 ± 0.44 | 1.0 ± 0.23 | −b |
| 15c | 2.2 ± 0.04 | 2.0 ± 0.59 | −b |
| 15d | 4.4 ± 1.0 | 6.3 ± 0.47 | −b |
| 15e | 1.9 ± 0.47 | 1.2 ± 0.29 | −b |
| 15f | 300 ± 57 | 258 ± 47 | −b |
| 15g | 248 ± 55 | 238 ± 37 | −b |
EC50 is the concentration that blocks HIV-1 infection by 50%.
No inhibitory activity was observed at 10 μM.
In conclusion, we developed novel potent cyclic pentapeptide-based CXCR4 antagonists containing amidine type peptide bond isosteres. Substitutions of four peptide bonds in FC131, except for the d-Tyr-Arg position, with an amidine motif, improved the inhibitory activity against SDF-1 binding and HIV-1 infection by X4 strains. It was also demonstrated that the analogues were selective antagonists for CXCR4 and not for CXCR7 and CCR5, which are the targets shared by SDF-1 (CXCR7) and HIV-1 (CCR5). Further studies to understand the binding mode of these peptidomimetics and to develop derivatives with multiple amidine motifs in a single molecule are in progress.
Glossary
Abbreviations
- CXCR4
CXC chemokine receptor type 4
- HIV-1
human immunodeficiency virus type 1
- Nal
3-(2-naphthyl)alanine
- DIC
N,N′-diisopropylcarbodiimide
- HOBt
N-hydroxybenzotriazole
- SDF-1
stromal cell-derived factor 1
Supporting Information Available
Experimental procedures and characterization data for all new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
This work was supported by Grants-in-Aid for Scientific Research and Targeted Protein Research Program from MEXT and Health and Labor Science Research Grants (Research on HIV/AIDS, Japan). E.I. was supported by a JSPS Research Fellowship for Young Scientists.
Supplementary Material
References
- Loetscher M.; Geiser T.; O'Reilly T.; Zwahlen R.; Baggiolini M.; Moser B. Cloning of a human seven-transmenbrane domain receptor, LESTR, that is highly expressed in leukocytes. J. Biol. Chem. 1994, 269, 232–237. [PubMed] [Google Scholar]
- Nagasawa T.; Kikutani H.; Kishimoto T. Molecular cloning and structure of a pre-B-cell growth-stimulating factor. Proc. Natl. Acad. Sci. U.S.A. 1994, 91, 2305–2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller A.; Homey B.; Soto H.; Ge N.; Catron D.; Buchanan M. E.; McClanahan T.; Murphy E.; Yuan W.; Wagner S. N.; Barrera J. L.; Mohar A.; Verástegui E.; Zlotnik A. Involvement of chemokine receptors in breast cancer metastasis. Nature 2001, 410, 50–56. [DOI] [PubMed] [Google Scholar]
- Feng Y.; Broder C. C.; Kennedy P. E.; Berger E. A. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science 1996, 272, 872–877. [DOI] [PubMed] [Google Scholar]
- O'Hayre M.; Salanga C. L.; Handel T. M.; Hamel D. Emerging concepts and approaches for chemokine-receptor drug discovery. Expert Opin. Drug Discovery 2010, 5, 1109–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamamura H.; Xu Y.; Hattori T.; Zhang X.; Arakaki R.; Kanbara K.; Omagari A.; Otaka A.; Ibuka T.; Yamamoto N.; Nakashima H.; Fujii N. A low-molecular-weight inhibitor against the chemokine receptor CXCR4: A strong anti-HIV peptide T140. Biochem. Biophys. Res. Commun. 1998, 253, 877–882. [DOI] [PubMed] [Google Scholar]
- Bridger G. J.; Skerlj R. T.; Thornton D.; Padmanabhan S.; Martellucci S. A.; Henson G. W.; Abrams M. J.; Yamamoto N.; De Vreese K.; Pauwels R.; De Clercq E. Synthesis and structure−activity relationships of phenylenebis(methylene)-linked bis-tetraazamacrocycles that inhibit HIV replication. Effects of macrocyclic ring size and substituents on the aromatic linker. J. Med. Chem. 1995, 38, 366–378. [DOI] [PubMed] [Google Scholar]
- Doranz B. J.; Grovit-Ferbas K.; Sharron M. P.; Mao S.-H.; Bidwell Goetz M.; Daar E. S.; Doms R. W.; O'Brien W. A. A small-molecule inhibitor directed against the chemokine receptor CXCR4 prevents its use as an HIV-1 coreceptor. J. Exp. Med. 1997, 186, 1395–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridger G. J.; Skerlj R. T.; Hernandez-Abad P. E.; Bogucki D. E.; Wang Z.; Zhou Y.; Nan S.; Boehringer E. M.; Wilson T.; Crawford J.; Metz M.; Hatse S.; Princen K.; De Clercq E.; Schols D. Synthesis and structure−activity relationships of azamacrocyclic C-X-C chemokine receptor 4 antagonists: Analogues containing a single azamacrocyclic ring are potent inhibitors of T-cell tropic (X4) HIV-1 replication. J. Med. Chem. 2010, 53, 1250–1260. [DOI] [PubMed] [Google Scholar]
- Skerlj R. T.; Bridger G. J.; Kaller A.; McEachern E. J.; Crawford J. B.; Zhou Y.; Atsma B.; Langille J.; Nan S.; Veale D.; Wilson T.; Harwig C.; Hatse S.; Princen K.; De Clercq E.; Schols D. Discovery of novel small molecule orally bioavailable C-X-C chemokine receptor 4 antagonists that are potent inhibitors of T-tropic (X4) HIV-1 replication. J. Med. Chem. 2010, 53, 3376–3388. [DOI] [PubMed] [Google Scholar]
- Zhan W.; Liang Z.; Zhu A.; Kurtkaya S.; Shim H.; Snyder J. P.; Liotta D. C. Discovery of small molecule CXCR4 antagonists. J. Med. Chem. 2007, 50, 5655–5664. [DOI] [PubMed] [Google Scholar]
- Rosenkilde M. M.; Gerlach L.-O.; Jakobsen J. S.; Skerlj R. T.; Bridger G. J.; Schwartz T. W. Molecular mechanism of AMD3100 antagonism in the CXCR4 receptor. J. Biol. Chem. 2004, 279, 3033–3041. [DOI] [PubMed] [Google Scholar]
- Zhang W.; Navenot J.-M.; Haribabu B.; Tamamura H.; Hiramatsu K.; Omagari A.; Pei G.; Manfredi J. P.; Fujii N.; Broach J. R.; Peiper S. C. A point mutation that confers constitutive activity to CXCR4 reveals that T140 is an inverse agonist and that AMD3100 and ALX40-4C are weak partial agonists. J. Biol. Chem. 2002, 277, 24515–24521. [DOI] [PubMed] [Google Scholar]
- Wu B.; Chien E. Y. T.; Mol C. D.; Fenalti G.; Liu W.; Katritch V.; Abagyan R.; Brooun A.; Wells P.; Bi F. C.; Hamel D. J.; Kuhn P.; Handel T. M.; Cherezov V.; Stevens R. C. Structures of the CXCR4 chemokine GPCR with small-molecule and cyclic peptide antagonists. Science 2010, 330, 1066–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii N.; Oishi S.; Hiramatsu K.; Araki T.; Ueda S.; Tamamura H.; Otaka A.; Kusano S.; Terakubo S.; Nakashima H.; Broach J. A.; Trent J. O.; Wang Z.; Peiper S. C. Molecular-size reduction of a potent CXCR4-chemokine antagonist using orthogonal combination of conformation- and sequence-based libraries. Angew. Chem., Int. Ed. 2003, 42, 3251–3253. [DOI] [PubMed] [Google Scholar]
- Ueda S.; Oishi S.; Wang Z.; Araki T.; Tamamura H.; Cluzeau J.; Ohno H.; Kusano S.; Nakashima H.; Trent J. O.; Peiper S. C.; Fujii N. Structure−activity relationships of cyclic peptide-based chemokine receptor CXCR4 antagonists: disclosing the importance of side-chain and backbone functionalities. J. Med. Chem. 2007, 50, 192–198. [DOI] [PubMed] [Google Scholar]
- Tamamura H.; Araki T.; Ueda S.; Wang Z.; Oishi S.; Esaka A.; Trent J. O.; Nakashima H.; Yamamoto N.; Peiper S. C.; Otaka A.; Fujii N. Identification of novel low molecular weight CXCR4 antagonists by structural tuning of cyclic tetrapeptide scaffolds. J. Med. Chem. 2005, 48, 3280–3289. [DOI] [PubMed] [Google Scholar]
- Tamamura H.; Hiramatsu K.; Ueda S.; Wang Z.; Kusano S.; Terakubo S.; Trent J. O.; Peiper S. C.; Yamamoto N.; Nakashima H.; Otaka A.; Fujii N. Stereoselective synthesis of [l-Arg-l/d-3-(2-naphthyl)alanine]-type (E)-alkene dipeptide isosteres and its application to the synthesis and biological evaluation of pseudopeptide analogues of the CXCR4 antagonist FC131. J. Med. Chem. 2005, 48, 380–391. [DOI] [PubMed] [Google Scholar]
- Narumi T.; Hayashi R.; Tomita K.; Kobayashi K.; Tanahara N.; Ohno H.; Naito T.; Kodama E.; Matsuoka M.; Oishi S.; Fujii N. Synthesis and biological evaluation of selective CXCR4 antagonists containing alkene dipeptide isosteres. Org. Biomol. Chem. 2010, 8, 616–621. [DOI] [PubMed] [Google Scholar]
- Inokuchi E.; Yamada A.; Hozumi K.; Tomita K.; Oishi S.; Ohno H.; Nomizu M.; Fujii N.. Org. Biomol. Chem. 2011, DOI: 10.1039/c0ob01193b. [DOI] [PubMed] [Google Scholar]
- Moser H.; Fliri A.; Steiger A.; Costello G.; Schreiber J.; Eschenmoser A. Poly(dipeptamidinium) salts: Definition and methods of preparation. Helv. Chim. Acta 1986, 69, 1224–1262. [Google Scholar]
- Jones R. C. F.; Ward G. J. Amide bond isosteres: imidazolines in pseudopeptide chemistry. Tetrahedron Lett. 1988, 29, 3853–3856. [Google Scholar]
- Epimerizations in the preparation of protected cyclic peptides 13b and 13f were verified by the comparative HPLC analysis of the amidine isomers 14b/14c and the amidoxime isomers 13f/13g, respectively (14b, 90% de; 13f, 95% de; see the Supporting Information for details).
- Våbenø J.; Nikiforovich G. V.; Marshall G. R. A minimalistic 3D pharmacophore model for cyclopentapeptide CXCR4 antagonists. Biopolymers 2006, 84, 459–471. [DOI] [PubMed] [Google Scholar]
- Våbenø J.; Nikiforovich G. V.; Marshall G. R. Insight into the binding mode for cyclopentapeptide antagonists of the CXCR4 receptor. Chem. Biol. Drug Des. 2006, 67, 346–354. [DOI] [PubMed] [Google Scholar]
- Oishi S.; Masuda R.; Evans B.; Ueda S.; Goto Y.; Ohno H.; Hirasawa A.; Tsujimoto G.; Wang Z.; Peiper S. C.; Naito T.; Kodama E.; Matsuoka M.; Fujii N. Synthesis and application of fluorescein- and biotin-labeled molecular probes for the chemokine receptor CXCR4. ChemBioChem 2008, 9, 1154–1158. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.