Abstract
A novel series of HCV replication inhibitors based on a pyrido[3,2-d]pyrimidine core were optimized for pharmacokinetics (PK) in rats. Several associations between physicochemical properties and PK were identified and exploited to guide the design of compounds. In addition, a simple new metric that may aid in the prediction of bioavailability for compounds with higher polar surface area is described (3*HBD-cLogP).
Keywords: Pharmacokinetics; HCV inhibitors; pyrido[3,2-d]pyrimidine core; 3*HBD-cLogP
Since the pioneering work by Lipinski and co-workers correlating the physical properties of a compound [molecular weight, lipophilicity, and the numbers of hydrogen bond donors and acceptors (HBDs and HBAs)] with good absorption properties,1 there has been significant effort within the field of medicinal chemistry to further define and develop methods that allow for the prediction of pharmacokinetics (PK) (especially oral bioavailability) from simple calculated properties. For example, Veber et al. analyzed a large and diverse set of compounds and proposed two separate rules based on either polar surface area (PSA) and rotatable bond count or HBDs and HBAs and rotatable bond count.2 Leeson and Davis compared the change in physical properties for approved oral drugs before and after 1983 and concluded that the most important oral druglike physical properties are lipophilicity, HBDs, and percent PSA.3 Johnson et al. proposed a model for optimizing permeability and clearance based on molecular weight and lipophilicity.4 Other contributions include a decision tree model based on logD, PSA, molecular weight, and number of HBDs,5 and a bioavailability scoring model based on PSA, charge, and the “rule-of-five”.6 Many other more complex prediction tools have been proposed,7 including a “statistical pattern recognition model” based on PSA and AlogP98.8
Chronic hepatitis C virus (HCV) infection represents a major unmet medical need with an estimated 130–170 million people affected worldwide.9 To address this, we recently began investigating a series of replication inhibitors based on a weakly basic 2-amino-pyrido[3,2-d]pyrimidine core. Herein, we show how the PK of compounds in this series was improved by analyzing and optimizing their physicochemical properties. By both lowering the PSA and raising cLogP, compounds were discovered with favorable potency and rat PK profiles. In addition, we note the difficulty in predicting the bioavailability of compounds with moderately high PSA and the significantly negative impact that HBDs appear to impart within this subgroup.
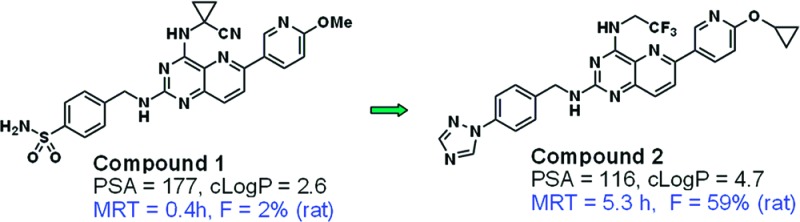
Consistent with the literature,2 compounds with PSA > 140 within this series tended to exhibit poor bioavailability, as illustrated by Figure 1 (in which the coloring scheme is chosen for ease of comparison with subsequent figures).10 For example, the replacement of fluorine by triazole (3 vs 4, Figure 2) results in a marked reduction in bioavailability.
Figure 1.
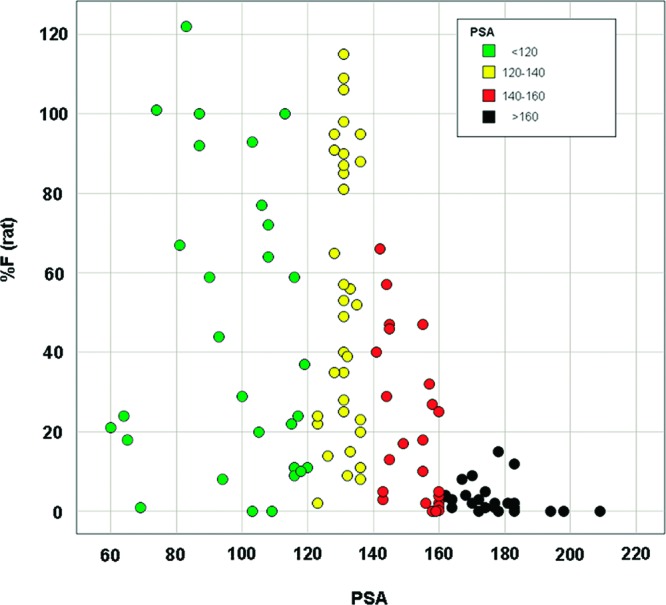
Relationship between rat bioavailability and PSA.
Figure 2.
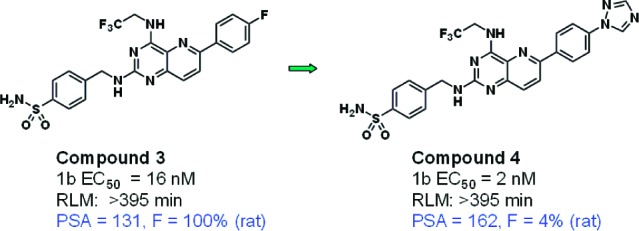
Example of increased PSA leading to reduced bioavailability.11
The relationship between low bioavailability and PSA is understood to be the result of poor passive permeability of more polar compounds across intestinal membranes.2,12 Because the cutoff for good predicted bioavailability is PSA < 140, it was somewhat surprising that a significant portion of the compounds in the PSA 140–160 range exhibited moderate to good bioavailability (see below).
A second notable feature of this series was an association between lipophilicity and mean residence time (MRT). Long MRTs were rare in compounds with cLogP < 3. Conversely, compounds of greater lipophilicity (cLogP > 4) were much more likely to have a long MRT in the rat.13
Figure 3.
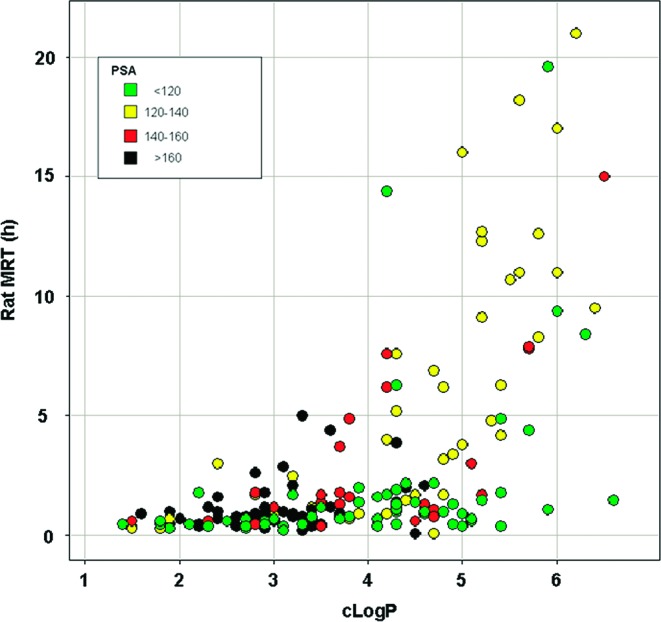
Relationship between MRT in the rat and cLogP.
Compounds 5–8 (Figure 4) illustrate this trend. Small changes (even in different parts of the molecule) that increase lipophilicity also significantly increase the MRT. Within this set, cLogP ranges from 2.8 to 6.0, and the MRT increases from less than 1 h to a very promising 11 h.
Figure 4.
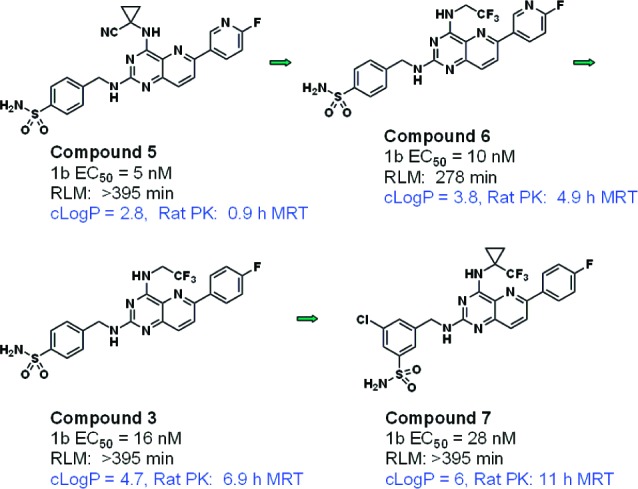
Examples of the association between MRT in the rat and cLogP.
Because MRT is a function of volume and clearance, the dependence of both on lipophilicity for this series was examined. No broad relationship emerged, although the data suggest that lipophilicity may, in some cases, be influencing volume, at least in the higher cLogP ranges. For example, in the series of compounds depicted in Figure 4, the increase in MRT is attributable to higher volumes (substantially above total body water) for the more lipophilic compounds 3 and 7. In contrast, the increase in MRT between compounds 5 and 6 is attributable entirely to reduced clearance, which was not predicted by hepatic microsomal stability (Table 1). Thus, in some cases within this data set and consistent with literature precedent,14 higher lipophilicity is associated with higher volume (due to increased tissue distribution). At the lower end of the cLogP range, it appears that further clearance mechanisms not predicted by hepatic microsomal stability assays (such as biliary excretion or renal elimination) are operative.
Table 1. cLogP and Observed PK Parameters for Compounds 5, 6, 3, and 7.
| compd no. | cLogP | predicted rat CL (L/h/kg) | rat CL (L/h/kg) | rat Vss (L/kg) | rat MRT (h) |
|---|---|---|---|---|---|
| 5 | 2.8 | <0.34 | 0.9 | 0.9 | 0.9 |
| 6 | 3.8 | 0.46 | 0.2 | 1 | 4.9 |
| 3 | 4.7 | <0.34 | 0.3 | 2.3 | 6.9 |
| 7 | 6.0 | <0.34 | 0.4 | 4.3 | 11 |
Through simultaneous manipulation of PSA and cLogP, analogues were identified with dramatically improved rat PK profiles without significant compromise in antiviral potency. Figure 5 illustrates two separate series in which controlled reduction of PSA and increase in cLogP resulted in two compounds with excellent overall characteristics. The rat plasma exposure of one such optimized compound (2) is illustrated in Figure 6.
Figure 5.
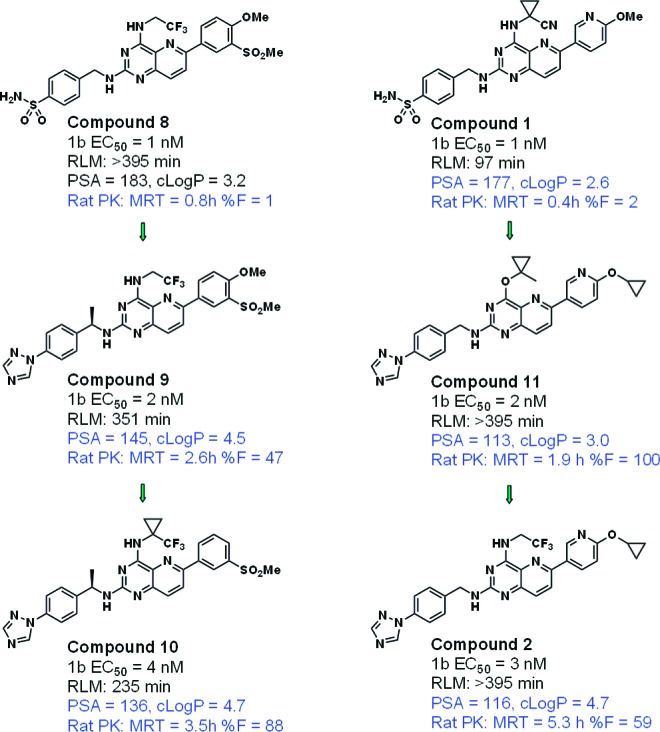
Two subseries in which PK was optimized through manipulation of PSA and cLogP.
Figure 6.
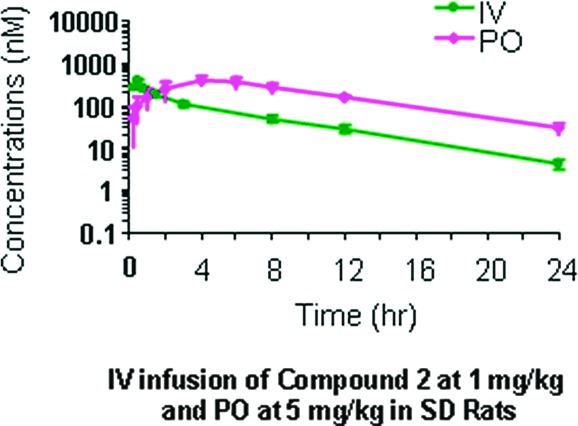
IV and oral rat PK for compound 2.
In the course of this optimization, it became evident that a number of compounds with PSA 140–160 still exhibited moderate bioavailability and that PSA alone was a poor predictor of bioavailability for this subset (Figure 1, red dots).
The presence of HBDs in drug candidates has frequently been cited as an important factor in bioavailability prediction, with high HBD count causing a significant impediment to good oral bioavailability.1−3,5 Indeed, the relative weighting of HBDs and HBAs in Lipinski's “rule of 5” (maximum of five HBDs and maximum of 10 HBAs) suggests that even small increases in HBD count can result in detrimental effects on absorption.1 Consistent with this paradigm, the bioavailability of compounds in this series with PSA 140–160 appears to be significantly influenced by HBD (as opposed to HBA) count (Figures 7 and 8).15 Still, HBD count alone is insufficient for the prediction of bioavailability.
Figure 7.
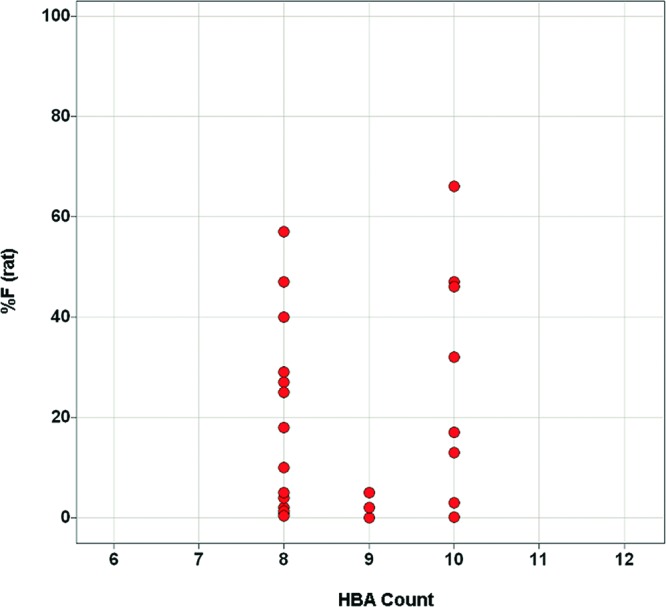
Relationship between % F (rat) and HBA count for analogues with PSA 140–160.
Figure 8.
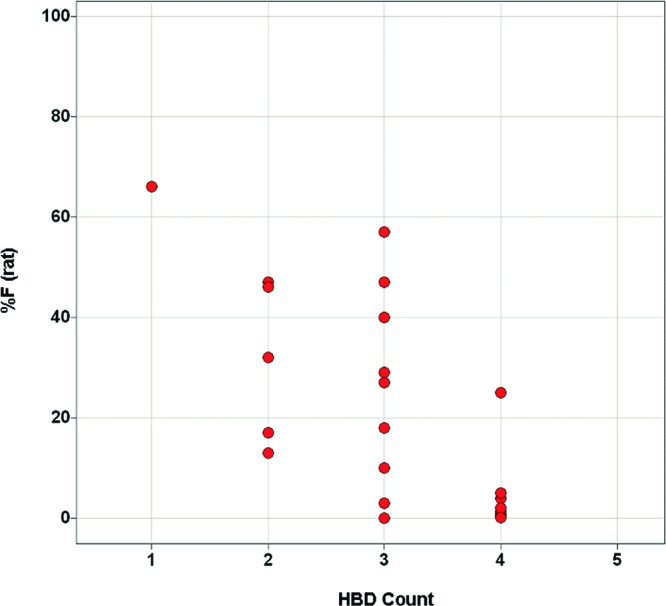
Relationship between % F (rat) and HBD count for analogues with PSA 140–160.
A comparison of 12 and 13 (Figure 9) suggests that lipophilicity might also influence bioavailability for compounds in this set. The bioavailability of 12, an analogue with high PSA (160) and HBD count (4), was very low.16 The introduction of two trifluoromethyl groups in 13, with the attendant increase in cLogP from 4.3 to 6.5, provided the desired improvement in % F.17
Figure 9.
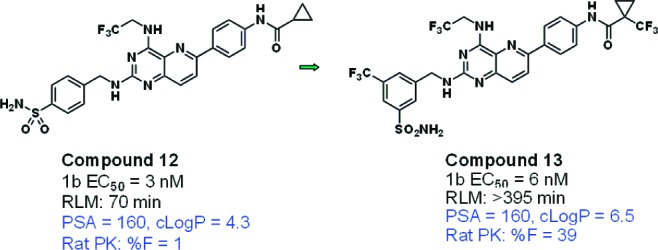
Example of a pair of high PSA analogues in which % F was improved by an increase in lipophilicity.
Thus, we became interested in exploring combinations of descriptors for both lipophilicity and polarity for the prediction of bioavailability of compounds within this series with PSA just above the range previously identified as preferred.2
Empirically, we found the weighted combination of HBD count and cLogP shown in Figure 10 (3*HBD-cLogP) to serve the purpose well: 10 out of 15 compounds with a 3*HBD-cLogP < 6 showed acceptable bioavailability (>20%), while all 10 compounds with a 3*HBD-cLogP > 6 showed poor bioavailability.
Figure 10.
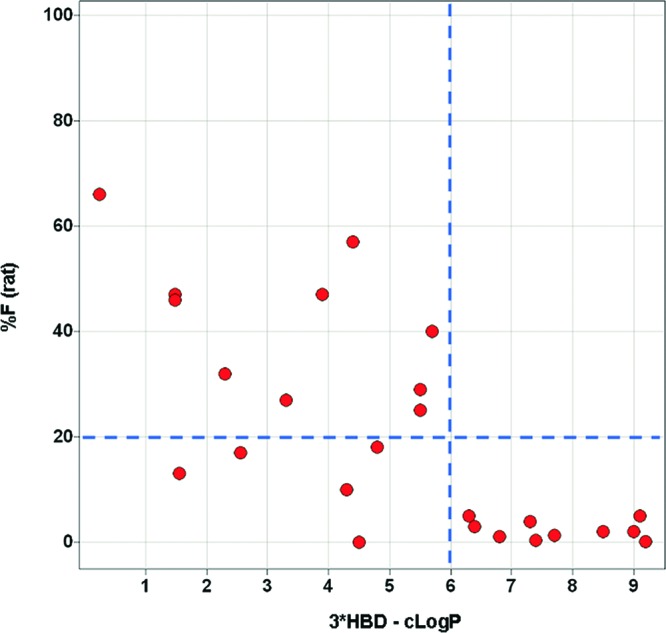
Relationship between % F (rat) and 3*HBD-cLogP for analogues with PSA 140–160.
Applying this metric to all of the compounds in the series, a similar and dramatic drop in % F is seen just above 3*HBD-cLogP = 6 (Figure 11). Similar combinations of HBD count and cLogP as predictors of bioavailability may be of value in other chemical series.
Figure 11.
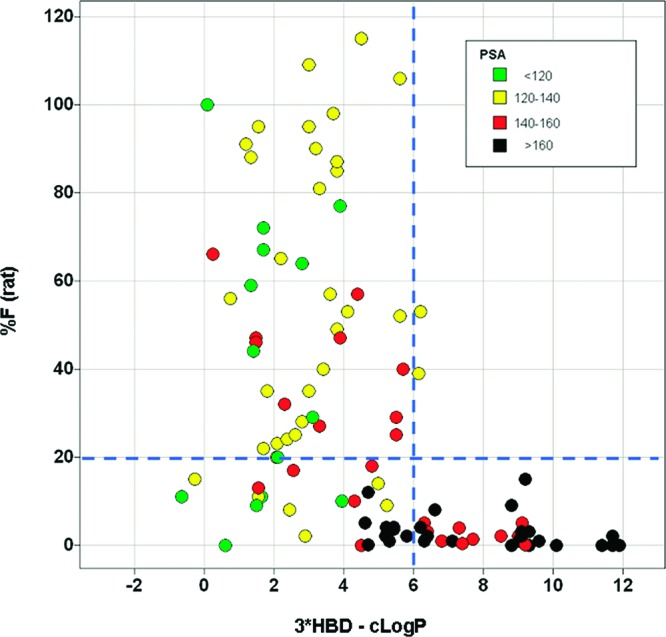
Relationship between % F (rat) and 3*HBD-cLogP for all of the pyridopyrimidines.
The significance of the metric 3*HBD-cLogP merits consideration. It is likely that in this “border-region” of high PSA compounds, low permeability limits bioavailability. Although permeability was not routinely measured in this study, the PAMPA Papp (apparent permeability) of 13 (Figure 9) was 0.09 × 10–6 cm/s, whereas that of the less bioavailable 12 was 0.004 × 10–6 cm/s. The 3-fold weighting of HBDs in this metric emphasizes its influence upon permeability and implies that the detrimental effect of an extra HBD is compensated for only by the addition of a large amount of lipophilicity. This study demonstrates the value of analyzing different components of PSA and suggests that an alternative calculation of molecular polarity incorporating the particular significance of HBDs would provide a better predictor of permeability (and bioavailability).
In summary, analysis of the associations between physicochemical properties and pharmacokinetics in a series of pyrido[3,2-d]pyrimidine HCV replication inhibitors resulted in three notable trends: (i) Compounds with high PSA (PSA >160) were unlikely to show good bioavailability in the rat, (ii) more lipophilic compounds generally had longer MRTs, and (iii) compounds with moderately high PSA (140–160) may show acceptable bioavailability if they are lipophilic enough. By exploiting these trends, several compounds (7, 10, and 2) with favorable rat PK profiles were discovered. Finally, the weighted combination of HBD count and cLogP (3*HBD-cLogP) was found to be a better predictor of bioavailability for the moderately high PSA compounds (140–160) in this series than any single descriptor.
Acknowledgments
We thank Kathy Brendza for high-resolution mass spectrometry and Michael Zhang for help with calculated properties.
Supporting Information Available
Experimental procedures for assay protocols, in vivo studies, synthesis, and characterization of compounds 1–13, as well as a complete set of calculated physicochemical properties and measured PK parameters for >180 compounds within the series described herein. This material is available free of charge via the Internet at http://pubs.acs.org.
Supplementary Material
References
- Lipinski C. A.; Lombardo F.; Dominy B. W.; Feeney P. J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Delivery Rev. 1997, 23, 4–25. [DOI] [PubMed] [Google Scholar]
- Veber D. F.; Johnson S. R.; Cheng H.-Y.; Smith B. R.; Ward K. W.; Kopple K. D. Molecular Properties That Influence the Oral Bioavailability of Drug Candidates. J. Med. Chem. 2002, 45, 2615–2623. [DOI] [PubMed] [Google Scholar]
- Leeson P. D.; Davis A. M. Time-Related Differences in the Physical Property Profiles of Oral Drugs. J. Med. Chem. 2004, 47, 6338–6348. [DOI] [PubMed] [Google Scholar]
- Johnson T. W.; Dress K. R.; Edwards M. Using the Golden Triangle to Optimize Clearance and Oral Absorption. Bioorg. Med. Chem. Lett. 2009, 19, 5560–5564. [DOI] [PubMed] [Google Scholar]
- Hou T.; Wang J.; Zhang W.; Xu X. ADME Evaluation in Drug Discovery. 7. Prediction of Oral Absorption by Correlation and Classification. J. Chem. Inf. Model. 2007, 47, 208–218. [DOI] [PubMed] [Google Scholar]
- Martin Y. C. A Bioavailability Score. J. Med. Chem. 2005, 48, 3164–3170. [DOI] [PubMed] [Google Scholar]
- For a review, see Egan W. J.; Lauri G. Prediction of intestinal permeability. Adv. Drug Delivery Rev. 2002, 54, 273–289. [DOI] [PubMed] [Google Scholar]
- Egan W. J.; Merz K. M. Jr.; Baldwin J. J. Prediction of Drug absorption Using Multivariate Statistics. J. Med. Chem. 2000, 43, 3867–3877. [DOI] [PubMed] [Google Scholar]
- Baldo V.; Baldovin T.; Trivello R.; Floreani A. Epidemiology of HCV Infection. Curr. Pharm. Des. 2008, 14, 1646–1654. [DOI] [PubMed] [Google Scholar]
- When bioavailability is normalized to remove the predicted effects of first pass metabolism [% F/(1 – Cl/QH)], the same trend is seen.
- RLM is rat liver microsomal stability, quantified as in vitro half-life.
- Clarke D. E. Rapid Calculation of Polar Molecular Surface Area and Its Application to the Prediction of Transport Phenomena. 1. Prediction of Intestinal Absorption. J. Pharm. Sci. 1997, 88, 807–814 and references therein. [DOI] [PubMed] [Google Scholar]
- This trend holds for compounds in this series with low predicted clearance based on rat liver microsomes.
- Obach R. S.Prediction of Human Volume of Distribution Using in vivo, in vitro, and in silico Approaches. In Annual Reports in Medicinal Chemistry; Macor J. E., Ed.; Academic Press: London, United Kingdom, 2007; pp 469–488. [Google Scholar]
- Similar analyses comparing % F with molecular weight and number of rotatable bonds for this subgroup also failed to produce useful relationships.
- Although microsomal stability differences between 12 and 13 would account for some of the bioavailability difference between these two compounds, the modest predicted metabolic stability in compound 12 should only reduce the observed % F by about 1/3 based on first-pass metabolism. Thus, metabolism in the liver is unlikely to be a significant cause of the large bioavailability difference seen here.
- Raising lipophilicity significantly as a strategy for the improvement of bioavailability should be approached with caution, as compounds with high cLogP also tend towards other undesirable properties, such as poor solubility and poor selectivity. See Leeson P. D.; Springthorpe B. The influence of drug-like concepts on decision-making in medicinal chemistry. Nature Rev. Drug Discovery 2007, 6, 881–890. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


