Abstract
Antagonism of the CRTH2 receptor represents a very attractive target for a variety of allergic diseases. Most CRTH2 antagonists known to date possess a carboxylic acid moiety, which is essential for binding. However, potential acid metabolites O-acyl glucuronides might be linked to idiosynchratic toxicity in humans. In this communication, we describe a new series of compounds that lack the carboxylic acid moiety. Compounds with high affinity (Ki < 10 nM) for the receptor have been identified. Subsequent optimization succeeded in reducing the high metabolic clearance of the first compounds in human and rat liver microsomes. At the same time, inhibition of the CYP isoforms was optimized, giving rise to stable compounds with an acceptable CYP inhibition profile (IC50 CYP2C9 and 2C19 > 1 μM). Taken together, these data show that compounds devoid of carboxylic acid groups could represent an interesting alternative to current CRTH2 antagonists in development.
Keywords: CRTH2 (DP2) receptor, antagonists, tetrazoles, asthma
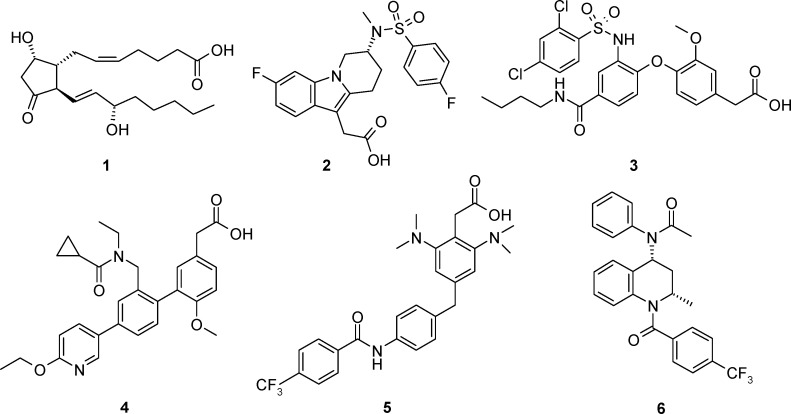
Prostaglandin D2 (PGD2) (1) (Figure 1) is the major prostanoid species produced by mast cells in response to stimulation by allergens and plays a key role in inflammatory processes. PGD2 exerts its effect through two high-affinity G protein-coupled receptors: the classical DP receptor, also called DP1, and the more recently discovered chemoattractant homologous receptor expressed on Th2 cells (CRTH2 also known as DP2). In humans, CRTH2 is predominantly expressed by Th2 cells, eosinophils, and basophils, all known to play a key role in allergic diseases.1 Activation of the Gi-coupled CRTH2 by PGD2 or the selective agonist DK-PGD2 stimulates chemotaxis of human Th2 cells, eosinophils, and basophils in vitro and in vivo,2,3 suggesting that the CRTH2 receptor may directly mediate the recruitment of inflammatory cells in allergic diseases such as asthma, allergic rhinitis, and atopic dermatitis. CRTH2 is expressed by antigen-specific Th2 cells in allergic individuals supporting this notion that the CRTH2 receptor is important in the recruitment of Th2 in allergic diseases in humans.4 Further data in animal models likewise point to a central role for CRTH2 in allergic disorders and support the promise of CRTH2 as a target for the treatment of atopic diseases.1 Moreover, the thromboxane A2 antagonist ramatroban, a drug marketed for allergic rhinitis, also has beeen shown to possess CRTH2 antagonistic properties;5 yet, it is not clear whether its clinical efficacy in allergic rhinitis is due to inhibition of TP, CRTH2, or both receptors. The relevance of CRTH2 as a target for allergic diseases spurred much effort in the pharmaceutical industry aimed at discovering selective agents to modulate its action.6,6b
Figure 1.
Structures of prostaglandin D2 (PGD2) (1), some CRTH2 antagonists in clinical development (2–5), and a tetrahydroquinoline derivative (6).
Most CRTH2 antagonists known to date, with the only exception being tetrahydroquinolines such as 6,7 possess a carboxylic acid moiety, which is essential for binding and which mimics the same moiety found in prostaglandins. Carboxylic acids can be metabolized in vivo into acyl-glucuronides. These potentially reactive metabolites have been linked to instances of idiosyncratic toxicity, which have led to the recall of several drugs.8 Given the chronic nature of the diseases for which CRTH2 antagonists could become useful drugs (e.g., asthma, atopic dermatitis), removing the carboxylic acid group could give rise to safer drug candidates.
A review of the literature on CRTH2 antagonists reveals that several research groups have attempted to replace the carboxylic acid moiety with a variety of groups that can be considered as bioisosteres, for example, using the tetrazole group. However, in all cases reported so far, a significant drop in affinity for the CRTH2 receptor has been observed when comparing tetrazole-containing compounds with the corresponding carboxylic acids.9−9d
During a recent high-throughput screening (HTS) campaign, we identified a series of potent CRTH2 antagonists that do not contain a carboxylic acid group and that on the other hand bear a tetrazole group. Even if tetrazoles can be metabolized to glucuronides in vivo, the adducts are not chemically reactive and have not been linked to toxic effects in man. As such, this chemical series was considered as being an interesting starting point for our drug discovery effort.
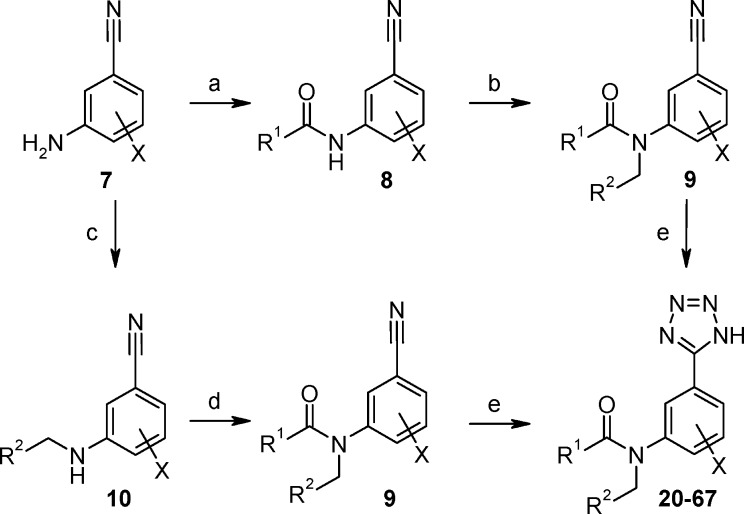
The tetrazoles 11–19 of the HTS pool were prepared as previously described.10 New analogues 20–67 were easily prepared as outlined in Scheme 1. Tetrazoles were prepared from the corresponding nitriles 9 at the final step, as this was generally found to make the purification of intermediates more straightforward. For this transformation, the reaction using the less toxic copper(I) oxide was generally preferred over the use of dibutyltin oxide.
Scheme 1.
Some early structure–activity relationship (SAR) could be gathered from the affinity data of the compounds present in the HTS pool (Table 1, 11–19): A longer, more lipophilic alkyl chain as R1 seemed to be more favorable than a smaller methyl group (see, for example, 13 vs 11 or 14 vs 12). The comparison of these pairs also showed that additional substitution with a halogen on the central aromatic also seemed to have a positive effect, at least in the 4-position. These findings could be confirmed when new analogues were tested. Chain lengths of three or four carbon atoms were optimal for potency, while the two-carbon chain led to a decrease in potency (39 and 38 vs 37), although not as marked as for the methyl group. The positive effect of the halogen atom could be confirmed with several analogues, with a particularly positive effect seen when the halogen was introduced at the 5-position (24 and 28). Appropriately substituted aromatic groups were favored in the R2 position. In particular, 3- and/or 4-alkoxy-substituted groups were found to confer additional affinity as compared to an unsubstituted phenyl (21, 22, or 37 vs 20). On the contrary, halogen-substituted aromatics (see, for example, 30–32) and heteroaromatics (ortho-, meta-, and para-pyridine, 1- and 2-thiophene, isoxazole, and oxazole, data not shown) did not increase the affinity. One notable exception was substitution with a benzofuran group (35).
Table 1. SAR of Tetrazole Compounds 11–67.
| compd | R1 | R2 | X | Ki (nM) | cLog D (pH 7.4) |
|---|---|---|---|---|---|
| 11 | methyl | 3-alkynyl-phenyl | H | 400 | 1.2 |
| 12 | methyl | 3-alkynyl-phenyl | 4-F | 140 | 1.6 |
| 13 | butyl | 3-alkynyl-phenyl | H | 45 | 2.7 |
| 14 | butyl | 3-alkynyl-phenyl | 4-F | 10 | 3.1 |
| 15 | butyl | benzodioxane-6-yl | 4-F | 3.3 | 2.1 |
| 16 | butyl | 4-methoxyphenyl | H | 50 | 1.9 |
| 17 | butyl | 2-naphthyl | H | 39 | 3.2 |
| 18 | butyl | 6-isoquinyl | H | 330 | 1.7 |
| 19 | butyl | 2-thienyl | H | 620 | 1.8 |
| 20 | ethyl | phenyl | H | 1800 | 1.0 |
| 21 | ethyl | 3-OMe-phenyl | H | 360 | 1.1 |
| 22 | ethyl | 4-OMe-phenyl | H | 220 | 0.9 |
| 23 | butyl | 3-OMe-phenyl | H | 44 | 2.1 |
| 24 | butyl | 3-OMe-phenyl | 5-F | 3.0 | 1.4 |
| 25 | ethyl | 3-OMe-phenyl | 5-F | 8.2 | 0.4 |
| 26 | ethyl | 4-OMe-phenyl | 5-F | 7.6 | 0.1 |
| 27 | butyl | 4-OMe-phenyl | 5-F | 3.0 | 1.1 |
| 28 | butyl | 4-OMe-phenyl | 5-Cl | 3.6 | 2.6 |
| 29 | ethyl | 3-isopropoxyphenyl | 5-F | 6.4 | 1.2 |
| 30 | ethyl | 2-F-phenyl | H | 950 | 1.1 |
| 31 | ethyl | 3-F-phenyl | H | 1400 | 1.0 |
| 32 | ethyl | 4-F-phenyl | H | 1200 | 1.1 |
| 33 | ethyl | 3-methylphenyl | H | 580 | 1.5 |
| 34 | butyl | 2-naphthyl | 4-F | 18 | 3.6 |
| 35 | butyl | 2-benzofuranyl | 4-F | 7.1 | 3.3 |
| 36 | ethyl | 2-quinolyl | 5-F | 56 | 0.3 |
| 37 | ethyl | 1,4-benzodioxane-6-yl | H | 42 | 0.8 |
| 38 | propyl | 1,4-benzodioxane-6-yl | H | 12 | 1.3 |
| 39 | butyl | 1,4-benzodioxane-6-yl | H | 7.0 | 1.8 |
| 40 | ethyl | 1,4-benzodioxane-6-yl | 4-F | 11 | 1.1 |
| 41 | isobutyl | 1,4-benzodioxane-6-yl | 4-F | 5.6 | 2.0 |
| 42 | propyl | 1,4-benzodioxane-6-yl | 4-F | 4.9 | 1.6 |
| 43 | butyl | 1,4-benzodioxane-6-yl | 5-F | 2.0 | 1.0 |
| 44 | butyl | 1,4-benzodioxane-6-yl | 4,6-difluoro | 45 | 1.7 |
| 45 | ethyl | 1,3-benzodioxol-5-yl | H | 130 | 1.0 |
| 46 | ethyl | 3-CF3-phenyl | H | 220 | 1.8 |
| 47 | ethyl | 4-CF3-phenyl | H | 110 | 1.8 |
| 48 | ethyl | 4-CF3-phenyl | 5-F | 5.7 | 1.1 |
| 49 | butyl | 4-CF3-phenyl | 5-F | 2.4 | 2.1 |
| 50 | butyl | 3-OCF3-phenyl | 5-F | 4.5 | 2.4 |
| 51 | ethyl | 4-OCF3-phenyl | 5-F | 6.9 | 1.2 |
| 52 | butyl | 4-OCF3-phenyl | 5-F | 7.7 | 2.2 |
| 53 | butyl | 4-OCF3-phenyl | 5-Cl | 19 | 3.6 |
| 54 | ethyl | 4-CF3SO2-phenyl | 5-F | 5.2 | 1.2 |
| 55 | ethyl | 3-hydroxyphenyl | 5-F | 26 | –0.3 |
| 56 | ethyl | 4-hydroxy-phenyl | 5-F | 22 | –0.4 |
| 57 | ethyl | 4-MeSO2-phenyl | H | 69 | –0.2 |
| 58 | ethyl | 4-MeSO2-phenyl | 5-F | 3.1 | –0.9 |
| 59 | isopropyl | 4-MeSO2-phenyl | 5-F | 17 | –0.6 |
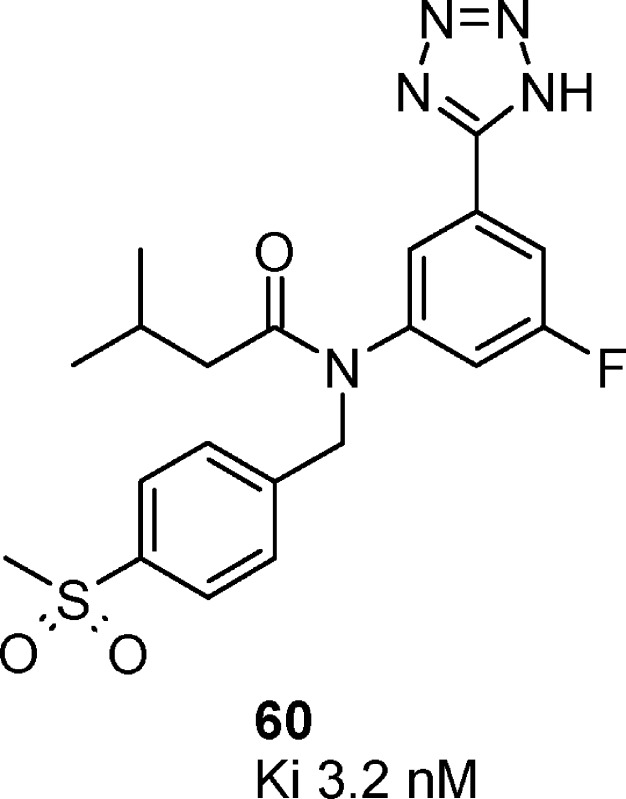
| 60 | isobutyl | 4-MeSO2-phenyl | 5-F | 3.2 | –0.1 |
| 61 | cyclopentyl | 4-MeSO2-phenyl | 5-F | 5.4 | 0.0 |
| 62 | phenyl | 4-MeSO2-phenyl | 5-F | 2.2 | –0.8 |
| 63 | benzyl | 4-MeSO2-phenyl | 5-F | 0.9 | –0.8 |
| 64 | 2-(4-tolyl)-ethyl | 4-MeSO2-phenyl | 5-F | 2.5 | 0.2 |
| 65 | dioxolan-4-yl | 4-MeSO2-phenyl | 5-F | 28 | –1.4 |
| 66 | 3-pyridyl | 4-MeSO2-phenyl | 5-F | 4.0 | –1.5 |
| 67 | 4-pyridyl | 4-MeSO2-phenyl | 5-F | 17 | –1.4 |
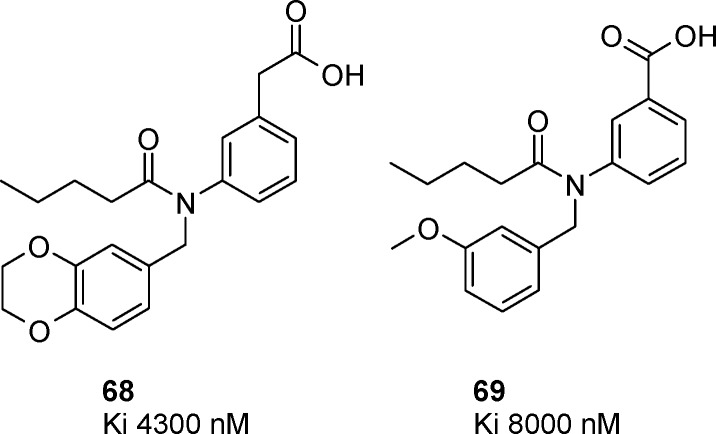
Substituting the tetrazole group with either a carboxylic acid or an acetic acid group led to compounds with Ki > 1 μM, with a loss of affinity of at least 2 orders of magnitude versus the corresponding tetrazoles with the same substitution (Figure 2, Ki = 4300 nM for 68 vs Ki = 7 nM for corresponding tetrazole 39, and Ki = 8000 nM for 69 vs Ki = 44 nM for tetrazole 23), confirming that for the purpose of CRTH2 antagonism tetrazoles and carboxylic acids cannot be considered as really bioisosteric.
Figure 2.
Activity of carboxylic acid analogues.
Some of the most potent analogues were also tested to test the functional activity of the compounds in physiological conditions, including the presence of plasma proteins. The assay selected was a flow cytometry-based detection of the change of shape of eosinophils in response to challenge with PGD2 (at a concentration of 10 nM) in whole blood from healthy volunteers.11,11b In this assay, compound 43 was the most potent of the series, with an IC50 of 40 nM. Several compounds of this series were also shown to be inverse agonists in a GTPγS binding assay (Table 3, entries 1–6).
Table 3. Activity of Selected Compounds in Functional Cellular Assays in Agonist.
| entry | compd | GTPγS agonist modea (Emax at 20 μM) | GTPγS antagonist modeb (IC50/inhibition at 2 μM) |
|---|---|---|---|
| 1 | 25 | –11% | 64 nM/107% |
| 2 | 28 | –10% | 10 nM/110% |
| 3 | 43 | –15% | 2.8 nM/102% |
| 4 | 48 | –8% | 37 nM/105% |
| 5 | 53 | –10% | 20 nM/112% |
| 6 | 55 | –17% | 85 nM/106% |
| 7 | 60 | no effectc | 46 nM/85% |
| 8 | 61 | +7% | 57 nM/80% |
| 9 | 63 | –10% | 3.7 nM/94% |
In the absence of PGD2, 100% represents the level of stimulation given by 2 μM PGD2.
In the presence of 80 nM PGD2.
No statistically significant effect (−5% < effect < 5%).
Unfortunately, all of the alkoxy-substituted compounds were found to be highly unstable in human microsomes and moderately unstable in rat microsomes (see, for example, 16, 29 ,and 39, Table 2). Analysis by LC-MS/MS of the metabolites obtained after treatment of compound 15 in human liver microsomes confirmed the prediction that oxidative metabolism was taking place on the alkoxy substituent (see the Supporting Information). To reduce oxidative metabolism, our first strategy involved blocking this weak spot. Compounds 50 and 51, bearing a trifluoromethoxy group, were found to retain potency on the target but were significantly less metabolized, in particular those with a C-2 chain on the acyl group, probably due to the lower overall lipophilicity. Quite surprisingly, considering the lower activity of the halogen-substituted compounds was the fact that the trifluoro-substituted 46–49 were also found to be highly potent. The stability in human and rat microsomes was also good (Table 2). Compound 48 was also tested in a mouse PK experiment and showed a low clearance (0.3 L/kg/h), with an acceptable half-life (1.4 h after intravenous dosing) and a medium volume of distribution (Vss 0.9 L/kg). The low in vivo clearance of this compound might indicate that limited conjugative metabolism might be taking place, at least in mouse. However, we have not studied the behavior of these compounds in hepatocytes (neither rodent nor human), so further studies would be warranted to make a definite statement on the matter. Despite a moderate permeability in a Caco-2 assay (Papp 3.0 × 10–6 cm/s), the oral bioavailability in mouse was found to be good (Fz 76%), resulting in an excellent exposure after oral dosing (AUC 11212 h ng/mL after a dose of 5 mg/kg), identifying the compound as a possible tool compound for pharmacology experiments.
Table 2. In Vitro Clearance in Human and Rat Liver Microsomes and Human CYP Inhibition Data for Selected Compounds.
| compd | Clint (HLM/RLM)a (μL/min/mg prot) | CYP inhibition (2C8/2C9/2C19) IC50 (μM) |
|---|---|---|
| 16 | >1000/43 | 0.32/0.84/0.26 |
| 29 | 1700/30 | 0.47/0.34/0.90 |
| 39 | >1000/125 | 0.57/0.64/0.68 |
| 48 | <10/<10 | 0.086/0.28/0.038 |
| 51 | <10/12 | 0.043/0.20/0.051 |
| 55 | 55/10 | 0.97/2.0/3.7 |
| 58 | <10/<10 | 0.054/>20/0.15 |
| 60 | 17/17 | 0.079/1.7/7.5 |
| 61 | 92/40 | 0.22/7.4/12 |
| 63 | 28/20 | 0.050/0.29/0.17 |
HLM, human liver microsomes; RLM, rat liver microsomes.
However, all CF3- and OCF3-containing compounds found to be stable in the microsomes were also found to be potent inhibitors of some CYP isoforms, in particular the 2C8, 2C9, and 2C19 isoforms (IC50 < 300 nM; see, for example, 48 and 51, Table 2). In contrast, no significant inhibition (IC50 > 10 μM) was found on isoforms 1A2, 2B6, 3A4, and 2D6. This could be rationalized as follows: modification to the structure had reduced the capability of the metabolizing enzymes to oxidize the compounds but not reduced the overall affinity of the compounds for these CYP isoforms; the high affinity coupled with the inability of the enzyme to process the compound resulted in the strong inhibition.
Given these results, two alternative strategies were pursued. First, it was decided to verify if the metabolites of compounds 25–26 would retain activity on the receptor. Phenolic compounds 55 and 56 were prepared, but their potency was lower than the corresponding parent compounds, albeit the CYP inhibition was indeed reduced.
As a second alternative, it was decided to increase the polarity of the compounds, to decrease affinity to the CYP isoforms, which in general show a preference for more lipophilic compounds.12 In particular, it was decided to introduce polar groups on the benzylic group, which is close to the main site of metabolism. Good results were obtained in particular with sulfone-containing compounds such as 58, which showed a low level of metabolism in the microsomes, coupled with a reduced level of inhibition of the CYP 2C9 isoform, as well as an excellent potency on the receptor. Keeping this R2 group fixed, we then examined a number of different R1 groups (59–67). A number of groups retained an excellent affinity for the receptor. The best combination in terms of in vitro profiling was the isobutyl group (60), which retained moderate metabolic stability and moderate 2C9 inhibition (IC50 = 1.7 μM) but also gave a significant improvement on the inhibition of the 2C19 isoform (IC50 = 7.5 μM), as compared to 58. All of these compounds still inhibited CYP2C8 with IC50 < 1 μM; however, it is interesting to note that an important asthma drug, Montelukast (Singulair), is also reported as a very potent inhibitor of CYP 2C8.13
The introduction of the polar sulfone moiety also had an effect on the functional behavior of the compounds. While earlier compounds had shown an inverse agonist behavior in the GTPγS assay, the sulfone-bearing compounds displayed different behaviors: a neutral antagonist behavior for compound 60, with no significant effect on the assay in agonist mode, even though the slightly reduced level of inhibition at the highest concentration in antagonist mode might suggest a weak partial agonist effect; compound 61 behaved as a weak partial agonist (7% Emax in agonist mode, with a corresponding reduction in the maximum inhibition in the assay in antagonist mode), whereas 63 had an inverse agonist behavior. This tendency for switch of functional behavior for CRTH2 agonists/antagonists, even with small structural changes such as methyl to ethyl, has been described in the literature.9d,14
In conclusion, the results presented in this communication show that it is possible to design potent tetrazole-based CRTH2 antagonists as a potentially safer alternative to the traditional carboxylic acid antagonists. Further investigations, aimed at further improving the metabolic stability, at improving cellular functional behavior, and at further reducing the CYP inhibition, are ongoing and will be communicated in due time.
Supporting Information Available
Experimental details for biological assays and for the synthesis and characterization of all compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
Supplementary Material
References
- Pettipher R.; Hansel T. T.; Armer R. Antagonism of the prostaglandin D2 receptors DP1 and CRTH2 as an approach to treat allergic diseases. Nature Rev. Drug Discovery 2007, 6, 313–325. [DOI] [PubMed] [Google Scholar]
- Hirai H.; Tanaka K.; Yoshie O.; Ogawa K.; Kenmotsu K.; Takamori Y.; Ichimasa M.; Sugamura K.; Nakamura M.; Takano S.; Nagata K. Prostaglandin D2 selectively induces chemotaxis in T helper type 2 cells, eosinophils and basophils via 7-transmembrane receptor CRTH2. J. Exp. Med. 2002, 193, 255–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraishi Y.; Asano K.; Nakajima T.; Oguma T.; Suzuki Y.; Shiomi T.; Sayama K.; Niimi K.; Wakaki M.; Kagyo J.; Ikeda E.; Hirai H.; Yamaguchi K.; Ishizaka A. Prostaglandin D2-induced eosinophilic airways inflammation is mediated by CRTH2 receptor. J. Pharmacol. Exp. Ther. 2005, 312, 954–960. [DOI] [PubMed] [Google Scholar]
- Cosmi L.; Annunziato F.; Galli M. I. G.; Maggi R. M. E.; Nagata K.; Romagnani S. CRTH2 is the most reliable marker for the detection of circulating human type 2 Th and type 2 T cytotoxic cells in health and disease. Eur. J. Immunol. 2000, 30, 2972–2979. [DOI] [PubMed] [Google Scholar]
- Hiromi Sugimoto H.; Shichijo M.; Iino T.; Manabe Y.; Watanabe A.; Shimazaki M.; Gantner F.; Bacon. K. B. An orally bioavailable small molecule antagonist of CRTH2, Ramatroban (BAY u3405), inhibits prostaglandin D2-induced eosinophil migration in vitro. J. Pharmacol. Exp. Ther. 2003, 305, 347–52. [DOI] [PubMed] [Google Scholar]
- Ly T. W.; Bacon K. B. Small-molecule CRTH2 antagonists for the treatment of allergic inflammation: An overview. Expert Opin. Invest. Drugs 2005, 14, 769–773. [DOI] [PubMed] [Google Scholar]
- Ulven T.; Kostenis E. Novel CRTH2 antagonists: A review of patents from 2006 to 2009. Expert Opin. Ther. Pat. 2010, 20, 1505–1530. [DOI] [PubMed] [Google Scholar]
- Liu J.; Wang Y.; Sun Y.; Marshall D.; Miao S.; Tonn G.; Anders P.; Tocker J.; Tang H. L.; Medina J. Tetrahydroquinoline derivatives as CRTH2 antagonists. Bioorg. Med. Chem. Lett. 2009, 19, 6840–6844. [DOI] [PubMed] [Google Scholar]
- Sawamura R.; Okudaira N.; Watanabe K.; Murai T.; Kobayashi Y.; Tachibana M.; Ohnuki T.; Masuda K.; Honma H.; Kurihara A.; Okazaki O. Predictability of idiosyncratic drug toxicity risk for carboxylic acid-containing drugs based on the chemical stability of acyl glucuronide. Drug Metab. Dispos. 2010, 38, 1857–1864. [DOI] [PubMed] [Google Scholar]
- Sandham D. A.; Aldcroft C.; Baettig U.; Barker L.; Beer D.; Bhalay G.; Brown Z.; Dubois G.; Budd D.; Bidlake L.; Campbell E.; Cox B.; Everatt B.; Harrison D.; Leblanc C. J.; Manini J.; Profit R.; Stringer R.; Thompson K. S.; Turner K. L.; Tweed M. F.; Walker C.; Watson S. J.; Whitebread S.; Willis J.; Williams G.; Wilson C. 2-Cycloalkyl phenoxyacetic acid CRTh2 receptor antagonists. Bioorg. Med. Chem. Lett. 2007, 17, 4347–4350. [DOI] [PubMed] [Google Scholar]
- Crosignani S.; Page P.; Missotten M.; Colovray V.; Cleva C.; Arrighi J.-F.; Atherall J.; Macritchie J.; Martin T.; Humbert Y.; Gaudet M.; Pupowicz D.; Maio M.; Pittet P.-A.; Golzio L.; Giachetti C.; Rocha C.; Bernardinelli G.; Filinchuk Y.; Scheer A.; Schwarz M. K.; Chollet A. Discovery of a new class of potent, selective and orally bioavailable CRTH2 (DP2) receptor antagonists for the treatment of allergic inflammatory diseases. J. Med. Chem. 2008, 51, 2227–2243. [DOI] [PubMed] [Google Scholar]
- Liu J.; Fu Z.; Wang Y.; Schmitt M.; Huang A.; Marshall D.; Tonn G.; Seitz L.; Sullivan T.; Tang H. L.; Collins T.; Medina J. Discovery and optimization of CRTH2 and DP dual antagonists. Bioorg. Med. Chem. Lett. 2009, 19, 6419–6423. [DOI] [PubMed] [Google Scholar]
- Luker T.; Bonnert R.; Schmidt J.; Sargent C.; Paine S. W.; Thom S.; Pairaudeau G.; Patel A.; Mohammed R.; Akam E.; Dougall I.; Davis A. M.; Abbott P.; Brough S.; Millichip I.; McInally T. Switching between agonists and antagonists at CRTh2 in a series of highly potent and selective biaryl phenoxyacetic acids. Bioorg. Med. Chem. Lett. 2011, 21, 3616–3621. [DOI] [PubMed] [Google Scholar]
- Desforges G.; Bombrun A.; Quattropani A. An efficient and expeditious synthesis of di- and trisubstituted amino-phenyl and -benzyl derivatives of tetrazole and [1,3,4]oxadiazol-2-one. J. Comb. Chem. 2008, 10, 671–680. [DOI] [PubMed] [Google Scholar]
- Sabroe I.; Hartnell A.; Jopling L. A.; Bel S.; Ponath P. D.; Pease J. E.; Collins P. D.; Williams T. J. Differential regulation of eosinophil chemokine signaling via CCR3 and non-CCR3 pathways. J. Immunol. 1999, 162, 2946–2955. [PubMed] [Google Scholar]
- Heinemann A.; Schuligoi R.; Sabroe I.; Hartnell A.; Peskar B. A. Delta 12-prostaglandin J2, a plasma metabolite of prostaglandin D2, causes eosinophil mobilization from the bone marrow and primes eosinophils for chemotaxis. J. Immunol. 2003, 170, 4752–8. [DOI] [PubMed] [Google Scholar]
- Lewis D. F. V.; Jacobs M. N.; Dickins M. Compound lipophilicity for substrate binding to human P450s in drug metabolism. Drug Discovery Today 2004, 9, 530–537. [DOI] [PubMed] [Google Scholar]
- Walsky R. L.; Gaman E. A.; Obach R. S. Examination of 209 drugs for inhibition of Cytochrome P450 2C8. J. Clin. Pharmacol. 2005, 45, 68–78. [DOI] [PubMed] [Google Scholar]
- Crosignani S.; Pretre A.; Jorand-Lebrun C.; Fraboulet G.; Seenisamy J.; Augustine J. K.; Missotten M.; Humbert Y.; Cleva C.; Abla N.; Daff H.; Schott O.; Schneider M.; Burgat-Charvillon F.; Rivron D.; Hamernig I.; Arrighi J.-F.; Gaudet M.; Zimmerli S. C.; Juillard P.; Johnson Z.. Discovery of potent, selective and orally bioavailable alkynyl-phenoxyacetic acid CRTH2 (DP2) receptor antagonists for the treatment of allergic inflammatory diseases J. Med. Chem. 2011, DOI: 10.1021/jm200866y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.