Abstract
Recently, there has been a strong interest in the ability to increase levels of high density lipoprotein-cholesterol (HDL-C). This interest stems from the hypothesis that such an elevation in HDL-C will decrease the likelihood of cardiovascular disease. Inhibition of cholesteryl ester transfer protein (CETP) has been shown to elevate HDL-C levels in human subjects. This letter describes the discovery of a novel and potent (<100 nM IC50 for the inhibition of CE transfer) CETP inhibitor scaffold containing an oxazolidinone core.
Keywords: Cholesteryl ester transfer protein, CETP, cardiovascular disease, high density lipoprotein, HDL, oxazolidinone
Despite advances in treatment, cardiovascular disease (CVD) remains a leading cause of death in developed countries. Two key risk factors for CVD are low-density lipoprotein-cholesterol (LDL-C) and high-density lipoprotein-cholesterol (HDL-C). Lower levels of LDL-C correlate with a decrease in deaths due to CVD. The opposite is true of HDL-C; CVD decreases at higher levels of HDL-C.1−1d In recent decades, great progress has been made in lowering LDL-C, particularly via medicines such as statins and with lifestyle changes. While much success has been achieved in decreasing LDL-C, increasing HDL-C still presents a medical challenge.1−1d Even after 50 years of use, niacin is still the leading therapy for raising HDL-C. However, the increase in HDL-C seen with niacin is modest (∼20%), and side effects/toxicity prohibit some patients from taking this drug.1−2b Thus, alternative avenues for raising HDL-C are being investigated.
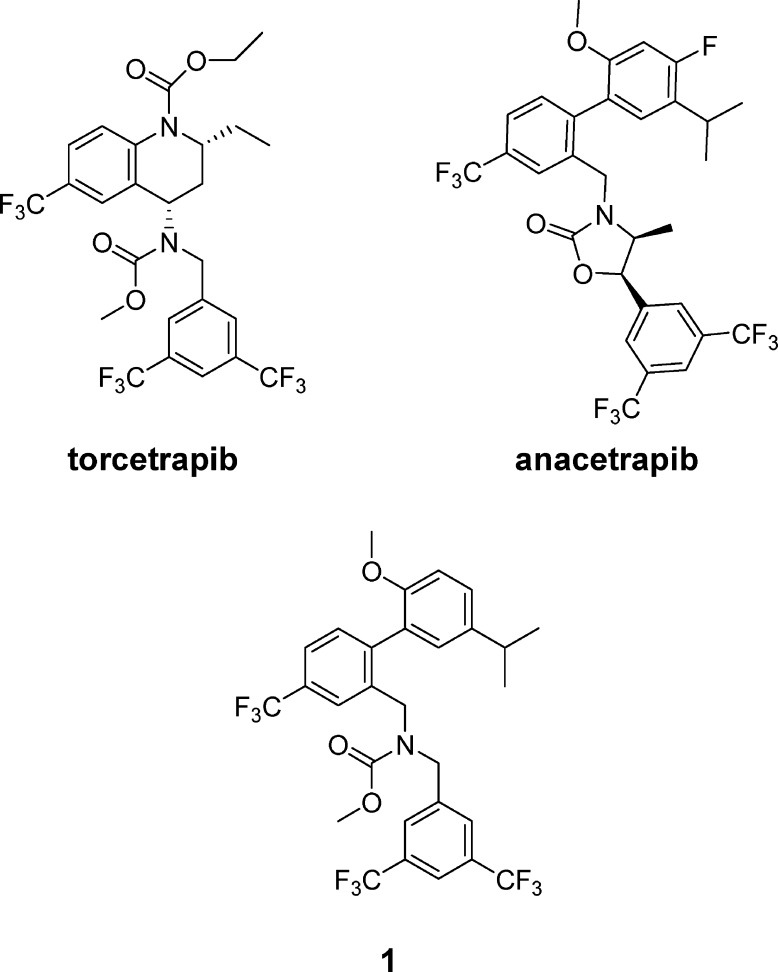
One target for raising HDL-C that has recently received great attention in the scientific community is cholesteryl ester transfer protein (CETP).1−1d,3−3d CETP exchanges cholesteryl ester (CE) and triglycerides between various lipoprotein particles. It has been shown that inhibition of CETP dramatically increases HDL-C, both in animal models and in the clinic.4,4b Unfortunately, the first CETP inhibitor to reach phase III trials, torcetrapib (Figure 1), failed to achieve the desired outcome of a reduction in coronary events in patients receiving treatment.5 In fact, treatment with torcetrapib increased the risk of death from both cardiovascular and noncardiovascular causes.5 The reasons for this failure have been the subject of intense debate, and further investigation of CETP inhibitors is necessary.6−6d The CETP inhibitor anacetrapib (Figure 1) has been shown to differ from torcetrapib in its off-target profile and is currently being studied in clinical trials.7,7b This letter describes the discovery of a novel CETP inhibitor scaffold, closely related to anacetrapib, which contains an oxazolidinone core.
Figure 1.
Structures of torcetrapib, anacetrapib, and lead compound 1.
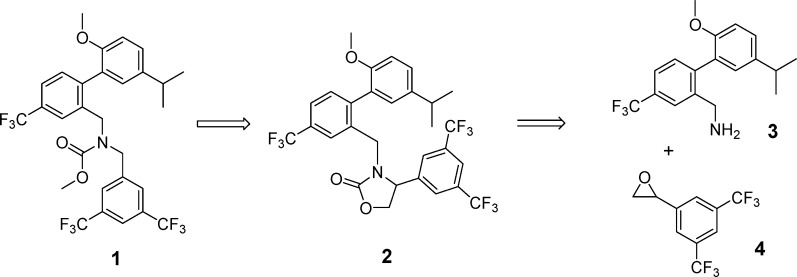
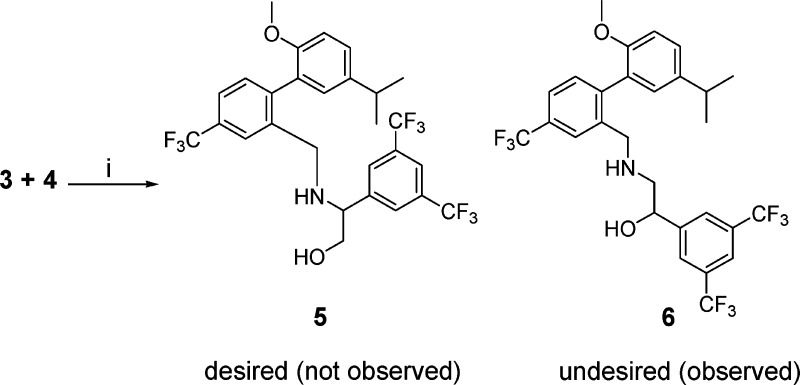
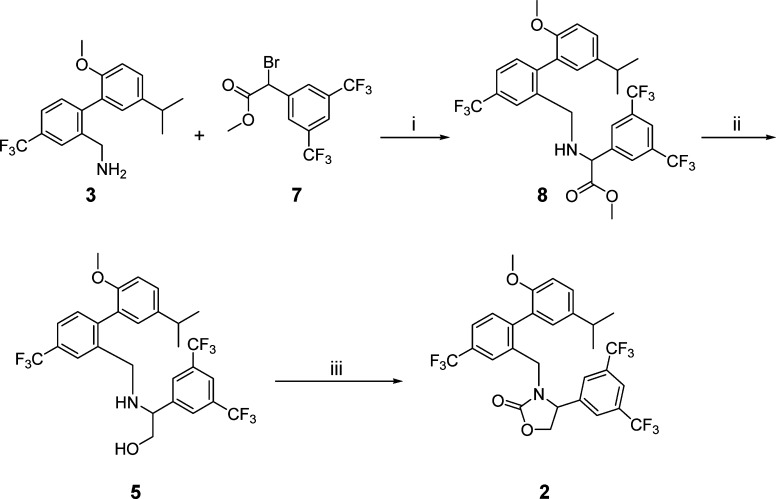
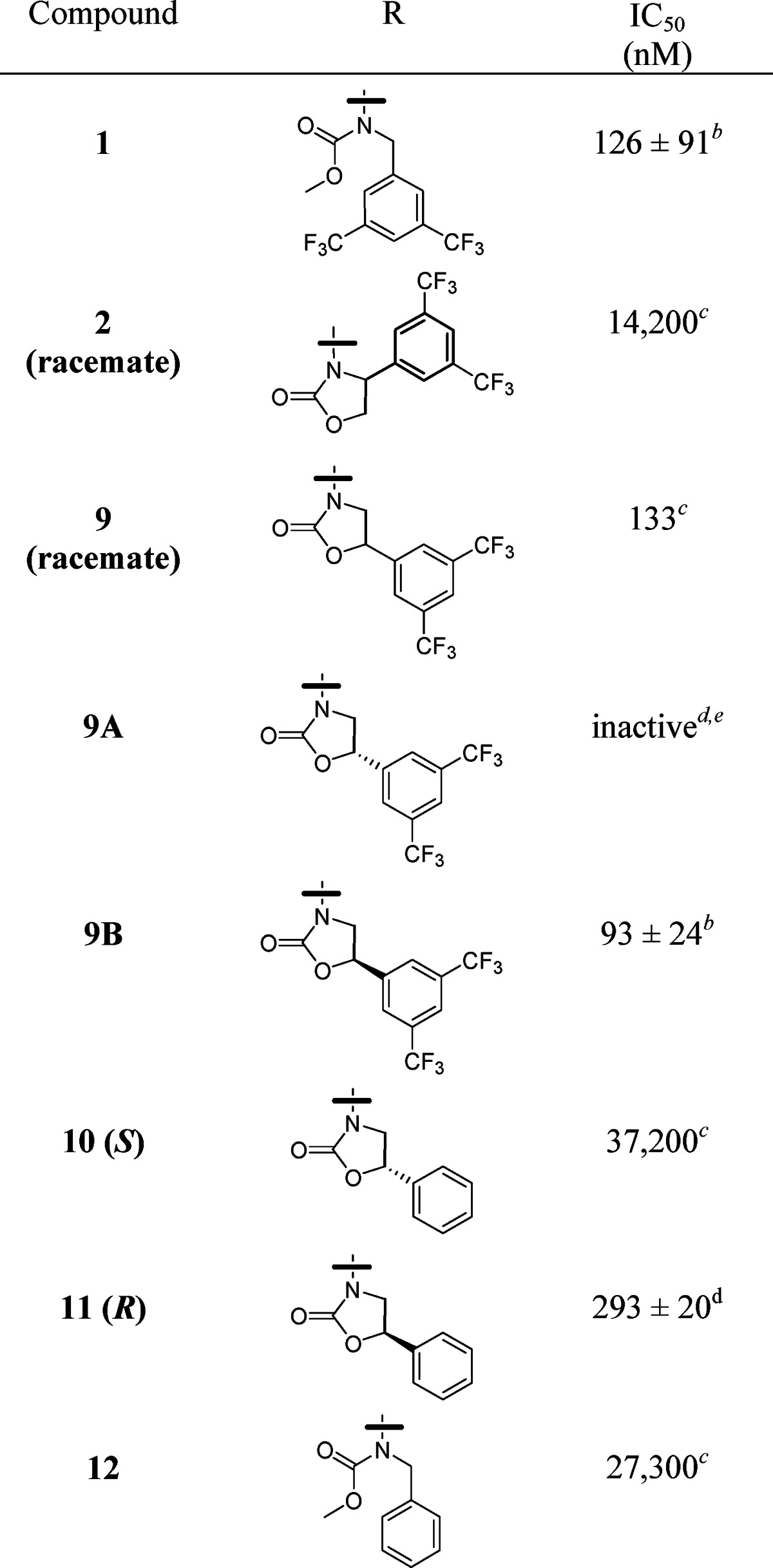
In the investigation of CETP inhibitors conducted at Merck, lead compound 1 (Figure 1) had emerged as a potent inhibitor of CE transfer by CETP.8,9 It was hypothesized that the potency and/or stability of 1 could be improved by constraining the acyclic carbamate into an oxazolidinone ring. Retrosynthetically, it was anticipated that the target molecule, 2, could be obtained by opening of epoxide 4 with amine 3 followed by closure of the resulting product to the oxazolidinone (Scheme 1). Although a mixture of attack at the terminal carbon and the benzylic position of epoxide 4 was expected, it was surprising to observe substitution exclusively at the less hindered carbon, leading to undesired amino-alcohol 6 (Scheme 2). With this first approach to compound 2 thwarted, an alternative synthesis was undertaken (Scheme 3). Alkylation of amine 3 with bromide 7 led to intermediate 8, which was reduced to desired amino alcohol 5 with LiAlH4. Cyclization led to the racemic oxazolidinone 2. Disappointingly, this constrained version of lead compound 1 was a very weak (14 μM) inhibitor of CETP (Table 1).10
Scheme 1. Retrosynthetic Analysis of Target Compound 2.
Scheme 2. Regioselective Epoxide Opening.
Reagents and conditions: (i) i-PrOH, reflux 12 h, 72% yield of 6.
Scheme 3. Synthesis of Compound 2.
Reagents and conditions: (i) CH2Cl2, room temperature, 5 h, 25% yield. (ii) LiAlH4, Et2O, room temperature, 1 h, 52% yield. (iii) Phosgene, (i-Pr)2EtN, CH2Cl2, room temperature, 10 min, 55% yield.
Table 1. IC50 Values for Inhibition of CE Transfera.
 |
IC50 values were determined using a standard bioassay described in ref (10). The IC50 value for torcetrapib was been determined by this method in ref (7a) and was reported to be 13 nM.
Average of three or more 10 point titrations.
Result of a single 10 point titration.
Average of two 10 point titrations.
Inactive indicates <50% inhibition of CETP at a concentration of 100 μM.
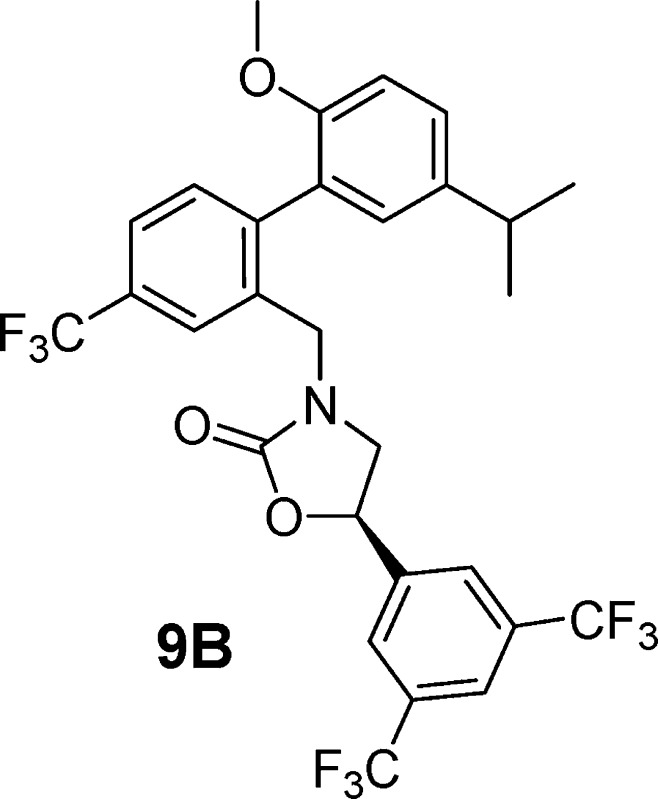
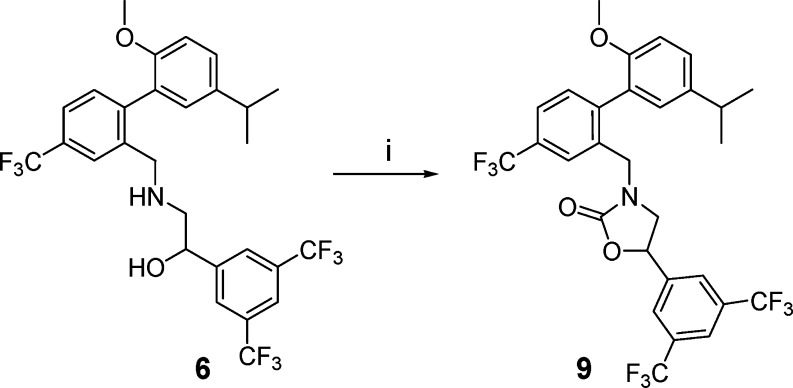
In an effort to ensure the thorough investigation of constrained analogues, amino alcohol 6 was also cyclized to the corresponding oxazolidinone, although it was not anticipated that this regioisomer would exhibit potent CETP inhibition.11 In the event, cyclization of 6 to 9 proceeded in good yield using triphosgene (Scheme 4). Surprisingly, racemic 9 was shown to be a potent inhibitor of CETP (Table 1). Separation of 9 into its two enantiomers (9A and 9B) demonstrated that all of the activity resided in a single enantiomer (9B).
Scheme 4. Synthesis of Compound 9.
Reagents and conditions: (i) Triphosgene, (i-Pr)2EtN, CH2Cl2, 0 °C, 1 h, 98% yield.
To determine the stereochemistry of compound 9B, related compounds 10 and 11 were synthesized following the route used in the synthesis of 9 (Schemes 2 and 4) and starting by opening the known, commercially available, S- and R-styrene oxides with amine 3.12 As in the case of compound 9, only one enantiomer (compound 11, R) was a potent inhibitor of CETP, and this observation forms the basis of the stereochemical assignment of 9A and 9B.13 Although compound 9B is only a slightly more potent inhibitor of CETP than original lead compound 1, it is interesting to note that 11 is much more potent than the comparable acyclic analogue, compound 12. It is also worth mentioning that the addition of a ring constraint did not improve phamacokinetics. Compound 1 has a clearance of 12.8 mL/min/kg in mice, while the clearance of 9B is 31.6 mL/mg/kg. Volumes of distribution (3.3 L/kg for 1 and 3.4 L/kg for 9B) and oral bioavailability (5% for 1 and 4% for 9B) in mice are similar for the two compounds.
In summary, a new class of CETP inhibitors containing an oxazolidinone core has been discovered. Surprisingly, the oxazolidinone does not have the substitution pattern anticipated from an earlier lead compound. Rather, it is an alternative regioisomer discovered through a serendipitous, regioselective epoxide opening. The novel molecule, 9B, is a potent inhibitor of CETP containing a single chiral center. Further developments from this program detailing the evolution of 9B into clinical candidate anacetrapib, which also contains an oxazolidinone core, will be forthcoming.
Acknowledgments
We acknowledge Cameron Smith for careful reading of this manuscript and suggestions.
Supporting Information Available
Synthetic procedures and spectral data for the preparation of intermediates 3−8 and final compounds 2, 9, 11, and 12. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
Compounds were synthesized by C.F.T. and N.Q. with guidance and helpful suggestions from A.A. and Z.L. Biological studies were done by S.S.E., Q.G., S.A.H., and D.P.M. Program guidance was provided by M.L.H., P.J.S., M.S.A., C.P.S., and S.D.W.
Supplementary Material
References
- Linsel-Nitschke P.; Tall A. R. HDL as a target in the treatment of atherosclerotic cardiovascular disease. Nature Rev. Drug Discovery 2005, 4, 193. [DOI] [PubMed] [Google Scholar]
- Rader D. J. Mechanisms of disease: HDL metabolism as a target for novel therapies. Nature Clin. Pract. Cardiovasc. Med. 2007, 4, 102. [DOI] [PubMed] [Google Scholar]
- Wang M.; Briggs M. R. HDL: The metabolism, function, and therapeutic importance. Chem. Rev. 2004, 104, 119. [DOI] [PubMed] [Google Scholar]
- Chapman M. J. Therapeutic elevation of HDL-cholesterol to prevent atherosclerosis and coronary heart disease. Pharmacol. Ther. 2006, 111, 893. [DOI] [PubMed] [Google Scholar]
- Birjmohun R. S.; Hutten B. A.; Kastelein J. J. P.; Stroes E. S. G. Efficacy and safety of high-density lipoprotein cholesterol-increasing compounds: A meta-analysis of randomized controlled trials. J. Am. Coll. Cardiol. 2005, 45, 185. [DOI] [PubMed] [Google Scholar]
- Taylor A. J.; Sullenberger L. E.; Lee H. J.; Lee J. K.; Grace K. A. Arterial biology for the investigation of the treatment effects of reducing cholesterol (ARBITER) 2: A double-blind, placebo-controlled study of extended-release niacin on atherosclerosis progression in secondary prevention patients treated with statins. Circulation 2004, 110, 3512. [DOI] [PubMed] [Google Scholar]
- Barter P. J.; Brewer H. B.; Chapman M. J.; Hennekens C. H.; Rader D. J.; Tall A. R. Cholesteryl ester transfer protein: A novel target for raising HDL and inhibiting atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 160. [DOI] [PubMed] [Google Scholar]
- Sikorski J. A. Oral cholesteryl ester transfer protein (CETP) inhibitors: A potential new approach for treating coronary artery disease. J. Med. Chem. 2006, 49, 1. [DOI] [PubMed] [Google Scholar]
- Shah P. K. Inhibition of CETP as a novel therapeutic strategy for reducing the risk of atherosclerotic disease. Eur. Heart J. 2007, 28, 5. [DOI] [PubMed] [Google Scholar]
- Ansell B.; Hobbs F. D. R. The potential for CETP inhibition to reduce cardiovascular disease risk. Curr. Med. Res. Opin. 2006, 22, 2467. [DOI] [PubMed] [Google Scholar]
- Brousseau M. E.; Schaefer E. J.; Wolfe M. L.; Bloedon L. T.; Digenio A. G.; Clark R. W.; Mancuso J. P.; Rader D. J. Effects of an inhibitor of cholesteryl ester transfer protein on HDL cholesterol. N. Engl. J. Med. 2004, 350, 1505. [DOI] [PubMed] [Google Scholar]
- Clark R. W.; Sutfin T. A.; Ruggeri R. B.; Willauer A. T.; Sugarman E. D.; Magnus-Aryitey G.; Cosgrove P. G.; Sand T. M.; Wester R. T.; Williams J. A.; Perlman M. E.; Bamberger M. J. Raising high-density lipoprotein in humans through inhibition of cholesteryl ester transfer protein: An initial multidose study of torcetrapib. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 490. [DOI] [PubMed] [Google Scholar]
- Barter P. J.; Caulfield M.; Eriksson M.; Grundy S. M.; Kastelein J. J. P.; Komajda M.; Lopez-Sendon J.; Mosca L.; Tardif J.-C.; Waters D. D.; Shear C. L.; Revkin J. H.; Buhr K. A.; Fisher M. R.; Tall A. R.; Brewer B. Effects of torcetrapib in patients at high risk for coronary events. N. Engl. J. Med. 2007, 357, 2109. [DOI] [PubMed] [Google Scholar]
- Tall A. R.; Yvan-Charvet L.; Wang N. The failure of torcetrapib: was it the molecule of the mechanism?. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 257. [DOI] [PubMed] [Google Scholar]
- Rader D. J. Illuminating HDL—Is it still a viable therapeutic target?. N. Engl. J. Med. 2007, 357, 2180. [DOI] [PubMed] [Google Scholar]
- Toth P. P. Torcetrapib and atherosclerosis: What happened and where do we go from here?. Future Lipidol. 2007, 2, 277. [Google Scholar]
- Nicholls S. J. HDL: Still a target for new therapies?. Curr. Opin. Invest. Drugs 2008, 9, 950. [PubMed] [Google Scholar]
- Forrest M. J.; Bloomfield D.; Briscoe R. J.; Brown P. N.; Cumiskey A-M; Ehrhart J.; Hershey J. C.; Keller W. J.; Ma X.; McPherson H. E.; Messina E.; Peterson L. B.; Sharif-Rodriguez W.; Siegl P. K. S.; Sinclair P. J.; Sparrow C. P.; Stevenson A. S.; Sun S-Y; Tsai C.; Vargas H.; Walker M. III; West S. H.; White V.; Woltmann R. F. Torcetrapib-induced blood pressure elevation is independent of CETP inhibition and is accompanied by increased circulating levels of aldosterone. Br. J. Pharmacol. 2008, 154, 1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon C. P.; Shah S.; Dansky H. M.; Davidson M.; Brinton E. A.; Gotto A. M.; Stephanavage M.; Liu S. X.; Gibbons P.; Ashraf T. B.; Zafarino J.; Mitchel Y.; Barter P. Safety of anacetrapib in patients with or at high risk for coronary heart disease. N. Engl. J. Med. 2010, 363, 2046. [DOI] [PubMed] [Google Scholar]
- Lu Z.; Napolitano J. B.; Theberge A.; Ali A.; Hammond M. L.; Tan E.; Tong X.; Xu S. X.; Latham M. J.; Peterson L. B.; Anderson M. S.; Eveland S. S.; Guo Q.; Hyland S. A.; Milot D. P.; Chen Y.; Sparrow C. P.; Wright S. D.; Sinclair P. J. Design of a novel class of biphenyl CETP inhibitors. Bioorg. Med. Chem. Lett. 2010, 20, 7469. [DOI] [PubMed] [Google Scholar]
- For a good overview of other approaches to CETP inhibitors, see ref (3b) and the following review article:Hunt J. A.; Lu Z. Cholesteryl ester transfer protein (CETP) inhibitors. Curr. Top. Med. Chem. 2009, 9, 419. [DOI] [PubMed] [Google Scholar]
- The ability of compounds to inhibit transfer of CE by CETP (IC50 values in Table 1) was determined using a fluorescence transfer assay that has been described in detail. See the following:Eveland S. S.; Milot D. P.; Guo Q.; Chen Y.; Hyland S. A.; Peterson L. B.; Jezequel-Sur S.; O'Donnell G. T.; Zuck P. D.; Ferrer M.; Strulovici B.; Wagner J. A.; Tanaka W. K.; Hilliard D. A.; Laterza O.; Wright S. D.; Sparrow C. P.; Anderson M. S. A high-precision fluorogenic cholestery ester transfer protein assay compatible with animal serum and 3456-well assay technology. Anal. Biochem. 2007, 368, 239. [DOI] [PubMed] [Google Scholar]
- Amino alcohol 6 is a weak inhibitor of CE transfer (IC50 value for the inhibition of CE transfer = 5.2 μM). Likewise, compound 5 has little activity (IC50 value for the inhibition of CE transfer = 13.7 μM).
- In the cases of R- and S-styrene oxide, attack at both the benzylic and the terminal positions of the epoxide was observed, as had been originally anticipated for 4. Attack at the benzylic carbon resulted in a less polar product (Rf = 0.32 in 25% ethyl acetate/hexanes) that was readily separated using silica gel chromatography from the desired, more polar product resulting from attack at the terminal carbon (Rf = 0.11 in 25% ethyl acetate/hexanes)
- The weak inhibitory activity seen for compound 10 is presumably due to the fact that the starting commercially available S-styrene oxide is 98% ee (purchased from Aldrich). The starting R-styrene oxide (also from Aldrich) leading to 11 is 95% ee. No erosion of stereochemical integrity was anticipated during the synthesis of 10 and 11, and the ee of the final products is assumed to be that of the starting materials.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.