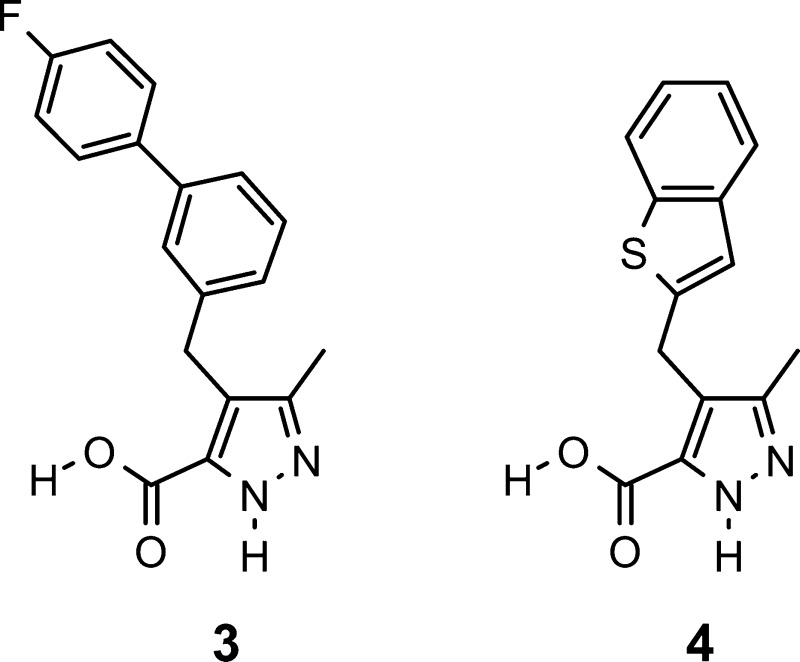
Abstract
l-2-Hydroxy acid oxidase (Hao2) is a peroxisomal enzyme with predominant expression in the liver and kidney. Hao2 was recently identified as a candidate gene for blood pressure quantitative trait locus in rats. To investigate a pharmacological role of Hao2 in the management of blood pressure, selective Hao2 inhibitors were developed. Optimization of screening hits 1 and 2 led to the discovery of compounds 3 and 4 as potent and selective rat Hao2 inhibitors with pharmacokinetic properties suitable for in vivo studies in rats. Treatment with compound 3 or 4 resulted in a significant reduction or attenuation of blood pressure in an established or developing model of hypertension, deoxycorticosterone acetate-treated rats. This is the first report demonstrating a pharmacological benefit of selective Hao2 inhibitors in a relevant model of hypertension.
Keywords: Hao2, hypertension, pyrazolecarboxylic acid, DOCA rat
Human hypertension is a complex, multifactorial disorder resulting from the interplay of multiple environmental and genetic factors, and this common disorder can lead to an increased risk of heart attack, stroke, and renal failure. The mechanisms underlying the initiation and maintenance of the hypertensive process remain unclear.1 Almost one-third of the U.S. adult population has high blood pressure (BP), which increases the risk of cardiovascular and renal disease and shortened life expectancy.2−23 Various antihypertensive drugs have been developed, including diuretics, beta blockers, calcium channel blockers (CCBs), renin inhibitors,3 angiotensin-converting enzyme (ACE) inhibitors, and angiotensin II receptor blockers (ARBs).4,62 However, these drugs either lack sufficient efficacy or are associated with significant adverse effects. In a search for a novel antihypertensive target, we have identified l-2-hydroxy acid oxidase (Hao2)5 as a potential target for pharmacological intervention.
l-2-Hydroxy acid oxidases are flavin mononucleotide (FMN)-dependent peroxisomal enzymes, which are members of the flavoenzyme family that are responsible for the oxidation of a number of l-2-hydroxy acids to ketoacids at the expense of molecular oxygen, resulting in the formation of hydrogen peroxide. Several examples of such enzymes have been identified in different organisms, e.g., glycolate oxidase from plants, lactate oxidase from Mycobacterium (l-lactate 2-monooxygenase), flavocytochrome b2 from yeasts (l-lactate cytochrome c oxidoreductase), and mandelate dehydrogenase from Pseudomonas putida.6 In mammals, this family of enzymes was first identified as an l-amino acid oxidase in the kidney and the liver of rats and later found to have activity similar to that of l-2-hydroxy acid.7,72 Two α-hydroxy acid oxidases were also reported from hog renal cortex, named long chain l-α-hydroxy acid oxidase (Hao2) and short chain l-α-hydroxy acid oxidase (Hao1), because of their substrate specificity toward long and short carbon chain l-α-hydroxy acids, respectively. In both prokaryotes and eukaryotes, all the members of the hydroxy oxidase family are highly conserved in terms of both nucleotide and amino acid sequences. Human Hao2 has 351 amino acids with a predicted molecular mass of 39 kDa, while human Hao1 comprises 370 amino acids and has a predicted molecular mass of 41 kDa. Human Hao2 shares ∼50% identity with human Hao1 and 72–74% identity with rodent (rat and mouse) Hao2. Hao2 is predominantly expressed in the liver and kidney, with greatest potency for long chain 2-hydroxy acid substrates (displays the highest activity toward 2-hydroxypalmitic acid). Hao1 is expressed primarily in liver and pancreas and shows greatest potency for the two-carbon 2-hydroxy acid substrate (glycolic acid) but also displays activity on long chain 2-hydroxy fatty acids. Both Hao2 and Hao1 are capable of oxidizing 2-hydroxy fatty acids, but the endogenous physiological substrates remain to be identified.
Hao2 has been identified as a candidate gene for the systolic BP quantitative trait locus (QTL) in rats.8−83 Genome-wide linkage analysis in humans locates a BP QTL in a defined region containing Hao2 (located in Ch. 1 at 119.6 cM), thus supporting a potential link between Hao2 and hypertension in humans, as well.9
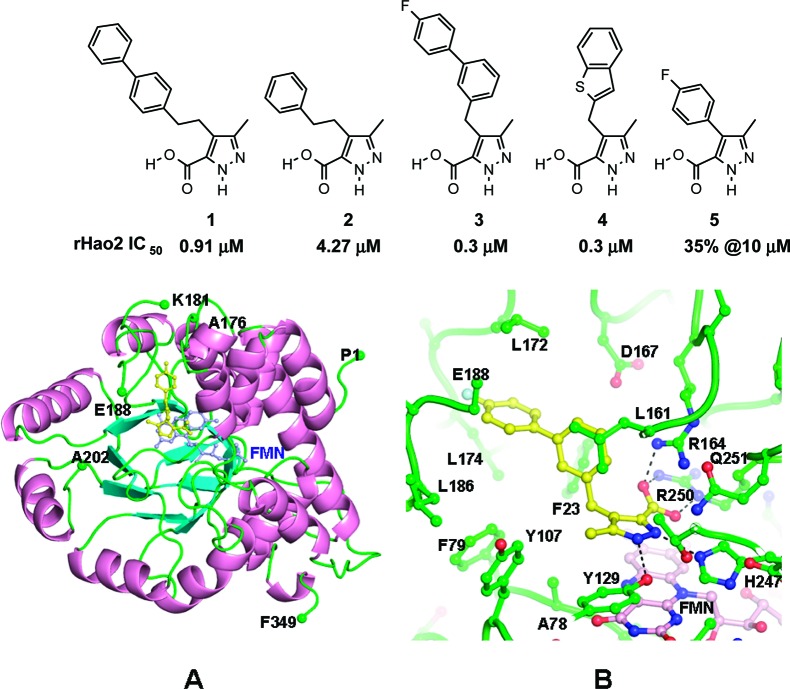
To establish a pharmacological validation of Hao2 in blood pressure regulation, potent and selective rat Hao2 inhibitors were developed. Pyrazole carboxylic acids, 1 and 2 (Figure 1), were first identified as inhibitors of Hao2 by a focused enzyme screen of our compound collection. Subsequent optimization of these hits resulted in the discovery of compounds 3 and 4 as potent inhibitors of rat Hao2, each exhibiting an IC50 value of 0.3 μM.10 These two compounds were further characterized in a spectrum of assays, including intervention studies in a well-established deoxycorticosterone acetate (DOCA) salt hypertension model.
Figure 1.
Structures of screening hits (1 and 2) and optimized leads (3 and 4). (A) Cartoon representation of docked compound 3 in the active site of rat Hao2 (Protein Data Bank entry 1TB3). The FMN ring is colored light blue. (B) Close-up view of docked compound 3 (yellow) in the active site of the enzyme. The FMN ring is colored light pink. The putative hydrogen bonds are shown as dashed lines.
Molecular modeling studies using the rat Hao2 crystal structure (Protein Data Bank entry 1TB3)11 were used to understand the potential binding mode of the inhibitor (3) in the enzyme active site. The modeling suggests that the carboxylic acid moiety binds in the active site by forming salt bridge interactions with basic residues R250 and R164 (Figure 1). The interaction of the carboxylic acid moiety with these residues has also been observed in homologous enzymes of Hao2.112,113 The NH group of the pyrazole moiety forms putative hydrogen bonds with the catalytic H247 and nearby Y129 residues. The biphenyl moiety resides in the hydrophobic pocket and is surrounded by residues such as F79, A185, L172, L174, E188, F23, and L161.
The model suggests that a one-carbon linker between the pyrazole and aromatic moiety (3 and 4) is optimal for filling the available space and placing the aromatic moiety in an appropriate orientation. A longer and flexible carbon linker may increase the conformational entropy of these structures (1 and 2), thereby decreasing their activity. Deletion of the carbon linker makes the molecules very rigid and leads to poor occupancy of the phenyl moiety in the active site. Hence, compound 5 without any linker shows substantially lower activity (Figure 1).
To assess selectivity and to demonstrate compounds 3 and 4 are selective inhibitors of rat Hao2, we profiled these two compounds in a range of in vitro assays. First, we tested their potential cross reactivity against a closely related enzyme, Hao1.10 Compound 3 inhibited rat Hao1 with an IC50 of 45.7 μM, while compound 4 was completely inactive at 10 μM, indicating that compounds 3 and 4 have a minimum 150-fold selectivity against rat Hao1. Compounds 3 and 4 were further profiled against a panel of 125 targets (MDS PanLab Drug Matrix Screen), which includes most targets known to regulate blood pressure, and were shown to be completely inactive at 10 μM. Additionally, compounds 3 and 4 were screened against the GPR109a12 receptor agonist assay, as they are structurally similar to the known high-affinity agonists 3-methylpyrazole-5-carboxylic acid and 3-n-butylpyrazole-5-carboxylic acid, and were found to be completely inactive at 10 μM.
Acknowledgments
This research was part of collaborative program between Advinus Therapeutics and Merck Research Laboratories. We thank Dr. Mahesh Mone for analytical support and Dr. Anup Ranade for managing intellectual property. We thank all the members of the team, business alliance leaders, and senior management from both organizations. Advinus Publication ADV-A-015.
Supporting Information Available
Experimental procedures, analytical data for compounds 3–5, expression and purification of recombinant proteins, in vitro screening protocol, protocol for the DOCA model, blood pressure measurement, and target engagement assay. This material is available free of charge via the Internet at http://pubs.acs.org.
Supplementary Material
References
- Biaggioni I. Sympathetic control of the circulation in hypertension: Lessons from antonomic disorders. Curr. Opin. Nephrol. Hypertens. 2003, 12, 175–180. [DOI] [PubMed] [Google Scholar]
- Martiniuk A. L.; Lee C. M.; Lawes C. M.; Ueshima H.; Suh I.; Lam T. H.; Gu D.; Feigin V.; Jamrozik K.; Ohkubo T.; Woodward M. Hypertension: Its prevalence and population attributable fraction for mortality from cardiovascular disease in the Asia-Pacific region. J. Hypertens. 2007, 25, 73–79. [DOI] [PubMed] [Google Scholar]
- Burt V. L.; Whelton P.; Roccella E. J.; Brown C.; Cutler J. A.; Higgins M.; Horan M. J.; Labarthe D. Prevalence of hypertension in the US adult population. Results from the Third National Health and Nutrition Examination Survey, 1988–1991. Hypertension 1995, 25, 305–313. [DOI] [PubMed] [Google Scholar]
- Hajjar I.; Theodore A.; Kotchen T. A. Trends in prevalence, awareness, treatment, and control of hypertension in the United States, 1988–2000. JAMA, J. Am. Med. Assoc. 2003, 290, 199–206. [DOI] [PubMed] [Google Scholar]
- Webb R. L.; Schiering N.; Sedrani R.; Maibaum J. Direct Renin Inhibitors as a New Therapy for Hypertension. J. Med. Chem. 2010, 53, 7490–7520. [DOI] [PubMed] [Google Scholar]
- Williams B. Drug treatment of hypertension. Br. Med. J. 2003, 326, 61–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chobanian A. V.; Bakris G. L.; Black H. R.; Cushman W. C.; Green L. A.; Izzo J. L. Jr.; Jones D. W.; Materson B. J.; Oparil S.; Wright J. T. Jr.; Roccella E. J. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 report. JAMA, J. Am. Med. Assoc. 2003, 289, 2560–2572. [DOI] [PubMed] [Google Scholar]
- Jones J. M.; Morrell J. C.; Gould S. J. Identification and characterization of HAOX1, HAOX2, and HAOX3, three human peroxisomal 2-hydroxy-acid oxidases. J. Biol. Chem. 2000, 275, 12590. [DOI] [PubMed] [Google Scholar]
- Diep Le K. H.; Florence L. Amino acid sequence of long chain α-hydroxy acid oxidase from rat kidney, a member of the family of FMN-dependent α-hydroxy acid-oxidizing enzymes. J. Biol. Chem. 1991, 266, 20877–20881. [PubMed] [Google Scholar]
- Blanchard M.; Green D. E. Isolation of l-amino acid oxidase. J. Biol. Chem. 1945, 161, 583–597. [PubMed] [Google Scholar]
- Robinson J. C.; Keay L.; Molinari R.; Sizer I. W. l-α-Hydroxy acid oxidases of hog renal cortex. J. Biol. Chem. 1962, 237, 2001–2010. [PubMed] [Google Scholar]
- Lee S. J.; Liu J.; Qi N.; Guarnera R. A.; Lee S. Y.; Cicila G. T. Use of a Panel of Congenic Strains to Evaluate Differentially Expressed Genes as Candidate Genes for Blood Pressure Quantitative Trait Loci. Hypertens. Res. 2003, 26, 75–87. [DOI] [PubMed] [Google Scholar]
- Rice T.; Rankinen T.; Province M. A.; Chagnon Y. C.; Pérusse L.; Borecki I. B.; Bouchard C.; Rao D. C. Genome-wide linkage analysis of systolic and diastolic blood pressure: The Québec family study. Circulation 2000, 102, 1956–1963. [DOI] [PubMed] [Google Scholar]
- Unpublished result from Merck Research Laboratories.
- Rice T.; Rankinen T.; Michael A.; Province M. A.; Chagnon Y. C.; Pérusse L. Quantitative trait loci for maximal exercise capacity phenotypes and their responses to training in the HERITAGE Family Study. Physiol. Genomics 2004, 16, 256–260. [DOI] [PubMed] [Google Scholar]
- See the Supporting Information.
- Cumane L. M.; Barton J. D.; Chen Z-w.; Diep Le K. H.; David A.; Lederer F.; Mathews F. S. Crystal structure analysis of recombinant rat kidney long chain hydroxy acid oxidase. Biochemistry 2005, 44, 1521–1531. [DOI] [PubMed] [Google Scholar]
- Xia Z.-x.; Mathews F. S. Molecular structure of Flavocytochrome b2 at 2.4 Å resolution. J. Mol. Biol. 1990, 212, 837–863. [DOI] [PubMed] [Google Scholar]
- Stenberg K.; Lindqvist Y. Three-dimensional structures of glycolate oxidase with bound active-site inhibitors. Protein Sci. 1997, 6, 1009–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharbaoui T.; Skinner P. J.; Shin Y. J.; Averbuj C.; Jung J. K.; Johnson B. R.; Duong T.; Decaire M.; Uy J.; Cherrier M. C.; Webb P. J.; Tamura S. Y.; Zou N.; Rodriguez N.; Boatman P. D.; Saga C. R.; Lindstrom A.; Xu J.; Schrader T. O.; Smith B. M.; Chen R.; Richman J. G.; Connolly D. T.; Colletti S. L.; Tata J. R.; Semple G. Agonist lead identification for the high affinity niacin receptor GPR109a. Bioorg. Med. Chem. Lett. 2007, 17, 4914–4919. [DOI] [PubMed] [Google Scholar]
- Scott C. Calcitonin gene related peptide protects against hypertension induced heart and kidney damage. Hypertension 2005, 45, 109–114. [DOI] [PubMed] [Google Scholar]
- Loch D.; Hoey A.; Morisseau C.; Hammock B. O.; Brown L. Prevention of hypertension in DOCA salt rats by an inhibitor of soluble epoxide hydrolase. Cell Biochem. Biophys. 2007, 47, 87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compound 3 was orally dosed with the same vehicle (0.5% Tween 80 and 0.5% sodium methylcellulose) at 3, 10, and 30 mg/kg, twice a day. Atenonol was used as the positive control in this study, with a dose of 10 mg/kg, twice a day.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.