Abstract
Combining chemotherapeutics is a promising method of improving cancer treatment; however, the clinical success of combination therapy is limited by the distinct pharmacokinetics of combined drugs, which leads to nonuniform distribution. In this study, we report a new robust approach to load two drugs with different hydrophilicities into a single cross-linked multilamellar liposomal vesicle (cMLV) to precisely control the drug ratio that reaches the tumor in vivo. The stability of cMLVs improves the loading efficiency and sustained release of doxorubicin (Dox) and paclitaxel (PTX), maximizing the combined therapeutic effect and minimizing the systemic toxicity. Furthermore, we show that the cMLV formulation maintains specific drug ratios in vivo for over 24 h, enabling the ratio-dependent combination synergy seen in vitro to translate to in vivo antitumor activity and giving us control over another parameter important to combination therapy. This combinatorial delivery system may provide a new strategy for synergistic delivery of multiple chemotherapeutics with a ratiometric control over encapsulated drugs to treat cancer and other diseases.
Keywords: cross-linked multilamellar liposomal vesicle, combination therapy, doxorubicin, paclitaxel, synergy, dose ratios, nanomedicine
Introduction
Target-based drug design has been successfully used to develop many drugs that can act on novel molecular targets; however, these drugs have shown poor efficacy in clinical trials. This can be attributed to the compensatory mechanism, or drug-mitigating response, enacted by complex diseases such as cancer.1,2 Overcoming this drug-mitigating response often requires high drug doses, which can induce drug resistance in target cells or side effects in other tissues,3 thus limiting the efficacy of many potential drugs in cancer therapy. These limitations of monotherapy can be overcome by synergistic combination of two or more agents, which can kill cells at lower drug doses by affecting multiple disease targets.4,5 However, current combination methods, through cocktail administration, have shown limited improvement over single drugs in clinical studies due to the distinctive pharmacokinetics of individual drugs, which lead to noncoordinated distribution after systemic administration.6,7 Moreover, unexpected adverse effects were reported in clinical trials using these cocktail combinations, raising concerns about the induction of synergistic systemic toxicities by combination therapies.8 For instance, although a combination of doxorubicin (Dox) and paclitaxel (PTX) has been widely used in the treatment of tumors, particularly in metastatic breast cancer, the clinical results were limited by increased cardiotoxicity.9−12 Clinical pharmacokinetic studies also revealed a noncoordinated plasma distribution of Dox and PTX when given in combination,13,14 rendering in vitro data ineffective in predicting in vivo therapeutic efficacy of combination therapy. A more effective combination strategy with the ability to coordinate the pharmacokinetics and biodistribution of various drug molecules is highly desirable to maximize the combinatorial effects without significant toxicity.
The development of nanotechnology has provided a novel combination strategy by enabling the simultaneous delivery of multiple drugs to a site of interest via a single vehicle.7 Nanoparticles are considered promising drug delivery vehicles for cancer therapy based on their ability to prolong drug circulation time, reduce systemic toxicity, and increase drug accumulation at tumor sites through the enhanced permeation and retention (EPR) effect.15−18 The pharmacokinetic behavior of the coformulated drugs can be determined by the pharmacokinetic behavior of the drug carriers. Thus, nanoparticle delivery systems offer the potential to coordinate the plasma elimination and biodistribution of multiple drugs, enabling dosage optimization to maximize cytotoxicity while minimizing the chances to develop drug resistance. Compared to other nanoparticle delivery systems, liposomes have shown superior ability to codeliver multiple drugs with vastly different hydrophobicities to the same site of action.19,20 However, the poor stability and limited loading efficiency of hydrophobic drugs remain the most significant concerns for conventional formulations of liposomes, limiting their clinical benefit in cancer therapy.21,22 For example, a number of studies reported that the maximal drug-to-lipid molar ratio of paclitaxel-encapsulated by a conventional liposome formulation was below 4%,23−26 thwarting the practical application of liposomal drug carriers. Moreover, fine-tuning of the comparative loading yield and release kinetics of multiple drugs in conventional liposomes remains an unmet need. Thus, a stable liposomal formulation that enables improved drug loading and drug release from the carrier in a controlled and sustained manner is necessary for combinatorial drug delivery.
To address such a need, we have previously reported the development of cross-linked multilamellar liposomal vesicles (cMLVs) and demonstrated their efficacy in achieving sustained delivery of doxorubicin both in vitro and in vivo.27 Herein, we extend the potential of cMLVs to facilitate synergistic combinatorial delivery of hydrophobic and hydrophilic drugs in a precisely controlled manner. Dox, a model hydrophilic drug, and PTX, a hydrophobic drug, were coencapsulated into the same cMLVs at predefined stoichiometric ratios. We show that the combination effects (antagonistic, additive, or synergistic) could be determined by controlling drug ratios of Dox and PTX in cMLVs. We also demonstrate that the drug ratio-dependent synergistic effect could be achieved via the cMLV codelivery system in a breast tumor model without significant cardiac toxicity. Moreover, cMLV particles are capable of prolonging maintenance of the synergistic ratios of combined drugs in vivo and, in turn, providing a significantly enhanced antitumor efficacy compared to free-drug cocktail administration. The results demonstrate the great potential of cMLVs as combinatorial drug delivery vesicles to induce synergy of antitumor therapeutics both in vitro and in vivo, thus setting a new paradigm in nanomedicine for combination therapies.
Experimental Section
Cell Lines, Antibodies, Reagents, and Mice
B16-F10 (ATCC number: CRL-6475) and 4T1 tumor cells (ATCC number: CRL-2539) were maintained in a 5% CO2 environment with Dulbecco’s modified Eagle’s medium (Mediatech, Inc., Manassas, VA) supplemented with 10% fetal bovine serum (FBS) (Sigma-Aldrich, St. Louis, MO) and 2 mM of l-glutamine (Hyclone Laboratories, Inc., Omaha, NE). Mouse anti-β-Actin and rabbit antibody against phospho-specific protein p44/42 MAPK (Erk 1/2) were purchased from Cell Signaling Technology (Danvers, MA). Goat anti-Rabbit IR dye680RD and goat anti-mouse IR Dye800CW were obtained from LI-COR BioSciences (Lincoln, Nebraska). Doxorubicin, paclitaxel, daunorubicin, and doxetaxel were purchased from Sigma-Aldrich (St. Louis, MO).
All lipids were obtained from NOF Corporation (Japan): 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC), 1,2-dioleoyl-sn-glycero-3-phospho-(10-rac-glycerol) (DOPG), and 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-[4-(p-maleimidophenyl) butyramide (maleimide-headgroup lipid, MPB-PE).
Female 6–10 week-old BALB/c mice were purchased from Charles River Breeding Laboratories (Wilmington, MA). All mice were held under specific pathogen-reduced conditions in the Animal Facility of the University of Southern California (Los Angeles, CA, USA). All experiments were performed in accordance with the guidelines set by the National Institute of Health and the University of Southern California on the Care and Use of Animals.
Synthesis of cMLVs
Liposomes were prepared based on the conventional dehydration–rehydration method. All lipids were obtained from NOF Corporation (Japan). DOPC, DOPG, and MPB-PE were combined in chloroform, at a molar lipid ratio of DOPC–DOPG–MPB = 4:1:5, and the organic solvent in the lipid mixture was evaporated under argon gas. The lipid mixture was further dried under vacuum overnight to form dried thin lipid films. To prepare cMLV (Dox+PTX), paclitaxel in organic solvent was mixed with the lipid mixture before formation of the dried thin lipid films. The resultant dried film was hydrated in 10 mM Bis-Tris propane at pH 7.0 with doxorubicin by vigorous vortexing every 10 min for 1 h and then applied with four cycles of 15 s sonication (Misonix Microson XL2000, Farmingdale, NY) on ice in 1 min intervals for each cycle. To induce divalent-triggered vesicle fusion, MgCl2 was added at a final concentration of 10 mM. The resulting multilamellar vesicles were further cross-linked by addition of dithiothreitol (DTT, Sigma-Aldrich) at a final concentration of 1.5 mM for 1 h at 37 °C. The resulting vesicles were collected by centrifugation at 14 000 g for 4 min and then washed twice with phosphate-buffered saline (PBS). For pegylation of cMLVs, the particles were incubated with 1 μmol of 2 kDa PEG-SH (Laysan Bio Inc. Arab, AL) for 1 h at 37 °C. The particles were then centrifuged and washed twice with PBS. The final products were stored in PBS at 4 °C.
Characterization of Physical Properties
The hydrodynamic size and size distribution of cMLVs were measured by dynamic light scattering (Wyatt Technology, Santa Barbara, CA).
In Vitro Drug Encapsulation and Release
To study the loading capacity of Dox, cMLV (Dox) and cMLV (Dox+PTX) were collected and washed twice with PBS, followed by lipid extraction of vesicles with 1% Triton X-100 treatment. Dox fluorescence (excitation 480 nm, emission 590 nm) was then measured by a Shimadzu RF-5301PC spectrofluorometer (Japan. The amount of paclitaxel incorporated in the cMLV(PTX) and cMLV(Dox+PTX) was determined by C-18 reverse-phase high-performance liquid chromatography (RP-HPLC) (Beckman Coulter, Brea, CA). The cMLV(PTX) and cMLV(Dox+PTX) suspensions were diluted by adding water and acetonitrile to a total volume of 0.5 mL. Extraction of paclitaxel was accomplished by adding 5 mL of tert-butyl methyl ether and votex-mixing the sample for 1 min. The mixtures were centrifuged, and the organic layer was transferred into a glass tube and evaporated to dryness under argon. Buffer A (95% water, 5% acetonitrile) was used to rehydrate the glass tube. To test PTX concentration, 1 mL of the solution was injected into a C18 column, and the paclitaxel was detected at 227 nm (flow rate 1 mL/min). To obtain the release kinetics of Dox and PTX from liposomes, the releasing media was removed from cMLVs incubated in 10% FBS-containing media at 37 °C and replaced with fresh media daily. The removed media was quantified for Dox fluorescence (by spectrofluorometer) and PTX fluorescence (by HPLC) every day.
In Vitro Drug Loading Efficiency
Loading efficiency was determined by the ratio of encapsulated drug to total phospholipid mass. A phospholipid phosphate assay was carried out to calculate the phospholipid mass. cMLVs were centrifuged, and 100 μL chloroform was added to the pellets to break down the lipid bilayers. The samples were transferred to glass tubes and evaporated to dryness. After adding 100 μL perchloric acid, the samples were boiled at 190 °C for 25 min. Samples will turn brown then clear as the lipids are digested. Samples were cooled to room temperature and diluted to 1 mL with distilled water. The amount of phospholipid phosphate was determined by the malachite green phosphate detection kit (R&D systems, Minneapolis, MN).
In Vitro Cytotoxicity and Data Analysis
B16-F10 and 4T1 cells were plated at a density of 5 × 103 cells per well in 10% FBS-containing media in 96-well plates and grown for 6 h. The cells were then exposed to a series of concentrations of cMLV (single drug) or cMLV (drug combinations), at different weight ratios of combined drugs, for 48 h. The cell viability was assessed using the Cell Proliferation Kit II (XTT assay) from Roche Applied Science according to the manufacturer’s instructions. The cell viability percentage was determined by subtracting absorbance values obtained from media-only wells from drug-treated wells and then normalizing to the control cells without drugs. The fraction of cells affected (fa) at each drug concentration was subsequently determined for each well. The data was analyzed by nonlinear regression to get the IC50 value. The combination index (CI) values were calculated by the equation: CI = CA,X/ICX,A + CB,X/ICX,B.28 Using this analysis method, a CI = 0.9–1.1 reflects additive activity, and a CI >1.1 indicates antagonism, while a CI < 0.9 suggests synergy.
Western Blot Analysis
Cells were collected 24 h after treatment and lysed in lysis buffer supplemented with protease inhibitors, incubated on ice for 15 min, and then cleared by centrifugation at 10 000 × g at 4 °C for 10 min. The protein concentration was determined using Micro BCA Protein Assay Kit (Thermo Scientific). Lysates (20 μg) were separated by reducing 12% polyacrylamide gel and then transferred to polyvinylidene difluoride membranes. Immunodetection of ERK was carried out with antibodies specific to rabbit phospho-specific protein p44/42 MAPK (Erk 1/2) and goat antirabbit IR dye 680RD. Immunodetection of β-actin was carried out with antibodies against β-actin and goat antimouse IR dye 800CW. Membranes were developed using Odyssey infrared fluorescent imager (LI-COR BioSciences, Lincoln, Nebraska).
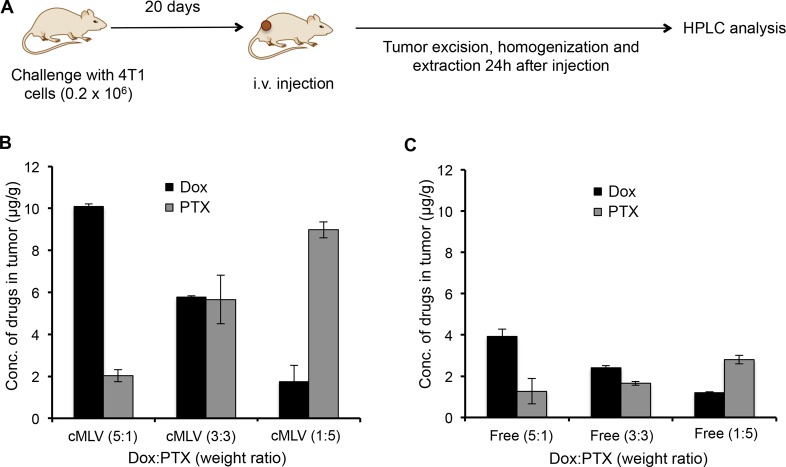
Determination of Doxorubicin and Paclitaxel Levels in Tumor
BALB/c female mice (6–10 weeks-old) were inoculated subcutaneously with 0.2 × 106 4T1 tumor cells. The tumors were allowed to grow for 20 days to a volume of ∼500 mm3 before treatment. On day 20, the mice were injected intravenously through the tail vein with 8.33 mg/kg Dox + 1.66 mg/kg PTX, 5 mg/kg Dox + 5 mg/kg PTX, or 1.66 mg/kg Dox + 8.33 mg/kg PTX either in solution or in cMLVs. Three days after injection, tumors were excised and frozen at −20 °C. Docetaxel (10 μL, 100 μg/mL) as an internal standard (IS) for paclitaxel, or 10 μL of daunorubicin (100 μg/mL) as an internal standard for doxorubicin, was added to the weighted tumor tissues. In order to extract paclitaxel and the internal standard (docetaxel), tumor tissue was homogenized in 1 mL ethyl acetate and then centrifuged at 5000 rpm for 10 min. In order to extract doxorubicin and its internal standard (daunorubicin), tumor tissue was homogenized in 1 mL of methanol and then centrifuged at 5000 rpm for 10 min. Then the organic layer was transferred to a clean glass tube and evaporated to dryness under a stream of argon. Buffer A (95% water, 5% acetonitrile) was used to rehydrate the sample in the glass tube. A portion of 1 mL of the solution was injected into C18 column, and the paclitaxel was detected at 227 nm (flow rate 1 mL/min), and doxorubicin was detected at 482 nm (flow rate 1 mL/min). Stock solutions of Dox and PTX (100, 10, and 1 μg/mL) and IS were prepared as calibration samples. Then 500 μL of tumor homogenates were spiked with 500 μL calibration samples with the internal standard at fixed concentration of 1 μg/mL. Calibration curves of doxorubicin and paclitaxel were constructed using the ratio of peak height of doxorubicin or paclitaxel and internal standard by weighted (1/y) linear regression analysis.
In Vivo Antitumor Activity Study
BALB/c female mice (6–10 weeks-old) were inoculated subcutaneously with 0.2 × 106 4T1 breast tumor cells. The tumors were allowed to grow for 8 days to a volume of ∼50 mm3 before treatment. On day 8, the mice were injected intravenously through the tail vein with 3.33 mg/kg Dox + 0.67 mg/kg PTX, 2 mg/kg Dox + 2 mg/kg PTX, or 0.67 mg/kg Dox + 3.33 mg/kg PTX, either in cMLVs or in solution every 3 days (six mice per group). The tumor growth and body weight were monitored until the end of an experiment. The length and width of the tumor masses were measured with a fine caliper every 3 days after injection. The tumor volume was expressed as 1/2 × (length × width2). The survival end point was set when the tumor volume reached 1000 mm3. The survival rates are presented as Kaplan–Meier curves. The survival curves of individual groups were compared by a log-rank test.
Immunohistochemistry of Tumors, Cardiac Toxicity, and Confocal Imaging
BALB/c female mice (6–10 weeks-old) were inoculated subcutaneously with 0.2 × 106 4T1 tumor cells. The tumors were allowed to grow for 20 days to a volume of ∼500 mm3 before treatment. On day 20, the mice were injected intravenously through tail vein with 8.33 mg/kg Dox + 1.66 mg/kgPTX, 5 mg/kg Dox + 5 mg/kg PTX, or 1.66 mg/kg Dox + 8.33 mg/kg PTX in solution or cMLVs. Three days after injection, tumors were excised, fixed, frozen, cryo-sectioned, and mounted onto glass slides. Frozen sections were fixed and rinsed with cold PBS. After blocking and permealization, the slides were washed by PBS and incubated with a terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) reaction mixture (Roche, Indianapolis, Indiana) for 1 h and counterstained with 4',6-diamidino-2-phenylindole (DAPI) (Invitrogen, Carlsbad, CA). Fluorescence images were acquired by a Yokogawa spinning-disk confocal scanner system (Solamere Technology Group, Salt Lake City, UT) using a Nikon Eclipse Ti-E microscope. Illumination powers at 405, 491, 561, and 640 nm solid-state laser lines were provided by an AOTF (acousto-optical tunable filter)-controlled laser-merge system with 50 mW for each laser. All images were analyzed using Nikon NIS-Elements software. For quantifying TUNEL positive cells, four regions of interest (ROI) were randomly chosen per image at ×2 magnification. Within one region, the area of TUNEL-positive nuclei and the area of nuclear staining were counted by Nikon NIS-Element software, with data expressed as % total nuclear area stained by TUNEL in the region.
For cardiac toxicity, heart tissues were harvested 3 days after injection and were fixed in 4% formaldehyde. The tissues were frozen and then cut into sections and mounted onto glass slides. The frozen sections were stained with hematoxylin and eosin. Histopathologic specimens were examined by light microscopy.
Statistics
The differences between two groups were determined with Student’s t test. The differences among three or more groups were determined with a one-way anaylsis of variance (ANOVA).
Results and Discussion
Characteristics of Combinatorial Drug Delivery via cMLVs
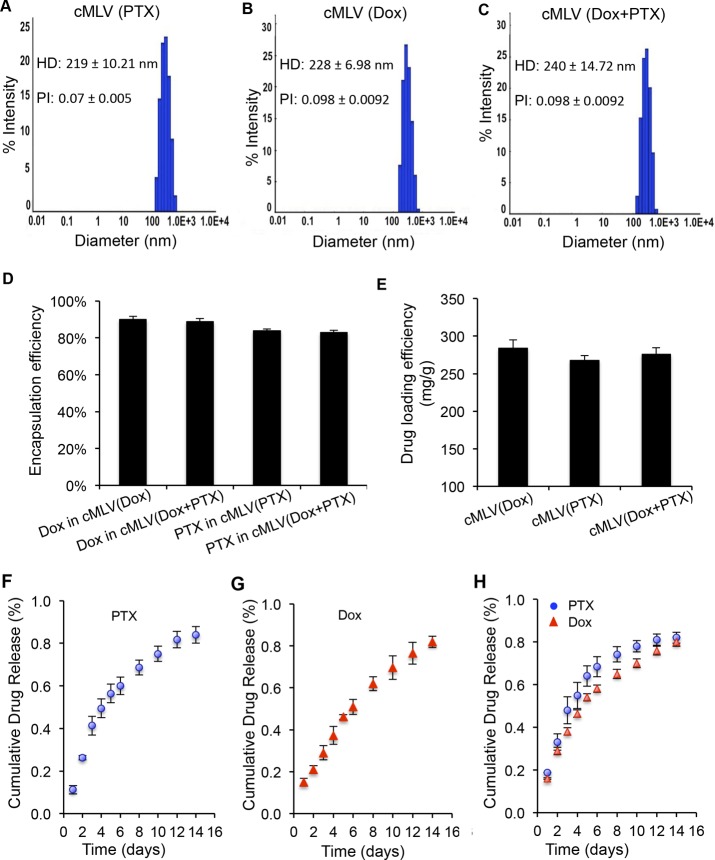
Our strategy of combination drug delivery via cross-linked multilayer liposomal vesicles was to incorporate the hydrophobic drug paclitaxel (PTX) into the lipid membranes and encapsulate the hydrophilic drug doxorubicin (Dox) in the aqueous core of liposomal vesicles, shown in Scheme 1. The cross-linked multilamellar liposomal vesicles (cMLVs) were formed by adding MgCl2 to trigger vesicle fusion and then stabilized by dithiothreitol (DTT) to form cross-linkers between adjacent liposomal vesicles.27,29 The surface of the cross-linked multilayer liposomes was further PEGylated with thiol-termineated PEG, which is known to enhance vesicle stability and elongate the blood circulation half-life.30,31 First, we characterized the physical properties of dual drug-loaded cMLVs compared to single drug-loaded cMLVs to determine whether drug combinations could change the physical properties of liposomal formulation. Dynamic light scattering (DLS) measurements showed that the resulting dual drug-loaded cMLVs had a similar average hydrodynamic diameter as single drug-loaded cMLVs (Figure 1A–C). We found no significant aggregation of particles during the cross-linking process in all three liposomal formulations, as evident by the narrow size distribution and similar polydispersity observed in both dual drug-loaded and single drug-loaded cMLVs. This suggests that the combination of Dox and PTX in a single nanoparticle has a negligible effect on the formation of cMLV particles.
Scheme 1. Schematic Illustration of the Codelivery of Hydrophobic Drug Paclitaxel (Green) and Hydrophilic Drug Doxorubicin (Red) via cMLVs.
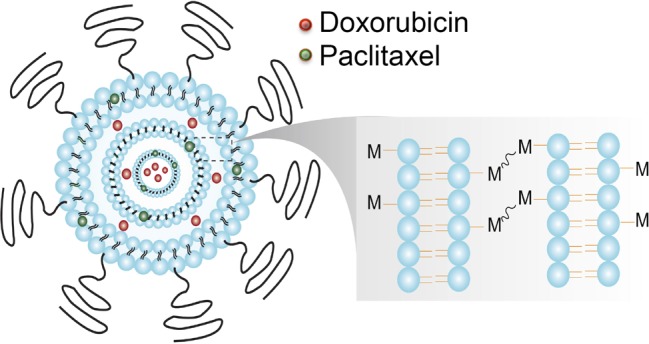
Figure 1.
Characteristics of cMLV (Dox+PTX). (A–C) The hydrodynamic size distribution of cMLV(Dox), cMLV(PTX), and cMLV(Dox+PTX) measured by dynamic light scattering. The mean hydrodynamic diameter (HD) and polydispersity index (PI) of cMLV(Dox), cMLV(PTX), and cMLV(Dox+PTX) are indicated on the graph. (D, E) Effects of coencapsulation of Dox and PTX on loading capability and drug release kinetic profiles of cMLVs. The encapsulation efficiency (D) and loading efficacy (E) of drugs in cMLV(combined drugs) and cMLV (single drug). (F–H) In vitro release kinetics of doxorubicin and paclitaxel from dual-drug loaded cMLVs and single-drug loaded cMLVs. Error bars represent the standard deviation of the mean from triplicate experiments.
We next determined whether the encapsulation efficiency or loading yield of cMLVs were affected by loading multiple therapeutics. Single drug-loaded and dual drug-loaded cMLVs were dissolved in organic solvents to free all encapsulated drugs (Dox and/or PTX). Dox and PTX concentrations were quantified by spectrofluorometer and/or HPLC, respectively. As shown in Figure 1D, the drug encapsulation efficiency of Dox and PTX in cMLV (Dox+PTX) was not significantly different from that in either cMLV (Dox) or cMLV (PTX). It was also shown that cMLV (Dox+PTX) had a comparable drug loading yield (∼270 mg drug per g of phospholipids) compared to single drug-loaded cMLVs (Figure 1E). The drug release profiles of Dox and PTX were also evaluated in dual drug-loaded cMLVs to investigate whether the cMLVs are able to release the individual drugs in a controlled manner. The results of in vitro drug release assay showed that cMLV (Dox+PTX) has slow and linearly sustained release kinetics of both Dox and PTX (up to 2 weeks), similar to that of single drug-loaded cMLVs (Figure 1F–H). These results confirm that this approach enables the loading of drugs with different hydrophobicity into the same nanoparticles with an efficient drug loading yield and sustained drug release profiles.
In Vitro Analysis of Doxorubicin: Paclitaxel for Drug Ratio-Dependent Synergy
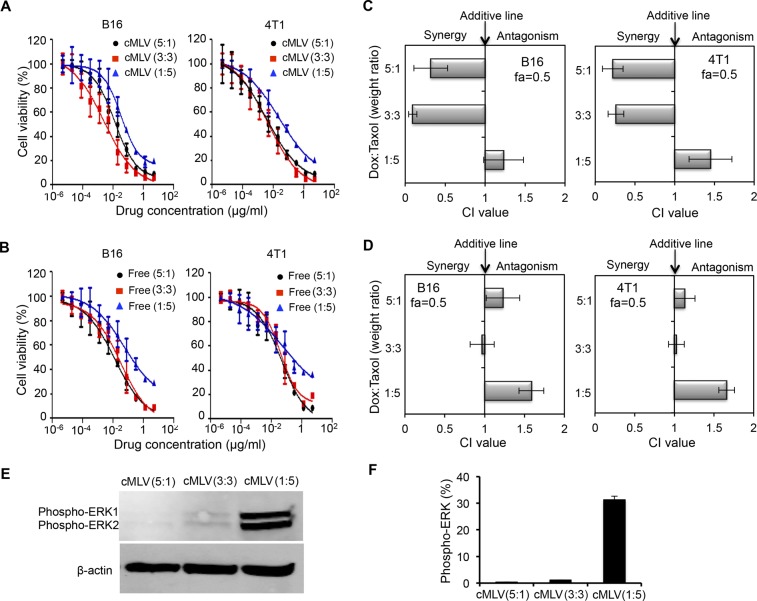
Certain cases of combinatorial drug delivery are able to induce synergistic effects, and it has been reported that the combination effect, synergy, additivity, or antagonism can be affected by the dose ratio.19,32 To test this hypothesis, the cytotoxicities of cMLV (Dox+PTX) encapsulating three different drug weight ratios (5:1, 3:3, and 1:5) were examined in B16 and 4T1 cell lines. The cytoxocicities of cMLVs were compared to the cytotoxicities of the same three ratio combinations in cocktail solutions. Figure 2A summarizes the results of IC50 measurements of the dual drug-loaded cMLVs with the three different dose ratios after 48 h of incubation with B16 and 4T1 cells. The IC50 values of cMLV (Dox+PTX) at Dox–PTX ratios of 3:3 and 5:1 were significantly smaller than that of the 1:5 ratio in the cell lines studied. A similar trend of IC50 values at the different dose ratios was observed for free Dox and PTX combinations (Figure 2B).
Figure 2.
Determination of the ratio of drug combinations to induce synergy. (A, B) In vitro cytotoxicity of three weight ratios (5:1, 3:3, and 1:5) of Dox and PTX in cMLV formulations (A) or solution (B) in B16 melanoma tumor or 4T1 breast tumor cell lines. The cytotoxicity was measured by a standard XTT assay. (C) Combination index (CI) histogram for cMLV (different drug combinations) exposed to cultured B16 and 4T1tumor cells. (D) Combination index histogram for different ratios of drug combination in solution exposed to culture B16 and 4T1 tumor cells. The surviving cell fraction from three replicates was averaged and analyzed by nonlinear regression. The histogram presents the CI values obtained at a fraction of 0.5. Error bars represent the standard deviation of the mean from triplicate experiments. (E) Immunoblot analysis of phosphorylated ERK in B16 cells treated by cMLV(Dox+PTX) with three dose ratios: 5:1, 3:3, and 1:5. β-actin was used as control. (F) Quantification of phosphorylated ERK shown in (E). Protein amounts were estimated by densitometry of immunoblots. Error bars represent SD.
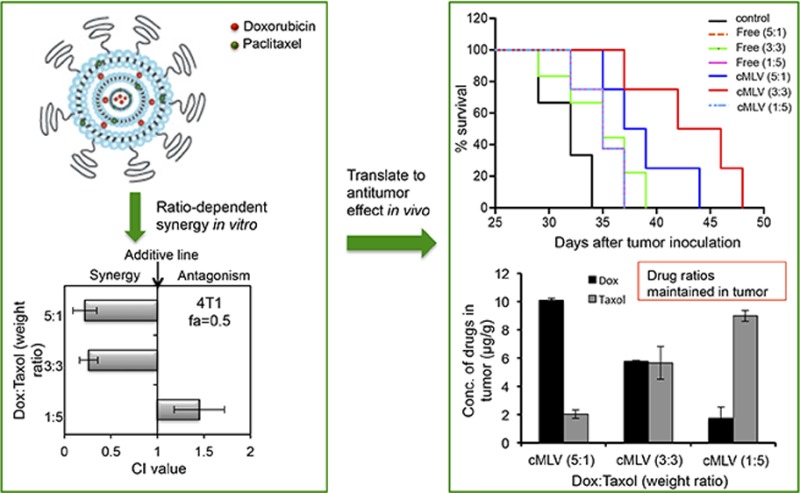
Moreover, combination index (CI) values were analyzed from in vitro cytotoxicity curves for Dox and PTX combinations either in cMLVs or cocktail solutions to assess the effects of combination. The IC50 values of individual drugs either in cMLVs or in solution are shown in Figure S1 of the Supporting Information. A CI of less than, equal to, and greater than 1 is known to indicate synergy, additivity, and antagonism, respectively.19,28,33,34 Although combination indexes are only shown for a 0.5 fraction of affected cells (fa) (50% cell growth inhibition relative to control cells) in Figure 2, the profile of synergy/antagonism was similar for other fa values. As shown in Figure 2C, at fa = 0.5, synergistic effects were observed in both B16 and 4T1 tumor cells for coloaded cMLVs at Dox–PTX ratios of 5:1 and 3:3 (Dox–PTX), while the combination at a 1:5 ratio was additive or antagonistic in B16 and 4T1 cells. In contrast, no synergistic effect was observed in B16 or 4T1 cells treated with three ratios of Dox and PTX in cocktail, as shown in Figure 2D, further confirming the potential of cMLVs to induce synergy by controlling dose ratios.
Our data indicated that combinatorial delivery via cMLVs with high ratio of PTX induced additivity or antagonism. In fact, some studies have shown that low concentrations of PTX can induce cell apoptosis more effectively than high concentrations, but the mechanism remains elusive.35,36 Further studies suggested that PTX could activate the extracellular signal regulated kinase (ERK), leading to cell proliferation and building drug resistance.37−39 It was also shown that inhibiting the ERK pathway dramatically enhanced cell apoptosis induced by PTX.37,39 These studies indicate that the high PTX concentration could be responsible for the antagonism seen between Dox and PTX at a 1:5 dose ratio. To investigate whether there is a difference in activation of ERK in melanoma cells treated by cMLV(Dox+PTX) at the three different dose ratios, phosphorylated ERK expression was detected by Western blot. As shown in Figure 2E, the combination of Dox and PTX at a 1:5 ratio showed significantly increased expression of phosphorylated ERK compared to the 3:3 and 5:1 ratios. Quantification of ERK phosphorylation (Figure 2F) showed a 30-fold enhancement in phosphorylated ERK in cells treated by the cMLV(Dox+PTX) 1:5 ratio. These data suggest that ratio-dependent combination effects are likely linked to the ERK activation caused by high concentrations of PTX.
Drug Ratio-Dependent Efficacy of cMLV(Dox+PTX) in Tumor Treatment
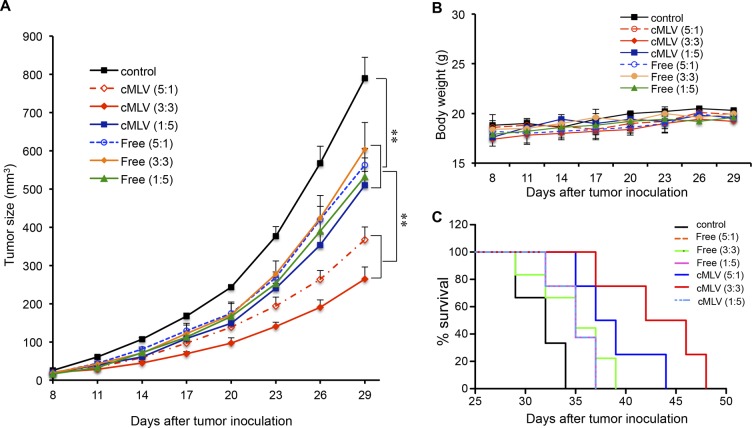
In order to assess whether the drug ratio-dependent in vitro cytotoxicity was also manifested in vivo, doxorubicin and paclitaxel were coencapsulated in cMLV particles at a weight ratios ranging from 5:1 to 1:5, while keeping the total drug mass encapsulated in cMLVs constant. This panel of fixed ratio cMLV formulations and the same fixed ratio combination in cocktail solutions were evaluated for their antitumor efficacy in an in vivo 4T1 breast tumor model. As shown in Figure 3A, tumor volume in the groups treated with drug combinations in solution decreased significantly compared to that in the control group (p < 0.01). The tumor volume between the groups treated with different ratios of drug combinations in solution did not show a significant difference (p > 0.05), consistent with the in vitro finding that free drug combinations did not show a synergistic effect. In comparison, administration of the 5:1 and 3:3 weight ratio of Dox to PTX in cMLV resulted in significantly enhanced antitumor activity compared to the 1:5 ratio, indicating the ability of cMLVs to induce a ratio-dependent synergistic effect in vivo. Moreover, no weight loss was observed for all treated groups during the experiment (Figure 3B), indicating that there was no significant toxicity from these dose combinations.
Figure 3.
Drug ratio-dependent efficacy of cMLV(Dox+PTX) in tumor treatment. (A) Tumor growth was measured after treatment with PBS, 3.33 mg/kg Dox + 0.67 mg/kg PTX, 2 mg/kg Dox + 2 mg/kg PTX, 0.67 mg/kg Dox + 3.33 mg/kg PTX, either in cMLVs or in solution every 3 days. Tumor growth and body weights were monitored until the end of the experiment. Error bars represent standard error of the mean, n = 6 for each treatment group (*p < 0.05, **p < 0.01). (B) Average mouse weight loss over the duration of the experiment. (C) Survival curves for 4T1 bearing mice treated with PBS, 3.33 mg/kg Dox + 0.67 mg/kg PTX, 2 mg/kg Dox + 2 mg/kg PTX, 0.67 mg/kg Dox + 3.33 mg/kg PTX either in cMLVs or in solution every 3 days. The survival rates are presented as Kaplan–Meier curves. The survival curves of individual groups were compared by a log-rank test.
The dose-dependent antitumor activity was further confirmed by survival test as shown in Figure 3C. Treatment with three ratios of drug combinations in cocktail solutions resulted in an increased survival time (35 days) compared to PBS treatment (28 days, p < 0.05). Administration of the 5:1 and 3:3 weight ratios in cMLV formulations resulted in a significant increased life span compared to 1:5 ratio in cMLVs (p < 0.05). These results confirmed a dose-dependent synergy of drug combinations in cMLV formulations and provide a positive correlation linking the combination effects in vitro to the degree of antitumor efficacy in vivo.
Drug Ratio-Dependent Efficacy of Coencapsulated Dox–PTX on Tumor Apoptosis
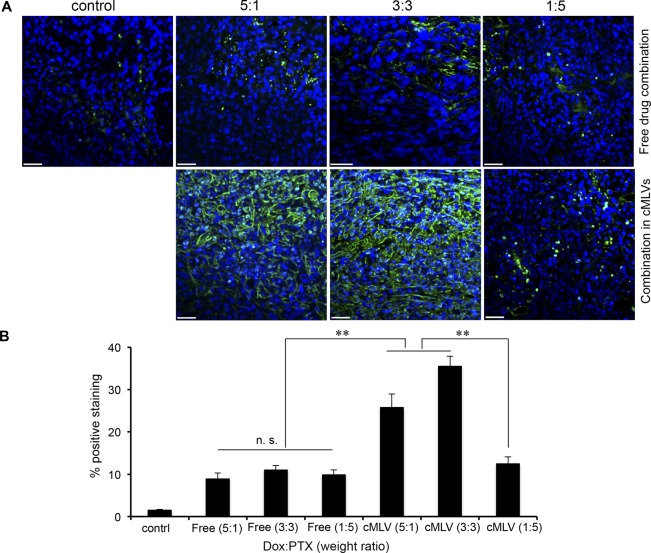
To investigate the ratio-dependent antitumor mechanism in vivo, a TUNEL assay was performed to detect apoptotic cells in 4T1 tumors treated with different ratios of Dox and PTX in cocktail and in cMLV formulations for 3 days. As shown in Figure 4A, 4T1 tumors treated with three different ratios (5:1, 3:3, and 1:5) of Dox and PTX in solution-induced cell apoptosis by a significant amount compared to controls. The apoptosis index was not remarkably different among different ratios of drug combination cocktails (p > 0.05), consistent with the similar effect on tumor growth between the cocktail treatments. Moreover, the 5:1 and 3:3 ratios of Dox and PTX in cMLVs promoted tumor cell apoptosis compared to the antagonistic ratio (1:5). The quantified data (Figure 4B) further confirm that drug ratio-dependent antitumor efficacy via cMLVs can contribute to different levels of tumor apoptosis.
Figure 4.
Drug ratio-dependent efficacy of coencapsulated Dox–PTX on tumor cell apoptosis. (A) 4T1 tumor-bearing mice were treated with PBS, 8.333 mg/kg Dox + 1.667 mg/kg PTX, 5 mg/kg Dox + 5 mg/kg PTX, or 1.667 mg/kg Dox + 8.33 mg/kg PTX, either in cMLVs or in solution. Three days after injection, tumors were excised. Apoptotic cells were detected by a TUNEL assay (green) and costained by nuclear staining DAPI (blue). The scale bar represents 50 μm. (B) Quantification of apoptotic positive cells in the 4T1 tumor. To quantify TUNEL positive cells, four regions of interest (ROI) were randomly chosen per image at ×2 magnification. Within one region, the area of TUNEL positive nuclei and the area of nuclear staining were counted by software. The data are expressed as % total nuclear area stained by TUNEL in the region. Data represented as mean ± SD (n = 3).
In Vivo Cardiac Toxicity Evaluation of Drug Combinations in cMLV Gormulations
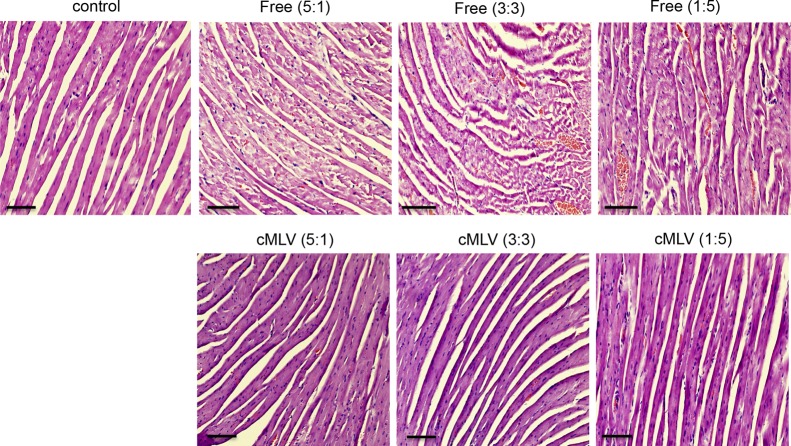
An unexpected clinical outcome of increased cardiotoxicity after combined treatments of Dox and PTX has been reported, thus limiting their clinical applications.40,41 To investigate whether the synergistic therapies could induce synergistic cardiac toxicity, three weight ratios of doxorubicin and paclitaxel in both cMLV formulations and cocktail solutions were evaluated for cardiac effects. Mice-bearing 4T1 tumors were injected intravenously through tail vein with 8.33 mg/kg Dox + 1.66 mg/kg PTX, 5 mg/kg Dox + 5 mg/kg PTX, or 1.66 mg/kg Dox + 8.33 mg/kg PTX in solution or in cMLVs. Hematoxylin and eosin staining of cardiac tissue sections from each treatment group were examined. As shown in Figure 5, all three dose ratios of Dox and PTX in cocktail solutions caused damage to cardiac tissue indicated by myofibrillary loss, disarray, and cytoplasmic vacuolization. No significant histopathologic changes in cardiac tissue were observed in three dose ratios of Dox and PTX in cMLV formulations compared to the control group, indicating that a reduction in systemic toxicity can be achieved when drugs are coencapsulated in cMLVs. Moreover, no synergistic toxicity was observed in the synergistic ratios (5:1 and 3:3) of Dox and PTX in cMLVs.
Figure 5.
In vivo toxicity. Histologic appearance of cardiac tissues obtained from C57/BL6 mice with no drug treatment or administered a single intravenous injection with three dose ratios of Dox and PTX (5:1, 3:3, and 1:5) in solutions or cMLV formulations at 10 mg/kg total drug equivalent. The scale bar represents 100 μm.
In Vivo Maintenance of Drug Ratios in cMLV Formulations
In order to determine if dose ratios of drugs delivered via cMLVs were well-maintained in vivo and to correlate the in vivo effects to the in vitro combination effect, the drug concentrations in tumor tissues were measured. Doxorubicin and paclitaxel were coencapsulated at the 5:1, 3:3, and 1:5 weight ratios inside cMLVs and administered i.v. to mice, while the same ratios of drug combinations in cocktail solutions were administrated as controls. Twenty-four hours after injection, tumors were excised and homogenized, and Dox and PTX were extracted and detected by HPLC analysis, as illustrated in Figure 6A. The HPLC results show that cMLVs maintain the doxorubicin–paclitaxel weight ratios at 5:1, 3:3, and 1:5, respectively, in tumors for over 24 h (Figure 6B). In comparison, the free-drug cocktail Dox–PTX weight ratio changed dramatically after administration, shown in Figure 6C. In addition, remarkably more doxorubicin and paclitaxel accumulated in tumors when administered via cMLV formulations compared to free-drug cocktails with equivalent amounts of Dox and PTX, thus maximizing their combinatorial effect. These results indicate that cMLVs can efficiently maintain dose ratio in vivo, thus translating the combination effects (synergy, additivity, and antagonism) from in vitro to in vivo.
Figure 6.
In vivo maintenance of Dox–PTX ratios in cMLV formulations. (A, B) Tumor-bearing mice were treated with PBS, 8.333 mg/kg Dox + 1.667 mg/kg PTX, 5 mg/kg Dox + 5 mg/kg PTX, or 1.667 mg/kg Dox + 8.33 mg/kg PTX, either in cMLVs (A) or in solution (B). Twenty-four h after injection, tumors were excised, and drug concentrations of Dox and PTX were measured by HPLC. All data are shown as the means of triplicate experiments.
To summarize, a robust approach for combinatorial chemotherapy was presented by encapsulating two different types of antitumor therapeutics, with ratiometric control over drug loading, into a cross-linked multilamellar liposomal formulation. Previously, we have demonstrated the superior ability of cMLVs as drug carriers to offer controllable and sustainable drug release profiles of doxorubicin with increased vesicle stability, enabling improved antitumor activity. In the present study, we explore the potential of cMLVs in combinatorial delivery of Dox and PTX, which have been widely used as a combined anthracycline–taxane regimen in metastatic breast cancer,42 to achieve synergistic antitumor activity. A number of studies suggest the noncoordinated biodistribution profiles of this combination when administered in cocktail solutions limit the efficacy of the combination.13,14 However, the versatile cross-linked multilamellar liposomes enabled codelivery of Dox and PTX via a single vesicle to the cancer site, thus coordinating the plasma elimination and tissue distribution of the combined drugs.
Recent studies revealed that the activity of antitumor drug combinations is determined by the ratio of the combined drugs exposed to cells.32,43−45 Therefore, it is highly desirable to maintain a synergistic ratio of combined drugs in vivo. Here, we demonstrate that the stability of cMLVs enables us to coload Dox and PTX with predefined ratios and induce a ratio-dependent synergy in tumor cells. It was previously reported by a number of studies that paclitaxel-containing liposomes could not maintain stability over a drug-to-lipid molar ratio of 3–4%. For example, one study showed that more than 8% PTX-to-lipid formulations (PG–PC 3:7 molar ratio) were not stable for 1 day.24 cMLVs can maintain a high stability up to 30% paclitaxel-to-lipid molar ratio. This is most likely due to the cross-linked multilamellar structure of cMLVs, which allows codelivery of Dox and PTX with high loading efficiency. In addition, enhanced vesicle stability of cMLVs enables these nanoparticles to maintain the dose ratios of Dox and PTX at tumor sites, translating the ratio-dependent synergy from in vitro to in vivo. This would be beneficial for predicting the efficacy of treatment in clinical trials and the optimal design of combination therapy based on in vitro cellular experiments. Our in vivo results also reveal that the enhanced combinatorial efficacy of cMLVs compared to cocktail combination is due to the augmented accumulation of drugs at tumor sties.
In clinical studies, Dox and PTX exhibit an increased cardiac toxicity when combined in cocktail,40,41 raising the concern that a significant side effects could be associated with the synergistic therapeutic efficacy. However, we previously demonstrated that the robust cMLV formulation greatly reduced systemic toxicity of Dox, most likely due to the sustained drug release profile of Dox. Here, we show that cMLVs can induce synergistic effects on tumor growth without causing cardiac toxicity, further demonstrating their potential in combinatorial drug delivery. These results, taken together, indicated that the superior ability of cMLVs in combination therapy is not only attributed to the prolonged exposure of drugs to tumor cells, but also to the maintenance of synergistic dose ratios at the site of action with no significant systemic toxicity.
Conclusions
In conclusion, we have demonstrated that the ratio-dependent synergy of drug combinations shown in vitro can be translated into the synergistic antitumor efficacy in vivo by coloading two types of drugs into cross-linked multilamellar liposomal formulations. Unlike the free-drug cocktail, cMLVs maintain dose ratios for prolonged times after administration in vivo due to the ability of cMLVs to coencapsulate and retain the combined drugs in a manner that coordinates their pharmacokinetics. In the present study two drugs (Dox and PTX) were chosen to demonstrate the advantage of this combination drug delivery system by cMLVs. In this regard, we believe this delivery system can offer the clinical possibility for improved synergistic delivery of multiple chemotherapeutics with a ratiometric control over drug encapsulation for combination cancer treatment.
Acknowledgments
We thank the USC NanoBiophysics Core Facility. We also thank Jennifer Rohrs for critical reading of the manuscript. This work was supported by National Institutes of Health grants (R01AI068978, R01CA170820, and P01CA132681), a translational acceleration grant from the Joint Center for Translational Medicine, the National Cancer Institute (P30CA014089), and a grant from the Ming Hsieh Institute for Research on Engineering Medicine for Cancer.
Supporting Information Available
IC50 values of Dox and PTX in cMLV formulation or free drug solution in B16 melanoma or 4T1 breast tumor cells. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Hartman J. L.; Garvik B.; Hartwell L. Principles for the Buffering of Genetic Variation. Science 2001, 291, 1001–1004. [DOI] [PubMed] [Google Scholar]
- Stelling J.; Sauer U.; Szallasi Z.; Doyle F. R.; Doyle J. Robustness of cellular functions. Cell 2004, 118, 675–85. [DOI] [PubMed] [Google Scholar]
- Hopkins A. L. Network pharmacology: the next paradigm in drug discovery. Nat. Chem. Biol. 2008, 4, 682–690. [DOI] [PubMed] [Google Scholar]
- Kaelin W. J. The concept of synthetic lethality in the context of anticancer therapy. Nat. Rev. Cancer 2005, 5, 689–98. [DOI] [PubMed] [Google Scholar]
- Keith C.; Borisy A.; Stockwell B. Multicomponent therapeutics for networked systems. Nat. Rev. Drug Discovery 2005, 4, 71–78. [DOI] [PubMed] [Google Scholar]
- Di M. M.; Chiodini P.; Georgoulias V.; Hatzidaki D.; Takeda K.; Wachters F. M.; Gebbia V.; Smit E. F.; Morabito A.; Gallo C.; Perrone F.; Gridelli C. Meta-analysis of single-agent chemotherapy compared with combination chemotherapy as second-line treatment of advanced non-small-cell lung cancer. J. Clin. Oncol. 2009, 27, 1836–1843. [DOI] [PubMed] [Google Scholar]
- Hu C. M.; Zhang L. Nanoparticle-based combination therapy toward overcoming drug resistance in cancer. Biochem. Pharmocol. 2012, 83, 1104–1111. [DOI] [PubMed] [Google Scholar]
- Farr M.; Bacon P. How and when should combination therapy be used? The role of an anchor drug. Br. J. Rheumatol. 1995, 34, 100–103. [PubMed] [Google Scholar]
- Lyman G. H.; Green S. J.; Ravdin P. M.; Geyer C. E.; Russell C. A.; Balcerzak S. P.; Thomas B. G.; Martino S. A Southwest Oncology Group randomized phase II study of doxorubicin and paclitaxel as frontline chemotherapy for women with metastatic breast cancer. Breast Cancer Res. Treat. 2004, 85, 143–150. [DOI] [PubMed] [Google Scholar]
- Sparano J. A.; Hu P.; Rao R. M.; Falkson C. I.; Wolff A. C.; Wood W. C. Phase II trial of doxorubicin and paclitaxel plus granulocyte colony-stimulating factor in metastatic breast cancer: an Eastern Cooperative Oncology Group Study. J. Clin. Oncol. 1999, 17, 3828–3834. [DOI] [PubMed] [Google Scholar]
- Duska L. R.; Penson R.; Supko J. G.; Finkelstein D. M.; Makastorsis T.; Gallagher J.; Borden K.; Goodman A.; Fuller A. F.; Nikrui N.; Seiden M. V. A Phase I Study of Continuous Infusion Doxorubicin and Paclitaxel Chemotherapy with Granulocyte Colony-Stimulating Factor for Relapsed Epithelial Ovarian Cancer. Clin. Cancer Res. 1999, 5, 1299–1305. [PubMed] [Google Scholar]
- Sledge G. W. Doxorubicin/paclitaxel combination chemotherapy for metastatic breast cancer: the Eastern Cooperative Oncology Group experience. Semin. Oncol. 1995, 22, 123–125. [PubMed] [Google Scholar]
- Grasselli G.; Viganò L.; Capri G.; Locatelli A.; Tarenzi E.; Spreafico C.; Bertuzzi A.; Giani A.; Materazzo C.; Cresta S.; Perotti A.; Valagussa P.; Gianni L. Clinical and pharmacologic study of the epirubicin and paclitaxel combination in women with meta-static breast cancer. J. Clin. Oncol. 2001, 19, 2222–2231. [DOI] [PubMed] [Google Scholar]
- Gustafson D. L.; Merz A. L.; Long M. E. Pharmacokinetics of combined doxorubicin and paclitaxel in mice. Cancer Lett. 2005, 220, 161–169. [DOI] [PubMed] [Google Scholar]
- Cho K.; Wang X.; Nie S.; Chen Z. G.; Shin D. M. Therapeutic nanoparticles for drug delivery in cancer. Clin. Cancer Res. 2008, 14, 1310–1316. [DOI] [PubMed] [Google Scholar]
- Ferrari M. Cancer nanotechnology: opportunities and challenges. Nat. Rev. Cancer 2005, 5, 161–171. [DOI] [PubMed] [Google Scholar]
- Hobbs S. K.; Monsky W. L.; Yuan F.; Roberts W. G.; Griffith L.; Torchilin V. P.; Jain R. K. Regulation of transport pathways in tumor vessels: Role of tumor type and microenvironment. Proc. Natl. Acad. Sci. U.S.A. 1998, 95, 4607–4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura Y.; Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46, 6387–6392. [PubMed] [Google Scholar]
- Tardi P.; Johnstone S.; Harasym N.; Xie S.; Harasym T.; Zisman N.; Harvie P.; Bermudes D.; Mayer L. In vivo maintenance of synergistic cytarabine:daunorubicin ratios greatly enhances therapeutic efficacy. Leuk. Res. 2009, 33, 129–139. [DOI] [PubMed] [Google Scholar]
- Lim W. S.; Tardi P. G.; Xie X.; Fan M.; Huang R.; Ciofani T.; Harasym T. O.; Mayer L. D. Schedule- and dose-dependency of CPX-351, a synergistic fixed ratio cytarabine:daunorubicin formulation, in consolidation treatment against human leukemia xenografts. Leuk. Lymphoma 2010, 51, 1536–1542. [DOI] [PubMed] [Google Scholar]
- Terwogt J. M.; Nuijen B.; Huinink W. W.; Beijnen J. H. Alternative formulations of paclitaxel. Cancer Treat. Rev. 1997, 23, 87–95. [DOI] [PubMed] [Google Scholar]
- Drummond D. C.; Noble C. O.; Hayes M. E.; Park J. W.; Kirpotin D. B. Pharmacokinetics and in vivo drug release rates in liposomal nanocarrier development. J. Pharm. Sci. 2008, 97, 4696–4740. [DOI] [PubMed] [Google Scholar]
- Sampedro F.; Partika J.; Santalo P.; Molins-Pujol A. M.; Bonal J.; Perez-Soler R. Liposomes as carriers of different new lipophilic antitumour drugs: a preliminary report. J. Microencapsul. 1994, 11, 309–318. [DOI] [PubMed] [Google Scholar]
- Sharma A.; Straubinger R. M. Novel taxol formula- tions: preparation and characterization of taxol-containing liposomes. Pharm. Res. 1994, 11, 889–896. [DOI] [PubMed] [Google Scholar]
- Bernsdorff C.; Reszka R.; Winter R. Interaction of the anticancer agent Taxol(TM) (paclitaxel) with phospholipid bilayers. J. Biomed. Mater. Res. 1999, 46, 141–149. [DOI] [PubMed] [Google Scholar]
- Campbell R. B.; Balasubramanian S. V.; Straubinger R. M. Influence of cationic lipids on the stability and membrane properties of paclitaxel-containing liposomes. J. Pharm. Sci. 2001, 90, 1095–1105. [DOI] [PubMed] [Google Scholar]
- Joo K.; Xiao L.; Liu S.; Liu Y.; Lee C.; Conti P.; Wong M.; Li Z.; Wang P. Crosslinked multilamellar liposomes for controlled delivery of anticancer drugs. Biomaterials 2013, 34, 3098–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L.; Wientjes M. G.; Au J. L.-S. Evaluation of Combination Chemotherapy: Integration of Nonlinear Regression, Curve Shift, Isobologram, and Combination Index Analyses. Clin. Cancer Res. 2004, 10, 7994–8004. [DOI] [PubMed] [Google Scholar]
- Moon J. J.; Suh H.; Bershteyn A.; Stephan M. T.; Liu H. P.; Huang B.; Sohail M.; Luo S.; Um S. H.; Khant H.; Goodwin J. T.; Ramos J.; Chiu W.; Irvine D. J. Interbilayer-crosslinked Multilamellar Vesicles as Synthetic Vaccines for Potent Humoral and Cellular Immune Responses. Nat. Mater. 2011, 10, 243–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torchilin V. P. Recent Advances with Liposomes as Pharmaceutical Carriers. Nat. Rev. Drug Discovery 2005, 4, 145–160. [DOI] [PubMed] [Google Scholar]
- Dos Santos N.; Allen C.; Doppen A. M.; Anantha M.; Cox K. A.; Gallagher R. C.; Karlsson G.; Edwards K.; Kenner G.; Samuels L.; Webb M. S.; Bally M. B. Influence of poly(ethylene glycol) grafting density and polymer length on liposomes: Relating plasma circulation lifetimes to protein binding. Biochim. Biophys. Acta 2007, 1768, 1367–1377. [DOI] [PubMed] [Google Scholar]
- Mayer L. D.; Harasym T. O.; Tardi P. G.; Harasym N. L.; Shew C. R.; Johnstone S. A.; Ramsay E. C.; Bally M. B.; Janoff A. S. Ratiometric dosing of anticancer drug combinations: controlling drug ratios after systemic administration regulates therapeutic activity in tumor-bearing mice. Mol. Cancer Ther. 2006, 5, 1854–63. [DOI] [PubMed] [Google Scholar]
- Chou T.-C.; Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv. Enzyme Regul. 1984, 22, 27–55. [DOI] [PubMed] [Google Scholar]
- Chou T.-C.; Talalay P. Analysis of combined drug effects: a new look at a very old problem. Trends Pharmacol. Sci. 1983, 4, 450–454. [Google Scholar]
- Das G. C.; Holiday D.; Gallardo R.; Haas C. Taxol-induced cell cycle arrest and apoptosis: dose–response relationship in lung cancer cells of different wild-type p53 status and under isogenic condition. Cancer Lett. 2001, 165, 147–53. [DOI] [PubMed] [Google Scholar]
- Jordan M. A.; Toso R. J.; Thrower D.; Wilson L. Mechanism of mitotic block and inhibition of cell proliferation by taxol at low concentrations. Proc. Natl. Acad. Sci. U.S.A. 1993, 90, 9552–9556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaid H. M.; Horwitz S. B. Selective potentiation of paclitaxel (taxol)-induced cell death by mitogen-activated protein kinase kinase inhibition in human cancer cell lines. Mol. Pharmacol. 2001, 60, 290–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selimovic D.; Hassan M.; Haikel Y.; Hengge U. R. Taxol-induced mitochondrial stress in melanoma cells is mediated by activation of c-Jun N-terminal kinase (JNK) and p38 pathways via uncoupling protein 2. Cell Signal. 2008, 20, 311–22. [DOI] [PubMed] [Google Scholar]
- Xu R.; Sato N.; Yanai K.; Akiyoshi T.; Nagai S.; Wada J.; Koga K.; Mibu R.; Nakamura M.; Katano M. Enhancement of paclitaxel-induced apoptosis by inhibition of mitogen-activated protein kinase pathway in colon cancer cells. Anticancer Res. 2009, 29, 261–270. [PubMed] [Google Scholar]
- Gehl J.; Boesgaard M.; Paaske T.; Jensen B. V.; Dombernowsky P. Combined doxorubicin and paclitaxel in advanced breast cancer: Effective and cardiotoxic. Ann. Oncol. 1996, 7, 687–693. [DOI] [PubMed] [Google Scholar]
- Gianni L.; Munzone E.; Capri G.; Fulfaro F.; Tarenzi E.; Villani F.; Spreafico C.; Laffranchi A.; Caraceni A.; Martini C.; Stefanelli M.; Valagussa P.; Bonadonna G. Paclitaxel by 3-h infusion in combination with bolus doxorubicin in women with untreated metastatic breast cancer: high antitumor efficacy and cardiac effects in a dose-finding and sequence-finding study. J. Clin. Oncol. 1995, 13, 2688–2699. [DOI] [PubMed] [Google Scholar]
- Valero V.; Hortobagyi G. N. Are anthracycline-taxane regimens the new standard of care in the treatment of metastatic breast cancer. J. Clin. Oncol. 2003, 21, 959–962. [DOI] [PubMed] [Google Scholar]
- Mayer L. D.; Janoff A. S. Optimizing combination chemotherapy by controlling drug ratios. Mol. Interv. 2007, 7, 216–223. [DOI] [PubMed] [Google Scholar]
- Pavillard V.; Kherfellah D.; Richard S.; Robert J.; Montaudon D. Effects of the combination of camptothecin and doxorubicin or etoposide on rat glioma cells and camptothecin-resistant variants. Br. J. Cancer 2001, 85, 1077–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaffar D. S.; Ang C. Y.; Desai P. B.; Rosenthal G. A.; Thomas D. A.; Crooks P. A.; John W. J. Combination therapy with 5-fluorouracil and L-canavanine: in vitro and in vivo studies. Anticancer Drugs 1995, 6, 586–593. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.